Abstract
Mammalian and avian torpor is highly effective in reducing energy expenditure. However, the extent of energy savings achieved and thus long-term survival appear to differ between species capable of multiday hibernation and species restricted to daily heterothermy, which could, however, be due to thermal effects. We tested how long-term survival on stored body fat (i.e. time to lean body mass), crucial for overcoming adverse periods, is related to the pattern of torpor expressed under different ambient temperatures (Ta: 7 °C typical of hibernation, 15 and 22 °C typical of daily torpor) in the small marsupial hibernator the pygmy-possum (Cercartetus nanus). Possums expressed torpor at all Tas and survived without food for 310 days on average at Ta 7 °C, 195 days at Ta 15 °C, and 127 days at Ta 22 °C. At Ta 7 and 15 °C, torpor bout duration (TBD) increased from < 1–3 to ~ 5–16 days over 2 months, whereas at Ta 22 °C, TBD remained at < 1 to ~ 2 days. At all Tas daily energy use was substantially lower and TBD and survival times of possums much longer (3–12 months) than in daily heterotherms (~ 10 days). Such pronounced differences in torpor patterns and survival times even under similar thermal conditions provide strong support for the concept that torpor in hibernators and daily heterotherms are physiologically distinct and have evolved for different ecological purposes.
Subject terms: Ecology, Evolution, Physiology, Zoology
Introduction
Daily torpor and multiday torpor (hibernation) in mammals and birds are crucial survival strategies because they can substantially reduce the high energy expenditure associated with endothermy. Both are employed by many species under a variety of conditions but they are either viewed as (1) two physiologically distinct patterns of torpor characteristic for certain species, selected for different ecological purposes and represented by clearly different physiological variables, or (2) as a continuum of functional variables of a single but temperature-dependent torpor pattern1.
Mammalian hibernation or multiday torpor is characterized by pronounced reductions of body temperatures (Tb) and energy expenditure2–4. The hibernation season, often from autumn to spring, typically is composed of a series of torpor bouts with a low Tb (~ 5 °C) and a torpor bout duration (TBD) of several days or weeks, which are interrupted by periodic, brief rewarming and normothermic periods with a high Tb (~ 37 °C) lasting for several hours3,5,6. Importantly and complicating terminology, although hibernators are capable of multiday torpor bouts, they also can express brief bouts of torpor lasting less than 1 day and these are typically observed at the beginning of the hibernation season, at high Ta, or when the animal is exposed to a fluctuating Ta7–11. Metabolic rates during torpor (TMR) in small hibernators can be reduced to as little as ~ 1–2% of the basal metabolic rate (BMR), whereas the energy expenditure during the entire hibernation season is usually ~ 5–20% of that in active individuals because of the energetically expensive periodic arousals that consume most of the energy during the hibernation season12–14. Nevertheless, many hibernators can survive for months relying entirely on stored body fat accumulated before the hibernation season3,12,15,16 and many species in cold climates show little or no foraging during the hibernation season.
In contrast, in mammalian daily heterotherms, which use daily torpor exclusively and seem incapable of expressing multiday torpor, TBD is only 0.34 days on average6. Daily heterotherms typically forage on a daily basis including during the torpor season, many show little or no fattening and some species, to reduce overall energy expenditure, even may lose body mass before the season they express torpor most regularly (e.g.17,18). The Tb during daily torpor is on average reduced to ~ 18 °C, the TMR to ~ 30% of BMR, much higher than in hibernators6, and the heart rates in torpid daily heterotherms are much higher than those of hibernators even at the same Tb1.
When functional variables of torpor or more specifically the physiologically possible capabilities of species are compared between the two groups of heterotherms, the mean and maximum TBD and the minimum TMR show strong bimodal distributions and differ significantly between the two groups6. The minimum Tbs also differ significantly, but show some overlap6. However, an important point to consider in this context is that variables of torpor are strongly temperature-dependent und affected by the Ta the animal is exposed to. Especially exposure to a high Ta is not likely to reveal the physiological capability of a species in the cold. This is most easily seen for the Tb, which in torpid animals follows the Ta often over a wide temperature range in both hibernators and daily heterotherms9,19 and because Tb approaches Ta but remains above Ta in both this can mask physiological differences. Moreover, during torpor entry and arousal from torpor the thermal response of Tb is to a large extent determined by the body mass of the animal and the prevailing Ta rather than the pattern of torpor expressed20–23. Nevertheless, it has been suggested that when the heterothermy index (HI) and other indices of heterotherms, based on such Tb measurements and quantified under different thermal conditions are compared, the two groups form a continuous single group with extreme values at either end24. This interpretation suggests there is only one functional group of heterothermic endotherms the physiology of which is simply a consequence of temperature and that the traditional classification of heterothermic mammals as hibernators and daily heterotherms in contrast to the homeotherms is clouded and possibly misleading24.
To resolve this controversy, crucial to the understanding of functional traits that have been selected to maximise survival in the wild, we quantified how TBD and loss of body mass of a small hibernator, reflecting energy expenditure and consequently long-term survival on stored body fat, are affected by Ta. We tested these variables under thermal conditions that are similar to the minimum Tb of hibernators (Ta 7 °C) in comparison to mild thermal conditions (Ta 15 and 22 °C) approximating the minimum Tbs of daily heterotherms. As long-term survival without food is one of the key adaptations of many hibernators, which have to survive on fat for months at low Tas in winter, this trait seems ultimately suited for tackling the question of whether there is one or two physiologically distinct groups of heterotherms.
