Summary
Background
Using country-specific surveillance data to describe influenza epidemic activity could inform decisions on the timing of influenza vaccination. We analysed surveillance data from African countries to characterise the timing of seasonal influenza epidemics to inform national vaccination strategies.
Methods
We used publicly available sentinel data from African countries reporting to the WHO Global Influenza Surveillance and Response FluNet platform that had 3–10 years of data collected during 2010–19. We calculated a 3-week moving proportion of samples positive for influenza virus and assessed epidemic timing using an aggregate average method. The start and end of each epidemic were defined as the first week when the proportion of positive samples exceeded or went below the annual mean, respectively, for at least 3 consecutive weeks. We categorised countries into five epidemic patterns: northern hemisphere-dominant, with epidemics occurring in October–March; southern hemisphere-dominant, with epidemics occurring in April–September; primarily northern hemisphere with some epidemic activity in southern hemisphere months; primarily southern hemisphere with some epidemic activity in northern hemisphere months; and year-round influenza transmission without a discernible northern hemisphere or southern hemisphere predominance (no clear pattern).
Findings
Of the 34 countries reporting data to FluNet, 25 had at least 3 years of data, representing 46% of the countries in Africa and 89% of Africa's population. Study countries reported RT-PCR respiratory virus results for a total of 503 609 specimens (median 12 971 [IQR 9607–20 960] per country-year), of which 74 001 (15%; median 2078 [IQR 1087–3008] per country-year) were positive for influenza viruses. 248 epidemics occurred across 236 country-years of data (median 10 [range 7–10] per country). Six (24%) countries had a northern hemisphere pattern (Algeria, Burkina Faso, Egypt, Morocco, Niger, and Tunisia). Eight (32%) had a primarily northern hemisphere pattern with some southern hemisphere epidemics (Cameroon, Ethiopia, Mali, Mozambique, Nigeria, Senegal, Tanzania, and Togo). Three (12%) had a primarily southern hemisphere pattern with some northern hemisphere epidemics (Ghana, Kenya, and Uganda). Three (12%) had a southern hemisphere pattern (Central African Republic, South Africa, and Zambia). Five (20%) had no clear pattern (Côte d'Ivoire, DR Congo, Madagascar, Mauritius, and Rwanda).
Interpretation
Most countries had identifiable influenza epidemic periods that could be used to inform authorities of non-seasonal and seasonal influenza activity, guide vaccine timing, and promote timely interventions.
Funding
None.
Translations
For the Berber, Luganda, Xhosa, Chewa, Yoruba, Igbo, Hausa and Afan Oromo translations of the abstract see Supplementary Materials section.
Introduction
Influenza disease causes approximately 28 000–163 000 deaths in Africa annually.1, 2, 3 Influenza vaccines remain the primary method to lower the risk of severe illness and mortality;4, 5 however, few countries in Africa use influenza vaccines. While influenza vaccines and antivirals are available and accessible in some African countries, influenza-related policy data from the WHO–UNICEF Joint Reporting Form show that only five (10%) of 47 African WHO member states have a national influenza immunisation policy (Algeria, Côte d'Ivoire, Kenya, Morocco, and South Africa).6 Of these five countries, only Morocco and Algeria target all WHO-recommended groups at increased risk (ie, children aged ≤5 years, people with chronic illness, pregnant people, health-care workers, and older adults aged >65 years).7
Ascertaining the appropriate vaccine formulation and epidemic timing are key to successful seasonal influenza vaccination and empirical antiviral treatment.8 However, assessing the timing of influenza activity can be challenging in tropical countries because epidemic activity can vary each year and might not align well with the traditional northern hemisphere (October–March) or southern hemisphere (April–September) epidemic patterns.9, 10 The current WHO transmission zones that inform seasonal influenza vaccination are geographical groups of countries, areas, or territories that have been defined to have similar influenza transmission patterns.11 A modelling study of peak timing for influenza activity for 125 countries found that 62% of northern hemisphere and 53% of southern hemisphere tropical countries used vaccines that seemed out of phase with their dominant epidemic patterns.9 Using a long time-series of country-specific surveillance data to model influenza epidemics, countries could improve decisions about which formulations to choose, when to vaccinate, and when to treat empirically with antivirals.8, 12
Research in context.
Evidence before this study
We searched PubMed, MEDLINE, and Embase with no language restrictions for relevant studies that were published until Jan 19, 2023, using the search terms “(Influenza OR avian influenza OR influenzas OR respiratory virus*) AND (surveillance OR survey OR outbreak* OR epidemic* OR epidemiolog* OR trend* OR pattern*) OR (AB (surveillance OR survey OR outbreak* OR epidemic* OR epidemiolog* OR trend* OR pattern* OR Seasonality*)) AND LMICs (exp Africa/ OR exp “south and central America”/ OR exp Asia/*)”. We identified 94 studies relevant for our analytical objectives and updated our literature review as relevant with manuscripts that were subsequently published and applicable to the analysis, of which six studies are summarised here. Although understanding the timing of influenza epidemics is useful in timing interventions, the last such analysis of the seasonality of influenza epidemics in Africa was based on data that were more than 5 years old, and 46% of countries in Africa were not included. In 2014, a study sought to describe the timing and peak of influenza detection in 125 countries. This study categorised countries by degree of seasonality (eg, minority, equivocal, and majority), but found it difficult to infer clear vaccination seasons for most tropical, mainly African, countries. In 2016, another WHO-led global study sought to describe and categorise influenza epidemics into a strict geographical paradigm for classifying northern and southern hemisphere vaccine formulation, but was unable to do so for 58% of the 14 African countries analysed. In 2021, another study attempted to classify influenza trends with data from 156 countries from 1996 to 2021, but could only partition region-specific trends and not national-level epidemics and transmission zones. In this study, we aim to fill these gaps in knowledge.
Added value of this study
The extent and quality of influenza surveillance data reported by African countries to the Global Influenza Surveillance and Response network has improved substantially in the past decades. Our analysis of 10 years of high-quality laboratory-confirmed influenza surveillance data from 25 African countries provides novel, policy-relevant information about when to anticipate and plan to mitigate influenza epidemics. We applied three WHO-endorsed analytical methods to investigate and categorise epidemic patterns into five groups: northern hemisphere-dominant (six countries), primarily northern hemisphere with some southern hemisphere (eight countries), southern hemisphere-dominant (three countries), primarily southern hemisphere with some northern hemisphere (three countries), and no clear pattern (five countries). This information can be used to recommend when to initiate vaccination campaigns with either northern or southern hemisphere influenza vaccine formulations, and when to treat empirically with antivirals.
