Abstract
Objective
To describe beliefs of physicians and patients in primary and secondary care about urate-lowering therapy (ULT), to examine differences in physicians’ medication beliefs and to examine the association of physicians’ medication beliefs with the prescribed dosage of ULT, gout outcomes and patients’ medication beliefs.
Methods
We conducted a cross-sectional study among rheumatologists and general practitioners (GPs) and their patients using ULT in The Netherlands. All participants filled out the Beliefs About Medication Questionnaire (BMQ). Demographics of physicians were collected through questionnaires. Patient and disease characteristics were collected through questionnaires and electronic medical records. Differences between rheumatologists and GPs in the BMQ subscales Necessity and Concern and the necessity–concern difference (NCD) score were analysed by two-sample t-tests. Multilevel analyses were performed to examine the association of physicians’ BMQ scores with the prescribed dosage of ULT, gout outcomes (number of gout flares, serum urate) and patients’ BMQ scores.
Results
A total of 28 rheumatologists, 443 rheumatology patients, 45 GPs and 294 GP patients were included. The mean NCD scores were 7.1 (s.d. 3.6), 4.0 (s.d. 4.0), and 4.2 (s.d. 5.0) for rheumatologists, GPs and patients, respectively. Rheumatologists scored higher on necessity beliefs [mean difference 1.4 (95% CI 0.0, 2.8)] and lower on concern beliefs [mean difference −1.7 (95% CI −2.7, −0.7)] compared with GPs. No associations between physicians’ beliefs and prescribed dosage of ULT, gout outcomes or patients’ beliefs were found.
Conclusion
Rheumatologists had higher necessity and lower ULT concern beliefs compared with GPs and patients. Physicians’ beliefs were not related to prescribed ULT dosage and patient outcomes. The role of physicians’ beliefs in gout management in patients using ULT seems limited. Future qualitative research can provide more insights into physicians’ views of gout management.
Keywords: gout, medication beliefs, rheumatologists, general practitioners, gout management
Key messages.
Rheumatologists reported a greater need and fewer concerns for prescribing ULT compared with GPs.
Physicians’ medication beliefs were not associated with the prescribed dosage of allopurinol, gout outcomes and patients’ beliefs.
Introduction
Gout is the most common form of inflammatory arthritis, with an estimated prevalence of 1.4% in the European population [1]. It is caused by the deposition of monosodium urate crystals within joints and soft tissue [1]. Urate-lowering therapy (ULT) effectively lowers serum urate (SU) levels below the proposed targets (<0.36 mmol/l, or in case of tophaceous gout <0.30 mmol/l) [2, 3]. This results in the resolution of gout symptoms and reduces the risk for recurrent gout flares [2, 3]. In a clinical setting with nurse-led care, personalized information and a treat-to-target strategy, SU targets can be reached in >90% of patients with gout [4]. However, despite proven effectiveness, a large proportion of patients do not reach SU targets in both primary and secondary care [5, 6], resulting in recurrent flares, tophi and, consequently, a higher disease burden for patients and societal costs [7].
Considerable attention has been paid to the barriers of effective treatment in patients with gout. Barriers that have been reported are patients’ lack of knowledge of the disease and the potential benefit of lifestyle adjustments and non-adherence to ULT medication [8–10]. A small number of educational and behavioural intervention studies in patients with gout addressed patients’ disease perceptions and were effective in improving knowledge on gout and adherence to ULT medication [4, 11].
Less is known about the potential impact of physician-related factors on the management of gout. Studies indicate the presence of various healthcare-related barriers to gout management, such as suboptimal guideline adherence, lack of physician’s knowledge about gout and ULT medication and underestimation of long-term gout complications [5, 6, 12].
Furthermore, at present, two different treatment strategies are being used in clinical practice, i.e. treat to target and treat to avoid symptoms. Although most guidelines advocate a treat-to-target strategy, including the Dutch gout guidelines for both primary and secondary care, the American College of Physicians promotes a treat-to-avoid-symptoms strategy [3, 13–16]. However, solid proof on which treatment strategy is superior is missing [13]. In the absence of a clear consensus on the therapeutic strategy, the individual beliefs of physicians can be a factor of importance in gout management.
According to the Theory of Planned Behaviour [17], physicians’ beliefs towards medication can shape prescribing behaviour and, in turn, gout management. Health beliefs models such as the Theory of Planned Behaviour [17] and the necessity–concern framework [18] postulate that beliefs about illness and medication can shape an individual’s intentions and behaviour. Hence physicians’ beliefs towards ULT medication (i.e. the necessity for and concerns with ULT medication) may influence gout management, specifically prescribed dosage of ULT.
Empirical findings about the relations between physicians’ medication beliefs, clinical management and patients’ beliefs and outcomes are scarce and inconclusive [19–24].
