Abstract
The health and disease of an individual is mediated by their genetics, a lifetime of environmental exposures, and interactions between the two. Genetic or biological sex, including chromosome composition and hormone expression, may influence both the types and frequency of environmental exposures an individual experiences, as well as the biological responses an individual has to those exposures. Gender identity, which can be associated with social behaviors such as expressions of self, may also mediate the types and frequency of exposures an individual experiences. Recent advances in exposome-level analysis have progressed our understanding of how environmental factors affect health outcomes; however, the relationship between environmental exposures and sex- and gender-specific health remains underexplored. The comprehensive, non-targeted, and unbiased nature of exposomic research provides a unique opportunity to systematically evaluate how environmental exposures interact with biological sex and gender identity to influence health. In this forward-looking narrative review, we provide examples of how biological sex and gender identity influence environmental exposures, discuss how environmental factors may interact with biological processes, and highlight how an intersectional approach to exposomics can provide critical insights for sex- and gender-specific health sciences.
Keywords: gender and sexual minorities, sex-specific medicine, gender-specific medicine, intersectionality, personal care products, occupational health
Introduction
Despite the evidence suggesting that sexual dimorphism—the systematic biological differences between males and females—is a crucial factor affecting health and disease, gender bias remains prevalent in biomedical and population health research. Gender bias occurs from the level of basic scientific research up to the level of human studies due to oversights in study design, failure to consider sexual dimorphism in the analysis and interpretation of results, and androcentrism and gender insensitivity in the translation of these findings into healthcare policy.1 In animal research, there has been a common misconception that inclusion of female animals will increase data variability and necessitate larger sample sizes.2 In fact, unstaged female mice (ie, not synchronized based on stage of estrous cycle) showed generally less variability across >9900 traits than their male counterparts.2 Similar bias is seen in human research, which has historically been dominated by studies on white cis-gender men. The exclusion of non-white, non-male, and non-cis-gender research subjects in human and clinical research has at times been justified to “minimize” extraneous variables. Such naïve attempts at minimizing confounding variables have resulted in the systematic exclusion of women, non-cis-gender, and Black, Indigenous, and People of Color (BIPOC) individuals in research. Together, these biases have created a deficiency in our ability to properly manage the health of individuals of all races and ethnicities across both the gender and sex spectra.
Health and disease are the result of interactions between an individual’s genetic composition and their cumulative lifetime environmental exposures. Few diseases result exclusively from genetic variation, and men and women are largely genetically identical aside from differences due to sex chromosomes. Only 10%–30% of chronic disease risk can be explained by genetics, meaning disease risk may be largely ascribed to environmental factors.3 Thus, environmental exposures represent a major contributor to health outcomes that requires a thorough characterization to understand health and disease. Many of the environmental exposures that humans experience are products of cultural and societal norms born out of pervasive heterosexism, classism, misogyny, patriarchy, and racism, meaning that biological sex and gender identity may influence the types and patterns of environmental exposures an individual experiences. Furthermore, given that many environmental exposures are governed by these cultural and societal norms, individuals who exist at the intersection of multiple marginalized groups are often subject to unique and compounded exposure patterns.
Unlike conventional analyses where only a few exposures or markers are targeted a priori, modern exposome analysis, or exposomics, sets out to characterize all exposures in an untargeted and comprehensive manner. Advancements in high-resolution mass spectrometry (HRMS) technologies have facilitated high-throughput detection and annotation of compounds or chemical patterns from complex and dynamic exposures.4 Furthermore, exposomics is amenable to intersectionality, which can be defined by a “theoretical framework for understanding how multiple social identities such as race, gender, sexual orientation, socioeconomic status (SES), and disability intersect at the micro level of individual experience to reflect interlocking systems of privilege and oppression (ie, racism, sexism, heterosexism, and classism) at the macro social–structural level.”5 A recent commentary outlines in detail how exposomics can utilize intersectionality to understand how environmental factors influence health and disease.6 In this forward-looking narrative review, we cover the historical lack of inclusion of biological sex and gender identity as factors that mediate health outcomes, provide examples of how both biological sex and gender identity may influence the types of environmental exposures an individual experiences as well as the biological response to those exposures, and discuss how intersectionality is a necessary factor to consider in sex- and gender-specific research. Thus, we present the exposome as a tool to analyze both environmental exposures and the associated biological effects of those exposures to understand how the intersection of environmental health and biological sex and gender identity impact health.
Biological sex and gender identity
Biological sex and gender identity have been increasingly recognized as playing significant roles in human health7,8; however, sex- and gender-associated environmental risk factors still require thorough investigation. When considering how the environment may confer sex- and gender-specific health outcomes, it is first important to define sex, gender, and the relationship between the two. While chromosomal sex is determined at the point of fertilization, all humans begin as undifferentiated zygotes with identical structures and indiscriminate biological sex until they undergo sexual differentiation in utero.9 Sexual differentiation results from the expression of sex hormones such as testosterone, estrogen, and progesterone, whose expression is regulated by the presence of the “SRY” (sex-determining region Y) gene on the Y chromosome.10 In addition to in utero sexual development, adolescent developmental stages where secondary sex organs develop are a key feature of sexual dimorphism and rely on hormonal signaling.
In Western societies, both biological sex and gender identity have historically been regarded as binary phenomena with assignment of male or female occurring at birth based on sex chromosome composition and the presentation of gonads and external genitalia. Thus, an individual possessing biological characteristics including XY chromosomes, testes, etc. would be assigned male, and an individual possessing biological characteristics including XX chromosomes, ovaries, etc. would be assigned female. Individuals with an ambiguous or atypical sexual characteristic presentation typically receive the assignment intersex; however, these individuals are frequently subject to medical interventions to conform to the male–female sex binary.11,12 The male–female sex binary is further reflected in the mainstream medical and scientific literature, which results in a lack of inclusion of people across the gender and sex spectra.
While biological sex can be defined by biological characteristics, gender is a social construct, not a biological construct, and individuals may have a gender identity that does not correspond with the biological sex to which they were assigned at birth. There are myriad examples throughout history of groups across Africa, Asia, Europe, Australia, New Zealand, and indigenous groups in the Americas who recognize both biological sex and gender identities outside of the male–female binary; however, the Western world has only recently begun to accept that gender exists on a spectrum that includes identities such as transgender, agender, non-binary, and genderqueer.13 This spectrum is also inclusive of individuals who choose to undergo gender-affirming treatments such as hormone therapy or sex-affirmation surgery, which allow individuals to have biological sexual characteristics consistent with their gender identity.
Since gender identity and biological sex are not inherently linked, it is important to make a distinction between gender-specific and sex-specific in the health science literature. Historically, the term gender-specific has been used in biomedical research to refer to sex-specific effects, or the terms have been used interchangeably with the assumption that people are cis-gender. In this manuscript, we distinguish biological sex from gender identity, where biological sex refers to the composition of chromosomes, sex hormones, gonads, and genitalia an individual has, and gender identity refers to the identity an individual possesses, which may be associated with social behaviors of traditional ideas of masculinity and femininity. Thus, we also employ gender-specific to refer to health as it relates to gender identity, and sex-specific to refer to health as it relates to biological sex.