Implications of all the available evidence
Our description of influenza epidemics can help public health officials to better identify untimely viral activity as might occur during the emergence of a novel influenza virus. Our results can also help countries better choose which WHO influenza vaccine formulation seems most appropriate for use within their jurisdictions. Such a contribution can be useful to the several African countries currently investing in influenza vaccination programmes. Our findings can also help health officials to better time handwashing and respiratory hygiene campaigns, vaccine procurement and roll-outs, and empirical antiviral treatment, so that these interventions are deployed to optimal effect.
Insufficient data in at least 22 of 48 WHO African region countries prevented their inclusion in previous analyses of influenza circulation using at least 90 weeks of data from 2011 to 2017.10, 13 In 2005, global influenza surveillance garnered momentum after an upswell of global investments to strengthen national surveillance platforms and reporting to WHO.1, 7 As a result, more surveillance data are now available to analyse patterns of influenza activity in countries across Africa.1 This analysis leverages data from these improved surveillance systems to identify and describe historical influenza epidemics, characterise typical yearly activity, and explore optimal times for interventions for countries in Africa.
Methods
Data source
We analysed publicly available, laboratory-confirmed, national-level surveillance data for 2010–19 on severe acute respiratory infections (SARIs) and influenza-like illnesses (ILIs) from the WHO Global Influenza Surveillance and Response System (GISRS) FluNet data platform.14 We evaluated data from 34 WHO member states on the African continent (including the WHO African and Eastern Mediterranean regions) that report surveillance data to the global monitoring platform, and included the 25 countries with at least 3 years of consecutive surveillance data in this analysis. Data were disaggregated by year, epidemiological week, number of specimens tested, and number of influenza-positive specimens. We described the cumulative number of specimens tested from cases of SARI and ILI and the number positive for influenza virus, as well as identifying epidemics and describing epidemic patterns, including their onset, peak, and end, using three methods detailed below.
Ascertaining influenza seasonality
Because there is no gold-standard method for ascertaining influenza seasonality, we used three published methods to identify influenza epidemics. First, we used the aggregate average method, which generates summary influenza epidemic curves by aggregating weekly data across years.15 The aggregate average method can be considered as an extension of the WHO method for ascertaining seasonal trends, outlined in WHO's 2013 Global Epidemiological Surveillance Standards for Influenza,16 which is the second method we used (hereafter referred to as the WHO method). The third method we used was the moving epidemic method.17 Although the methodology to calculate epidemic thresholds differs across methods, we defined epidemic thresholds as the proportional positivity above which a country is in an influenza season. We present our results based on the aggregate average method; however, all three methods are described and compared below. Results from the WHO and moving epidemic methods are included in appendix 9.
The aggregate average method generates a summary curve by summing weekly data across years without requiring manual assessment and alignment of yearly peak influenza activity. With this method, we generated summary epidemic curves of all years with surveillance data for each country by first calculating the sum of influenza virus-positive specimens and the sum of total specimens tested for each epidemiological week, using the following formulas:
and
where n is the epidemiological week, x is the first year with available influenza surveillance data, y is the last year with available influenza surveillance data, p[n] is the number of influenza virus-positive specimens in week n, pn is the total number of influenza virus-positive specimens for week n in years x–y of influenza surveillance, s[n] is the number of specimens tested for influenza virus in week n, and sn is the total number of specimens tested for influenza virus for week n in years x–y of influenza surveillance.
We then calculated a centre-aligned 3-week moving influenza proportional positivity using the following formula:
where mn was the 3-week moving proportional positivity for week n=1 … 52. We defined p0=p52, s0=s52, p53=p1, and s53=s1 to account for the temporal continuity between years. We calculated 95% binomial proportion CIs for the 3-week moving proportional positivity using the Wilson method. Weeks were excluded from smoothed analyses if the number of specimens tested for that week represented less than 0·1% of the total specimens tested during all 52 weeks, because of low confidence in proportional positivity data. We calculated the epidemic threshold as the mean of the 3-week moving proportional positivity across all 52 epidemiological weeks. We defined the onset of an epidemic or season as the first week when the weekly proportional positivity exceeded the annual mean influenza proportional positivity for at least 3 consecutive weeks. The end of an epidemic or season was the first week when the weekly proportional positivity fell below the annual mean influenza proportional positivity and stayed below the annual mean for at least 3 consecutive weeks. Influenza transmission peaks during a year were defined as the highest proportional positivity during the periods of time when proportional positivity was above the epidemic threshold.
The WHO method generates an average epidemic curve for each country by aligning yearly influenza epidemic curves. It calculates proportional positivity followed by a 3-week moving proportional positivity for each week, and generates a yearly seasonal epidemic curve from these data. We identified the week with the highest proportional positivity for each surveillance year, calculated the median week of peak activity from these yearly peak proportional positivity data, and manually aligned yearly curves so that proportional positivity peaks aligned with the median week. We then generated a summary curve by calculating the average weekly proportional positivity across all surveillance years. Seasonal epidemic thresholds for summary epidemic curves were determined on the basis of the mean of weekly proportional positivity for all 52 epidemiological weeks for all years of data included in the study. The start, peak, and end of the epidemic season were calculated from these summary curves using the same definitions as those used for the aggregate average method.
To ascertain influenza seasonality with the moving epidemic method, we used the moving epidemic method web application R4.1.0 v2.18 This method calculates influenza baseline and epidemic thresholds by identifying pre-epidemic, epidemic, and post-epidemic periods from historical data. The epidemic threshold calculations in the moving epidemic method application were calculated as the upper limit of the 95% one-sided CI of the 30 highest pre-epidemic weekly proportional positivity values for that country. Medium, high, and very high intensity thresholds were estimated as the upper limits of the 40%, 90%, and 97·5% one-sided CIs of the geometric mean of the 30 highest epidemic weekly rates.17, 19, 20 To ensure comparability to the other two methods, we uploaded source data for each country separately, with precalculated 3-week moving proportional positivity, to the moving epidemic method web application. We maintained all the predefined default settings of the moving epidemic method application and optimised the slope parameters to maximise the sensitivity and specificity of the epidemic thresholds. To optimise slope parameters, the moving epidemic method calculates the goodness of fit of the model and the optimal windows parameter for detecting the epidemic. For countries that showed more than one annual epidemic season, as ascertained by the aggregate average method or WHO method, we applied the application's two-wave detection algorithm option. The start of the epidemic season was defined as the first week when weekly proportional positivity exceeded this calculated epidemic threshold.