A systematic review in low back pain (LBP) showed that the attitudes and beliefs of the health professional were associated with the attitudes and beliefs of their consulting patients with LBP. In addition, the attitudes and beliefs of healthcare professionals (HCPs) were associated with clinical management and guideline adherence [23]. Regarding cholesterol-lowering medication, Foley et al. [24] showed that physicians’ attitudes and beliefs about hyperlipidaemia were associated with the decision to increase the statin dose in high-risk patients on lipid therapy. However, another study among physicians, pharmacy staff and patients found no association between HCPs beliefs about statins and patients’ statin beliefs and their medication-taking behaviour [20]. In rheumatology, only one study has examined the relations between physicians (implicit and explicit) beliefs about DMARDs and the attitudes and beliefs, medication taking behaviour and disease activity of patients with RA and found no associations [18]. Considering the variety of gout treatment strategies in clinical practice and the high non-adherence rates for ULT among gout patients, insights into physicians’ beliefs about ULT and whether these beliefs influence prescribed dosages of ULT is warranted.
Therefore, the aim of this study was to describe the medication beliefs of physicians and patients in both primary and secondary gout care, examine differences in beliefs between rheumatologists and GPs and assess the association of physicians’ medication beliefs with their prescribed dosages of ULT, gout outcomes and their patients’ medication beliefs.
Patients and methods
Study design and participants
A cross-sectional study among physicians and their patients with gout was conducted. Physicians and patients were recruited simultaneously in the period May–December 2020. The local ethical review board (CMO region Arnhem-Nijmegen, dossier number: 2019-5268) exempted the study from ethical approval since the study was not subject to the Dutch Medical Research Involving Human Subjects act (WMO). The study was approved by the internal review board of the Sint Maartenskliniek, Nijmegen, The Netherlands. All participants gave written informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology checklist was used to ensure complete and transparent reporting [25].
Participating physicians
All physicians (including trainees and physicians assistants) at the rheumatology department of the Sint Maartenskliniek Nijmegen and general practitioners (GPs) participating in the Family Medicine practice-based research network (PBRN) in the Nijmegen region were invited to participate. The PBRN consists of 17 primary care practices with 75 GPs in the east of the Netherlands (Nijmegen and surrounding area). There were no additional eligibility criteria for physicians.
Participating patients
Retrospectively, patients (≥18 years) with a clinical diagnosis of gout according to the treating physician and use of ULT were identified and extracted from the electronic medical record in both the Sint Maartenskliniek and the PBRN. To be eligible, patients had to use ULT in the year prior to inclusion and were still on ULT, were able to understand the Dutch language and had no cognitive impairments.
Procedure and measures
Demographic characteristics and medication beliefs of physicians and patients were collected by a questionnaire. Clinical characteristics of the rheumatology patients were extracted from the electronic medical record by the researchers. For GP patients, the data were provided by the PBRN network. Questionnaires for all physicians (digital format) and patients (paper-and-pencil format) from the rheumatology department were sent by the researchers; questionnaires for GP patients (paper-and-pencil format) were sent through their GP practice.
Demographic and clinical characteristics
For the physicians, the following demographic characteristics were collected by questionnaire: sex, age, years of working experience, estimated hours of direct patient contact per week and gout consultations per week. For the patients, sex, age and number of self-reported flares over the past 3 months were collected by questionnaire. GP patients were asked if they were currently under treatment by a rheumatologist for their gout.
From the electronic medical records, the following patient data were extracted: latest ULT use, including type and dosage, latest available lab history on serum urate and renal function over the past 2 years and all known comorbidities.
Medication beliefs: Beliefs About Medicines Questionnaire (BMQ)
To assess medication beliefs the BMQ, reflecting/quantifying the underlying necessity–concern framework, was used [26]. The BMQ consists of a specific part regarding the medication of interest (BMQ-specific) and a part about medications in general (BMQ-general) [26]. The BMQ-specific consists of two subscales, a necessity and concern scale, both with five items. All items are scored on a 5-point Likert scale of 1 (strongly disagree) to 5 (strongly agree), resulting in a sum score range of 5–25. The BMQ-general consists of two subscales, a harm and an overuse scale, both with four items. All items are scored on a 5-point Likert scale 1of (strongly disagree) to 5 (strongly agree), resulting in a sum score range of 4–20.
In addition, the necessity–concern difference (NCD) score can be derived from the BMQ-specific. For this score, the concern score is subtracted from the necessity score (range −20–20). A positive score reflects that the necessity score outweighs the concern score and a negative score reflects that the concern score outweighs the necessity score.
Finally, four attitudinal profiles can be derived from the BMQ-specific: acceptant, ambivalent, sceptic and indifferent [27]. Respondents are classified into these attitudinal groups according to the median cut-off score of the necessity and concern subscales. The attitudinal profiles were calculated only for physicians. For patients, the Dutch version of the BMQ was used [28]. For physicians, an adapted version of the BMQ was used [21]. Only complete BMQs were applicable for analyses.
