Abstract
Copper deficiency (CD) is a rare complication of long-term treatment of Wilson's disease (WD) and is usually accompanied by high serum zinc levels. A 57-year-old woman with WD presented with limb weakness and sensory disturbance due to myeloneuropathy and macrocytic anemia after 36 years of treatment. Markedly reduced serum free copper values confirmed CD, which was considered to be caused by progressive dysphagia and severe diarrhea rather than zinc overdose because of the normal serum zinc levels. Discontinuing copper-reducing therapy and increasing copper intake improved her symptoms. Physicians should be alert for the risk of CD in WD patients, especially those with dysphagia.
Keywords: Wilson's disease, copper deficiency, myeloneuropathy, macrocytic anemia, dysphagia
Introduction
Wilson's disease (WD) is characterized by excessive copper deposition in multiorgan systems due to its transport impairment, mainly resulting in liver and neurological dysfunction (1). Treatment options include zinc salts and copper chelators. Zinc salts inhibit copper absorption in the intestine. Copper chelators, such as D-penicillamine and trientine, promote copper excretion into the urine.
Since WD requires lifelong treatment, physicians must be alert for long-term complications. Among these, copper deficiency (CD) is a rare but severe condition that can lead to macrocytic anemia and myeloneuropathy (2-10).
We herein report a case of CD in a WD patient supposedly due to synergistic effects of the insufficient intake of copper, the inhibition of intestinal absorption by zinc salt, and the increased urinary excretion of copper by D-penicillamine.
Case Report
A 57-year-old woman presented with muscle weakness and sensory disturbance in her extremities. She had first presented to our department at 20 years old with dysarthria and progressive dystonia of her left arm that had continued for 2 years. Kayser-Fleischer corneal rings, low serum ceruloplasmin, low serum copper, increased urinary copper excretion, and compound heterozygous mutations in ATP7B [NM_000053:c.(3104 G>T);(3155C>T) predicting NP_000044: p.((G1035V));((P1052 L))] led to the diagnosis of WD, so she started taking zinc acetate (80 mg/day as elemental zinc) and D-penicillamine (1,000 mg/day).
Progressive dysphagia that appeared at 35 years old left her able to eat only a small amount of jelly by 55 years old. Undertreatment of WD was suspected, and the zinc dose was increased to 150 mg/day as elemental zinc. She underwent gastrostomy to start tube feeding at 56 years old, but severe diarrhea prevented her from receiving sufficient nutrition. Gastrointestinal overload due to post-fasting enteral nutrition and mucosal stress caused by the hyperosmotic nutrition were suspected to be the causes of her diarrhea. We first prescribed intestinal regulators and reduced the amount of intake to correct the overload on the gastrointestinal mucosa, only to find a poor response. Her daily caloric intake was approximately 500-600 kcal/day, which was administered almost exclusively by tube feeding. Her estimated daily copper intake was about 0.5-0.6 mg/day. She lost 5 kg in weight 1 year before admission. Fifteen months after the surgery, distal weakness and numbness in the four extremities appeared. We also started to prescribe albumin tannate three months before the admission, which finally improved her diarrhea. However, her neurological symptoms worsened subacutely, placing her in a bedridden state when she was admitted to our hospital.
Neurological examinations revealed dysarthria, dysphagia, and finger deformities due to long-term dystonia, as shown at the previous admission. In addition, she presented with moderate distal weakness, hypotonia, absent tendon reflexes, sensory ataxia, and severe sensory deficits in all four extremities. Dystonia, noted at previous admissions, was not apparent on admission. Blood tests revealed macrocytic anemia (hemoglobin concentration, 6.9 g/dL and mean corpuscular volume, 101.7 fL). Total serum copper and ceruloplasmin levels were 11 μg/dL and 5.5 mg/dL, respectively. The concentration of free copper was too low to be calculated. Her serum free copper level had been 6.2 μg/dL at the most recent laboratory follow-up at 55 years old, indicating that copper deficiency had progressed over the course of a year. The 24-hour urinary copper excretion was 74 μg, which was presumably appropriate for patients with WD. Her serum zinc concentration was 127 μg/dL (normal range, 80-130 μg/dL), and this value had also been within the normal limits 5 and 10 years before admission (103 μg/dL and 122 μg/dL, respectively).