Describing dominant epidemic patterns
We used the summary curves from all three methods to describe dominant influenza seasonality patterns for each country. We categorised countries as having northern hemisphere-dominant patterns if their influenza epidemic period occurred during October–March, and southern hemisphere-dominant patterns if their epidemic period occurred during April–September. If a country had two epidemic periods, with one period during the northern hemisphere months and the other during the southern hemisphere months, the dominant pattern was assigned to the period that had the highest peak proportional positivity, while noting the occurrence of a second peak during the other period (ie, primarily southern hemisphere with some northern hemisphere, or primarily northern hemisphere with some southern hemisphere). Countries that had epidemic periods that bridged the northern hemisphere and southern hemisphere months were similarly classified as primarily southern hemisphere or northern hemisphere with some northern hemisphere or southern hemisphere, depending on how much of their epidemic periods occurred during northern hemisphere or southern hemisphere months. Countries whose influenza seasons occurred outside the typical northern hemisphere or southern hemisphere bounds and without a predominant pattern were labelled as having no clearly defined seasonality. Year-round activity was defined as influenza being detected and ascertained to be circulating throughout the year for at least 8 months. Out-of-season activity was defined as epidemics each year that occurred outside the dominant pattern for a country.
Comparison of epidemic activity between methods
We compared the outputs from the three methods for each country's dominant seasonality pattern to calculate concordance across methods. We tabulated the number of times each method found the same dominant pattern across two and all three methods, respectively, and calculated concordance by dividing this sum by the number of countries being assessed across all three methods. We also assessed concordance using Cohen's κ coefficient as a measure of inter-rater agreement of epidemic patterns between each method. The κ scale ranges from 0 (amount of agreement to be expected by chance) to 1 (perfect agreement).21
Role of the funding source
There was no funding source for this study.
Results
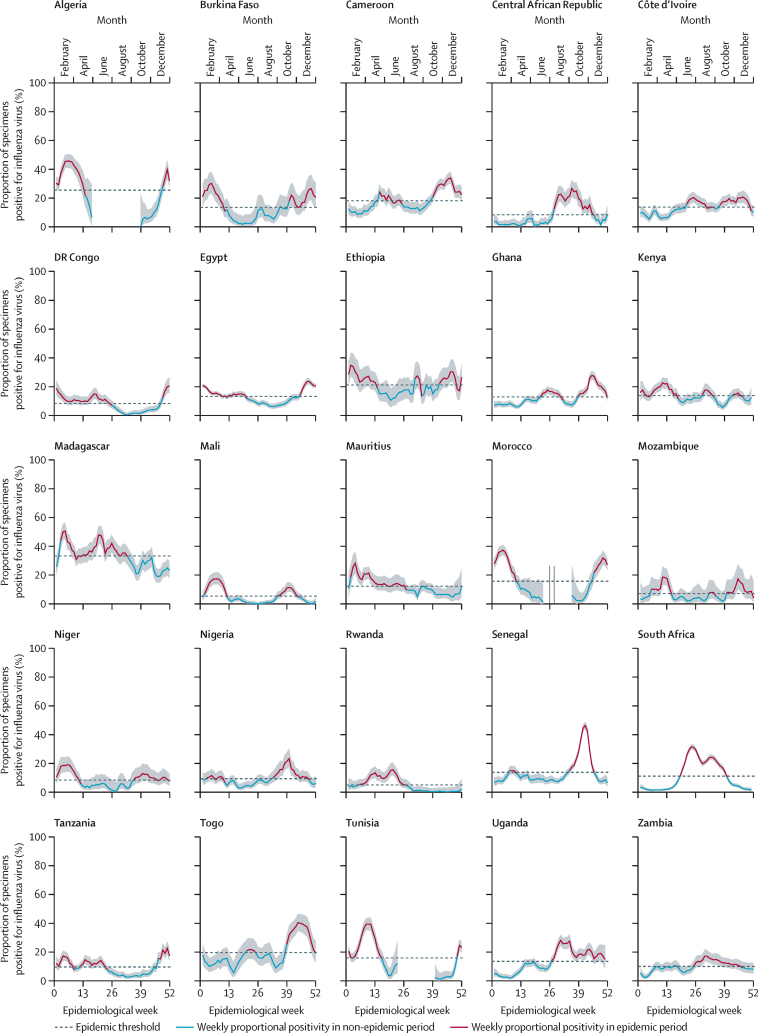
We reviewed data from the 34 African countries that submit data to FluNet and excluded nine countries that had insufficient data to assess their influenza epidemic timing (three countries with <3 years of data from 2010–19 and six countries that did not submit data to FluNet from 2010–19). The 25 countries included in our analysis represent 46% of the 54 countries in Africa and contain 1·1 billion (89%) of Africa's total population of 1·2 billion people. Of the included study countries, 16 (64%) countries had all 10 study years of surveillance data, five (20%) countries had 9 years, and three (12%) countries had 8 years of influenza surveillance during 2010–19 (table 1). Study countries reported RT-PCR respiratory virus results for a cumulative total of 503 609 specimens (median 12 971 [IQR 9607–20 960] per country-year), of which 74 001 (15%; median 2078 [IQR 1087–3008]) were positive for influenza viruses. We identified 248 epidemics during the 236 country-years of data. 122 epidemics occurred in northern hemisphere months, 74 in southern hemisphere months, and 52 epidemics were between the northern and southern hemisphere months. We excluded 38 (1·3%) of 3000 weeks, which represented less than 0·001% of the yearly sampled specimens across all countries. We identified a clear northern hemisphere-dominant or southern hemisphere-dominant epidemic period for nine (36%) of 25 countries. 16 (64%) countries consistently had one annual influenza epidemic and nine (36%) countries had multiple influenza epidemics per year during the study period (figure 1). We have also presented yearly data by proportional positivity and weekly samples tested per 100 000 people (appendix 9 pp 2–3). Start, peak, and end months for primary and secondary influenza seasons and tertiary seasons where applicable in study countries are described in table 2. Epidemic thresholds ranged from 5% influenza-positive specimens in Rwanda to 34% influenza-positive specimens in Madagascar.