Statistical analyses
Descriptive statistics were used to describe participant characteristics. For normally distributed data, means (s.d.s) were calculated, otherwise medians and interquartile ranges (IQRs; 25th–75th percentile) were calculated. BMQ scores and attitudinal profiles were grouped according to primary vs secondary care and described for both physicians and patients.
Differences between rheumatologists and GPs regarding the BMQ subscales, necessity, concern, harm and overuse and the NCD score were analysed by two-sample t-tests. Differences in attitudinal groups between rheumatologists and GPs was assessed by the chi-squared test.
Series of multilevel analyses were performed, as patient data (level 0) was nested within physicians (level 1), to examine the association of physicians’ medication beliefs (i.e. necessity and concern scores as independent variables) with the following dependent variables: prescribed dosage of ULT (measured as the maximum dosage of allopurinol, the most common ULT) per patient, the latest SU levels in their patients, patients’ necessity or concern score, the proportion of patients who reached the SU target of <0.36 mmol/l and the presence of gout flares (yes/no) in the past 3 months. To perform the multilevel analyses with patients nested within physicians, it was necessary that both responded in order to be matched. Furthermore, only complete BMQs were included in these analyses.
First, collinearity between potential physician-related and patient-related covariates was assessed. Physicians’ age and years of work experience had an r-value >0.7, therefore only work experience was taken into further analyses. Next, bivariate analyses were performed to determine which physician-related (primary vs secondary care, sex, years of work experience, hours of direct patient contact/week and number of gout consults/week) and patient-related [age, sex, diabetes mellitus, hypertension, renal failure and start ULT past year (yes/no)] factors were associated with the necessity or concern score of physicians. Covariates with a P-value <0.157 (according Akaike information criterion [29]) were included in their respective full adjusted multilevel models. A linear multilevel model was used for the following continuous dependent variables: dosage of allopurinol, SU level, patients’ necessity score and patients’ concern score, presenting the unstandardized beta coefficient. A logistic multilevel model was used for the following binary dependent variables: proportion of patients who reached the SU target and the presence of gout flares (yes/no) in the past 3 months, presenting the corresponding odds ratio (OR). Likelihood ratio tests were used to assess multilevel model fit. For all models, a multilevel model with a random intercept for physician level (patients nested within physicians) was deemed to be most suitable. The intraclass correlation coefficient (ICC), which quantifies the degree to which data at the lower level are correlated, is presented as well [30]. Post hoc analyses without patients who received both GP and rheumatology care were performed with the same full models.
No formal sample size calculation was made, as a convenience sample was used. Data were analysed using Stata version 17 (StataCorp, College Station, TX, USA).
Results
Characteristics of study participants
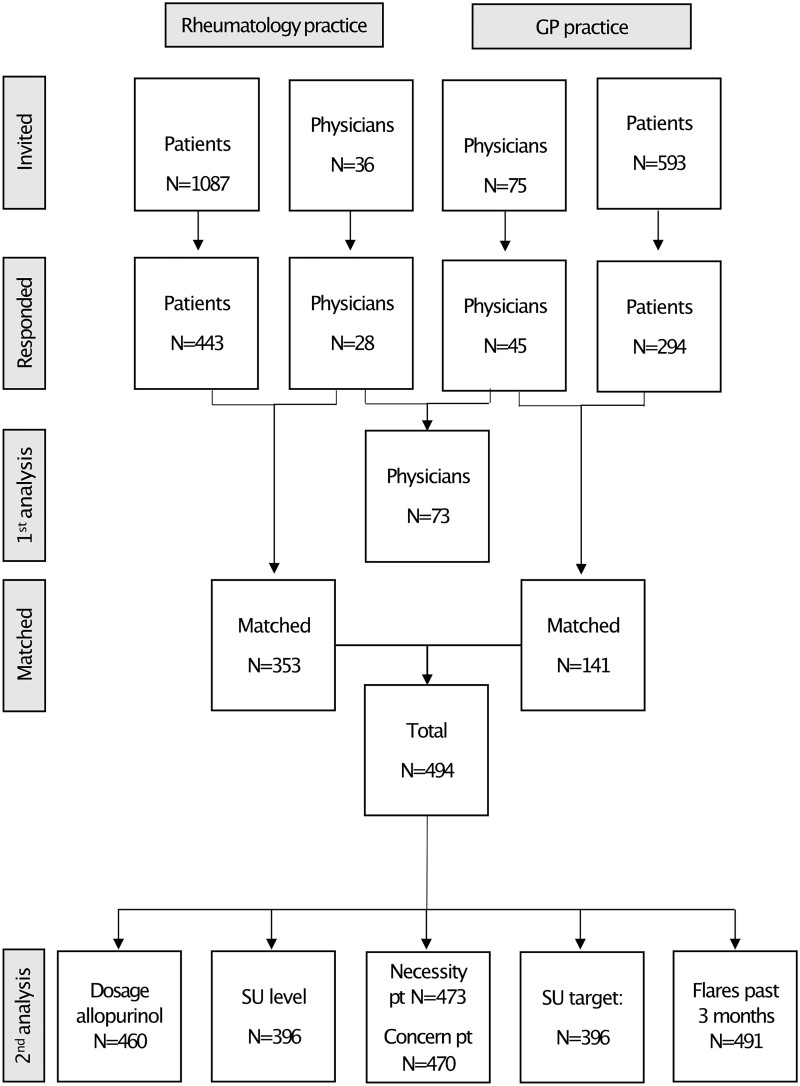
In Fig. 1, the study flow diagram of participants is presented. In total, 111 physicians were invited to participate, 28/36 from the rheumatology department (78%), including 19 rheumatologists, 7 residents and 2 physician’s assistants and 45/75 GPs (60%) from 16 of 17 general practices responded. Their characteristics are described in Table 1.
