Abstract
Background
The clinical value of the controlling nutritional status (CONUT) score has been widely reported in multiple malignancies. The aim of this study is to investigate the association between the CONUT score and clinical outcomes in patients with gastric cancer.
Methods
A comprehensive literature search of electronic databases including PubMed, Embase, and Web of Science was performed up to December 2022. The primary endpoints were survival outcomes and postoperative complications. Subgroup analysis and sensitivity analysis were performed during the pooled analysis.
Results
Nineteen studies including 9,764 patients were included. The pooled results indicated that patients in the high CONUT group had a worse overall survival (HR = 1.70 95%CI: 1.54–1.87; P < 0.0001; I2 = 33%) and recurrence-free survival (HR = 1.57; 95%CI: 1.36–1.82; P < 0.0001; I2 = 30%), and a higher risk of complications (OR = 1.96; 95%CI: 1.50–2.57; P < 0.0001; I2 = 69%). In addition, a high CONUT score was significantly associated with larger tumor size, higher percentage of microvascular invasion, later TNM stage and fewer patients receiving adjuvant chemotherapy, but not with tumor differentiation.
Conclusion
Based on existing evidence, the CONUT score could act as a valuable biomarker to predict clinical outcomes in patients with gastric cancer. Clinicians could use this useful indicator to stratify patients and formulate individual treatment plans.
Keywords: controlling nutritional status score, postoperative complications, survival outcomes, meta-analysis, gastric cancer
1. Background
Gastric cancer (GC) remains the fifth most frequently diagnosed cancer and the third leading cause of cancer-related deaths in the world (1, 2). Despite advances in perioperative therapies and surgical techniques for GC patients, the clinical prognosis for GC has not significantly improved until now, mainly due to early recurrence and metastasis (3, 4). It is important to formulate treatment plans based on the expected survival time of patients to improve the cure rate for GC. Currently, the treatment of GC is mainly based on the AJCC TNM staging system. However, the staging system alone does not support treatment selection and prognosis assessment of GC well (5, 6). Therefore, it is essential to explore novel prognostic biomarkers to guide treatment of GC.
As indicated by growing evidence, host’s nutrition status plays a critical role in the progression and survival of cancer patients (7). Based on these insights, several nutritional indicators have been successfully constructed to predict outcomes in cancer patients (8, 9). Among these, the controlling nutritional status (CONUT) score, which is calculated using peripheral albumin level, total cholesterol level and total lymphocyte count, has been developed as a nutritional screening tool (Table 1) (10). Recently, the clinical value of the CONUT score for predicting short-term and long-term outcomes has been widely reported in solid tumors and hematologic malignancies (11). The impact of the CONUT score on outcomes in GC patients was first reported in 2017 (12). After that, a growing number of studies have further explored the relationship between the CONUT score and clinical outcomes in GC patients (13–18). In 2019, Takagi et al. (19) preliminarily confirmed the prognostic value of CONUT score in GC by pooling five studies. Nevertheless, the authors acknowledge that the included studies are limited, and the role of the CONUT score in GC patients is actually unclear. Given that additional reports have been published in recent years, we therefore performed a meta-analysis based on available evidence to further investigate the association between the CONUT score and outcomes in patients with GC.
TABLE 1.
The scoring criteria for the CONUT score.
| Variables | Degree | |||
| Normal | Mild | Moderate | Severe | |
| Albumin level (g/dl) | ≥3.50 | 3.00–3.49 | 2.50–2.99 | <2.50 |
| Score | 0 | 2 | 4 | 6 |
| Cholesterol level (mg/dl) | ≥1,600 | 1,200–1,599 | 800–1,199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Total lymphocyte count (/ml) | ≥180 | 140–179 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
| CONUT score | 0–1 | 2–4 | 5–8 | 9–12 |
2. Methods
2.1. Search strategy
The current study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to identify studies that assess the association of the CONUT score with clinical outcomes in GC patients. Relevant studies from PubMed, Embase, and Web of Science were comprehensively examined up to 1 December 2022. The following combination of key words was used to search the potential related studies: (“CONUT”) AND (“gastric cancer” OR “stomach cancer” OR “stomach tumor”). Language restriction was not applied during the search process. In addition, the references of the included studies were further scanned for extra reports. The search was independently performed by two investigators (HL and X-CY).
2.2. Study selection
The inclusion criteria were presented as follows: (1) studies examined the relationship between the CONUT score and clinical outcomes of GC patients; (2) the outcomes including survival outcomes and/or complications were available; (3) the cut-off value of the CONUT was clearly reported; and (4) studies were published in any language.
The exclusion criteria were as follows: (1) studies did not report data for GC patients separately; (2) studies were reported as case reports, reviews, conferences and letters; (3) duplicated data; and (4) studies was not peer reviewed.