Table 1.
Influenza activity and hemisphere detection by season for 25 African countries
| Population, n (% of Africa) | Years of data* | Samples tested, n | Influenza positive, n (%) | Samples tested per 100 000 population | Epidemic threshold, %† | |
|---|---|---|---|---|---|---|
| Algeria | 43 851 043 (4%) | 10 | 7020 | 2351 (33%) | 16·009 | 22% |
| Burkina Faso | 20 903 278 (2%) | 8 | 4987 | 735 (15%) | 23·858 | 13% |
| Cameroon | 26 545 864 (2%) | 10 | 18 804 | 3730 (20%) | 70·836 | 18% |
| Central African Republic | 4 829 764 (<1%) | 9 | 9607 | 817 (9%) | 198·912 | 8% |
| Côte d'Ivoire | 26 378 275 (2%) | 10 | 21 193 | 2985 (14%) | 80·343 | 14% |
| DR Congo | 89 561 404 (8%) | 10 | 17 643 | 1426 (8%) | 19·699 | 8% |
| Egypt | 102 334 403 (9%) | 10 | 82 895 | 12 489 (15%) | 81·004 | 13% |
| Ethiopia | 114 963 583 (10%) | 8 | 5954 | 1284 (22%) | 5·179 | 21% |
| Ghana | 31 072 945 (3%) | 10 | 39 256 | 5014 (13%) | 126·335 | 13% |
| Kenya | 53 771 300 (5%) | 9 | 20 183 | 2775 (14%) | 37·535 | 14% |
| Madagascar | 27 691 019 (3%) | 10 | 12 971 | 4403 (34%) | 46·842 | 34% |
| Mali | 20 250 834 (2%) | 8 | 17 434 | 929 (5%) | 86·090 | 6% |
| Mauritius | 1 265 740 (<1%) | 9 | 10 863 | 1400 (13%) | 858·233 | 12% |
| Morocco | 39 910 558 (4%) | 10 | 10 228 | 2688 (26%) | 25·627 | 14% |
| Mozambique | 31 255 435 (3%) | 7 | 4400 | 347 (8%) | 14·078 | 7% |
| Niger | 24 206 636 (2%) | 9 | 7088 | 677 (10%) | 29·281 | 8% |
| Nigeria | 206 139 587 (19%) | 10 | 12 368 | 1087 (9%) | 6·000 | 9% |
| Tanzania | 59 734 213 (5%) | 10 | 20 960 | 2078 (10%) | 35·089 | 10% |
| Rwanda | 12 952 209 (1%) | 10 | 10 147 | 601 (6%) | 78·342 | 5% |
| Senegal | 16 743 930 (2%) | 10 | 35 442 | 6298 (18%) | 211·671 | 14% |
| South Africa | 59 308 690 (5%) | 10 | 75 609 | 11 132 (15%) | 127·484 | 11% |
| Togo | 8 278 737 (1%) | 9 | 7505 | 1664 (22%) | 90·654 | 20% |
| Tunisia | 11 818 618 (1%) | 10 | 10 811 | 2353 (22%) | 91·474 | 11% |
| Uganda | 45 741 000 (4%) | 10 | 22 940 | 3008 (13%) | 50·152 | 13% |
| Zambia | 18 383 956 (2%) | 10 | 17 301 | 1730 (10%) | 94·109 | 10% |
Number of complete years of surveillance data available.
Epidemic thresholds calculated using the aggregate average method.
Figure 1.
Average influenza activity and seasonal thresholds for 25 African countries based on data from 2010 to 2019, according to the aggregate average method
Shaded areas are 95% CI.
Table 2.
Start, peak, and end months of influenza seasons and dominant epidemic patterns for 25 African countries
| Number of peaks |
Season duration (months) |
Dominant season |
Secondary season |
Tertiary season |
Transmission zones |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dominant | Secondary | Tertiary | Start | Peak | End | Start | Peak | End | Start | Peak | End | Current WHO vaccination zones11 | Dominant epidemic pattern* | ||
| Algeria | 1 | 4 | .. | .. | Dec | Feb | April | .. | .. | .. | .. | .. | .. | Northern | NH |
| Burkina Faso | 1 | 7 | .. | .. | Oct | Feb | March | .. | .. | .. | .. | .. | .. | Western | NH |
| Egypt | 1 | 7 | .. | .. | Nov | Dec | May | .. | .. | .. | .. | .. | .. | Northern | NH |
| Morocco | 1 | 5 | .. | .. | Nov | Feb | March | .. | .. | .. | .. | .. | .. | Northern | NH |
| Niger | 1 | 7 | .. | .. | Sept | Feb | March | .. | .. | .. | .. | .. | .. | Western | NH |
| Tunisia | 1 | 5 | .. | .. | Dec | March | April | .. | .. | .. | .. | .. | .. | Northern | NH |