Figure 1.
Study flowchart from invitation to analyses. First analysis: beliefs about medication physicians. Second analysis: multilevel analyses including all patient–physicians matches. Pt: patient
Table 1.
Characteristics of physicians
| Characteristics | Rheumatologists (n = 28) | GPs (n = 45) |
|---|---|---|
| Male, n (%) | 9 (32.1) | 20 (44.4) |
| Age, years, mean (s.d.) | 43.1 (10.6) | 46.1 (8.3) |
| Working experience, years, median (IQR) | 8.5 (3–14) | 13 (8–20) |
| Direct patient contact, hours/week, median (IQR) | 15 (8.5–20) | 24 (20–30) |
| Gout consults/week, median (IQR) | 4 (1–6.5) | 1 (0.2–1) |
In total, 443/1087 (40.8%) of the invited rheumatology patients and 294/593 (49.6%) of the invited GP patients responded. The patients’ characteristics are shown in detail in Supplementary Table S1, available at Rheumatology Advances in Practice online. Most patients were male (85.3% in rheumatology patients vs 88.1% in GP patients) with a mean age of 68.3 years (s.d. 10.52) and 68.6 years (s.d. 10.32) for rheumatology patients and GP patients, respectively. There were no differences in relevant chronic comorbidities between rheumatology patients and GP patients.
Of the rheumatology patients, 25.7% reported one or more flares in the previous 3 months, whereas 17.7% of GP patients reported one or more flares. The mean SU levels in rheumatology patients were 0.30 (s.d. 0.08) vs 0.37 (s.d. 0.08) in GP patients. Target SU (<0.36 mmol/l) was reached in 79% of the rheumatology patients, whereas 48.6% of GP patient reached the SU target. The most frequently used ULT for both groups was allopurinol, with a median dosage of 300 mg (IQR 50–900) for rheumatology patients and 200 mg (IQR 100–700 mg) for GP patients.
Physicians’ and patients’ beliefs about medication
The BMQ scores for physicians are displayed in Table 2. Rheumatologists scored higher on the BMQ necessity scale [17.5 (s.d. 2.4) vs 16.1 (3.2)] and lower on the BMQ concern scale [10.4 (s.d. 2.0) vs 12.1 (2.1)] compared with GPs. Rheumatologists scored lower on both overuse [9.9 (s.d. 2.1) vs 11.3 (2.1)] and harms [7.1 (s.d. 1.6) vs 8.3 (1.4)] subscales compared with GPs. Rheumatologists were mostly classified in the attitudinal group acceptant (46.4%), whereas the GPs were mostly classified in two attitudinal groups: sceptic (31.1%) and indifferent (33.3%) (χ2 = 9.0, P = 0.029). The NCD difference score was 3.1 (95% CI 1.2, 5.0), reflecting that for rheumatologists the necessity beliefs outweighed the concern beliefs more than they did for GPs.
Table 2.
Medication beliefs and attitudinal medication profiles of rheumatologists and GPs regarding ULT
| Variable | Rheumatologists (n = 28) | GPs (n = 45) | Difference (95% CI) |
|---|---|---|---|
| Necessity score, mean (s.d.) | 17.5 (2.4) | 16.1 (3.2) | 1.4 (0.0, 2.8) |
| Concern score, mean (s.d.) | 10.4 (2.0) | 12.1 (2.1) | −1.7 (−2.7, −0.7) |
| Overuse score, mean (s.d.) | 9.9 (2.1) | 11.3 (2.1) | −1.5 (−2.5, −0.5) |
| Harm score, mean (s.d.) | 7.1 (1.6) | 8.3 (1.4) | −1.2 (−1.9, −0.5) |
| Attitudinal profilesa,b, n (%) | |||
| Acceptant | 13 (46.4) | 7 (15.6) | |
| Ambivalent | 5 (17.9) | 9 (20.0) | |
| Sceptic | 6 (21.4) | 14 (31.1) | |
| Indifferent | 4 (14.3) | 15 (33.3) | |
| NCD score, mean (s.d.) | 7.1 (3.6) | 4.0 (4.0) | 3.1 (1.2, 5.0) |
Necessity score range: 5–25; concern score range: 5–25; overuse score range: 4–20; harm score range: 4–20.