2.3. Data extraction and quality assessment
Two reviewers (HL and X-CY) conducted the data extraction independently and cross-checked all the results. The extracted data included first author, publication year, study interval, country, study design and sample size, selection method, cut-off value, clinicopathological features like age, sex, tumor size, tumor differentiation, microvascular invasion, tumor stage and adjuvant chemotherapy, and clinical outcomes including postoperative complications, survival data, and follow-up time. When necessary, the authors would be contacted to provide relevant data.
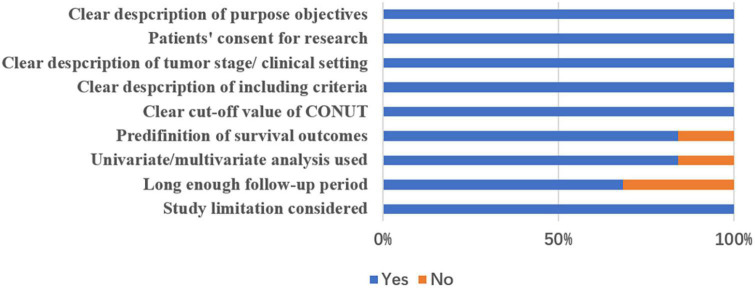
The quality assessment of included studies was performed following the method described by Lin et al. (20) with the following nine items: (1) clear description of purpose/objectives, (2) clear ethical statements, (3) clear description of tumor stage and/or clinical setting, (4) clear description of inclusion criteria, (5) clear description of the cutoff value, (6) predefinition of outcome measurements, (7) whether or not use multivariate analysis and/or univariate analysis, (8) long enough follow-up period, and (9) limitations considered. Finally, a study could get a final score from 0 to –9 after assessment. Quality assessment was not used as exclusion criterion for these 19 included studies.
2.4. Outcomes assessment
In this study, the primary outcomes were postoperative complications and survival outcomes including overall survival (OS), recurrence-free survival (RFS), progression-free survival (PFS), disease-free survival (DFS), and cancer-specific survival (CSS). The postoperative complications were defined as any morbidities occurred with 30 days after gastrectomy and graded by Clavien-Dindo (CD) (21, 22) system. Since RFS, PFS, DFS, and CSS share similar endpoints, they were analyzed together as one outcome, RFS, as previously suggested (23, 24). The secondary outcomes were other postoperative oncological parameters, including tumor size (<5 cm), tumor differentiation (poor differentiation), and TNM stage (Stage III/IV), microvascular invasion (Yes), and adjuvant chemotherapy (Yes).
2.5. Statistical analysis
The odds ratios (ORs) and hazard ratios (HRs) with their 95% confidence intervals (CIs) were used as the effect size for postoperative complications and survival outcomes, respectively. Statistical heterogeneity among enrolled studies was assessed using I2 statistic. When I2 is less than 50%, a fixed-effect model was used to calculate the pooled estimates; otherwise, a random-effects model was performed. Subgroup analysis and sensitivity analysis were utilized to evaluate the credibility of pooled results. Begg’s funnel plot was applied to assess the possibility of publication bias. A two-tailed P-value <0.05 was considered statistically significant. All of these statistical analyses were performed by Review Manager Software, version 5.3 (Cochrane, London, UK) and Stata, version 12.0 (Statacorp, College Station, TX, USA).
3. Results
3.1. Study characteristics
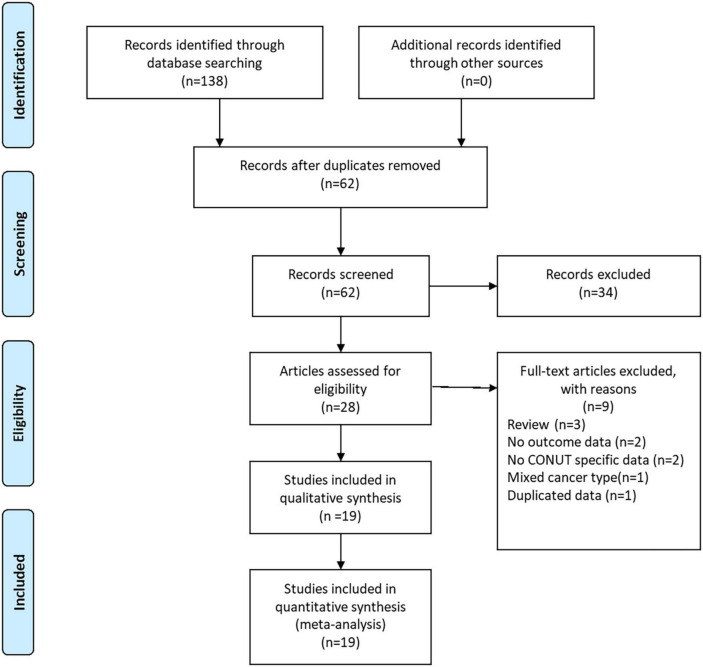
As shown in Figure 1, a total of 138 records were yielded after searching the databases. Through careful title, abstract assessment and full text assessment, 19 studies (12–18, 25–36) with 21 cohorts were finally included in the present study. The basic information and clinical characteristics of the included studies were summarized in Tables 2, 3, respectively. A total of 9,764 patients from China, Japan, Korea, and Turkey were included in this study. These studies were published from 2017 to 2022 with a sample size ranging from 33 to 2182. In terms of primary treatment, surgery was performed in 16 studies, neoadjuvant therapy was performed in two studies and mixed treatment including immunotherapy was performed in one study. The quality of the included studies was good with a median score of 9 (range: 6–9, Figure 2 and Supplementary Table 1).