We tested two hypotheses:
Hypothesis 1
Survival to lean body mass, TBD and body mass loss of a small hibernator are similar to those of daily heterotherms at least at mild Tas. If this is the case the interpretation of a single torpor pattern with a temperature-dependent continuum of functional variables rather than different patterns of torpor is supported.
Hypothesis 2
Survival to lean body mass, TBD and body mass loss of a small hibernator differ substantially from those of daily heterotherms including at mild Tas. If this is the case, the classification into two functionally different torpor patterns seems justified.
The species used for the experimental part of the study was the eastern pygmy-possum (Cercartetus nanus), referred to ‘possums’ in the text, a hibernator shown previously to be able express torpor under a variety of thermal conditions25 and with a lean body mass (~ 22 g) similar to that of the median (~ 26 g) of mammalian daily heterotherms6. This hibernating possum is well suited for the study because they fatten substantially and at high Ta they may express brief torpor bouts of < 1 day, temporally similar to the pattern in daily heterotherms. Consequently if functionally similar especially with regard to energy expenditure, this should be reflected in similar survival times. However, at low Ta they can remain torpid for up to 35 days26. The data on possums were compared with those of hibernators and daily heterotherms from the literature.
Results
Possums maintained under natural autumn photoperiod, at Ta 20 °C and ad libitum food fattened extensively (Table 1). In all experimental groups, body mass reached around 2.5-fold the lean body mass, and the mean body masses when the torpor experiments began at the three experimental Tas of 7, 15, and 22 °C and food was withheld did not differ (p = 0.65) among the experimental groups (range of means: 53.8–56.6 g). The body mass when most stored fat was depleted at a lean body mass of ~ 22 g and torpor experiments were terminated was also similar (p = 0.13) among the experimental groups (range of means: 20.1–22.7 g). Consequently, the loss of body mass (range of means: 32.0–34.5 g) also was similar among the groups (p = 0.89). However, the loss of body mass per day without access to food differed significantly (p < 0.0001) among the experimental groups (range of means 0.107–0.27 g/day) and increased by ~ 1.7-fold from Ta 7–15 °C and by ~ 1.5-fold from Ta 15–22 °C (Table 1). The loss of body mass per day after the gut was voided and when TBD was near stable was lower than that measured over the entire torpor period (range of means 0.051–0.18 g/day), but also differed significantly (p < 0.0001) among the experimental groups (Table 1); it increased by 2.5-fold from Ta 7–15 °C and by 1.4-fold from Ta 15–22 °C.
Table 1.
Body masses of pygmy possums (Cercartetus nanus) before and after the period of food withdrawal and the loss of body mass during that time.
| Ambient temperature (°C) | Body mass | Body mass | Body mass | Body mass | Body mass |
|---|---|---|---|---|---|
| Start (g) | End (g) | Loss (g) | Loss all (g/day) | Loss after > 38 days (g/day) | |
| 7 | 53.8 (1.4) | 20.1 (0.2) | 32.9 (1.4) | 0.107 (0.004) | 0.051 (0.006) |
| 15 | 56.6 (2.7) | 22.1 (0.5) | 34.5 (2.2) | 0.183 (0.010) | 0.131 (0.013) |
| 22 | 56.4 (2.1) | 22.7 (0.7) | 33.7 (2.5) | 0.271 (0.014) | 0.180 (0.009) |
| ANOVA F2,12 | 0.44 | 2.41 | 0.89 | 49.31 | 32.81 |
| p = 0.65 | p = 0.13 | p = 0.89 | p < 0.0001 | p < 0.0001 |
Body mass loss after > 38 days when the gut content was voided and TBD was stable is also shown for calculation of energy expenditure. Values are means with SE (in brackets) for n = 5 individuals at each Ta. P values for differences in body mass among experimental groups at the beginning and end of the experiment and loss of body mass are provided.
Daily energy expenditure, estimated from body mass loss when TBD of possums was steady, was 2.0 kJ/day or 2% of the field metabolic rate (FMR) of a 30-g marsupial mammal (83.7 kJ/day27) at Ta 7 °C, 5.1 kJ/day or 6.1% of FMR at Ta 15 °C, and 7.0 kJ/day or 8.4% of FMR at Ta 22 °C. The daily energy expenditure of possums at the different Tas was only ~ 7–28% of the average daily metabolic rate of a 30-g daily heterotherm (~ 30 kJ/day) using daily torpor.
All possums expressed torpor at all Tas examined and both Ta (p < 0.0001) and month (p < 0.0001) had highly significant effects on TBD. Initially, animals rewarmed frequently and TBD was brief (< 1–3 days, Fig. 1). During the first month of torpor use, TBD was indistinguishable among thermal groups (1.1 ± 0.2 days at Ta 7 °C, 1.1 ± 0.2 days at Ta 15 °C; 1.1 ± 0.2 days at Ta 22 °C). The TBD at Ta 7 °C increased over the next two months to mean values ranging from 11.3 days to 16.1 days. All individuals at Ta 7 °C were able to hibernate on stored fat to month 9, four individuals to month 10, three individuals to month 12 and one individual to month 13. At Ta 15 °C, the TBD also increased over the first two months after food was withheld, but unlike at Ta 7 °C, the TBD increased only to mean values ranging from 4.8 to 6.0 days. All individuals at Ta 15 °C were able to hibernate on stored fat to month 5, four individuals to month 6, three individuals to month 7, two individuals to month 8 and one individual to month 10 with a mean TBD of 7.7 days in that month. At Ta 22 °C, TBD was similar to that of the other experimental groups on month 1 and lasted for 1.1 days. However, unlike for the other experimental groups, the TBD did not change substantially over the next months and mean TBDs at Ta 22 °C ranged between 1.1 and 1.6 days, but TBDs of < 1 day were observed in all months. Nevertheless, all individuals at Ta 22 °C were able to hibernate on stored fat to month 3, four individuals to month 5 and one individual to month 6 when its mean TBD was 0.9 days.