Attitudinal profiles of physicians based on median cut-off scores: acceptant (necessity >17, concern ≤11), ambivalent (necessity >17, concern >11), sceptic (necessity ≤17, concern >11) and indifferent (necessity ≤17, concern ≤11).
P = 0.029.
Overall, patients had a mean necessity score of 16.8 (s.d. 4.2) and a mean concern score of 12.6 (s.d. 3.7). The mean NCD score of patients was 4.2 (s.d. 5.0). They had the following attitudinal profile distribution: 29.0% acceptant, 25.8% ambivalent, 14.5% sceptic and 30.6% indifferent. For specific group scores see Table 3.
Table 3.
Medication beliefs and attitudinal medication profiles of patients on ULT
| Variable | Patients treated by a rheumatologist | Patients treated by a GP |
|---|---|---|
| Necessity score, mean (s.d.) | 17.3 (4.2) | 16.1 (4.1) |
| Concern score, mean (s.d.) | 13.1 (3.7) | 11.9 (3.7) |
| Overuse score, mean (s.d.) | 11.2 (2.7) | 10.8 (2.6) |
| Harm score, mean (s.d.) | 10.1 (2.4) | 9.9 (2.4) |
| Attitudinal profilesa, n (%) | ||
| Acceptant | 93 (19.8) | 61 (18.0) |
| Ambivalent | 177 (37.6) | 113 (33.3) |
| Sceptic | 107 (22.7) | 69 (20.4) |
| Indifferent | 94 (20.0) | 96 (28.3) |
| NCD score, mean (s.d.) | 4.2 (5.1) | 4.2 (4.9) |
Necessity score range: 5–25; concern score range: 5–25; overuse score range: 4–20; harm score range: 4–20.
Attitudinal profiles of patients based on median cut-off scores: acceptant (necessity >17, concern <13), ambivalent (necessity >17, concern ≥13), sceptic (necessity ≤17, concern ≥13) and indifferent (necessity ≤17, concern <13).
Association of physicians’ beliefs with allopurinol dosage, gout outcomes and patients’ beliefs
Tables 4 and 5 show the results of the multilevel analyses exploring the association of physicians’ medication beliefs (separate for necessity and concern scores) with their highest prescribed dosage of allopurinol, the latest SU levels in their patients, patients’ necessity or concern score, the proportion of patients who reached the SU target of <0.36 mmol/l and the presence of gout flares (yes/no) in the past 3 months. Unadjusted, a higher physicians’ concern score was associated with a lower dosage of allopurinol. Adjusted models, as seen in Tables 4 and 5, did not show any associations between physicians’ beliefs and the outcome measures. Similar results were found for the association between the NCD of physicians and prescribed dosage of allopurinol, gout outcomes and patients’ NCD (see Supplementary Table S2, available at Rheumatology Advances in Practice online). Post hoc analyses without 32 patients who received both GP and rheumatology care did not show any differences compared with the primary analyses (see Supplementary Table S3 and S4, available at Rheumatology Advances in Practice online).
Table 4.
The association between physicians’ necessity beliefs and prescribed dosage of allopurinol, gout outcomes and patients’ necessity beliefs
| Variable | Allopurinol dosage (mg) |
SU level (mmol/l) |
Patients’ necessity score |
Target SU (yes/no) |
Flares in the past 3 months (yes/no) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | Adja (95% CI) | β (95% CI) | Adjb (95% CI) | β (95% CI) | Adjc (95% CI) | OR (95% CI) | Adjd (95% CI) | OR (95% CI) | Adje (95% CI) | |
| Physicians’ necessity score | 3.83 (−3.00, 10.65) | 0.56 (−4.49, 5.82) | 0.00 (−0.01, 0.01) | 0.00 (−0.00, 0.01) | 0.03 (−0.11, 0.17) | 0.00 (−0.15, 0.15) | 1.01 (0.81, 1.25) | 0.95 (0.83, 1.10) | 1.03 (0.96, 1.11) | 1.00 (0.92, 1.08) |
| ICC | 0.18 | 0.06 | 0.43 | 0.20 | 0.01 | 0.02 | 0.48 | 0.11 | 0.00 | 0.00 |
Adjusted for primary vs secondary care, hours of patient contact, number of gout consultations, age of the patient, sex of the patient, diabetes mellitus, renal failure and started ULT in the past year.
Adjusted for primary vs secondary care, work experience, hours of patient contact, number of gout consultations, age of the patient, sex of the patient, renal failure and started ULT in the past year.
Adjusted for primary vs secondary care, hours of patient contact and hypertension.
Adjusted for primary vs secondary care, sex of the physician, work experience, hours of patient contact, number of gout consultations, age of the patient, sex of the patient, renal failure and started ULT in the past year.
Adjusted for primary vs secondary care, work experience, number of gout consultations and started ULT in the past year.