FIGURE 1.
The PRISMA flowchart of study selection.
TABLE 2.
Basic information of included studies.
| Author | Publication year | Country | Study design | Study interval | Sample size | Age, years | Sex (male/female) | TNM stage |
| Akagunduz | 2021 | Turkey | R; S | 2017–2021 | 161 | 58.7 (range,32–80) | 110/51 | I–III |
| Aoyama | 2022 | Japan | R; S | 2013–2017 | 331 | NA | 219/112 | I–III |
| Chen | 2022 | China | R; S | 2016–2020 | 146 | 59 (range,34–82) | 102/44 | I–IV |
| Hirahara | 2019 | Japan | R; S | 2010–2016 | 210 | NA | 146/64 | I–III |
| Huang | 2019 | China | P; S | 2014–2016 | 357 | 73.29 ± 5.24 | 275/82 | I–III |
| Jeon | 2020 | Korea | R; S | 2009–2015 | 1,307 | NA | 862/445 | I–III |
| Jin | 2021 | China | R; S | 2004–2015 | 272 | 61 (range, 32–80) | 201/71 | 0–III |
| Kudou | 2019 | Japan | R; S | 2005–2016 | 144 | 65 (range,35–91) | 104/40 | I–III |
| Kuroda | 2018 | Japan | R; S | 2005–2014 | 416 | 67.2 (range 25–94) | 276/149 | I–III |
| Lin | 2019 | China | R; S | 2009–2014 | 2,182 | 60.8 (IQR, 54–68.3) | 1,643/539 | I–III |
| Liu | 2018 | China | R; S | 2000–2012 | 697 | 57 (range, 21–86) | 457/230 | II–III |
| Mimatsu | 2017 | Japan | R; S | 2006–2016 | 33 | NA | 28/6 | IV |
| Qian | 2021 | China | R; S | 2016–2019 | 309 | 63.4 ± 0.6 | 228/81 | I–IV |
| Ryo | 2019 | Japan | R; M | 2010–2014 | 626 | 67.9 ± 10.9 | 435/191 | II–III |
| Sun | 2021 | China | R; S | 2016–2018 | 1,479 | 60.4 ± 17.3 | 1,083/396 | I–IV |
| Suzuki | 2021 | Japan | R; S | 2000–2015 | 211 | ≥75 | 141/70 | I–III |
| Xiao | 2022 | China | R; S | 2014–2019 | 106 | 67 (range,43–85) | 84/22 | I–IV |
| Zheng | 2018 | China | R; S | 2010–2011 | 532 | 61.1 ± 11.5 | 403/129 | I–III |
| Zhu | 2021 | China | R; S | 2005–2015 | 245 | NA | 179/66 | I–IV |
R, retrospective; S, single center; M, multiple center; NA, not available; IQR, inter-quartile range; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival; DFS, disease-free survival.
TABLE 3.
Survival information of included studies.
| Author | Publication year | Sample size | Low group | High group | Primary treatment | Selection method | Cut-off value | Multivariate analysis | Survival outcomes | Median follow-up time, months |
| Akagunduz | 2021 | 161 | 56 | 105 | Neoadjuvant chemotherapy | ROC | ≥4 | Yes | OS | 11.2 (range:2.3–32.3) |
| Aoyama | 2022 | 331 | 221 | 110 | Curative surgery | NA | ≥2 | Yes/Yes | OS; RFS | NA |
| Chen | 2022 | 146 | 75 | 71 | PD-1/PD-L1 inhibitors or chemotherapy | NA | >0 | Yes/Yes | OS; PFS | NA |
| Hirahara | 2019 | 210 | 105 | 105 | Curative surgery | ROC | ≥3 | Yes | OS | 35.3 (range:4.0–97.0) |
| Huang | 2019 | 357 | 153 | 204 | Curative surgery | NA | ≥2 | NA | NA | NA |