Figure 1.
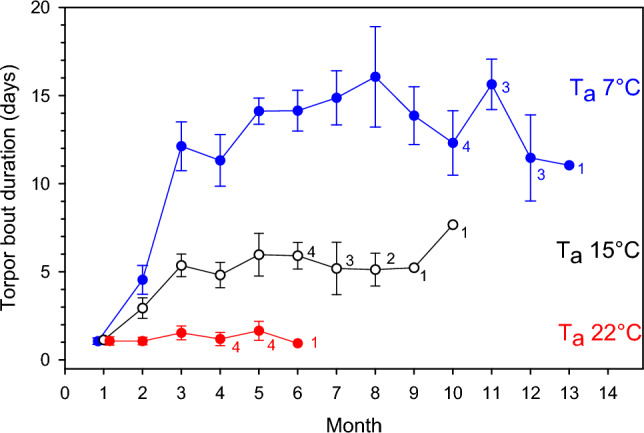
Torpor bout duration (TBD) as a function of time of pygmy-possums (Cercartetus nanus) hibernating at three different ambient temperatures (Ta, 7 °C blue, 15 °C black and white, 22 °C red). Means with SE for n = 5 individuals at each Ta are shown, or numbers for individuals are shown next to the values. Both Ta (F = 69.1, p < 0.0001) and month (F = 37.0, p < 0.0001) had highly significant effects on TBD.
The mean TBD was strongly and negatively affected by Ta (Fig. 2). The mean TBD was 11.2 ± 1.9 days at Ta 7 °C, 4.6 ± 0.8 days at Ta 15 °C, and 1.2 ± 0.1 days at Ta 22 °C. TBD increased 9.3-fold from Ta 22 to 7 °C, 2.4-fold from Ta 15 to 7 °C, and 3.8-fold from Ta 22 to 15 °C. The mean TBD of possums at Ta 7 °C was slightly above that of mammalian hibernators (8.25 days, n = 70 species) at their mean Tb of 6.2 °C, but within the 95% confidence interval of hibernators6. In contrast at Ta 18.1 °C, equalling the mean minimum Tb of mammalian daily heterotherms, the TBD of possums was 9.9-fold of the mean TBD of daily heterotherms (0.34 days, n = 50 species) and well above the 95% confidence interval of daily heterotherms6.
Figure 2.
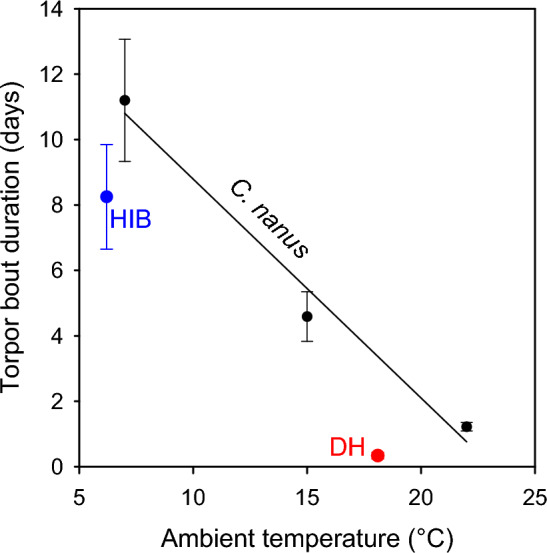
Mean torpor bout duration (TBD) of pygmy-possums (Cercartetus nanus) as a function of ambient temperature (Ta). Mean TBD was strongly affected by Ta: y = 15.48–0.67 x; r2 = 0.98. The mean TBD of other hibernators at the mean minimum Tb of hibernators (HIB blue dot, n = 70 species) and the mean TBD at the mean Tb of mammalian daily heterotherms (DH red dot, n = 50 species) are shown for comparison (data from6). The minimum Tb is shown on the Ta axis because the Tb-Ta differential is small during steady-state torpor.
The mean time possums were able to survive on stored fat or the time to lean body mass differed significantly (p < 0.001) among the three experimental groups (Fig. 3). At Ta 7 °C, mean survival time was 310 days, 1.6-fold the survival time of possums at Ta 15 °C, where they survived on stored fat for 195 days on average. At Ta 22 °C, mean survival time was 127 days, 40% of that at Ta 7 °C and 65% of that at Ta 15 °C. The 9.3-fold increase in TBD from 1.2 days at Ta 22 °C to 11.2 days at Ta 7 °C was accompanied by a 2.43-fold increase in survival time. The mean survival time of three small hibernators (322 ± 31 days) at the mean minimum Tb of hibernators was similar to that of possums (Fig. 3). In contrast, the mean survival times of four small daily heterotherms (9.5 ± 3.9 days) at the mean minimum Tb of 18.1 °C was only 5.7% of possums at that temperature.
Figure 3.