Covariates denoted in bold are significantly associated with the outcome parameter (P < 0.05).
β: unstandardized β; adj: adjusted.
Table 5.
The association between physicians’ concern beliefs and prescribed dosage of allopurinol, gout outcomes and patients’ concern beliefs
| Variable | Allopurinol dosage (mg) |
SU level (mmol/l) |
Patients’ concern score |
Target SU (yes/no) |
Flares in the past 3 months (yes/no) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | Adja (95% CI) | β (95% CI) | Adjb (95% CI) | β (95% CI) | Adjc (95% CI) | β (95% CI) | Adjd (95% CI) | β (95% CI) | Adje (95% CI) | |
| Physicians’ concern score | −11.42 (−19.58, −3.25) | −1.66 (−8.48, 5.16) | 0.01 (−0.00, 0.02) | 0.00 (−0.01, 0.01) | −0.11 (−0.28, 0.05) | −0.02 (−0.18, 0.14) | 0.84 (0.65, 1.10) | 1.05 (0.87, 1.25) | 1.01 (0.92, 1.11 | 1.04 (0.94, 1.16) |
| ICC | 0.16 | 0.07 | 0.41 | 0.20 | 0.02 | 0.00 | 0.46 | 0.11 | 0.00 | 0.00 |
β: unstandardized β; adj: adjusted.
Adjusted for: primary vs secondary care, work experience, hours of patient contact, number of gout consultations, age of the patient, sex of the patient, diabetes mellitus, renal failure and started ULT in the past year.
Adjusted for: primary vs secondary care, work experience, hours of patient contact, number of gout consultations, age of the patient, sex of the patient, renal failure and started ULT in the past year.
Adjusted for: primary vs secondary care, work experience, number of gout consultations and started ULT in the past year.
Adjusted for: primary vs secondary care, sex physician, work experience, hours of patient contact, number of gout consultations, age of the patient, sex of the patient, renal failure and started ULT in the past year.
Adjusted for: primary vs secondary care, work experience, hours of patient contact, number of gout consultations and started ULT in the past year.
Covariates denoted in bold are significantly associated with the outcome parameter (P < 0.05).
Discussion
In this cross-sectional study, the role of medication beliefs in gout management was examined. In both physicians (rheumatologists and GPs) and patients, the need for ULT outweighed the concern. Rheumatologists reported higher medication necessity beliefs and lower concern beliefs than GPs and patients. Physicians’ medication beliefs were not associated with the dosage of prescribed allopurinol, treatment outcomes or medication beliefs of patients.
In line with other studies, we found that physicians reported a greater need and fewer concerns for their prescribed medication [19–21]. The average necessity score falls in the range of previous studies regarding beliefs regarding statins and DMARDs (13.9–20.9) [19–21]. The average concern score of rheumatologists, however, is slightly lower than the range of previous studies of prescribing DMARDs and statins (11.5–13.5) [19–21]. A plausible explanation for this is that ULT is relatively safe and well tolerated [2].
Rheumatologists had higher necessity beliefs and lower concern beliefs compared with GPs. There are a few explanations that could account for these subgroup differences. First, rheumatologists in our study treated more gout patients individually and therefore are likely to have more accurate knowledge of gout management [12]. Second, the Dutch gout population in secondary care tends to have a more severe gout phenotype, which often necessitates more intensive treatment. Although we did not collect specific data on gout severity, such as the presence of erosions or tophi, more flares and higher dosage of allopurinol were reported in the population treated by a rheumatologist.
We found no associations between physicians’ medication beliefs and their prescribed dosage of allopurinol, gout outcomes or the medication beliefs of patients. This is in line with earlier research in rheumatology [19]. Of note, in our unadjusted model, a higher concern score was associated with a lower prescribed dosage of allopurinol. This is in line with other studies [23, 24]. In our adjusted model, however, the relationship between concern beliefs and the prescribed dosage of allopurinol disappeared. The concern beliefs were outweighed by other covariate factors, including primary vs secondary care, age of the patient and recent start of ULT, influencing the prescribed dosage of allopurinol. Overall, in our adjusted multilevel analyses, the covariate primary vs secondary care was the strongest factor independently associated with the prescribed dosage of allopurinol and clinical outcomes.