Sex- and gender-specific health
Biological sex and gender identity can mediate an individual’s health through endogenous physiological processes regulated by genetics and hormones, and exogenous processes including behaviors influenced by social factors.14 Many major chronic diseases are characterized by sex and gender disparities in terms of prevalence, diagnosis, treatment, and mortality.7 Although there is significant evidence that sex and gender are key mediators of health, it was not until 1993 that the National Institute of Health (NIH) issued guidelines to ensure inclusion of women and minorities in clinical research, and only launched an initiative to include sex as a biologic variable in preclinical research in 2014.15,16 This has subsequently been followed by a 2019 initiative to include sexual and gender minorities (SGMs) in research, including those “who identify as lesbian, gay, bisexual, asexual, transgender, Two-Spirit, queer, and/or intersex… with same-sex or -gender attractions or behaviors and those with a difference in sex development… [and] those who do not self-identify with one of these terms but whose sexual orientation, gender identity or expression, or reproductive development is characterized by non-binary constructs of sexual orientation, gender, and/or sex.”17 It is important to acknowledge that although the umbrella term SGM refers to both sexual and gender minorities, sexual orientation is distinct from biological sex and gender identity, which are the focus of this article.
Despite these initiatives, both preclinical and clinical research continue to suffer from a lack of inclusion and diversity. The exclusion of women and SGM from preclinical and clinical research studies has led to a lack of understanding of sex- and gender-specific health and disease outcomes. For example, managing health with prescription medication represents a key area of health. However, insufficient representation of people across the gender and sex spectra in clinical trials has resulted in a lack of understanding of the sex- and gender-specific effects of newly developed therapeutics.18 This lack of inclusion likely contributes to the fact that 8 out of 10 drugs removed from the US market have more adverse effects in cis-gender women.19 Biological differences including body size and body composition can influence drug dosing and distribution. Cis-gender women typically have smaller body mass than cis-gender men, but a higher body fat percentage, which may result in a higher bodily concentration and distribution of a drug taken at the same dose.20,21
Furthermore, there is significant evidence that sex hormones can influence drug pharmacokinetics and/or pharmacodynamics.18 For instance, sex hormones may compete with medications for drug targets, interact with the enzymes responsible for drug metabolism, or alter the transcription of these enzymes.22 Thus, the interaction between sex hormones and drug pharmacokinetics and/or pharmacodynamics is particularly important to consider for the health of people utilizing hormone therapy. For example, people experiencing menopause may take estrogen as hormone replacement therapy to mitigate negative symptoms.23 Additionally, some SGM individuals experiencing gender dysphoria and/or body dysmorphia as a result of the dissonance between their gender identity and biological sex may utilize hormone therapy and/or sex-affirming surgeries, which have been shown to improve health outcomes for these individuals.24-29 While it is known that sex hormones may influence drug metabolism, there is currently a lack of data investigating the effects of hormone therapy on drug effects including dosage and metabolism.22
Sex- and gender-specific exposures
Environmental exposures are diverse and complex, including both physicochemical and social–structural stressors, and are experienced continuously across locations such as our homes, schools, workplaces, and in ambient indoor and outdoor environments. Physicochemical exposures include both manmade and naturally occurring compounds in sources such as personal care products (PCPs), food and food packaging, household cleaners, and environmental pollutants. Social–structural stressors include social, political, and economic factors that facilitate and constrain health and involve structural forces such as systemic racism, sexism, and heterosexism; state and federal policies; and residential segregation.30 Exposure patterns may vary significantly depending on biological sex and gender identity, and exposures may exert sex-specific effects by interacting with biological factors such as sex hormones.
The types of exposures an individual experiences can be influenced by biological sex-based needs, cultural norms, societal norms, and pressure to conform to accepted gender roles, which are shaped by patriarchal structures. Thus, the ability to define and characterize one’s exposome based on gender identity and biological sex would provide critical insight into factors influencing an individual’s health. For example, the history of occupations and household responsibilities being segregated due to gender identity and/or racialized identities represents a potential source of difference of environmental exposures.31 Furthermore, biological sex can influence use of PCPs, such as menstrual and intimate care products (MICPs) and hormonal contraceptives for people with uteruses. Textbox 1 presents examples to demonstrate how PCPs and occupational exposure may mediate sex- and gender-specific health.
Textbox 1. Sex- and gender-specific PCP and occupational exposures.
Personal care products: MICPs, including tampons, panty liners, sanitary napkins/pads, sprays, douches, anti-itch creams, wipes, and powders, are an example of a sex-specific exposure experienced by menstruating people. The mucosal membranes of the vagina and vulva are more permeable than many dermal surfaces, and the vaginal walls contain ample vasculature, allowing for the rapid absorption of chemicals into the system.109 This has major implications for toxicant exposure around these sensitive tissues, with suspected enhanced absorption of these compounds. As such, greater oversight should be placed on the manufacturing of these products to ensure the safety of their users. However, the FDA considers MICPs, such as tampons and pads, medical devices requiring much less regulation in their composition than food, drugs, or cosmetics.110
Multiple studies have revealed hazardous chemical components in MICPs that have been linked with health effects and disease outcomes, including a variety of EDCs, which can disrupt hormonal signaling, such as phthalates, bisphenols, parabens, dioxins, and furans.111-113 Tampons, which use cotton, confer a risk of pesticide exposure due to pesticides being sprayed directly on the cotton crops, and a risk of metal exposure due to the bioaccumulation of metals in cotton plants.114 Further, dioxins and furans, chemicals used in the cotton bleaching process, have been reported in tampons and sanitary napkins sold in the United States.115,116 A complete list of chemicals of concern in MICPs and their associated health effects has been curated and summarized.117
Evaluating MICP and occupational exposures through an intersectional approach reveals racial/ethnic disparities. The most commonly used MICP products include tampons, panty liners, and pads, with up to 85%, 75%, and 73% of US women reporting usage, respectively. Race and ethnicity can have a large impact on usage of these products, with Black and Latina women reporting increased use of douches, wipes, sprays, and powders.118 Historically, there have been racist olfactory stereotypes utilized to dehumanize and oppress people of color, which can increase pressure to use MICP products such as vaginal deodorants and douches in effort to challenge these stereotypes and gain equality.119 Capitalizing on these stereotypes, people of color have been disproportionally targeted by the marketing of these products.119 Furthermore, use of these products has been associated with lower education levels and SES.120
Occupational exposures: Domestic work is an example of a traditionally gendered responsibility, and occupation, dominated by female individuals. According to the US Bureau of Labor Statistics 2021 data, 88.7% of maids/housekeeping cleaners were women, almost half of which were Hispanic or Latino.121 Although cleaning practices promote overall health and hygiene, they can expose individuals heavily utilizing these cleaners to a variety of chemical hazards. The most common routes of chemical exposure for household cleaners are dermal, specifically the hands, and respiratory, through the use of aerosols and sprays.122 In line with these exposure routes, a meta-analysis of the literature surrounding occupational exposures in household cleaners and janitorial staff reported respiratory diseases and dermatologic diseases as the most common ailments suffered by this population.123
Some of the compounds found in household cleaners include volatile organic compounds (VOCs), which are chemicals that are emitted as gas at ambient temperatures such as limonene, toluene, benzene, chloroform, ethylbenzene, trichloroethene, and carbon tetrachloride.124,125 Passive samplers to characterize VOC exposures for hotel housekeepers discovered twice the total target VOC concentration levels compared with indoor air samplers.126 Occupational exposure to VOCs and chemical dust has been associated with female infertility, potentially due to the endocrine disrupting properties of some of these chemicals.127 Furthermore, disorders and diseases of the lung, including poor asthma control, chronic obstructive pulmonary disease and lung cancer, have been associated with occupational exposure to cleaners.128-131 Reduced lung function due to occupational and household cleaning exposure has been found in women, but not men, most likely due to the disproportionate number of women in this study who reported cleaning.132 The health risks associated with occupational exposure to cleaners go beyond those directly exposed, with one study showing the children of exposed mothers to be at increased risk of developing pulmonary disorders, including asthma, allergies, and wheezing.133
Gender identity can likewise influence use of PCPs including cosmetics and other beauty products, which is in part due to the marketing of products targeted to women, female-identifying, and feminine-presenting consumers, who are the main targets of unrealistic beauty standards. Studies have shown that women use on average twice as many PCPs each day compared with men, which results in higher chemical exposures to carcinogens, nanoparticles, metals, and endocrine disrupting chemicals (EDCs).32-40 EDCs, which are exogenous compounds whose structures mimic or resemble those of endogenously occurring sex hormones, can interfere with the hormone actions while increasing health risks.41 EDCs are prevalent in PCPs, but are also found in manufacturing waste, food and food packaging, flame retardants, and plastics.42 As exposure to EDCs is ubiquitous throughout our environments, they represent an universal exposure; however, depending on which hormone EDCs resemble or interact with, they will have different molecular effects, and thus may exhibit sex-specific health effects.