| Jeon | 2020 | 1,307 | Normal: 893 | Light:396; Moderate:18; Severe:1 |
Curative surgery | NA | NA | Yes | OS | 59.0 (range: 1–109) |
| Jin | 2021 | 272 | 182 | 85 | Neoadjuvant chemotherapy | ROC | ≥4 | Yes/Yes | OS; PFS | NA |
| Kudou | 2019 | 144 | 118 | 26 | Curative surgery | ROC | ≥3 | No/No | OS; RFS | NA |
| Kuroda | 2018 | 416 | 354 | 62 | Curative surgery | ROC | ≥4 | Yes/No | OS; RFS | 61.2 (range: 1–134) |
| Lin | 2019 | 2,182 | 1704 | 478 | Curative surgery | X-tile | >2 | No | OS | 52 (range: 1–118) |
| Liu | 2018 | 697 | 480 | 217 | Curative surgery | ROC | ≥3 | Yes | CSS | 36 (range: 3–162) |
| Mimatsu | 2017 | 33 | 16 | 17 | Non-curative surgery | NA | >4 | No | OS | NA |
| Qian | 2021 | 309 | 214 | 95 | Curative surgery | ROC | 2.5 | NA | NA | NA |
| Ryo | 2019 | 626 | 337 | 289 | Curative surgery | ROC | ≥2 | Yes/No | OS; DFS | 49.2 |
| Sun | 2021 | 1,479 | 627 | 852 | Curative surgery | ROC | ≥2 | NA | NA | NA |
| Suzuki | 2021 | 211 | 175 | 36 | Curative surgery | NA | >4 | Yes/Yes | OS; CSS | 47 (range: 5–185) |
| Xiao | 2022 | 106 | 43 | 63 | Curative surgery | NA | >4 | No | OS | 30 (range:7–64) |
| Zheng | 2018 | 532 | Normal:291 | Light: 183; Moderate or severe: 58 |
Curative surgery | NA | NA | Yes/Yes | OS; RFS | 60 (range: 2- 76) |
| Zhu | 2021 | 245 | 104 | 141 | Curative surgery | ROC | ≥4 | Yes/Yes | OS; DFS | NA |
ROC, receiver operating characteristic curve; NA, not available; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival; DFS, disease-free survival; CSS, cancer specific survival.
FIGURE 2.
Quality assessment of included studies.
3.2. Relationship between the CONUT and OS
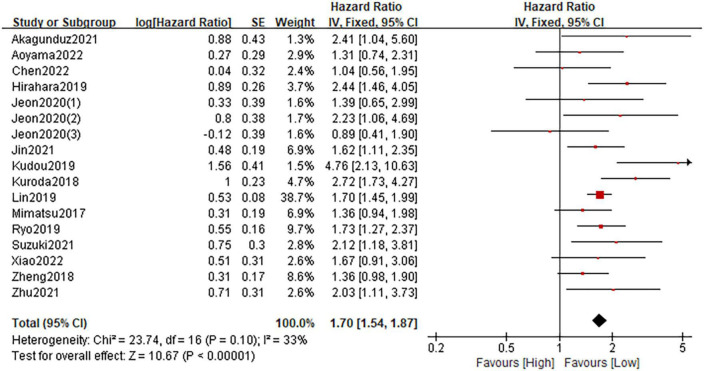
Fifteen studies involving 6,922 patients described the association between the CONUT and OS. The fixed-effect model was applied due to the low heterogeneity (I2 = 33%; P = 0.10). The pooled HR was 1.70 (95%CI: 1.54–1.87; P < 0.0001), which indicated that a high CONUT score was significantly associated with worse OS in patients with gastric cancer (Figure 3). Furthermore, subgroup analyses based on country, sample size, primary treatment, cut-off method, cut-off value and analysis method were performed. As shown in Table 4 and Supplementary Figure 1, the pooled results from all subgroup analyses revealed that patients in the high CONUT group had a substantially reduced OS when compared to these in the low CONUT group. In addition, sensitivity analysis by omitting one study at a time demonstrated that the combined outcome was not significantly changed (Supplementary Figure 3A).
FIGURE 3.
Forest plot assessing the relationship between the CONUT and OS.
TABLE 4.
Subgroup analyses for OS and RFS of CONUT-high patients vs. CONUT-low patients.