In this study, some limitations must be considered. First, only patients treated with ULT were included. Medication beliefs may also influence a physician’s decision whether to initiate ULT. In hindsight, including patients who are not treated with ULT would have given a broader perspective. However, in our opinion, specific beliefs on medication are stable and are therefore not likely to change in different contexts. Second, not all patients could be paired with their physician, due to non-response from either of them, resulting in a slightly smaller sample size for the multilevel analyses, particularly in the GP setting. However, we do not think that this had any major influence on our results, as the unadjusted results show differences that are expected and disappearing after correction for potential confounding factors. Third, we were unable to identify possible duplication between GP and rheumatology patients. A total of 32 GP patients stated that they were being treated by a rheumatologist (not necessarily our included rheumatologists) as well. Post hoc analyses excluding these 32 patients did not show any different results. Fourth, participation bias may have occurred, as physicians who responded to the questionnaire might be more involved with their gout patients and therefore were more willing to participate in this study. If beliefs of responding and non-responding physicians differed, this may have led to biased estimates of our study findings. Similarly, participation bias may also have occurred in responding patients and may have led to a non-representative group. However, our response rate can be considered as high and the patient characteristics reflect the average gout population. Fifth, no formal power calculation was performed, as the primary objective was descriptive. A post hoc power analysis for the second objective showed a slightly underpowered sample (73 responders included where 87 were needed). Therefore, these results should be interpreted with caution. For the third objective, the study was sufficiently powered. Last, physicians from one specialized hospital were included in this cross-sectional study, limiting study generalizability. A multisite study is needed to confirm or refute our findings. Furthermore, a qualitative study (e.g. focus groups, interviews) can provide a more in-depth understanding of the beliefs of physicians and their possible influencing role in gout management. Also, a longitudinal study is needed to firmly ascertain the influence of physicians’ medication beliefs on gout management. This first study might be a starting point for further studies on the role of physicians’ beliefs regarding gout.
Despite the limitations, there are strengths as well. To our knowledge, this is the first study wherein the beliefs of physicians regarding ULT for gout are subject of study. With previous studies focusing on patient barriers in effective treatment [8–10], it is important to know what role the beliefs of physicians play in effective gout management. Second, both primary and secondary care physicians involved in gout management were included in this study, covering the entire spectrum of gout patients. Last, the response rates of 76% and 60% in rheumatologists and GPs and 40.8% and 49.6% in their patients, respectively, can be considered as high.
In conclusion, the results show that rheumatologists scored higher on necessity and lower on concern beliefs compared with GPs. We found no associations between physicians’ beliefs with the prescribed dosage of ULT and clinical outcomes in their patients. The role of physicians’ beliefs in gout management in patients being treated with ULT seems limited. Future qualitative research can provide more insights into physicians’ views of gout management.
Supplementary Material
Contributor Information
Frouwke Veenstra, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands; Department of Research and Innovation, Sint Maartenskliniek, Nijmegen, The Netherlands.
Johanna E Vriezekolk, Department of Research and Innovation, Sint Maartenskliniek, Nijmegen, The Netherlands.
Bart J F van den Bemt, Department of Pharmacy, Sint Maartenskliniek, Nijmegen, The Netherlands; Department of Pharmacy, Radboud University Medical Center, Nijmegen, The Netherlands.
Henk J Schers, Department of Primary and Community Care Medicine, Radboud University Medical Center, Nijmegen, The Netherlands.
Bart Sloot, Department of Research and Innovation, Sint Maartenskliniek, Nijmegen, The Netherlands.
Cornelia H M van den Ende, Department of Research and Innovation, Sint Maartenskliniek, Nijmegen, The Netherlands; Department of Rheumatology, Radboud University Medical Center, Nijmegen, The Netherlands.
Noortje van Herwaarden, Department of Pharmacology-Toxicology, Radboud University Medical Center, Nijmegen, The Netherlands; Department of Rheumatology, Sint Maartenskliniek, Nijmegen, The Netherlands.
Marcel Flendrie, Department of Rheumatology, Sint Maartenskliniek, Nijmegen, The Netherlands.
Supplementary material
Supplementary material is available at Rheumatology Advance in Practice online.
Data availability
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.