| Cameroon | 2 | 4 | 3 | .. | Sept | Nov | Dec | April | April | June | .. | .. | .. | Middle | NH (some SH) |
| Mozambique | 3 | 3 | 3 | 1 | Feb | March | April | Oct | Nov | Dec | Aug | Aug | Aug | Eastern | NH (some SH) |
| Ethiopia | 2 | 7 | 1 | .. | Oct | Jan | April | Aug | Aug | Aug | .. | .. | .. | Eastern | NH (some SH) |
| Mali | 2 | 3 | 3 | .. | Jan | Feb | March | Aug | Oct | Oct | .. | .. | .. | Western | NH (some SH) |
| Nigeria | 2 | 5 | 3 | .. | Aug | Oct | Dec | Jan | Feb | March | .. | .. | .. | Western | NH (some SH) |
| Senegal | 2 | 4 | 2 | .. | Aug | Oct | Nov | Feb | March | March | .. | .. | .. | Western | NH (some SH) |
| Togo | 2 | 4 | 2 | .. | Sept | Oct | Dec | May | June | June | .. | .. | .. | Western | NH (some SH) |
| Tanzania | 2 | 5 | 4 | .. | Nov | Dec | March | March | April | June | .. | .. | .. | Eastern | NH (some SH) |
| Kenya | 3 | 5 | 2 | 2 | Jan | March | May | July | Aug | Aug | Oct | Nov | Nov | Eastern | SH (some NH) |
| Uganda | 1 | 6 | .. | .. | July | Aug | Dec | .. | .. | .. | .. | .. | .. | Eastern | SH (some NH) |
| Ghana | 2 | 4 | 3 | .. | Sept | Nov | Dec | June | June | Aug | .. | .. | .. | Western | SH (some NH) |
| Central African Republic | 1 | 5 | .. | .. | July | Sept | Nov | .. | .. | .. | .. | .. | .. | Middle | SH |
| South Africa | 1 | 6 | .. | .. | May | June | Oct | .. | .. | .. | .. | .. | .. | Southern | SH |
| Zambia | 1 | 6 | .. | .. | June | Aug | Nov | .. | .. | .. | .. | .. | .. | Eastern | SH |
| Côte d'Ivoire | 2 | 4 | 3 | .. | Sept | Nov | Dec | June | June | Aug | .. | .. | .. | Western | No clear pattern |
| DR Congo | 1 | 7 | .. | .. | Dec | Dec | June | .. | .. | .. | .. | .. | .. | Middle | No clear pattern |
| Rwanda | 1 | 6 | .. | .. | Feb | May | July | .. | .. | .. | .. | .. | .. | Eastern | No clear pattern |
| Madagascar | 1 | 8 | .. | .. | Jan | Feb | Aug | .. | .. | .. | .. | .. | .. | Eastern | No clear pattern |
| Mauritius | 1 | 7 | .. | .. | Jan | Jan | July | .. | .. | .. | .. | .. | .. | Eastern | No clear pattern |
NH=northern hemisphere. SH=southern hemisphere.
Generated with the aggregate average method.
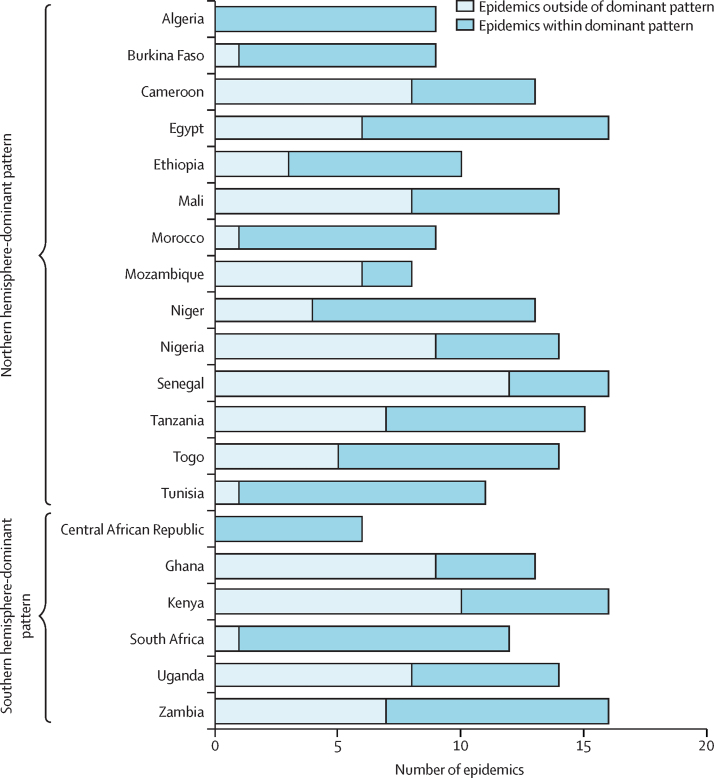
We also identified out-of-season epidemic periods in many countries (figure 2). Although most countries had clear epidemics consistently in the dominant epidemic season, some epidemics occurred outside of the dominant epidemic season. For example, we found Egypt to align with a northern hemisphere pattern, with a peak occurring every year during the northern hemisphere season. However, there were an additional 6 years with epidemics also occurring during the southern hemisphere season. Overall, 106 (43%) of 248 epidemics occurred outside of the dominant epidemic season for 20 countries with identified seasonal patterns (figure 2).
Figure 2.
Total number of epidemics within or outside of dominant or expected epidemic patterns in study countries* during 2010–19, according to the aggregate average method
*Epidemics from countries with no clearly defined influenza epidemic pattern are not included.
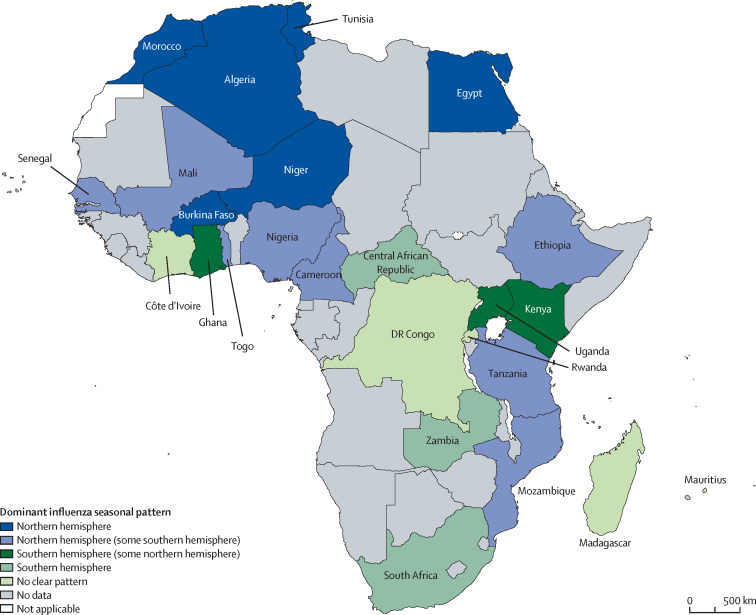
We identified five categories of epidemic pattern from our analysis using the aggregate average method (figure 3): northern hemisphere-dominant, primarily northern hemisphere with some southern hemisphere, primarily southern hemisphere with some northern hemisphere, southern hemisphere-dominant, and no clear pattern of southern or northern hemisphere epidemic activity.