There is ample evidence to suggest that women of color, including Black, Latinx, and Asian women, are exposed to greater cumulative chemical exposures than white women, with studies identifying higher levels of some EDCs in the circulation of women of color compared with white women.43 These differences in exposure may, in part, be driven by the environmental injustice of beauty.44 BIPOC individuals experience a greater pressure to conform to Eurocentric beauty standards including light skin, straight hair, and clean and fresh scent which may influence PCP use. In fact, a recent community-based research study by members of our study team demonstrated that racialized beauty norms can impact personal decisions to use chemical straighteners and skin lighteners among women and femme-identifying individuals in Northern Manhattan and the South Bronx.45 A related study from 2022 provided the first epidemiologic evidence of an association between the use of chemical hair straighteners and the development of uterine cancer, amplifying the need for education initiatives and public policy reform for the regulation of these products.46 More comprehensive reviews on the environmental injustice of beauty can be seen in these reports.47,48
The exposome
As the environment itself and the environment to which an individual is exposed are in a constant state of flux, measuring the environment itself provides critical but limited insight into cumulative exposures or exposures during key developmental periods. People are exposed to a mixture of environmental factors every day, some of which may interact directly and transform into a new exposure, or some of which may have additive or incremental effects in the body. Exposures are also cumulative both in the sense that individuals are exposed to multiple compounds in their lives, and that individuals are exposed to the same compounds repeatedly as they age. Exposome-wide characterization, which features the use of HRMS-based assays, can profile a variety of biospecimens in an untargeted and unbiased way to simultaneously identify both exogenous factors and the endogenous responses to those exposures.49 By encompassing both the entirety of external factors as well as internal processes, the exposome provides the most relevant insight into the functional consequences of exposures on health.4
While exposomics analysis has not yet been widely applied to investigate biological sex and gender identity in health, studies have been performed to interrogate differences in the metabolomic profiles of men and women (as defined by biological sex) in healthy and disease states.50-57 One type of exposome measure uses HRMS to probe the metabolome, which focuses on the holistic profile of small molecules and metabolites found in a biosample including both endogenously produced compounds and exogenously experienced compounds.58 The findings of these studies have revealed baseline differences based on biological sex such as creatinine content, steroid hormones, and branched chain amino acids.50,51 Functional pathway analysis, such as over-representation analysis, can be applied to the metabolomic findings, which revealed in one study differences in redox homeostasis as well as steroid and purine nucleotide metabolism.53
Sex hormones, menstruation, menopause, and the use of hormonal contraceptives are important to consider in metabolomics research. The phase of the menstrual cycle a participant is in when samples are taken can influence metabolomic profiles. For instance, one study identified 67 differences in metabolites during the luteal phase including amino acids, lipids, carbohydrates, and metabolites associated with energy and vitamin metabolism.52 Furthermore, a study analyzing plasma samples from patients with metabolic syndrome identified 97 differences in the metabolomic profiles of women who were and were not using hormonal contraceptives including differences in amino acids, free and acyl-carnitine, and non-esterified fatty acids.55 In addition to differences in metabolomic profiles based on biological sex, gender identity may also impact metabolomic and exposomic profiles due to an individual’s social behaviors, which can affect the types and frequency of environmental exposures an individual experiences.
Exposomics can help disentangle whether the differences in the effects of environmental exposures are due to differences in the patterns and frequencies of exposures themselves, or differences due to sex-specific biological responses to those exposures. For example, although it has been widely believed that individuals living in the same household are “environmentally matched,” this is not necessarily the case. Some initiatives have begun to examine this in more detail, using male–female partners living in the same households, to determine the effect of sex on one’s environmental exposures. Using an exposome experimental framework, Chung et al.59 assessed levels of 128 EDCs in the blood sera and urine of 501 heterosexual couples via HRMS. Strikingly, they found eight EDCs correlated with sex, including five per- and polyfluoroalkyl substances (PFASs), cotinine, blood lead, and mercury.59 Additionally, shared household explained less than 20% of the total variance of 11 EDC classes, with PFASs and blood metals being the only two classes of EDCs where the total variance was explained largely by shared living conditions, 43% and 41%, respectively.59 These findings further highlight the variability in exposure between pair-members suggesting that sex-specific practices play an important role in an individuals’ exposure profile. This study provides an important proof-of-concept showing not only that sex-specific environmental exposures exist but can be sensitively distinguished between members of the same household using currently available technological and methodological platforms.
Incorporating sex and gender in exposome research
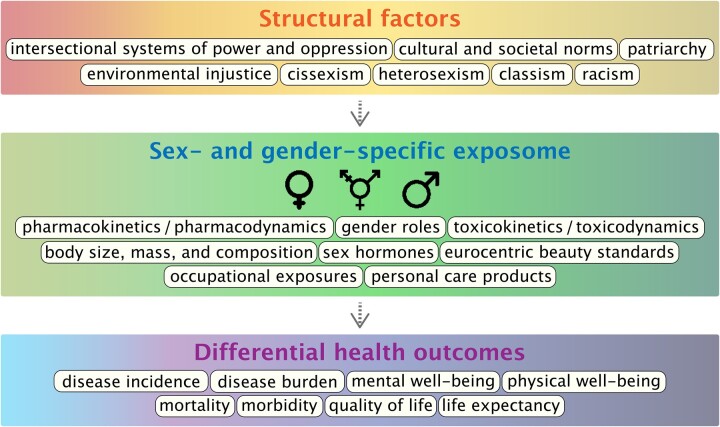
As illustrated in Figure 1, upstream structural factors including intersectional systems of power and oppression and cultural and societal norms create systems of injustice including cissexism, heterosexism, classism, and racism.6 These upstream factors dictate accepted gender roles and beauty standards, which can in turn mediate sex- and gender-specific exposome by influencing PCP use and occupational exposures. An individual’s biological responses to these exposures are mediated by several factors including sex hormone expression, body composition, and overall pharmaco- and toxico-dynamics and kinetics. The interaction of exposures with biological factors results in differential health outcomes for individuals across the gender- and sex-spectra.
Figure 1.
Overarching structural factors create systems that mediate sex- and gender-specific exposome. Exposures interact with biological processes in a sex- and gender-dependent manner to influence health outcomes.