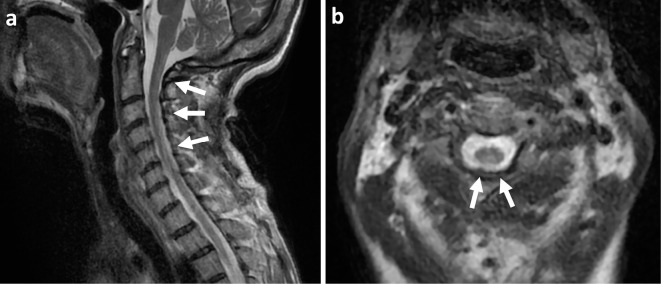
Nerve conduction studies showed severe sensorimotor axonal polyneuropathy. Somatosensory evoked potential studies demonstrated the absence of nerve root and scalp potentials in both the median and tibial nerves. Brain magnetic resonance imaging (MRI) showed no evidence of WD progression. The key findings of spinal MRI were longitudinal hyperintensity lesions in the posterior cord at the cervical level on T2-weighted imaging (Figure).
Figure.
T2-weighted magnetic resonance imaging of the cervical spinal cord. Sagittal (a) and axial (b) views show longitudinal hyperintense lesions in the dorsal column from C1 to C6 (arrows).
Her myeloneuropathy and macrocytic anemia were thus attributed to CD after excluding vitamin B6 or B12 deficiency, folate deficiency, monoclonal gammopathy, human immunodeficiency virus or human T-lymphotropic virus type 1 infection, syphilis, and collagen diseases. Whole-body computed tomography revealed no malignancy. We discontinued both zinc and D-penicillamine and increased her copper intake to 1.67 mg/day to correct the insufficient intake. The anemia stopped progressing immediately after the cessation of decoppering therapy, and mean corpuscular volume improved to 93.1 fL after 3 weeks. The concentration of hemoglobin improved to 10.8 g/dL after about 3 months. Two months later, her dysesthesia had been reduced to 40% of the value at admission, and she became able to walk and maintain a standing position using parallel bars. However, despite her good clinical response, the dorsal cord lesions on MRI and neurophysiological abnormalities remained.
Discussion
We encountered a case of CD in a WD patient with normal zinc levels. Eleven cases of CD in WD have been reported previously (2-10). Zinc was administered in all cases, and serum zinc values were high in most cases (Table).
Table.
Clinical Characteristics of CD in WD Patients.
| no. | Age (yo) | Sex | Age at Dx. (yo) | Medication | Neurological sign | Electrophysiological finding | MRI finding | Laboratory test | References | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn (mg/day) | Other (mg/day) | M | S | R | Neuropathy | Myelopathy | Dorsal cord lesion | Serum tCu (µg/dL) | Serum CPN (mg/dL) | Calculated serum fCu (µg/dL) | Urine Cu (µg/24h) | Serum Zn (µg/dL) | |||||
| Normal values | 68-128 | 21-37 | 10-15 | 4.2-33.0 | 80-130 | ||||||||||||
| 1 | 41 | M | 25 | 275 | + | + | N | N/A | + | - | Low | Low | N/A | N/A | N/A | (2) | |
| 2 | 43 | M | 15 | 170 | T; 900 | + | - | N | + | - | - | 3 | <10 N/A | 108 | 251 | (3) | |
| 3 | 51 | F | 27 | 480 | + | + | ↓ | + | + | - | 5 | N/A | <5 | 20 | N/A | (4) | |
| 4 | 37 | F | 21 | 180 | - | - | - | - | + | + | <5 | 0.92 | <2.1 | 11 | 474 | (5) | |
| 5 | 46 | F | 41 | 180 | N/A | N/A | N/A | N/A | N/A | N/A | <5 | 0.5 | <3.4 | 6 | 192 | (5) | |
| 6 | 18 | F | 12 | 180 | N/A | N/A | N/A | - | + | N/A | 7 | 0.9 | 4.2 | 12 | 247 | (5) | |
| 7 | 56 | F | 18 | 150 | - | + | ↑ | N/A | + | + | 3 | Low | N/A | Low | 179 | (6) | |
| 8 | 44 | F | 29 | 160 | - | + | ↑ | N/A | N/A | + | 3 | 8 | 0 | 7.4 | 311 | (7) | |
| 9 | 17 | F | 17 | 225 | + | + | ↑ | + | N/A | + | N/A | N/A | N/A | Low | N/A | (8) | |
| 10 | 56 | M | 25 | 480 | N/A | N/A | N/A | N/A | N/A | N/A | 5 | N/A | N/A | N/A | N/A | (9) | |
| 11 | 36 | M | 20 | 150 | DP; 600 | - | + | N | + | N/A | + | 13.3 | 3.0 | 3.9 | 40.5 | 96.1 | (10) |