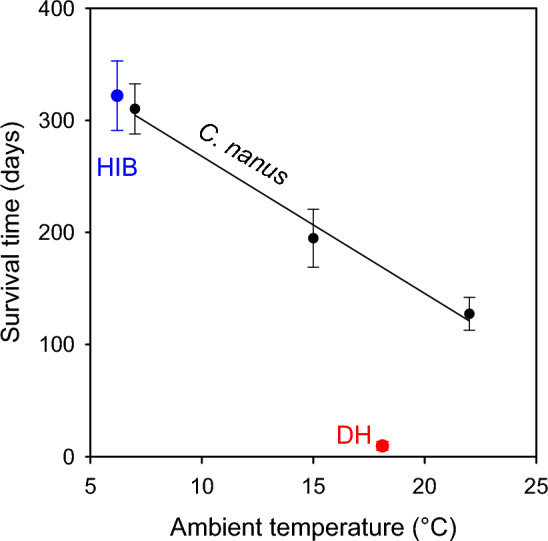
The survival times (time to lean body mass) without food of pygmy-possums (Cercartetus nanus) held at three ambient temperatures (Ta). Means with SE for n = 5 individuals at each Ta are shown. Survival times differed significantly among the three groups (F2,12 = 18.52; p < 0.001). Mean survival times were a linear function of Ta: y = 390.3–12.34 x; r2 = 0.99. The mean survival time without food of other hibernators (3 species at a mean body mass of 50 g17) at the mean minimum Tb of hibernators (HIB blue dot) and the mean survival time at the mean Tb of mammalian daily heterotherms (DH red dot) are shown for comparison (Tb data from6; survival times of daily heterotherms were for 4 species (Apus apus juvenile, Sminthopsis crassicaudata, Dasycercus cristicauda, Peromyscus maniculatus bairdi) at a mean body mass of 48 g from33,44,45). The minimum Tb is shown on the Ta axis because the Tb − Ta differential is small during steady-state torpor.
Considering the relationships between Ta and TBD, and Ta and survival time, it is not surprising that TBD and mean survival times were strongly correlated (Fig. 4). A mean TBD of 1.2 days in possums was accompanied by a mean survival time of 127 days, whereas a mean TBD of 11.2 days by a mean survival time of 310 days. Thus a 1-day increase in TBD resulted in a 0.24-fold increase in possum survival time. While the survival times of small hibernators at the mean TBD was similar to that of possums, the survival times of daily heterotherms at the mean TBD was only 7% of that in possums.
Figure 4.
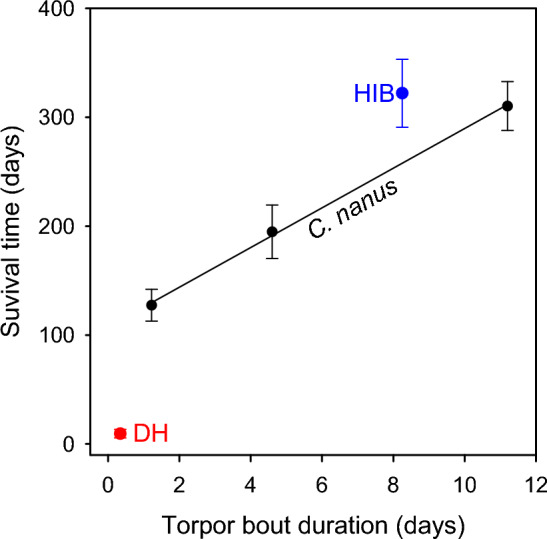
The survival times (time to lean body mass) without food of pygmy-possums (Cercartetus nanus) as a function of torpor bout duration (TBD). Mean survival times were a linear function of TBD: y = 107.5 + 18.2 x; r2 = 0.99. The mean survival times of small hibernators and daily heterotherms as in Fig. 3 at the mean TBDs for the groups from6.
Discussion
Our study shows that irrespective of the thermal conditions, possums capable of hibernation, were able to survive for months on stored fat including when exposed thermal conditions experienced by daily heterotherms during daily torpor. The TBD of possums was strongly temperature-dependent, but even when exposed to a high Ta, torpor bouts, similar to 13-lined ground squirrels5, were much longer than in daily heterotherms, resulting in comparatively lower mass loss, lower energy expenditure and much longer survival times in the small hibernator.
Thus, Hypothesis 1, which tested whether TBD, survival to lean body mass and mass loss of the small hibernator are similar to that of daily heterotherms at least at mild Tas, is not supported. Both torpor patterns and survival differed between possums capable of multiday hibernation and daily heterotherms even at high Tas when TBDs of possums were relatively short. In contrast, Hypothesis 2, which predicted that the small hibernator differs from daily heterotherms with regard to torpor patterns and survival times even at high Tas is supported by the observation of clearly distinct patterns of torpor pattern of possums and consequently much longer survival times than those expressed by daily heterotherms at all Tas investigated. This provides strong support for the view that daily torpor and hibernation are physiologically distinct. Rather than forming a continuum, the two patterns of heterothermy differ functionally from each other and also from the homeotherms. Thus, from a thermal energetics perspective, the three groups of endotherms, hibernators, daily heterotherms and homeotherms appear to represent ‘punctuated equilibria’.
With regard to the two groups of heterotherms, although torpor in both serves as energy and water conserving mechanism, the patterns appear to have been selected for different purposes. Hibernation in fat-storing hibernators aids primarily long-term survival of adverse conditions without or limited access to food3, whereas daily torpor assists in minimising daily energy expenditure and foraging requirements often under any environmental and trophic conditions and for adjustment of energy expenditure to energy availability28.