The inclusion of diverse and inclusive populations of research participants is of utmost importance to better understand sex- and gender-based health outcomes. Not only will this ensure data regarding health and disease outcomes are collected on these individuals, but it will also provide insight into how to adequately address specific issues experienced by these subgroups. One obstacle health researchers must overcome is the historical exploitation and mistreatment of BIPOC communities in health research, which has sown a justified mistrust of health researchers.60 One way to address this mistrust is through engagement of local communities in the research process. Community-based participatory research is a critical research framework, which can be used to better understand multiple forms of intersectional discrimination experienced by marginalized communities including specific exposure pathways that drive environmental health burdens, as well as systemic solutions for environmental health equity.61 Several community-based research collaboratives, such as the Taking Stock Study and the Beauty Inside Out Campaign, are working to reduce risks from unregulated chemicals in consumer products among Black, Latinx, and Asian women and femme-identifying individuals.62,63
Once study participants are recruited, however, it is vital that questionnaires do not inadvertently exclude individuals based on survey response options that are limited to cis-gender identities within the male–female binary.64 One method to ensure inclusivity is the “two-step method,” which asks study participants to identify their current gender identity as well as separately identifying the sex to which they were assigned at birth.65,66 Surveys can include options for identity including non-binary, genderqueer, agender, gender non-conforming, transgender, and two-spirit. The inclusion of culturally specific identities is imperative and can be accomplished with phrasing such as the example suggested by Bauer et al.67 as “Indigenous or other cultural gender minority (eg, two-spirit).” Expansions on the “two-step method” have resulted in a multidimensional sex/gender measure, which can include subsequent questions for study participants with non-cis-gender identities to create a more complete picture of the individual.67 These expanded questionnaires may include questions regarding the use of hormone therapy, which is not only relevant to non-cis-gender individuals, but also important in the context of biological sex- and gender-specific health as a consideration for post-menopausal individuals. As a result, several documents with recommendations for inclusive language have been created as resources to researchers.63,67-69
There are several examples of researchers centering and prioritizing marginalized groups in environmental health research at both the preclinical and clinical levels. For instance, there have been significant efforts to be inclusive of SGMs in toxicology research and medicine, with researchers further outlining how animal models can be used to address the health of individuals participating in gender affirming hormone therapies.70,71 Additionally, in the realm of environmental justice, focus is being dedicated to the disparity in environmental exposures for marginalized populations such as LGBTQIA+ individuals.72 Concepts such as the public health exposome can guide these efforts to translate the findings from exposomic research into tangible changes.73 Once associations between exposures and effects are elucidated, interventions at the individual, local, national, and/or global level can be implemented to decrease disease risk and improve health. For example, as a result of extensive research demonstrating how exposure to mercury in beauty products such as skin lighteners negatively impacts health, New York became the third state to ban the sale of mercury-containing beauty products in late 2022.74
Additional efforts should be made to understand the specific environmental justice concerns of different marginalized communities through a sex and gender exposome lens. For example, rural and indigenous communities are disproportionately impacted by mining and other extractive industries, with severe consequences on local livelihoods, community cohesion, and the environment.75 When extractive industries intrude on indigenous populations, men in the community may be provided compensation or jobs; however, women bare a larger burden of responsibility for subsistence farming, which can be affected due to land displacement and pollution.76 Furthermore, colonization results in increased violence against indigenous women and girls, resulting in forced sterilization and widespread instances of missing and murdered women and girls.77 More information regarding issues surrounding indigenous feminism and environmental justice can be found in the special issue titled “Women’s Everyday Resistance to the Extractive Industry” of Human Geography (Volume 13, Issue 1).78
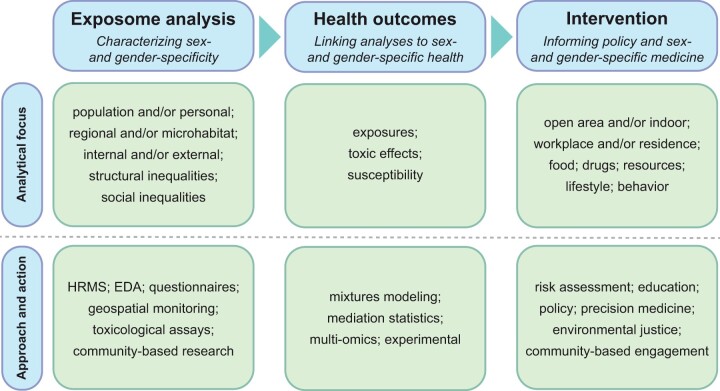
New agendas and frameworks, as summarized in Figure 2, can facilitate the systematic conduct of research revolving around exposome characterization, health outcomes, and interventions. For instance, new infrastructure needs to be constructed, which spans digital databases (eg, environmental data, chemicals, and toxicokinetics), biobanking (eg, BioBank procedures, silicone passive samplers, analytical platforms [eg, HRMS]), and computing power (eg, cloud computing services).79-83 The exposome first needs to be characterized on multiple levels depending on the research questions asked, including population and/or personal and regional and/or microhabitat. Both internal and external compounds can be profiled through untargeted HRMS platforms, which can be further coupled to effect-directed analysis (EDA), and geospatial monitoring as well as traditional methods of questionnaires, surveys, and toxicological assays.84,85 The exposomics data obtained through these novel platforms will provide solid data that supplement the data obtained through questionnaires, surveys, and geospatial monitoring. Once exposome profiles are obtained, research should be focused on how the differences in exposome profiles identified lead to altered health outcomes. Data science approaches, such as mixtures modeling and mediation statistics, may aid in the association of markers of exposures and genetic susceptibility with health outcomes or disease phenotypes.86,87 The exposome data may also be integrated with data from other -omics such as genomics and proteomics to examine relevant gene-by-environment interactions and outstanding mediating factors such as gut microbiota.88 Basic scientists can follow up on the findings of exposomic-level studies to perform in vitro and in vivo studies to validate the findings and move from association toward causation.89
Figure 2.
Toward sex- and gender-specific exposome: (1) characterizing sex- and gender-specificity in exposomics, (2) determining how sex- and gender-specific exposome are linked to health outcomes, and (3) utilizing the findings from exposomics to inform policy and enable sex- and gender-specific medicine and improved health sciences.
Previous efforts, such as the NIH-funded Human Genome Project, have dedicated enormous amounts of funding and research effort to understanding the human genome at a fundamental level, and how it relates to health and disease. With successful completion of the Human Genome Project in 2003 and upgraded genomic sequencing capabilities, subfields of genetics—including molecular genetics, epigenetics, and population genetics—have been fast expanding to further advance our understanding of sex-selective toxicity and disease susceptibility while laying critical foundations for incorporating biological sex as a key variable in exposome analysis.90-92 The technique of genome-wide association studies (GWASs) to identify gene variants associated with health and disease can likewise be applied to exposomics in exposome-wide association studies (XWASs), which can identify markers of environmental exposures that are associated with health and disease. While a parallel project for the exposome might initially seem intractable, the NIH has recently launched a new initiative titled “All of Us,” which could provide an excellent resource of data from participants located across the United States from which to build a more complete understanding of the exposome. Globally, there are initiatives and projects to characterize the exposome and establish databases93 including ATHLETE,94 EPHOR,95 Equal-life,96 EXIMIOUS,97 HEDIMED,98 longITools,99 REMEDIA,100 EXPOsOMICS,101 HBM4EU,102 HEALS,103 the Human Exposome Project,104 the European Human Exposome Network,105 the “HERCULES” center,105,106 and the “EXPANSE” project.107
Exposome research, with new designs, infrastructures, and approaches, provides a framework by which sex- and gender-specific environmental factors can be evaluated for their impacts on health. An intersectional approach with a concerted effort to not only include but center women, SGM, and BIPOC individuals in health research will help to remedy our current dearth of understanding regarding sex- and gender-specific health outcomes. The findings of this research can be translated into educational efforts on regional and local levels, among stakeholders, scientists, and the public, to increase awareness of the role of the environment in sex- and gender-specific health.108 This can in turn inform policymaking regarding the regulation of environmental factors and exposures. Ultimately, this research will help to manage individual health risk assessment and precision medicine, where individual behaviors may be geared to improve health.