| Cohorts, n | Patients, n | HR (95%CI) | P-value | I2 (%) | ||
| Overall survival | ||||||
| Total | 17 | 6,922 | 1.70 (1.54–1.87) | <0.0001 | 33 | |
| Country | China | 6 | 3,483 | 1.62 (1.43–1.83) | <0.0001 | 0 |
| Japan | 7 | 1,971 | 1.90 (1.60–2.26) | <0.0001 | 56 | |
| Others | 4 | 1,468 | 1.58 (1.07–2.33) | 0.02 | 26 | |
| Sample size | >200 | 12 | 6,332 | 1.72 (1.54–1.91) | <0.0001 | 16 |
| ≤200 | 5 | 590 | 1.61 (1.25–2.08) | <0.0001 | 62 | |
| Primary treatment | Surgery | 14 | 6,343 | 1.72 (1.55–1.91) | <0.0001 | 37 |
| Others | 3 | 579 | 1.54 (1.14–2.07) | 0.005 | 25 | |
| Cut-off method | ROC | 7 | 2,074 | 2.06 (1.72–2.46) | <0.0001 | 33 |
| Others | 10 | 4,848 | 1.57 (1.39–1.76) | <0.0001 | 0 | |
| Cut-off value | ≥4 | 7 | 1,444 | 1.82 (1.51–2.19) | <0.0001 | 10 |
| <4 | 10 | 5,478 | 1.66 (1.48–1.86) | <0.0001 | 45 | |
| Analysis method | Univariate | 4 | 2,465 | 1.70 (1.48–1.95) | <0.0001 | 61 |
| Multivariate | 13 | 4,457 | 1.70 (1.49–1.95) | <0.0001 | 25 | |
| Recurrence free survival | ||||||
| Total | 10 | 3,620 | 1.57 (1.36–1.82) | <0.0001 | 30 | |
| Country | China | 5 | 1,892 | 1.50 (1.25–1.81) | <0.0001 | 0 |
| Japan | 5 | 1,728 | 1.70 (1.34–2.16) | <0.0001 | 53 | |
| Sample size | >200 | 8 | 3,330 | 1.56 (1.34–1.82) | <0.0001 | 13 |
| ≤200 | 2 | 290 | 1.69 (1.02–2.79) | 0.04 | 79 | |
| Primary treatment | Surgery | 8 | 3,202 | 1.60 (1.36–1.89) | <0.0001 | 40 |
| Others | 2 | 418 | 1.47 (1.07–2.02) | 0.02 | 0 | |
| Cut-off method | ROC | 6 | 2,400 | 1.64 (1.36–1.97) | <0.0001 | 39 |
| Others | 4 | 1,220 | 1.47 (1.16–1.87) | 0.002 | 29 | |
| Cut-off value | ≥4 | 4 | 1,144 | 1.99 (1.47–2.70) | <0.0001 | 24 |
| <4 | 6 | 2,476 | 1.47 (1.24–1.73) | <0.0001 | 15 | |
| Analysis method | Univariate | 3 | 1,186 | 1.60 (1.22–2.12) | 0.0008 | 67 |
| Multivariate | 7 | 2,434 | 1.56 (1.31–1.86) | <0.0001 | 10 | |
3.3. Relationship between the CONUT and RFS
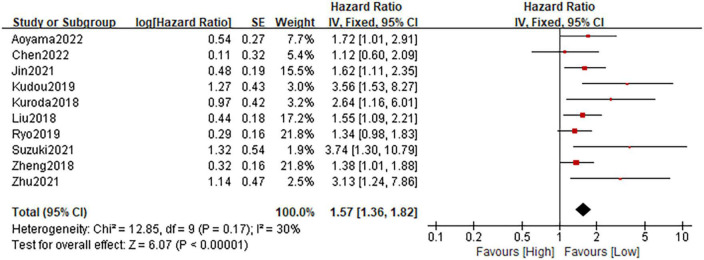
A total of ten studies consisting of 3,620 patients reported on RFS. The heterogeneity test showed a low heterogeneity among studies (I2 = 30%; P = 0.17), and the fixed-effect model was performed. The pooled HR was 1.57 (95%CI: 1.36–1.82; P < 0.0001), which suggested that patients in the high CONUT group had a significantly poorer RFS when compared with patients in the low CONUT group (Figure 4). Similarly, Stratification by country, sample size, primary treatment, cut-off method, cut-off value, and analysis method showed that the incorporated results were consistent in each subgroup (Table 4 and Supplementary Figure 2). Sensitivity analysis demonstrated that the pooled result remained unchanged (Supplementary Figure 3B).
FIGURE 4.
Forest plot accessing the relationship between the CONUT and RFS.
3.4. Relationship between the CONUT and postoperative complications
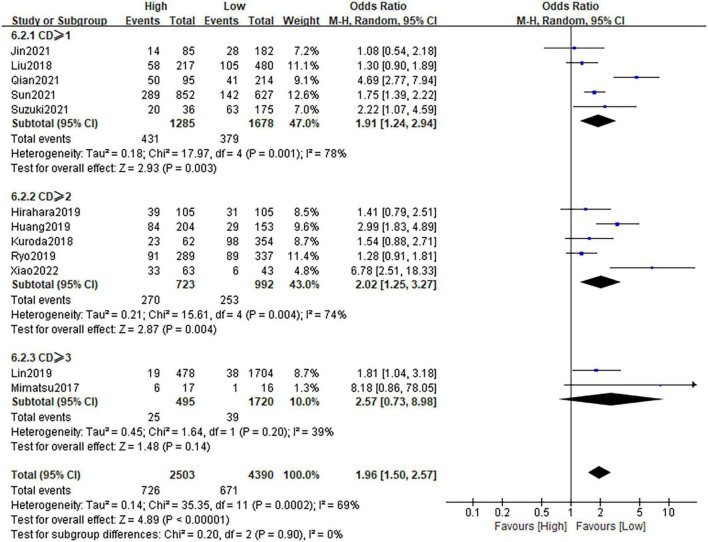
Twelve studies, comprising 6,893 patients, investigated postoperative complications in patients with gastric cancer. Following the result of heterogeneity test (I2 = 69%; P = 0.0002), the random-effect model was applied. The pooled OR was 1.96 (95%CI: 1.50–2.57; P < 0.0001), which suggested that a high CONUT score was a risk factor of postoperative complications for gastric cancer patients (Figure 5). Stratification by CD grade showed that the pooled results were almost unchanged in each subgroups. Sensitivity analysis confirmed the credibility of the combined result (Supplementary Figure 3C).