At all Tas examined, once possums had settled into a pattern of steady torpor bouts, which importantly was not instant, but took ~ 2 months at Ta 7 and 15 °C (Fig. 1), mass loss and consequently energy expenditure were very low. Although the values for daily energy expenditure observed here were affected by Ta and increased with Ta, daily energy use of possums, despite periodic rewarming was a small fraction of the FMR of normothermic individuals, but similar to or at the low end of that observed in other hibernators13,14. Daily energy use of possums was however substantially lower (by ~ 70–90%) than in daily heterotherms expressing daily torpor under comparable thermal conditions.
Some of the differences observed for physiological variables between species expressing different patterns of torpor may raise the question as to why the interpretation of a single temperature-dependent pattern of torpor was developed. As outlined above this is largely related to the variables compared and their temperature-dependence. If Tb is compared it is important to consider that during torpor it is to some extent a function of Ta and, although Tb often may be a reasonable proxy for metabolic processes during torpor, it is the reduction of metabolism and water loss that are major purposes of torpor. Animals do not primarily enter torpor to reduce Tb, but to lower energy and water use and odour towards predators29. Although a low Tb contributes to the reduction of these vital processes, its fall is unavoidable at Tas below the thermo-neutral zone because metabolism is reduced first at torpor entry and Tb typically follows.
Relying on Tb measurements to quantify torpor may be logistically the most feasible approach to quantifying temporal and thermal aspects of torpor in free-ranging animals, but its reliability as a proxy for physiological functions and especially energy expenditure is not as precise as is often assumed or implied. Energy savings are typically viewed to differ between hibernators and daily heterotherms because, in addition to longer TBDs, the former use metabolic inhibition more extensively and can reduce metabolism substantially with only small reductions in Tb26,30,31. Differences in heart rates reflecting metabolic rate also differ substantially between hibernators and daily heterotherms even at the same Tb1. Another potential problem of relying on Tb measures for estimating energy expenditure is passive rewarming from torpor which has been observed in many species. For the same increase of Tb when it is raised passively, metabolism changes only little, which is in sharp contrast to endothermic rewarming which requires an enormous increase in metabolism with substantial consequences for energy budgets (see e.g.32).
With regard to comparisons of long-term survival on stored body fat it is of course important to consider the substantial differences in fattening in hibernators and specifically possums in contrast to many daily heterotherms. Different fat stores could be used to argue that simply the extent of fat storage is responsible for the observed differences and reject our arguments. However, all possums at all Tas examined were able to survive a ~ 60% reduction in body mass over > 120 days without food, whereas obese deer mice (Peromyscus maniculatus), typical daily heterotherms of similar size to possums, died after a ~ 30–50% reduction in body mass, but within only 3–5 days of food withdrawal33. Although fat storage is important in this context, the combined effects of prolonged torpor bouts and extremely low TMRs in hibernators are the traits that are mainly responsible for the observed differences with daily heterotherms and permit the slow use of stored fat. Interestingly, TMR and TBD also appear to be functionally linked34 and in turn likely contribute to the exceptional longevity observed for many hibernators35. Considering that both survival and TBD are strongly affected by Ta in possums it may not be especially surprising that survival is strongly affected by TBD, what is surprising is how strong the relationship is (Fig. 4).
Possums are not exceptional. For example, small (~ 10-g) insectivorous bats exhibit both short and multiday bouts of torpor often within the same season. Nevertheless, their minimum TMRs during torpor bouts of < 1 day are similar to those of similar-sized hibernators10,36–38. Even much larger hibernating dormice (Glis glis, ~ 150 g), exhibit both long and short bouts of torpor7,16,39. During short bouts TMR of G. glis was as low as 0.05 mlO2/g/h similar to that predicted for the minimum TMR of hibernators and much lower than that of daily heterotherms. Generally, the minimum TMR in hibernators, including during short bouts of torpor1,10,37,38 is less than 20% of that during daily torpor6, and one would therefore expect that fat reserves last proportionally longer in hibernators. This also could be one of the reasons why on average hibernators are larger than daily heterotherms6 to permit increased fat stores40.
Our data raise a conundrum in terminology with regard to the use of the term ‘daily torpor’. If we consider torpor solely from a temporal point of view, a Tb reduction for less than 1 day could be called ‘daily torpor’ for all species, but this would not reflect the same physiological response in all. In small bats, discussed above, torpor bouts in summer often last less than 1 day and are often referred to ‘daily torpor’ in the literature, but as stated above it has been shown for several species that even though they may appear thermally and temporally similar, metabolically they differ substantially from daily heterotherm (e.g.1,37). Thus, energetic estimates based on temporal variations of Tb only will be erroneous because short bouts of torpor in hibernators differ metabolically from those in daily heterotherms. Consequently, using ‘daily torpor’ to describe torpor bout of < 1 day in hibernators seems imprecise at best and this term should be reserved to the daily heterotherms, whereas in hibernators it should be called short bouts of torpor or similar to acknowledge the functional differences.
So why should we care whether there are 1 or 2 patterns of torpor? We should for several reasons. If all species were part of the same physiological group and only Tb or temperature effects were responsible for an observed heterothermic continuum, one would expect all species to perform the same under the same thermal conditions. Clearly, as we have shown here, this is not the case. Since the primary goal of hibernators seems to be survival without food for prolonged periods, whereas in daily heterotherms it is the reduction of energy expenditure to reduce food requirements during periods that include regular foraging, different physiological and behavioural mechanisms are required. Such behavioural, ecological and physiological differences will profoundly affect seasonal survival in relation to temporal changes in food availability and thermal conditions. Further, they are also crucial in relation to climate change with a predicted increase in temperature and the resulting new functional challenges that will require appropriate responses depending on the physiological capabilities of a species.