Contributor Information
Meghan L Bucher, Department of Environmental Health Sciences, Mailman School of Public Health at Columbia University, New York, NY, USA.
Faith L Anderson, Department of Environmental Health Sciences, Mailman School of Public Health at Columbia University, New York, NY, USA.
Yunjia Lai, Department of Environmental Health Sciences, Mailman School of Public Health at Columbia University, New York, NY, USA.
Jocelyn Dicent, Department of Environmental Health Sciences, Mailman School of Public Health at Columbia University, New York, NY, USA.
Gary W Miller, Department of Environmental Health Sciences, Mailman School of Public Health at Columbia University, New York, NY, USA.
Ami R Zota, Department of Environmental Health Sciences, Mailman School of Public Health at Columbia University, New York, NY, USA.
Author contributions
Meghan L. Bucher (Conceptualization [equal], Funding acquisition [supporting], Visualization [equal], Writing—original draft [lead], Writing—review & editing [lead]), Faith L. Anderson (Conceptualization [equal], Funding acquisition [supporting], Writing—original draft [equal], Writing—review & editing [supporting]), Yunjia Lai (Conceptualization [equal], Visualization [supporting], Writing—original draft [equal], Writing—review & editing [supporting]), Jocelyn Dicent (Conceptualization [supporting], Funding acquisition [supporting], Visualization [supporting], Writing—original draft [supporting], Writing—review & editing [supporting]); Gary W. Miller (Conceptualization [equal], Funding acquisition [lead], Supervision [equal], Writing—review & editing [equal]), and Ami R. Zota (Conceptualization [equal], Funding acquisition [lead], Supervision [lead], Writing—original draft [equal], Writing—review & editing [equal])
Data availability
No new data were generated or analyzed in support of this research.
Funding
This work was funded by the National Institutes of Health P30ES009089. M.L.B., F.L.A., and J.D. received support from the National Institute of Environmental Health Sciences T32 ES007322. G.W.M. was supported by the National Institutes of Health grants RF1AG066107, R01AG067501, U2CES030163, R01ES023839, and UL1TR00187. A.R.Z is supported by grants from the National Institutes of Health (R01ES031065, R21HD096248, R24ES029489), and the California Breast Cancer Research Program (23UB6511, B28TP5728).
Conflict of interest statement
Dr. G.W.M. receives royalties from his books “The Exposome: A Primer” and “The Exposome: A New Paradigm for the Environment and Health.” He also receives an annual stipend for his service as Editor-in-Chief of Exposome.
References
- 1. Ruiz-Cantero MT, Vives-Cases C, Artazcoz L, et al. A framework to analyse gender bias in epidemiological research. J Epidemiol Community Health. 2007;61(Supplement 2):ii46–ii53. 10.1136/jech.2007.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beery AK. Inclusion of females does not increase variability in rodent research studies. Curr Opin Behav Sci. 2018;23:143–149. 10.1016/j.cobeha.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wheelock CE, Rappaport SM.. The role of gene–environment interactions in lung disease: the urgent need for the exposome. Eur Respir J. 2020;55(2):1902064. 10.1183/13993003.02064-2019. [DOI] [PubMed] [Google Scholar]
- 4. Vermeulen R, Schymanski EL, Barabási A-L, et al. The exposome and health: where chemistry meets biology. Science. 2020;367(6476):392–396. 10.1126/science.aay3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowleg L. The problem with the phrase women and minorities: intersectionality—an important theoretical framework for public health. Am J Public Health. 2012;102(7):1267–1273. 10.2105/AJPH.2012.300750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zota AR, VanNoy BN.. Integrating intersectionality into the exposome paradigm: a novel approach to racial inequities in uterine fibroids. Am J Public Health. 2021;111(1):104–109. 10.2105/AJPH.2020.305979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–582. 10.1016/S0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nielsen MW, Stefanick ML, Peragine D, et al. Gender-related variables for health research. Biol Sex Differ. 2021;12(1):23. 10.1186/s13293-021-00366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rey R, Josso N, Racine C. Sexual Differentiation. In: Feingold KR, Anawalt B, Blackman MR, (eds). Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2020.
- 10. Berta P, Hawkins JR, Sinclair AH, et al. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348(6300):448–450. 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- 11. Dickens BM. Management of intersex newborns: legal and ethical developments. Int J Gynaecol Obstet. 2018;143(2):255–259. 10.1002/ijgo.12573. [DOI] [PubMed] [Google Scholar]
- 12. Warne GL, Raza J.. Disorders of sex development (DSDs), their presentation and management in different cultures. Rev Endocr Metab Disord. 2008;9(3):227–236. 10.1007/s11154-008-9084-2. [DOI] [PubMed] [Google Scholar]
- 13. Dess NK, Marecek J, Bell L.C.. Gender, Sex, and Sexualities: psychological Perspectives. Oxford University Press; 2018. [Google Scholar]
- 14.WHO Team. Gender and Health; © Copyright World Health Organization (WHO), 2021. https://www.who.int/news-room/questions-and-answers/item/gender-and-health.
- 15. NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research; 2022. https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm.
- 16. Clayton JA, Collins FS.. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sexual & Gender Minority Research Office. Sexual and Gender Minority Populations in NIH-Supported Research. 2019. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-19-139.html.
- 18. Liu KA, Mager NA.. Women’s involvement in clinical trials: historical perspective and future implications. Pharm Pract (Granada). 2016;14(1):708. 10.18549/PharmPract.2016.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gahart MTR, Emily J, Bradley L, Heinrich J.. Drug Safety: Most Drugs Withdrawn in Recent Years Had Greater Health Risks for Women; U.S. Government Accountability Office, 2001. https://www.gao.gov/products/gao-01-286r.
- 20. Parekh A, Fadiran EO, Uhl K, et al. Adverse effects in women: implications for drug development and regulatory policies. Expert Rev Clin Pharmacol. 2011;4(4):453–466. 10.1586/ecp.11.29. [DOI] [PubMed] [Google Scholar]
- 21. Pinn VW. Sex and gender factors in medical studies: implications for health and clinical practice. JAMA 2003;289(4):397–400. 10.1001/jama.289.4.397. [DOI] [PubMed] [Google Scholar]
- 22. Moyer AM, Matey ET, Miller VM, et al. Individualized medicine: sex, hormones, genetics, and adverse drug reactions. Pharmacol Res Perspect. 2019;7(6):e00541. 10.1002/prp2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin KAB, Robert L.. Treatment of Menopausal Symptoms with Hormone Therapy. 2022. https://www.uptodate.com/contents/treatment-of-menopausal-symptoms-with-hormone-therapy.