| 12 | 57 | F | 20 | 150 | DP; 1000 | + | + | ↓ | + | + | + | 11 | 5.5 | 0 | 74 | 127 | *** |
No.: number, Dx: diagnosis, F: female, M: male, yo: years old, tCu: total copper, fCu: free copper, Zn: zinc, T: trientine, DP: D-penicillamine, CPN: ceruloplasmin, M: motor symptoms, S: sensory impairment, R: tendon reflex, N: normal, N/A: not available, MRI: magnetic resonance imaging
fCu(µg/dL)=tCu(mg/dL)-3.15(mg/µg)×CPN(mg/dL). We set fCu as 0 when it is calculated to be a negative value.
***Current case.
Orally administered zinc induces metallothionein in intestinal enterocytes. Metallothionein inhibits excessive copper absorption in the intestine by binding to copper in food and excreting itself into the feces (1). Inappropriate zinc dosage leads to metallothionein over-expression, which causes zinc-induced CD. Previous cases have suggested a common etiology wherein zinc over-inhibits copper absorption in these patients. However, our patient had a normal serum zinc concentration.
Most WD patients under zinc medication had serum zinc levels maintained within the range of 150-200 μg/dL (11). In our case, the serum zinc level was normal for healthy subjects (80-130 μg/dL) both on admission and 10 years earlier, but the values were below the normal range for WD patients. This implies that zinc medication was not the only cause of CD in our case and that a few other factors contributed to it.
Considering the subacute onset of her CD soon after the initiation of tube feeding and the onset of chronic diarrhea, we hypothesized that an insufficient intake of copper might be the main cause of CD. Dysphagia, a common manifestation affecting more than half of patients in the long course of WD (1), often leads to malnutrition. Our patient had been suffering from dysphagia for 22 years, and her oral intake had become scarce in the last few years. Her estimated copper intake was lower than the daily requirement of 0.7-0.8 mg/day for Japanese adults (12), even after the initiation of enteral tube feeding. Previous reports of CD without WD under zinc medication also support our hypothesis. In these reports, some patients had normal serum zinc levels. They had either diarrheal diseases, such as celiac disease (13), or a history of upper gastrointestinal surgery, such as small bowel resection (14). This implies that zinc administration can exacerbate copper malabsorption collaboratively.
Urinary excretion of copper due to D-penicillamine may also have contributed to her CD with a normal zinc level. The same dose of D-penicillamine throughout the 36-year disease course in this case suggested that it was not the primary cause of CD with a subacute onset. However, D-penicillamine increases the urinary excretion of zinc as well as copper (15), which may have reduced the serum zinc level. Because zinc preparations inhibit copper absorption in the intestinal tract, evaluating the effect of zinc preparations by serum zinc values can lead to underestimation. In our case, D-penicillamine may have worked together with increased zinc preparations to accelerate copper deficiency while suppressing the rise in serum zinc levels due to the increase in zinc dosage. Given the difficulty in assessing the combined effects of D-penicillamine and zinc salts, physicians should consider more frequent copper monitoring when both drugs are prescribed.