Methods
Study species and procedures
Possums captured at ~ 800 m elevation on the cool-temperate New England Tablelands (30° 22′ S, 152° 45′ E) near Dorrigo, New South Wales, Australia, were maintained at a Ta of 20 °C under natural photoperiod. Animals were held individually in cages provided with wood shavings and bedding, and were offered a surplus of food consisting of a mixture of high protein baby cereal, honey and water with added multivitamins and minerals, nuts and apples.
In austral autumn when body mass was high (> 50 g, ~ 2.5-fold that of lean adult mass, Table 1), individually caged animals (n = 5 for each thermal condition) and supplied with wood shavings, were transferred to temperature-controlled cabinets at one of three Tas: 7.3 ± 0.3 °C (SD), 15.0 ± 0.3 °C, and 21.7 ± 0.8 °C, referred to in the text as: Ta 7, 15, and 22 °C. A Ta of 7 °C is slightly above the mean minimum Tb (6.2 °C) of hibernating mammals and above the minimum Tb of C. nanus6. Ta 15 °C is slightly below the minimum Tb (16.9 °C) of a 30-g mammalian daily heterotherm and Ta 22 °C is similar to the minimum Tb (21.8 °C) of a 30-g avian daily heterotherm6. The photoperiod was L10:D14, which approximates the shortest yearly photoperiod within the species' range in south-eastern Australia; artificial light was provided by a dim incandescent bulb (10W). Water was freely available and food was withheld.
Periodic arousals from torpor were quantified non-invasively by passive infrared detectors that determined when each animal rewarmed periodically and re-entered torpor. For this purpose, passive infrared detectors (Jaycar Electronics LA-5017), which monitored the temperature profile of the cage and animal surface over an angle of 90º, were placed on top of each cage; activity events and thus arousals and normothermic periods were summed over 30 min and stored on a data logger (Electronic Services Unit, University of New England). Arousals were confirmed by checking daily whether fine sawdust that had been placed on animals when they first entered torpor had been removed12. Possums were weighed (to the nearest 0.1 g) at regular intervals to determine the loss of body mass over time and were removed from the experiment and offered food when their body mass reached ~ 22 g (Table 1), the lean mass of adults.
Animal ethics declaration
This study was conducted under a scientific license provided by the NSW Parks and Wildlife Authority (SL100791) under the guidelines of the Environment Protection and Biodiversity Act 1999. Animal Ethics approval was granted by the animal ethics committee of the University of New England. The study was carried out in compliance with the ARRIVE guidelines on animal research and the Australian Code for the Care and Use of Animals for Scientific Research.
Analyses
We measured and analysed body mass before and after the experiment, overall body mass loss, as well as body mass loss after the initial weeks of hibernation. The body mass loss after the initial hibernation phase (i.e. after > 38 days) when gut content was voided and TBD was steady was assumed to be entirely the result of fat metabolism2 because animals had access to water and likely maintained body water content at a steady percentage41,42. The energy consumption during this time was assumed to be equivalent to 38.9 kJ/g of fat lost43. Mean monthly TBD was determined and when a torpor bout extended over two months it was assigned to the month in which the greater part of the bout occurred. Survival times were calculated from the day food was removed and the animals were transferred to the temperature cabinet to the day they had reached their lean body mass (Table 1). Some of the data at Ta 7 °C were used in a paper12 with a different emphasis to the present study and all data were re-analysed for the purpose of the present study.
Measured values of TBD in possums were compared with those of daily heterotherms (n = 50 species) and hibernators (n = 70 species) as reported6. Survival times of hibernators without food were averaged for three species with a mean body mass of 50 g on average (from17). Survival times of daily heterotherms without food were obtained for four species (Apus apus, Sminthopsis crassicaudata, Dasycercus cristicauda and Peromyscus maniculatus) weighing 48 g on average33,44,45. Importantly, the three hibernators survived the food restrictions whereas the daily heterotherms died (as reflected by the times these experiments were permitted) and although there are data on physiological variables on many heterothermic species, data on survival times without food are scarce.
Numerical values in the results are expressed as means ± SE for n = 5 individuals at each Ta. Residuals from all statistical models were normally distributed, as confirmed by visual inspection (quantile–quantile plots). Differences in body mass among experimental groups at the beginning and end of the experiment and loss of body mass were compared using one-way ANOVAs. Repeated measurements of TBD were analysed with a mixed effects model using the intercept as a random effect (R package lme446 followed by ANOVA using package car47). Least squares regressions were used for linear fits.
Acknowledgements
We would like to thank Rebecca Drury and Wendy Westman for technical assistance. We thank Renate Hengsberger for her help with citations, formatting and submission of the article. The Australian Research Council supported the work.
Author contributions
F.G. designed and conducted the experiments, analysed the data and wrote drafts of the manuscript. T.R. analysed the data and contributed to and revised the manuscript.
Funding
Open access funding provided by University of Veterinary Medicine Vienna. The work was supported by the Australian Research Council.