- 24. Jones BA, Haycraft E, Murjan S, et al. Body dissatisfaction and disordered eating in trans people: a systematic review of the literature. Int Rev Psychiatry. 2016;28(1):81–94. 10.3109/09540261.2015.1089217. [DOI] [PubMed] [Google Scholar]
- 25. Zamantakis A, Lackey D.. Dying to be (a)gendered: an exploratory content analysis of trans/nonbinary people’s experiences with eating disorders. Sociol Inq. 2022;92(S1):870–893. 10.1111/soin.12425. [DOI] [Google Scholar]
- 26. Nisly NL, Imborek KL, Miller ML, Kaliszewski SD, Williams RM, Krasowski MD.. Unique primary care needs of transgender and gender non-binary people. Clin Obstet Gynecol. 2018;61(4):674–686. 10.1097/GRF.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 27. Radix A. Hormone therapy for transgender adults. Urol Clin North Am. 2019;46(4):467–473. 10.1016/j.ucl.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 28. Nguyen HB, Chavez AM, Lipner E, et al. Gender-affirming hormone use in transgender individuals: impact on behavioral health and cognition. Curr Psychiatry Rep. 2018;20(12):110. 10.1007/s11920-018-0973-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heylens G, Verroken C, De Cock S, et al. Effects of different steps in gender reassignment therapy on psychopathology: a prospective study of persons with a gender identity disorder. J Sex Med. 2014;11(1):119–126. 10.1111/jsm.12363. [DOI] [PubMed] [Google Scholar]
- 30. Blankenship KM, Bray SJ, Merson MH, et al. Structural interventions in public health. Aids. 2000;14(Suppl 1):S11–21. 10.1097/00002030-200006001-00003. [DOI] [PubMed] [Google Scholar]
- 31. Alonso-Villar O, del Río C.. The occupational segregation of African American women: its evolution from 1940 to 2010. Fem Econ. 2017;23:108–134. 10.1080/13545701.2016.1143959. [DOI] [Google Scholar]
- 32. Malinauskiene L, Blaziene A, Chomiciene A, et al. Formaldehyde may be found in cosmetic products even when unlabelled. Open Med (Wars). 2015;10(1):323–328. 10.1515/med-2015-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wong KH, Durrani TS.. Exposures to endocrine disrupting chemicals in consumer products—a guide for pediatricians. Curr Probl Pediatr Adolesc Health Care. 2017;47(5):107–118. 10.1016/j.cppeds.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 34. Fytianos G, Rahdar A, Kyzas GZ, et al. Nanomaterials in cosmetics: recent updates. Nanomaterials (Basel). 2020;10(5):979. 10.3390/nano10050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borowska S, Brzoska MM.. Metals in cosmetics: implications for human health. J Appl Toxicol. 2015;35(6):551–572. 10.1002/jat.3129. [DOI] [PubMed] [Google Scholar]
- 36. Robinson VC, Bergfeld WF, Belsito DV, et al. Final report of the amended safety assessment of sodium laureth sulfate and related salts of sulfated ethoxylated alcohols. Int J Toxicol. 2010;29(Suppl 4):151S–161S. 10.1177/1091581810373151. [DOI] [PubMed] [Google Scholar]
- 37. Koo HJ, Lee BM.. Estimated exposure to phthalates in cosmetics and risk assessment. J Toxicol Environ Health A. 2004;67(23–24):1901–1914. 10.1080/15287390490513300. [DOI] [PubMed] [Google Scholar]
- 38. Mendelsohn E, Hagopian A, Hoffman K, et al. Nail polish as a source of exposure to triphenyl phosphate. Environ Int. 2016;86:45–51. 10.1016/j.envint.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim MK, Kim KB, Yoon S, et al. Risk assessment of unintentional phthalates contaminants in cosmetics. Regul Toxicol Pharmacol. 2020;115:104687. 10.1016/j.yrtph.2020.104687. [DOI] [PubMed] [Google Scholar]
- 40. Voller LM, Persson L, Bruze M, et al. Formaldehyde in “nontoxic” nail polish. Dermatitis. 2019;30(4):259–263. 10.1097/DER.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 41. La Merrill MA, Vandenberg LN, Smith MT, et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol. 2020;16(1):45–57. 10.1038/s41574-019-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kumar M, Sarma DK, Shubham S, et al. Environmental endocrine-disrupting chemical exposure: role in non-communicable diseases. Front Public Health. 2020;8:553850. 10.3389/fpubh.2020.553850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. James-Todd TM, Chiu Y-H, Zota AR, et al. Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep. 2016;3(2):161–180. 10.1007/s40471-016-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zota AR, Shamasunder B.. Environmental health equity: moving toward a solution-oriented research agenda. J Expo Sci Environ Epidemiol. 2021;31(3):399–400. 10.1038/s41370-021-00333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Edwards L, Ahmed L, Martinez L, et al. Beauty inside out: examining beauty product use among diverse women and femme-identifying individuals in Northern Manhattan and South Bronx through an environmental justice framework. Environ Justice. 2023;00(00):1–12. 10.1089/env.2022.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chang CJ, et al. Use of straighteners and other hair products and incident uterine cancer. J Natl Cancer Inst. 2022;114(12):1636–1645. 10.1093/jnci/djac165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McDonald JA, Llanos AAM, Morton T, et al. The environmental injustice of beauty products: toward clean and equitable beauty. Am J Public Health. 2022;112(1):50–53. 10.2105/AJPH.2021.306606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zota AR, Shamasunder B.. The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern. Am J Obstet Gynecol. 2017;217:418 e411–418 e416. 10.1016/j.ajog.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. David A, Chaker J, Price EJ, et al. Towards a comprehensive characterisation of the human internal chemical exposome: challenges and perspectives. Environ Int. 2021;156:106630. 10.1016/j.envint.2021.106630. [DOI] [PubMed] [Google Scholar]
- 50. Krumsiek J, Mittelstrass K, Do KT, et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics. 2015;11(6):1815–1833. 10.1007/s11306-015-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Costanzo M, Caterino M, Sotgiu G, et al. Sex differences in the human metabolome. Biol Sex Differ. 2022;13(1):30. 10.1186/s13293-022-00440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brennan L, Gibbons H.. Sex matters: a focus on the impact of biological sex on metabolomic profiles and dietary interventions. Proc Nutr Soc. 2020;79(2):205–209. 10.1017/S002966511900106X. [DOI] [PubMed] [Google Scholar]
- 53. Saito K, Maekawa K, Kinchen JM, et al. Gender- and age-associated differences in serum metabolite profiles among Japanese populations. Biol Pharm Bull. 2016;39(7):1179–1186. 10.1248/bpb.b16-00226. [DOI] [PubMed] [Google Scholar]
- 54. Zarzar TG, et al. Sex differences in the metabolome of Alzheimer’s disease progression. Front Radiol. 2022;2:1–14. 10.3389/fradi.2022.782864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rauschert S, Uhl O, Koletzko B, et al. Sex differences in the association of phospholipids with components of the metabolic syndrome in young adults. Biol Sex Differ. 2017;8:10. 10.1186/s13293-017-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Escarcega RD, Honarpisheh P, Colpo GD, et al. Sex differences in global metabolomic profiles of COVID-19 patients. Cell Death Dis. 2022;13(5):461. 10.1038/s41419-022-04861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chary S, Amrein K, Lasky-Su JA, et al. Metabolomic differences between critically Ill women and men. Sci Rep. 2021;11(1):3951. 10.1038/s41598-021-83602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wishart DS, Feunang YD, Marcu A, et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018;46(D1):D608–D617. 10.1093/nar/gkx1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chung MK, Kannan K, Louis GM, et al. Toward capturing the exposome: exposure biomarker variability and coexposure patterns in the shared environment. Environ Sci Technol. 2018;52(15):8801–8810. 10.1021/acs.est.8b01467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scharff DP, Mathews KJ, Jackson P, et al. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21(3):879–897. 10.1353/hpu.0.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Van Horne YO, Alcala CS, Peltier RE, et al. An applied environmental justice framework for exposure science. J Expo Sci Environ Epidemiol. 2023;33(1):1. 10.1038/s41370-022-00422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dodson RE, Cardona B, Zota AR, et al. Personal care product use among diverse women in California: taking stock study. J Expo Sci Environ Epidemiol. 2021;31(3):487–502. 10.1038/s41370-021-00327-3. [DOI] [PubMed] [Google Scholar]
- 63. Office of Research Ethics. Participant inclusivity in research: gender, sex and sexual orientation. The University of British Columbia. 2022.