FIGURE 5.
Forest plot assessing the relationship between the CONUT and postoperative complications.
3.5. Relationship between the CONUT and other postoperative oncological parameters
As shown in Table 5 and Supplementary Figure 4, the pooled results revealed that a higher CONUT score was associated with larger tumor size (OR = 0.60; 95%CI:0.49–0.75; P < 0.0001; I2 = 0%), higher percentage of microvascular invasion (OR = 0.67; 95%CI:0.50–0.89; P = 0.006; I2 = 0%), later TNM stage (OR = 0.63; 95%CI:0.55–0.72; P < 0.0001; I2 = 35%) and fewer patients receiving adjuvant chemotherapy (OR = 1.44; 95%CI:0.98–2.12; P = 0.06; I2 = 68%). Nevertheless, no significant association was found in tumor differentiation (OR = 1.03; 95%CI:0.88–1.20; P = 0.72; I2 = 33%).
TABLE 5.
Secondary outcomes in terms of CONUT-high patients vs. CONUT-low patients.
| Variables | Cohorts, n | Patients, n | OR (95%CI) | P-value | I2 (%) |
| Tumor size (<5 cm) | 3 | 1,469 | 0.60 (0.49–0.75) | <0.0001 | 0 |
| Tumor differentiation (Poor) | 11 | 3,544 | 1.03 (0.88–1.20) | 0.72 | 33 |
| Microvascular invasion (Yes) | 3 | 1,645 | 0.67 (0.50–0.89) | 0.006 | 0 |
| TNM stage (Stage III/IV) | 12 | 4,122 | 0.63 (0.55–0.72) | <0.0001 | 35 |
| Adjuvant chemotherapy (Yes) | 6 | 1,913 | 1.44 (0.98–2.12) | 0.06 | 68 |
3.6. Publication bias
The Begg’s funnel plots of the primary outcomes were displayed in Supplementary Figure 5. Begg’s test revealed that there was no significant publication bias in the present study about CONUT score and OS (P = 0.680), RFS (P = 0.602), and postoperative complications (P = 0.304).
4. Discussion
Malnutrition is common in cancer patients, which is further exacerbated in gastric cancer patients due to additional factors such as malabsorption and obstructive syndrome (37). Numerous pieces of evidence have illustrated that malnutrition can lead to increased length of hospital stays and deteriorate the prognosis of cancer patients (38, 39). Therefore, early screening and proper treatment of malnourished patients is extremely important in clinical practice. Currently, although several tumor-related nutrition assessment tools like NRS2002 and PG-SGA have been developed (40, 41), the utilization of these tools is controversial due to their complexity and subjectivity. Ideally, the screening tool should be simple, convenient, sensitive and objective.
In this context, the CONUT score was constructed by González-Madroño et al. (42) in 2012 as a potential tool to make clinical undernutrition screening using three peripheral blood parameters (albumin level, total cholesterol level, and total lymphocyte count). Since then, the CONUT has been gradually used to assess the prognosis of various cancers due to its easy availability and convenient calculation (43–45). Niu et al. in a meta-analysis of 12 studies have reported that high CONUT score is associated with worse survival OS and CSS in urological cancers (46). Another meta-analysis by Takagi et al. also confirmed that the CONUT score is a practical prognostic factor associated with the prognosis of colorectal cancer (45). Additionally, the clinical value of the CONUT score has been successfully validated in other malignancies, such as lung cancer (47) and hepatocellular carcinoma (48). However, since each cancer type varies a lot, it is important to explore the applicability of the CONUT score in gastric cancer.
We conducted a comprehensive literature search and identified 19 studies with 9,764 GC patients. Relative to previous studies (19), this update has several strengths. First, by including all patients in our study, the generalizability of the CONUT score as a predictive marker in GC patients is enhanced compared to previous studies that only included patients undergoing radical resection. Second, by including an adequate number of samples, the heterogeneity of the pooled survival outcomes is significantly reduced. Third, due to the full inclusion of all studies, we are able to perform adequate subgroup analyses to fully explore the ability of the CONUT score as a nutritional screening metric to predict clinical outcomes in different kinds of GC patients. Through our pooled analyses, we found that patients in the high CONUT score group had 1.70, 1.57, and 1.96 times increased risk of the poor OS and RFS, as well as higher incidence of postoperative complications, compared to those with low CONUT score. Besides, we noted that high COUNT score was significantly associated with larger tumor size, higher percentage of microvascular invasion, later TNM stage and fewer patients receiving adjuvant chemotherapy. On examination of all subgroup analyses, it can be seen that all of the pooled outcomes supported the efficacy of the CONUT score in the primary outcomes prediction. Meanwhile, the pooled outcomes remained their significance on sensitivity analyses, and no evidence of publication bias was observed through Begg’s tests. The results were robust and therefore increase the credibility of our conclusions.