Data availability
The datasets generated and analyzed during the current study will be made available by request to F. Geiser (fgeiser@une.edu.au).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Currie SE, Körtner G, Geiser F. Pronounced differences in heart rate and metabolism distinguish daily torpor and short-term hibernation in two bat species. Sci. Rep. 2022;12:21721. doi: 10.1038/s41598-022-25590-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyer BB, Barnes BM. Molecular and metabolic aspects of mammalian hibernation. BioScience. 1999;49:713–724. doi: 10.2307/1313595. [DOI] [Google Scholar]
- 3.French AR. Allometries of the durations of torpid and euthermic intervals during mammalian hibernation: A test of the theory of metabolic control of the timing of changes in body temperature. J. Comp. Physiol. B. 1985;156:13–19. doi: 10.1007/BF00692921. [DOI] [PubMed] [Google Scholar]
- 4.Lyman CP, Willis JS, Malan A, Wang LCH. Hibernation and Torpor in Mammals and Birds. Academic Press; 1982. [Google Scholar]
- 5.MacCannell ADV, Staples JF. Elevated ambient temperature accelerates aspects of torpor phenology in an obligate hibernator. J. Therm. Biol. 2021;96:102839. doi: 10.1016/j.jtherbio.2021.102839. [DOI] [PubMed] [Google Scholar]
- 6.Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol. Rev. 2015;90:891–926. doi: 10.1111/brv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieber C, Ruf T. Summer dormancy in edible dormice (Glis glis) without energetic constraints. Naturwissenschaften. 2009;96:165–171. doi: 10.1007/s00114-008-0471-z. [DOI] [PubMed] [Google Scholar]
- 8.Blanco MB, Greene LK, Schopler R, Williams CV, Lynch D, Browning J, et al. On the modulation and maintenance of hibernation in captive dwarf lemurs. Sci. Rep. 2021;11:5740. doi: 10.1038/s41598-021-84727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dausmann KH, Warnecke L. Primate torpor expression: Ghost of the climatic past. Physiology. 2016;31:398–408. doi: 10.1152/physiol.00050.2015. [DOI] [PubMed] [Google Scholar]
- 10.Geiser F, Brigham RM. Torpor, thermal biology, and energetics in Australian long-eared bats (Nyctophilus) J. Comp. Physiol. B. 2000;170:153–162. doi: 10.1007/s003600050270. [DOI] [PubMed] [Google Scholar]
- 11.Nowack J, Cooper CE, Geiser F. Cool echidnas survive the fire. Proc. R. Soc. B. 2016;283:20160382. doi: 10.1098/rspb.2016.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geiser F. Yearlong hibernation in a marsupial mammal. Naturwissenschaften. 2007;94:941–944. doi: 10.1007/s00114-007-0274-7. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DW, Dorais M, Bergeron J-M. Winter energy budgets and cost of arousals for hibernating little brown bats, Myotis lucifugus. J. Mammal. 1990;71:475–479. doi: 10.2307/1381967. [DOI] [Google Scholar]
- 14.Wang LCH. Energetic and field aspects of mammalian torpor: The Richardson's ground squirrel. In: Wang LCH, Hudson JW, editors. Strategies in Cold: Natural Torpidity and Thermogenesis. Academic Press; 1978. pp. 109–145. [Google Scholar]
- 15.Bieber C, Lebl K, Stalder G, Geiser F, Ruf T. Body mass dependent use of hibernation: Why not prolong the active season, if they can? Funct. Ecol. 2014;28:167–177. doi: 10.1111/1365-2435.12173. [DOI] [Google Scholar]
- 16.Hoelzl F, Bieber C, Cornils JS, Gerritsmann H, Stalder GL, Walzer C, et al. How to spend the summer? Free-living dormice (Glis glis) can hibernate for 11 months in non-reproductive years. J Comp. Physiol. B. 2015;185:931–939. doi: 10.1007/s00360-015-0929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiser F. Ecological Physiology of Daily Torpor and Hibernation. Springer; 2021. [Google Scholar]
- 18.Steinlechner S, Heldmaier G, Becker H. The seasonal cycle of body weight in the Djungarian hamster: Photoperiodic control and the influence of starvation and melatonin. Oecologia. 1983;60:401–405. doi: 10.1007/BF00376859. [DOI] [PubMed] [Google Scholar]
- 19.Nowack J, Levesque DL, Reher S, Dausmann KH. Variable climates lead to varying phenotypes: “Weird” mammalian torpor and lessons from non-holarctic species. Front. Ecol. Evol. 2020;8:60. doi: 10.3389/fevo.2020.00060. [DOI] [Google Scholar]
- 20.Geiser F, Baudinette RV. The relationship between body-mass and rate of rewarming from hibernation and daily torpor in mammals. J. Exp. Biol. 1990;151:349–359. doi: 10.1242/jeb.151.1.349. [DOI] [PubMed] [Google Scholar]
- 21.McKechnie AE, Wolf BO. The energetics of the rewarming phase of avian torpor. In: Barnes BM, Carey HV, editors. Life in the Cold V: Evolution, Mechanism, Adaptation, and Application. Twelfth International Hibernation Symposium Biological Papers of the University of Alaska, number 27. Institute of Arctic Biology, University of Alaska; 2004. pp. 265–273. [Google Scholar]
- 22.Menzies AK, Webber QMR, Baloun DE, McGuire LP, Muise KA, Coté D, et al. Metabolic rate, latitude and thermal stability of roosts, but not phylogeny, affect rewarming rates of bats. Physiol. Behav. 2016;164:361–368. doi: 10.1016/j.physbeh.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Nicol SC, Andersen NA, Arnold W, Ruf T. Rewarming rates of two large hibernators: Comparison of a monotreme and a eutherian. J. Therm. Biol. 2009;34:155–159. doi: 10.1016/j.jtherbio.2009.01.003. [DOI] [Google Scholar]