- 64. Cortina CS. Inclusion and reporting of transgender and nonbinary persons in clinical trials and tumor registries—the time is now. JAMA Oncol. 2022;8(8):1097–1098. 10.1001/jamaoncol.2022.1638. [DOI] [PubMed] [Google Scholar]
- 65. Meerwijk EL, Sevelius JM.. Transgender population size in the United States: a meta-regression of population-based probability samples. Am J Public Health. 2017;107(2):e1–e8. 10.2105/AJPH.2016.303578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tate CC, Ledbetter JN, Youssef CP, et al. A two-question method for assessing gender categories in the social and medical sciences. J Sex Res. 2013;50(8):767–776. 10.1080/00224499.2012.690110. [DOI] [PubMed] [Google Scholar]
- 67. Bauer GR, Braimoh J, Scheim AI, et al. Transgender-inclusive measures of sex/gender for population surveys: mixed-methods evaluation and recommendations. PLoS ONE. 2017;12(5):e0178043. 10.1371/journal.pone.0178043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. California State University San Marcos. Inclusive Language Guidelines: Gender Identity; 2023. https://www.csusm.edu/ipa/surveys/inclusive-language-guidelines.html.
- 69. Malatino H, Stoltzfus-Brown L.. Best Practices for Gender Inclusion in Research . Pennsylvania State University; 2020. [Google Scholar]
- 70. King DE. The inclusion of sex and gender beyond the binary in toxicology. Front Toxicol. 2022;4:929219. 10.3389/ftox.2022.929219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Aghi K, et al. Centering the needs of transgender, nonbinary, and gender-diverse populations in neuroendocrine models of gender-affirming hormone therapy. Biol Psychiatry Cogn Neurosci Neuroimaging.2022;7(12):1268–1279. 10.1016/j.bpsc.2022.07.002. [DOI] [PMC free article] [PubMed]
- 72. Goldsmith L, Bell ML.. Queering environmental justice: unequal environmental health burden on the LGBTQ+ community. Am J Public Health. 2022;112(1):79–87. 10.2105/AJPH.2021.306406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Juarez PD, Matthews-Juarez P, Hood DB, et al. The public health exposome: a population-based, exposure science approach to health disparities research. Int J Environ Res Public Health. 2014;11(12):12866–12895. 10.3390/ijerph111212866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vol. Rule Number: S8291A. In: New York State Senate, ed., 2022.
- 75. Cirefice VC, Sullivan L.. Women on the frontlines of resistance to extractivism. Policy Pract Dev Educ Rev. 2019;29:78–99. [Google Scholar]
- 76. Macdonald C. In: Addison T, Roe A, eds. Extractive Industries: The Management of Resources as a Driver of Sustainable Development. Ch. 21, Oxford University Press; 2018:442–459. [Google Scholar]
- 77. Dorries H, Harjo L.. Beyond safety: refusing colonial violence through indigenous feminist planning. J Plann Educ Res. 2020;40(2):210–219. 10.1177/0739456x19894382. [DOI] [Google Scholar]
- 78. Caretta MA, Zaragocin S.. Women’s resistance against the extractive industry: embodied and water dimensions. Hum Geogr. 2020;13(1):3–5. 10.1177/1942778620910893. [DOI] [Google Scholar]
- 79. McEachran AD, Sobus JR, Williams AJ, et al. Identifying known unknowns using the US EPA’s CompTox chemistry dashboard. Anal Bioanal Chem. 2017;409(7):1729–1735. 10.1007/s00216-016-0139-z. [DOI] [PubMed] [Google Scholar]
- 80. Lermen D, Gwinner F, Bartel-Steinbach M, et al. Towards harmonized biobanking for biomonitoring: a comparison of human biomonitoring-related and clinical biorepositories. Biopreserv Biobank. 2020;18(2):122–135. 10.1089/bio.2019.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koelmel JP, Lin EZ, Guo P, et al. Exploring the external exposome using wearable passive samplers—the China BAPE study. Environ Pollut. 2021;270:116228. 10.1016/j.envpol.2020.116228. [DOI] [PubMed] [Google Scholar]
- 82. Hu X, Walker DI, Liang Y, et al. A scalable workflow to characterize the human exposome. Nat Commun. 2021;12(1):5575. 10.1038/s41467-021-25840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Merino Martinez R, Müller H, Negru S, et al. Human exposome assessment platform. Environ Epidemiol. 2021;5(6):e182. 10.1097/EE9.0000000000000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dong H, Cuthbertson AA, Richardson SD, et al. Effect-directed analysis (EDA): a promising tool for nontarget identification of unknown disinfection byproducts in drinking water. Environ Sci Technol. 2020;54(3):1290–1292. 10.1021/acs.est.0c00014. [DOI] [PubMed] [Google Scholar]
- 85. Schootman M, Nelson EJ, Werner K, et al. Emerging technologies to measure neighborhood conditions in public health: implications for interventions and next steps. Int J Health Geogr. 2016;15(1):20. 10.1186/s12942-016-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Patel CJ. Analytic complexity and challenges in identifying mixtures of exposures associated with phenotypes in the exposome era. Curr Epidemiol Rep. 2017;4(1):22–30. 10.1007/s40471-017-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Blum MGB, Valeri L, François O, et al. Challenges raised by mediation analysis in a high-dimension setting. Environ Health Perspect. 2020;128(5):55001. 10.1289/EHP6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Miller GW. Integrating the exposome into a multi-omic research framework. Exposome. 2021;1(1):1–3. 10.1093/exposome/osab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fang M, Hu L, Chen D, et al. Exposome in human health: utopia or wonderland? Innovation (Camb). 2021;2(4):100172. 10.1016/j.xinn.2021.100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Balik-Meisner M, Truong L, Scholl EH, et al. Elucidating gene-by-environment interactions associated with differential susceptibility to chemical exposure. Environ Health Perspect. 2018;126(6):067010. 10.1289/EHP2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Migliore L, Nicolì V, Stoccoro A, et al. Gender specific differences in disease susceptibility: the role of epigenetics. Biomedicines. 2021;9(6):652. 10.3390/biomedicines9060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mederos N, Friedlaender A, Peters S, et al. Gender-specific aspects of epidemiology, molecular genetics and outcome: lung cancer. ESMO Open. 2020;5(Suppl 4):e000796. 10.1136/esmoopen-2020-000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Huhn S, Escher BI, Krauss M, et al. Unravelling the chemical exposome in cohort studies: routes explored and steps to become comprehensive. Environ Sci Eur. 2021;33(1):17. 10.1186/s12302-020-00444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. ATHLETE: The Exposome from Evidence to Translation; 2023. https://athleteproject.eu/.
- 95. The EPHOR Project; 2023. https://www.ephor-project.eu/.
- 96. Equal-Life: Studying the Exposome for a Healthier Future for All Children; 2023. https://www.equal-life.eu/en.
- 97. EXIMIOUS: Mapping Exposure-Induced Immune Effects: Connecting the Exposome and the Immunome; 2023. https://www.eximious-h2020.eu/. [DOI] [PMC free article] [PubMed]
- 98. The HEDIMED Project; 2023. https://www.hedimed.eu/.