The good discriminatory value of the CONUT score could be explained as follows: Firstly, each of the components of the CONUT score has been demonstrated to be associated with outcomes in cancer patients. Albumin as a recognized indicator has been widely used to reflect a patient’s nutritional status. Hypoalbuminemia has been demonstrated to be significantly associated with poor wound healing, increased risk of infections and reduced survival of cancer patients (49, 50). In addition, serum albumin plays an important role in inhibiting the production of pro-inflammatory cytokines and enhancing cell-mediated immunity (51). And low levels of albumin thereby reduce response to adjuvant therapy. Secondly, cholesterol, as an important component of the cell membrane, plays an essential role in maintaining the cellular function. Low levels of cholesterol have been suggested to prompt tumor progression and deteriorate patient prognosis in various cancers (52, 53). The underlying mechanism may be a consequence of the requirement of cholesterol consumption for tumor growth (54). In addition, a recent study based on animal models showed that high serum cholesterol levels can enhance the antitumor effect of natural killer cells in mice (55). Finally, lymphocyte count, an important indicator of immune and nutritional status in cellular immunity, has been confirmed to inhibit tumor progression by inducing its lysis and apoptosis (56). Numerous studies have demonstrated that lymphopenia is strongly associated with early recurrence and poor survival in cancer patients (57). Secondly, our pooled results further revealed that higher COUNT score was significantly related to larger tumor size, higher percentage of microvascular invasion, later TNM stage and fewer patients receiving adjuvant chemotherapy, even though it remains unclear whether the results of the CONUT score were a cause or a consequence of these advanced tumor characteristics.
The present meta-analysis had several limitations. First, all of these studies were retrospective in nature, which may increase the risk of selection bias, and more prospective studies are thereby required to further investigate this issue. Second, most included studies were from Asian countries, which may affect the applicability of the CONUT score in Western populations. Third, the cut-off value of the CONUT score varies greatly among studies, which might affect the clinical utility of these findings.
5. Conclusion
Our results suggested that the CONUT score could be a valuable prognostic biomarker for patients with gastric cancer. Patients in the high CONUT score group have poor OS, RFS, and a higher rate of complications. Clinicians could use this useful indicator to stratify patients and formulate individual treatment plans. However, further research is still required to validate the value of this index in gastric malignancy.
Author contributions
HL wrote the manuscript. HL and X-CY performed the data search and data analysis. HL, X-CY, and D-CL prepared the figures and tables. WW and R-HC approved the final manuscript. All authors reviewed the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1156006/full#supplementary-material
References
- 1.Cao W, Chen H, Yu Y, Li N, Chen W. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. (2021) 134:783–91. 10.1097/CM9.0000000000001474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.Pang H, Zhang W, Liang X, Zhang Z, Chen X, Zhao L, et al. Prognostic score system using preoperative inflammatory, nutritional and tumor markers to predict prognosis for gastric cancer: a two-center cohort study. Adv Ther. (2021) 38:4917–34. 10.1007/s12325-021-01870-z [DOI] [PubMed] [Google Scholar]
- 4.Sexton R, Al Hallak M, Diab M, Azmi A. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. (2020) 39:1179–203. 10.1007/s10555-020-09925-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bando E, Ji X, Kattan M, Seo H, Song K, Park C, et al. Development and validation of a pretreatment nomogram to predict overall survival in gastric cancer. Cancer Med. (2020) 9:5708–18. 10.1002/cam4.3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma L, Chen G, Wang D, Zhang K, Zhao F, Tang J, et al. A nomogram to predict survival probability of gastric cancer patients undergoing radical surgery and adjuvant chemotherapy. Front Oncol. (2022) 12:893998. 10.3389/fonc.2022.893998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis? Nutr Cancer. (2017) 69:1151–76. 10.1080/01635581.2017.1367947 [DOI] [PubMed] [Google Scholar]
- 8.Kubota K, Ito R, Narita N, Tanaka Y, Furudate K, Akiyama N, et al. Utility of prognostic nutritional index and systemic immune-inflammation index in oral cancer treatment. BMC Cancer. (2022) 22:368. 10.1186/s12885-022-09439-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fruchtenicht A, Poziomyck A, Kabke G, Loss S, Antoniazzi J, Steemburgo T, et al. Nutritional risk assessment in critically ill cancer patients: systematic review. Rev Bras Ter Intensiva. (2015) 27:274–83. 10.5935/0103-507X.20150032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignacio de Ulíbarri J, González-Madroño A, de Villar N, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. (2005) 20:38–45. [PubMed] [Google Scholar]