- 24.Boyles JG, Thompson AB, McKechnie AE, Malan E, Humphries MM, Careau V. A global heterothermic continuum in mammals. Glob. Ecol. Biogeogr. 2013;22:1029–1039. doi: 10.1111/Geb.12077. [DOI] [Google Scholar]
- 25.Turner JM, Körtner G, Warnecke L, Geiser F. Summer and winter torpor use by a free-ranging marsupial. Comp. Biochem. Physiol. A. 2012;162:274–280. doi: 10.1016/j.cbpa.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Song X, Körtner G, Geiser F. Thermal relations of metabolic rate reduction in a hibernating marsupial. Am. J Physiol. Reg. Int. Comp. Physiol. 1997;273:R2097–R2104. doi: 10.1152/ajpregu.1997.273.6.R2097. [DOI] [PubMed] [Google Scholar]
- 27.Nagy KA, Girard IA, Brown TK. Energetics of free-ranging mammals, reptiles, and birds. Annu. Rev. Nutr. 1999;19:247–277. doi: 10.1146/annurev.nutr.19.1.247. [DOI] [PubMed] [Google Scholar]
- 28.Shankar A, Cisneros INH, Thompson S, Graham CH, Powers DR. A heterothermic spectrum in hummingbirds. J. Exp. Biol. 2022;225:jeb243208. doi: 10.1242/jeb.243208. [DOI] [PubMed] [Google Scholar]
- 29.Brown LN. Population dynamics of the western jumping mouse (Zapus princeps) during a four-year study. J. Mammal. 1970;51:651–658. doi: 10.2307/1378291. [DOI] [Google Scholar]
- 30.Reher S, Dausmann KH. Tropical bats counter heat by combining torpor with adaptive hyperthermia. Proc. R. Soc. B. 2021;288:20202059. doi: 10.1098/rspb.2020.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tøien Ø, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: Independence of metabolic suppression from body temperature. Science. 2011;331:906–909. doi: 10.1126/science.1199435. [DOI] [PubMed] [Google Scholar]
- 32.Currie SE, Noy K, Geiser F. Passive rewarming from torpor in hibernating bats: Minimizing metabolic costs and cardiac demands. Am. J Physiol. Reg. Int. Comp. Physiol. 2015;308:R34–R41. doi: 10.1152/ajpregu.00341.2014. [DOI] [PubMed] [Google Scholar]
- 33.Howard WE. Relation between low temperatures and available food to survival of small rodents. J. Mammal. 1951;32:300–312. doi: 10.2307/1375662. [DOI] [Google Scholar]
- 34.Ruf T, Giroud S, Geiser F. Hypothesis and theory: A two-process model of torpor-arousal regulation in hibernators. Front. Physiol. 2022;13:901270. doi: 10.3389/fphys.2022.901270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turbill C, Bieber C, Ruf T. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B. 2011;278:3355–3363. doi: 10.1098/rspb.2011.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Currie SE, Stawski C, Geiser F. Cold-hearted bats: Uncoupling of heart rate and metabolism during torpor at sub-zero temperatures. J. Exp. Biol. 2018;221:jeb170894. doi: 10.1242/jeb.170894. [DOI] [PubMed] [Google Scholar]
- 37.Stawski C, Geiser F. Do season and distribution affect thermal energetics of a hibernating bat endemic to the tropics and subtropics? Am. J. Physiol. Reg. Int. Comp. Physiol. 2011;301:R542–R547. doi: 10.1152/ajpregu.00792.2010. [DOI] [PubMed] [Google Scholar]
- 38.Turbill C, Körtner G, Geiser F. Timing of the daily temperature cycle affects the critical arousal temperature and energy expenditure of lesser long-eared bats. J. Exp. Biol. 2008;211:3871–3878. doi: 10.1242/Jeb.023101. [DOI] [PubMed] [Google Scholar]
- 39.Wilz M, Heldmaier G. Comparison of hibernation, estivation and daily torpor in the edible dormouse, Glis glis. J. Comp. Physiol. B. 2000;170:511–521. doi: 10.1007/s003600000129. [DOI] [PubMed] [Google Scholar]
- 40.Calder WA. Size, Function, and Life History. 2. Dover Publications; 1996. [Google Scholar]
- 41.Fisher KC, Manery JF, et al. Water and electrolyte metabolism in heterotherms. In: Fisher KC, et al., editors. Mammalian Hibernation III. Oliver and Boyd; 1967. pp. 235–279. [Google Scholar]
- 42.Thomas DW, Geiser F. Periodic arousals in hibernating mammals: Is evaporative water loss involved? Funct. Ecol. 1997;11:585–591. doi: 10.1046/j.1365-2435.1997.00129.x. [DOI] [Google Scholar]
- 43.Withers PC, Cooper CE, Maloney SK, Bozinovic F, Cruz Neto AP. Ecological and Environmental Physiology of Mammals. Oxford University Press; 2016. [Google Scholar]
- 44.Kennedy PM, Macfarlane WV. Oxygen consumption and water turnover of the fat-tailed marsupials Dasycercus cristicauda and Sminthopsis crassicaudata. Comp. Biochem. Physiol. A. 1971;40:723–732. doi: 10.1016/0300-9629(71)90257-X. [DOI] [PubMed] [Google Scholar]
- 45.Koskimies J. On temperature regulation and metabolism in the swift, Micropus apus L., during fasting. Experientia. 1948;4:274–276. doi: 10.1007/BF02164408. [DOI] [PubMed] [Google Scholar]
- 46.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 47.Fox J, Weisberg S. An R Companion to Applied Regression. 3.0. Sage Publications; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study will be made available by request to F. Geiser (fgeiser@une.edu.au).