Figure 3.
Dominant influenza patterns during 2010–19, identified by the aggregate average method
Data are from FluNet. Map boundaries are based on WHO GIS Centre for Health data. Dotted lines represent approximate border lines for which there might not yet be full agreement.
Six (24%) countries (Algeria, Burkina Faso, Egypt, Morocco, Niger, and Tunisia) aligned strongly with a northern hemisphere-dominant pattern of epidemic activity. On average, epidemic activity began in November (±1 month), peaked in February (±1 month), and ended in April (±1 month). Epidemic activity in these countries lasted a mean 6 months (SD 1). Countries in this cluster consistently had one distinct and dominant peak of influenza epidemic activity.
Eight (32%) countries (Cameroon, Ethiopia, Mali, Mozambique, Nigeria, Senegal, Tanzania, and Togo) aligned primarily with the northern hemisphere epidemic pattern with some southern hemisphere epidemic activity. We detected bimodal (two peaks) and trimodal (three peaks) influenza epidemics in this subtropical group. The dominant epidemic typically began during northern hemisphere seasonal months and lasted a mean 4 months (SD 1). The secondary epidemic typically began during southern hemisphere seasonal months and lasted a mean 3 months (SD 1).
Three (12%) countries (Ghana, Kenya, and Uganda) aligned primarily with a southern hemisphere epidemic pattern with some northern hemisphere epidemic activity. Uganda had a single 6-month season beginning in June or July, peaking in August, and ending in November or December. Ghana had two seasons and Kenya had three seasons, which occurred primarily during southern hemisphere seasonal months (table 2). The dominant epidemic in these countries lasted a mean 5 months (SD 1).
Three (12%) countries (Central African Republic, South Africa, and Zambia) had a southern hemisphere-dominant pattern of epidemic activity. Epidemics typically began in June and ended in October or November. Epidemic activity in these countries lasted a mean 5 months (SD 1).
Five (20%) countries (Côte d'Ivoire, DR Congo, Madagascar, Mauritius, and Rwanda) had both northern hemisphere and southern hemisphere epidemic activity, without a clear predominance between these two patterns. Epidemic activity in these countries lasted a mean 7 months (SD 2).
We compared the results from the analyses of epidemic patterns across all three methods (figure 1; appendix 9 pp 4–5) to assess concordance of dominant patterns. 16 (64%) countries (κ=0·2322, p=0·0027) were concordant between the WHO method and aggregate average method, 15 (60%) countries (κ=0·2274, p=0·0061) were concordant between the moving epidemic method and the WHO method, and 12 (48%) countries (κ=0·1905, p=0·0050) were concordant between the aggregate average method and moving epidemic method.
Discussion
We categorised and described the start, peak, and end of seasonal influenza activity for 25 countries covering 89% of the population of Africa, based on these countries’ own surveillance data. Our analysis included 13 additional countries in Africa where more data are now available compared with previous timeseries analyses to define influenza seasonality and when to vaccinate.10, 13 We identified five predominant epidemic patterns, which could help public health officials better monitor influenza in groups of countries with similar epidemic patterns, differentiate typical versus atypical influenza activity, anticipate the start of epidemics, and time the launch of influenza prevention and control interventions. Although influenza epidemic patterns in northern and southern Africa predictably coincided with northern hemisphere22 and southern hemisphere periods, some countries in subtropical and especially western and eastern Africa had less predictable epidemic patterns that did not neatly align with classical northern hemisphere or southern hemisphere patterns. Five countries had both northern hemisphere and southern hemisphere epidemics with some influenza detections during both periods, which is consistent with findings from previous attempts to describe seasonal patterns for Madagascar, Rwanda, and Mauritius.23 We also note between-country differences in the epidemic threshold, which was highest in Madagascar (34%) and lowest in Rwanda (5%), reflecting different influenza surveillance and testing strategies. Madagascar has a well established surveillance system and consistently collects influenza samples throughout the year, whereas Rwanda does not. For Rwanda, low proportions of influenza-positive specimens were observed overall.
The northern hemisphere versus southern hemisphere geographical paradigm for classification of predominance of influenza activity is imperfect, as it is largely defined by influenza trends in countries with temperate climates but might be less applicable to other climates.10 Our analysis showed that the standard northern hemisphere or southern hemisphere dichotomy might not describe influenza activity well in tropical and subtropical countries, not even in those with single annual epidemics. For instance, we identified that the Central African Republic generally had a distinct single epidemic each year from July to November, which starts in the middle of the southern hemisphere epidemic period and ends well into the start of the northern hemisphere epidemic period. In addition, Tanzania and Mozambique are geographically in the southern hemisphere, yet their influenza surveillance data suggest some epidemic activity in the northern hemisphere months.
These findings have important implications for recommendations about which hemisphere vaccine formulation countries should purchase. Our findings suggest that countries could benefit from using dominant seasonal influenza patterns, ascertained through yearly surveillance data, to guide the choice of southern hemisphere versus northern hemisphere vaccine, rather than choosing on the basis of their geographical location or proximity to another country with a clearly defined season. Although it might be logistically and financially challenging, countries with multiple epidemics in a year could choose to use both northern hemisphere and southern hemisphere vaccine formulations, or to use the most recent vaccine formulation recommended at any time of the year.10, 16 For example, in our analysis, Kenya had two distinct peaks of increased influenza activity and could invest in vaccine purchases on the basis of the dominant epidemic, rather than secondary waves, to maximise the effect of vaccine campaigns. In some tropical countries, however, the season started before the availability of the recommended vaccine formulation, making it difficult for countries to use that formulation to protect their populations against influenza in the weeks before the start of the influenza season. The growing ability to rely on surveillance data rather than geography to guide vaccine formulation decisions underscores the importance of strengthened year-round sentinel surveillance to generate evidence-based classifications of epidemic patterns.
Subregional variability in influenza virus circulation, especially in the tropics, compounded by non-uniform weekly sampling across the year, as evident in Kenya,24 makes it challenging to ascertain seasonality patterns for many countries. Moreover, despite numerous methodological approaches, no gold-standard method for ascertaining influenza epidemic activity exists.16, 25 Notably, countries that showed concordance of influenza epidemic patterns across all three methods had at least 9 years of consistent surveillance data reported to WHO GISRS. Two of these countries had the longest-established sentinel surveillance platforms in Africa: Madagascar (whose platform was established in 1978) and South Africa (1984).1 Nevertheless, all three methods used in this report present different strengths and weaknesses.