Authors’ contributions
F.V., J.V., B.B., H.S., B.S., N.H. and M.F. were involved in the conception and design of the study. F.V., J.V., H.S. and M.F. were involved in the data collection. F.V., J.V., C.E. and M.F. contributed to the data analysis. F.V., J.V. and M.F. drafted the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by an unrestricted grant from Grünenthal B.V.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Kuo CF, Grainge MJ, Zhang W, Doherty M.. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol 2015;11:649–62. [DOI] [PubMed] [Google Scholar]
- 2. Dalbeth N, Gosling AL, Gaffo A, Abhishek A.. Gout. Lancet 2021;397:1843–55. [DOI] [PubMed] [Google Scholar]
- 3. Richette P, Doherty M, Pascual E. et al. Updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42. [DOI] [PubMed] [Google Scholar]
- 4. Doherty M, Jenkins W, Richardson H. et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet 2018;392:1403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oderda GM, Shiozawa A, Walsh M. et al. Physician adherence to ACR gout treatment guidelines: perception versus practice. Postgrad Med 2014;126:257–67. [DOI] [PubMed] [Google Scholar]
- 6. Jeyaruban A, Larkins S, Soden M.. Management of gout in general practice–a systematic review. Clin Rheumatol 2015;34:9–16. [DOI] [PubMed] [Google Scholar]
- 7. Roddy E, Zhang W, Doherty M.. Concordance of the management of chronic gout in a UK primary-care population with the EULAR gout recommendations. Ann Rheum Dis 2007;66:1311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spencer K, Carr A, Doherty M.. Patient and provider barriers to effective management of gout in general practice: a qualitative study. Ann Rheum Dis 2012;71:1490–5. [DOI] [PubMed] [Google Scholar]
- 9. Reach G. Treatment adherence in patients with gout. Joint Bone Spine 2011;78:456–9. [DOI] [PubMed] [Google Scholar]
- 10. Harrold LR, Mazor KM, Velten S, Ockene IS, Yood RA.. Patients and providers view gout differently: a qualitative study. Chronic Illn 2010;6:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramsubeik K, Ramrattan LA, Kaeley GS, Singh JA.. Effectiveness of healthcare educational and behavioral interventions to improve gout outcomes: a systematic review and meta-analysis. Ther Adv Musculoskelet Dis 2018;10:235–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abhishek A, Doherty M.. Education and non-pharmacological approaches for gout. Rheumatology (Oxford) 2018;57:i51–8. [DOI] [PubMed] [Google Scholar]
- 13. Dalbeth N, Bardin T, Doherty M. et al. Discordant American College of Physicians and international rheumatology guidelines for gout management: Consensus statement of the Gout, Hyperuricemia and Crystal-Associated Disease Network (G-CAN). Nat Rev Rheumatol 2017;13:561–8. [DOI] [PubMed] [Google Scholar]
- 14. Janssens HL, Schaafstra A, Shackleton DP. et al. NHG-STANDAARD: Artritis [November 2017]. https://richtlijnen.nhg.org/standaarden/artritis#volledige-tekst-beleid-jichtartritis (19 May 2022, date last accessed).
- 15. NVR. Richtlijn jicht 2013 [updated 03-02-2014]. https://richtlijnendatabase.nl/richtlijn/jicht/jicht_-_startpagina.html (27 June 2022, date last accessed).
- 16. FitzGerald JD, Dalbeth N, Mikuls T. et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken) 2020;72:744–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179–211. [Google Scholar]
- 18. Horne R, Weinman J.. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999;47:555–67. [DOI] [PubMed] [Google Scholar]
- 19. van Heuckelum M, Hebing RCF, Vandeberg L. et al. Are health care professionals’ implicit and explicit attitudes toward conventional disease-modifying antirheumatic drugs associated with those of their patients? Arthritis Care Res (Hoboken) 2021;73:364–73. [DOI] [PubMed] [Google Scholar]
- 20. Huiskes VJB, van den Ende CHM, van Dijk L, Burger DM, van den Bemt BJF.. Association between healthcare practitioners' beliefs about statins and patients’ beliefs and adherence. Br J Clin Pharmacol 2021;87:1082–8. [DOI] [PubMed] [Google Scholar]
- 21. Zwikker HE, Lesuis N, Den Broeder AA. et al. Rheumatologists’ beliefs about medication barely differ from patients’ medication beliefs. Eur J Person Center Healthcare 2017;5:308–14. [Google Scholar]
- 22. Ramström H, Afandi S, Elofsson K, Petersson S.. Differences in beliefs between patients and pharmaceutical specialists regarding medications. Patient Educ Couns 2006;62:244–9. [DOI] [PubMed] [Google Scholar]
- 23. Darlow B, Fullen BM, Dean S. et al. The association between health care professional attitudes and beliefs and the attitudes and beliefs, clinical management, and outcomes of patients with low back pain: a systematic review. Eur J Pain 2012;16:3–17. [DOI] [PubMed] [Google Scholar]
- 24. Foley KA, Denke MA, Kamal-Bahl S. et al. The impact of physician attitudes and beliefs on treatment decisions: Lipid therapy in high-risk patients. Med Care 2006;44:421–8. [DOI] [PubMed] [Google Scholar]
- 25. Cuschieri S. The STROBE guidelines. Saudi J Anaesth 2019;13(Suppl 1):S31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horne R, Weinman J, Hankins M.. The beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1–24. [Google Scholar]
- 27. van Geffen EC, Philbert D, van Boheemen C. et al. Patients’ satisfaction with information and experiences with counseling on cardiovascular medication received at the pharmacy. Patient Educ Couns 2011;83:303–9. [DOI] [PubMed] [Google Scholar]
- 28. de Ridder D, Theunissen N.. De rol van ziektepercepties in therapietrouw bij hypertensie. Gedrag Gezondheid: 2003;31:237–49. [Google Scholar]
- 29. Teräsvirta T, Mellin I.. Model selection criteria and model selection tests in regression models. Scand J Stat 1986;13:159–71. [Google Scholar]
- 30. Monsalves MJ, Bangdiwala AS, Thabane A, Bangdiwala SI.. LEVEL (Logical Explanations & Visualizations of Estimates in Linear mixed models): recommendations for reporting multilevel data and analyses. BMC Med Res Methodol 2020;20:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). All data relevant to the study are included in the article.