- 99. longITools: Health & Environment Dynamics; 2023. https://longitools.org/.
- 100. REMEDIA: Impact of Exposome on the Course of Lung Disease; 2023. https://h2020-remedia.eu/.
- 101.EXPOsOMICS. 2023. https://exposomics-project.eu/live-exposome.pantheonsite.io/index.html.
- 102. HBM4EU: Science and Policy for a Healthy Future; 2023. https://www.hbm4eu.eu/.
- 103. Health and Environment-Wide Associations Based on Large Population Surveys; 2023. https://cordis.europa.eu/project/id/603946.
- 104. The Human Exposome Project; 2023. https://humanexposomeproject.com/.
- 105. European Human Exposome NETWORK; 2023. www.humanexposome.eu.
- 106. HERCULES Exposome Research Center; 2023. https://emoryhercules.com/.
- 107. EXPANSE Project; 2023. https://expanseproject.eu/.
- 108. Deguen S, Amuzu M, Simoncic V, et al. Exposome and social vulnerability: an overview of the literature review. Int J Environ Res Pub Health. 2022;19(6):3534. 10.3390/ijerph19063534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Farage M, Maibach HI.. The vulvar epithelium differs from the skin: implications for cutaneous testing to address topical vulvar exposures. Contact Derm. 2004;51(4):201–209. 10.1111/j.0105-1873.2004.00444.x. [DOI] [PubMed] [Google Scholar]
- 110. Center for Devices and Radiological Health. Menstrual Tampons and Pads: Information for Premarket Notification Submissions (510(s)s)—Guidance for Industry and FDA Staff. 2005. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/menstrual-tampons-and-pads-information-premarket-notification-submissions-510ks-guidance-industry.
- 111. Park CJ, Barakat R, Ulanov A, et al. Sanitary pads and diapers contain higher phthalate contents than those in common commercial plastic products. Reprod Toxicol. 2019;84:114–121. 10.1016/j.reprotox.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gao C-J, Wang F, Shen H-M, et al. Feminine hygiene products—a neglected source of phthalate exposure in women. Environ Sci Technol. 2020;54(2):930–937. 10.1021/acs.est.9b03927. [DOI] [PubMed] [Google Scholar]
- 113. Upson K, Shearston JA, Kioumourtzoglou M-A, et al. Menstrual products as a source of environmental chemical exposure: a review from the epidemiologic perspective. Curr Environ Health Rep. 2022;9(1):38–52. 10.1007/s40572-022-00331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Angelova V, Ivanova R, Delibaltova V, et al. Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Ind Crops Prod. 2004;19(3):197–205. 10.1016/j.indcrop.2003.10.001. [DOI] [Google Scholar]
- 115. DeVito MJ, Schecter A.. Exposure assessment to dioxins from the use of tampons and diapers. Environ Health Perspect. 2002;110(1):23–28. 10.1289/ehp.0211023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Shin JH, Ahn YG.. Analysis of polychlorinated dibenzo-p-dioxins and dibenzo-furans in sanitary products of women. Text Res J. 2007;77:597–603. 10.1177/0040517507078786. [DOI] [Google Scholar]
- 117. Scranton A. Chem Fatale: Potential Health Effects of Toxic Chemicals in Feminine Care Products; Women’s Voices for the Earth, 2013. https://womensvoices.org/menstrual-care-products/chem-fatale-report/chem-fatale-fact-sheet/#:~:text=Unregulated%20toxic%20chemicals%20in%20feminine,%2C%20asthma%2C%20and%20allergic%20reactions.
- 118. Branch F, Woodruff TJ, Mitro SD, et al. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001–2004. Environ Health. 2015;14:57. 10.1186/s12940-015-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ferranti M. An odor of racism: vaginal deodorants in African-American beauty culture and advertising. Advert Soc Rev. 2011;11. 10.1353/asr.2011.0003. [DOI] [Google Scholar]
- 120. Aral SO, Mosher WD, Cates W, et al. Vaginal douching among women of reproductive age in the United States: 1988. Am J Public Health. 1992;82(2):210–214. 10.2105/ajph.82.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.U.S. Bureau of Labor Statistics. Labor Force Statistics from the Current Population Survey; 2022. https://www.bls.gov/cps/cpsaat11.htm.
- 122. Bello A, Quinn MM, Perry MJ, et al. Characterization of occupational exposures to cleaning products used for common cleaning tasks—a pilot study of hospital cleaners. Environ Health. 2009;8:11. 10.1186/1476-069X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Charles LE, Loomis D, Demissie Z, et al. Occupational hazards experienced by cleaning workers and janitors: a review of the epidemiologic literature. Work. 2009;34(1):105–116. 10.3233/WOR-2009-0907. [DOI] [PubMed] [Google Scholar]
- 124. Kwon K-D, Jo W-K, Lim H-J, et al. Volatile pollutants emitted from selected liquid household products. Environ Sci Pollut Res Int. 2008;15(6):521–526. 10.1007/s11356-008-0028-x. [DOI] [PubMed] [Google Scholar]
- 125. Odabasi M, Elbir T, Dumanoglu Y, Sofuoglu SC.. Halogenated volatile organic compounds in chlorine-bleach-containing household products and implications for their use. Atmos Environ. 2014;92:376–383. 10.1016/j.atmosenv.2014.04.049. [DOI] [Google Scholar]
- 126. Lin N, Rosemberg M-A, Li W, Meza-Wilson E, Godwin C, Batterman S.. Occupational exposure and health risks of volatile organic compounds of hotel housekeepers: field measurements of exposure and health risks. Indoor Air. 2021;31(1):26–39. 10.1111/ina.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Smith EM, Hammonds-Ehlers M, Clark MK, Kirchner HL, Fuortes L.. Occupational exposures and risk of female infertility. J Occup Environ Med. 1997;39(2):138–147. 10.1097/00043764-199702000-00011. [DOI] [PubMed] [Google Scholar]
- 128. Dumas O, Wiley AS, Quinot C, et al. Occupational exposure to disinfectants and asthma control in US nurses. Eur Respir J. 2017;50(4):1700237. 10.1183/13993003.00237-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. De Matteis S, Jarvis D, Hutchings S, et al. Occupations associated with COPD risk in the large population-based UK Biobank cohort study. Occup Environ Med. 2016;73(6):378–384. 10.1136/oemed-2015-103406. [DOI] [PubMed] [Google Scholar]
- 130. Dumas O, Varraso R, Boggs KM, et al. Association of occupational exposure to disinfectants with incidence of chronic obstructive pulmonary disease among US female nurses. JAMA Netw Open. 2019;2(10):e1913563. 10.1001/jamanetworkopen.2019.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Atramont A, Guida F, Mattei F, et al. ; iCARE Study Group. Professional cleaning activities and lung cancer risk among women: results from the iCARE study. J Occup Environ Med. 2016;58(6):610–616. 10.1097/JOM.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 132. Svanes Ø, Bertelsen RJ, Lygre SHL, et al. Cleaning at home and at work in relation to lung function decline and airway obstruction. Am J Respir Crit Care Med. 2018;197(9):1157–1163. 10.1164/rccm.201706-1311OC. [DOI] [PubMed] [Google Scholar]
- 133. Tjalvin G, Svanes Ø, Igland J, et al. Maternal preconception occupational exposure to cleaning products and disinfectants and offspring asthma. J Allergy Clin Immunol. 2022;149(1):422–431 e425. 10.1016/j.jaci.2021.08.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.