The aggregate average and WHO methods are similar in how the surveillance data are translated and examined, although the aggregate average method makes fewer underlying assumptions about what influenza epidemics in the tropics should look like. By aggregating proportional positivity over many years of data, the aggregate average method reduces the magnitude of epidemic peaks but extends the duration of seasons overall. The WHO and aggregate average methods were both useful for exploring data for countries with multiple peaks of increased influenza activity with some year-round activity, whereas the moving epidemic method had greater utility for countries with distinct single peak seasons. The moving epidemic method yielded consistent results when applied to countries with complete and robust surveillance data, whereas the WHO and aggregate average methods were useful for examining data for countries with less robust or complete data. The appropriateness of the moving epidemic method for some countries with less robust and complete surveillance data, including those with year-round virus circulation or bimodal peaks, should be further investigated, given that influenza viruses change year to year and that surveillance capacities to detect and mitigate the effect of influenza disease vary by region. Our analysis does not portend that one method is superior to the other, but attests that completeness of surveillance data could factor into the choice of analytical methods applied to examine and accurately describe seasonal patterns to recommend vaccine timing.
Identifying a gold-standard method for characterising influenza epidemic activity could be useful; however, given the variability of available and robust surveillance data,26 partner countries and research teams might consider applying methods for analyses that best suit the epidemic variability and local factors (eg, weather or migration).26 Our study findings suggest the value of strengthening influenza surveillance to identify epidemics in subtropical and tropical countries, such as those in Africa. These findings are updates to the ongoing discourse about the applicability of different approaches to re-examining national respiratory virus epidemic activity in the tropics. Other analytical methods—such as Wavelet, Serfling, or other time-series methods27, 28, 29, 30—could be explored for replicating the results of this study.
Our findings are subject to several limitations. First, we used national surveillance data for our analyses where sampling was not uniform across the year. While we used a 3-week moving proportional positivity to smooth weekly fluctuations, the uneven sampling might nevertheless have resulted in overestimating or underestimating influenza activity during specific periods of the year. Second, we acknowledge that we could not assess whether multiple peaks were caused by the aggregation of data from within-country regions with distinct epidemic periods into a national dataset. Therefore, for countries with multiple peaks of influenza epidemics, it is suggested that subnational data be examined to help inform whether more regionally targeted vaccine strategies are required. Third, we could not disaggregate FluNet data by SARI and ILI specimens, age, case definitions, case severity, or sentinel surveillance sites, which might have obscured the magnitude of peaks and threshold levels, especially if SARI and ILI reporting was not systematic across the study period and by country. Finally, we did not explore the role of virus subtypes or antigenic characterisation on seasonality, nor did we examine the effect of year-to-year variability of data. We acknowledge that timing of the season is only one aspect of deciding on the vaccine formulation and that more data on antigenic characterisation of circulating viruses are important. We recommend that such analyses should be implemented when more viruses are characterised and available from African countries.
African nations have generated surveillance data to identify typical influenza epidemic periods by strengthening their ILI and SARI surveillance and laboratory capacity and through decades of partnership building and funding.7 Vaccination strategies for countries in the tropics should be aligned to both the beginning of expected epidemic activity and the dominant pattern to have the maximum public health impact. Based on findings from this analysis, countries should anticipate which formulations would be optimal to protect against influenza during the anticipated primary or dominant epidemic period and better time procurement, distribution, and vaccination campaigns. Furthermore, countries could investigate, sequence, and antigenically characterise seasonal and non-seasonal influenza viruses to assess their use as candidate vaccine viruses. Future analyses could investigate real-world vaccine effectiveness against circulating viruses to inform the yearly vaccine composition decisions.31, 32 Anticipating peak epidemic periods can help health authorities better time the mobilisation of antiviral stockpiles to the point of care and release risk communication messages to prompt clinicians to empirically treat people at increased risk of influenza complications with antivirals (eg, people in hospital or with pre-existing conditions that predispose them to severe influenza).
Data sharing
The data used in this analysis are publicly available and accessible through WHO's Global Influenza Surveillance and Response System.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We acknowledge all members of the African Network for Influenza Surveillance and Epidemiology Working Group, partner countries, and national reference laboratories for their data contributions to national influenza-like illness and severe acute respiratory infection surveillance, sharing of data to global monitoring platforms, and continued long-term collaborations. We thank our US Centers for Disease Control and Prevention (CDC) colleagues Ndahwouh Talla Nzussouo, Joelle Kabamba, Kathryn E Lafond, and Margaret McCarron for their contributions to the development of this manuscript, and Gabriele Richardson for guidance and support for mapping. The findings and conclusions in this Article are those of the authors and do not necessarily represent the official position of the CDC. The designations employed in map images and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of WHO concerning the legal status of any country, territory, city, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps.
Contributors
LSI and EA-B conceived the study. LSI and EA-B contacted and engaged potential partners for collaboration in the regional network, communicated progress of the planned seasonality analysis methods and design, and requested feedback. LSI, KR, PMar, and EA-B compiled, managed, cleaned, evaluated, analysed, and vetted country-specific data. LSI, KR, and PMar drafted the manuscript, created the tables and figures, compiled feedback, and addressed all comments from coauthors. All other authors reviewed country-specific results and contributed to and approved results, manuscript drafts, and this publication. All authors had full access to all the data in the study, verified the underlying data reported, provided statistical and methodological feedback on manuscript drafts, and were responsible for the decision to submit for publication.