In conclusion, this report reveals that CD can be caused by a synergistic mechanism in WD patients, especially those with a long course. Some argue that clinicians should plan the initial and maintenance therapies separately, and that the latter should be milder in order to prevent CD. Physicians need to examine whether or not patients have any risk of CD continually, such as an insufficient copper intake and malabsorption. Given that CD can progress in a subacute manner, more frequent monitoring of serum free copper is necessary, especially in high-risk patients.
Author's disclosure of potential Conflicts of Interest (COI).
Jun Mitsui: Research funding, Nobel Pharma.
Financial Support
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant-in-Aid for Scientific Research (B) 18H02739] and a research grant from the Takeda Science Foundation to J.M.
References
- 1.Pfeiffer RF. Wilson's disease. Handb Clin Neurol 100: 681-709, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Horvath J, Beris P, Giostra E, Martin PY, Burkhard PR. Zinc-induced copper deficiency in Wilson disease. J Neurol Neurosurg Psychiatry 81: 1409-1410, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Foubert-Samier A, Kazadi A, Rouanet M, et al. Axonal sensory motor neuropathy in copper-deficient Wilson's disease. Muscle Nerve 40: 294-296, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Cortese A, Zangaglia R, Lozza A, Piccolo G, Pacchetti C. Copper deficiency in Wilson's disease: peripheral neuropathy and myelodysplastic syndrome complicating zinc treatment. Mov Disord 26: 1361-1362, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Dzieżyc K, Litwin T, Sobańska A, Członkowska A. Symptomatic copper deficiency in three Wilson's disease patients treated with zinc sulphate. Neurol Neurochir Pol 48: 214-218, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Lozano J, Muñoz Bertrán E, Ortega González I, Gómez Espín R, López Espín MI. Myelopathy secondary to copper deficiency as a complication of treatment of Wilson's disease. Gastroenterol Hepatol 35: 704-707, 2012. [DOI] [PubMed] [Google Scholar]
- 7.da Silva-Júnior FP, Machado AAC, Lucato LT, Cançado ELR, Barbosa ER. Copper deficiency myeloneuropathy in a patient with Wilson disease. Neurology 76: 1673-1675, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Narayan SK, Kaveer N. CNS demyelination due to hypocupremia in Wilson's disease from overzealous treatment. Neurol India 54: 110-111, 2006. [DOI] [PubMed] [Google Scholar]
- 9.van den Hamer CJA, Hoogeraad TU. Copper deficiency in Wilson's disease. Lancet 2: 442, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Teodoro T, Neutel D, Lobo P. Recovery after copper-deficiency myeloneuropathy in Wilson's disease. J Neurol 260: 1917-1918, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu N, Fujiwara J, Ohnishi S, et al. Effects of long-term zinc treatment in Japanese patients with Wilson disease: efficacy, stability, and copper metabolism. Transl Res 156: 350-357, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Ministry of Health, Labour and Welfare. Dietary Reference Intakes for Japanese (2015) [Internet]. 2015 [cited 2022 Apr 21]. Available from: mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Full_DRIs2015.pdf
- 13.Porter KG, Mcmaster D, Elmes ME, Love AH. Anæmia and low serum-copper during zinc therapy. Lancet 310: 774, 1977. [DOI] [PubMed] [Google Scholar]
- 14.Todd LM, Godber IM, Gunn IR. Iatrogenic copper deficiency causing anaemia and neutropenia. Ann Clin Biochem 41: 414-416, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Van Caillie-Bertrand M, Degenhart HJ, Luijendijk I, Bouquet J, Sinaasappel M. Wilson's disease: assessment of D-penicillamine treatment. Arch Dis Child 60: 652-655, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]