Supplementary Materials
References
- 1.Igboh LS, McMorrow M, Tempia S, et al. Influenza surveillance capacity improvements in Africa during 2011–2017. Influenza Other Respir Viruses. 2021;15:495–505. doi: 10.1111/irv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafond KE, Nair H, Rasooly MH, et al. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982–2012: a systematic analysis. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lafond KE, Porter RM, Whaley MJ, et al. Global burden of influenza-associated lower respiratory tract infections and hospitalizations among adults: a systematic review and meta-analysis. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAnerney JM, Walaza S, Tempia S, et al. Estimating vaccine effectiveness in preventing laboratory-confirmed influenza in outpatient settings in South Africa, 2015. Influenza Other Respir Viruses. 2017;11:177–181. doi: 10.1111/irv.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antunes JL, Waldman EA, Borrell C, Paiva TM. Effectiveness of influenza vaccination and its impact on health inequalities. Int J Epidemiol. 2007;36:1319–1326. doi: 10.1093/ije/dym208. [DOI] [PubMed] [Google Scholar]
- 6.Palache A, Oriol-Mathieu V, Abelin A, Music T. Seasonal influenza vaccine dose distribution in 157 countries (2004–2011) Vaccine. 2014;32:6369–6376. doi: 10.1016/j.vaccine.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Morales KF, Brown DW, Dumolard L, et al. Seasonal influenza vaccination policies in the 194 WHO member states: the evolution of global influenza pandemic preparedness and the challenge of sustaining equitable vaccine access. Vaccine X. 2021;8 doi: 10.1016/j.jvacx.2021.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman LP, Bhat N, Fleming JA, Neuzil KM. Global influenza seasonality to inform country-level vaccine programs: an analysis of WHO FluNet influenza surveillance data between 2011 and 2016. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alonso WJ, Yu C, Viboud C, et al. A global map of hemispheric influenza vaccine recommendations based on local patterns of viral circulation. Sci Rep. 2015;5 doi: 10.1038/srep17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirve S, Newman LP, Paget J, et al. Influenza seasonality in the tropics and subtropics—when to vaccinate? PLoS One. 2016;11 doi: 10.1371/journal.pone.0153003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Influenza transmission zones. Sept 14, 2018. https://cdn.who.int/media/docs/default-source/influenza/influenza-updates/2020/influenza_transmission_zones20180914.pdf?sfvrsn=dba8eca5_3&download=true
- 12.Ampofo WK, Azziz-Baumgartner E, Bashir U, et al. Strengthening the influenza vaccine virus selection and development process: report of the 3rd WHO Informal Consultation for Improving Influenza Vaccine Virus Selection held at WHO headquarters, Geneva, Switzerland, April 1–3, 2014. Vaccine. 2015;33:4368–4382. doi: 10.1016/j.vaccine.2015.06.090. [DOI] [PubMed] [Google Scholar]
- 13.Muscatello DJ. Redefining influenza seasonality at a global scale and aligning it to the influenza vaccine manufacturing cycle: a descriptive time series analysis. J Infect. 2019;78:140–149. doi: 10.1016/j.jinf.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 14.WHO FluNet. https://www.who.int/tools/flunet
- 15.Durand LO, Cheng PY, Palekar R, et al. Timing of influenza epidemics and vaccines in the American tropics, 2002–2008, 2011–2014. Influenza Other Respir Viruses. 2016;10:170–175. doi: 10.1111/irv.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO Global epidemiological surveillance standards for influenza. 2013. https://apps.who.int/iris/handle/10665/311268
- 17.Vega T, Lozano JE, Meerhoff T, et al. Influenza surveillance in Europe: comparing intensity levels calculated using the moving epidemic method. Influenza Other Respir Viruses. 2015;9:234–246. doi: 10.1111/irv.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lozano JE. The moving epidemic method shiny web application. 2022. https://github.com/lozalojo/memapp
- 19.AbdElGawad B, Vega T, El Houssinie M, et al. Evaluating tools to define influenza baseline and threshold values using surveillance data, Egypt, season 2016/17. J Infect Public Health. 2020;13:430–437. doi: 10.1016/j.jiph.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Biggerstaff M, Kniss K, Jernigan DB, et al. Systematic assessment of multiple routine and near real-time indicators to classify the severity of influenza seasons and pandemics in the United States, 2003–2004 through 2015–2016. Am J Epidemiol. 2018;187:1040–1050. doi: 10.1093/aje/kwx334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 22.Caini S, El-Guerche Séblain C, Ciblak MA, Paget J. Epidemiology of seasonal influenza in the Middle East and north Africa regions, 2010–2016: circulating influenza A and B viruses and spatial timing of epidemics. Influenza Other Respir Viruses. 2018;12:344–352. doi: 10.1111/irv.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alonso WJ, Guillebaud J, Viboud C, et al. Influenza seasonality in Madagascar: the mysterious African free-runner. Influenza Other Respir Viruses. 2015;9:101–109. doi: 10.1111/irv.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emukule GO, Mott JA, Spreeuwenberg P, et al. Influenza activity in Kenya, 2007–2013: timing, association with climatic factors, and implications for vaccination campaigns. Influenza Other Respir Viruses. 2016;10:375–385. doi: 10.1111/irv.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rguig A, Cherkaoui I, McCarron M, et al. Establishing seasonal and alert influenza thresholds in Morocco. BMC Public Health. 2020;20 doi: 10.1186/s12889-020-09145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vega T, Alonso J, Lozano A, Raul OdL, Perez M. Modelling influenza epidemic—can we detect the beginning and predict the intensity and duration? Int Congr Ser. 2004;1263:281–283. [Google Scholar]
- 27.Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Rep. 1963;78:494–506. [PMC free article] [PubMed] [Google Scholar]
- 28.Imai C, Armstrong B, Chalabi Z, Mangtani P, Hashizume M. Time series regression model for infectious disease and weather. Environ Res. 2015;142:319–327. doi: 10.1016/j.envres.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 29.Yang W, Cummings MJ, Bakamutumaho B, et al. Transmission dynamics of influenza in two major cities of Uganda. Epidemics. 2018;24:43–48. doi: 10.1016/j.epidem.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health. 2019;7:e1031–e1045. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 31.Sofia Arriola C, El Omeiri N, Azziz-Baumgartner E, et al. Influenza vaccine effectiveness against hospitalizations in children and older adults—data from South America, 2013–2017. A test negative design. Vaccine X. 2019;3 doi: 10.1016/j.jvacx.2019.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edoka I, Kohli-Lynch C, Fraser H, et al. A cost-effectiveness analysis of South Africa's seasonal influenza vaccination programme. Vaccine. 2021;39:412–422. doi: 10.1016/j.vaccine.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this analysis are publicly available and accessible through WHO's Global Influenza Surveillance and Response System.