- 11.Kheirouri S, Alizadeh M. Prognostic potential of the preoperative controlling nutritional status (CONUT) score in predicting survival of patients with cancer: a systematic review. Adv Nutr. (2021) 12:234–50. 10.1093/advances/nmaa102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mimatsu K, Fukino N, Ogasawara Y, Saino Y, Oida T. Utility of Inflammatory marker- and nutritional status-based prognostic factors for predicting the prognosis of stage IV gastric cancer patients undergoing non-curative surgery. Anticancer Res. (2017) 37:4215–22. 10.21873/anticanres.11812 [DOI] [PubMed] [Google Scholar]
- 13.Kudou K, Saeki H, Nakashima Y, Kamori T, Kawazoe T, Haruta Y, et al. C-reactive protein/albumin ratio is a poor prognostic factor of esophagogastric junction and upper gastric cancer. J Gastroenterol Hepatol. (2019) 34:355–63. 10.1111/jgh.14442 [DOI] [PubMed] [Google Scholar]
- 14.Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, et al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. (2018) 21:204–12. 10.1007/s10120-017-0744-3 [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Zhang D, Lin E, Chen Y, Li W, Chen Y, et al. Preoperative controlling nutritional status (CONUT) score as a predictor of long-term outcome after curative resection followed by adjuvant chemotherapy in stage II-III gastric Cancer. BMC Cancer. (2018) 18:699. 10.1186/s12885-018-4616-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryo S, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. The controlling nutritional status score serves as a predictor of short- and long-term outcomes for patients with stage 2 or 3 gastric cancer: analysis of a multi-institutional data set. Ann Surg Oncol. (2019) 26:456–64. 10.1245/s10434-018-07121-w [DOI] [PubMed] [Google Scholar]
- 17.Suzuki S, Kanaji S, Yamamoto M, Oshikiri T, Nakamura T, Kakeji Y. Controlling nutritional status (CONUT) score predicts outcomes of curative resection for gastric cancer in the elderly. World J Surg. (2019) 43:1076–84. 10.1007/s00268-018-04889-6 [DOI] [PubMed] [Google Scholar]
- 18.Zheng Z, Lu J, Xie J, Wang J, Lin J, Chen Q, et al. Preoperative skeletal muscle index vs the controlling nutritional status score: which is a better objective predictor of long-term survival for gastric cancer patients after radical gastrectomy? Cancer Med. (2018) 7:3537–47. 10.1002/cam4.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagi K, Domagala P, Polak W, Buettner S, Wijnhoven B, Ijzermans J. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing gastrectomy for gastric cancer: a systematic review and meta-analysis. BMC Surg. (2019) 19:129. 10.1186/s12893-019-0593-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y, Liu Z, Qiu Y, Zhang J, Wu H, Liang R, et al. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. (2018) 44:1494–503. 10.1016/j.ejso.2018.07.052 [DOI] [PubMed] [Google Scholar]
- 21.Clavien P, Barkun J, de Oliveira M, Vauthey J, Dindo D, Schulick R, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 22.Pang H, Zhao L, Wang H, Chen X, Liu K, Zhang W, et al. Impact of type of postoperative complications on long-term survival of gastric cancer patients: results from a high-volume institution in China. Front Oncol. (2021) 11:587309. 10.3389/fonc.2021.587309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang H, Yan M, Chen L, Chen X, Chen Z, Zhang S, et al. Detection of asymptomatic recurrence following curative surgery improves survival in patients with gastric cancer: a systematic review and meta-analysis. Front Oncol. (2022) 12:1011683. 10.3389/fonc.2022.1011683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Liu H, Liu D, Tong C, Liang X, Chen R. Prognostic value of pan-immune-inflammation value in colorectal cancer patients: a systematic review and meta-analysis. Front Oncol. (2022) 12:1036890. 10.3389/fonc.2022.1036890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akagunduz B, Demir M, Atcı M. Controlling nutritional status (CONUT) score is a prognostic factor for patients with gastric cancer treated by perioperative FLOT. J Gastrointest Cancer. (2022) 53:571–80. 10.1007/s12029-021-00664-4 [DOI] [PubMed] [Google Scholar]
- 26.Aoyama T, Komori K, Nakazano M, Hara K, Tamagawa H, Kazama K, et al. The clinical influence of the CONUT score on survival of patients with gastric cancer receiving curative treatment. In Vivo. (2022) 36:942–8. 10.21873/invivo.12784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Sun H, Zhao R, Huang R, Pan H, Zuo Y, et al. Controlling nutritional status (CONUT) predicts survival in gastric cancer patients with immune checkpoint inhibitor (PD-1/PD-L1) outcomes. Front Pharmacol. (2022) 13:836958. 10.3389/fphar.2022.836958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirahara N, Tajima Y, Fujii Y, Kaji S, Kawabata Y, Hyakudomi R, et al. Controlling nutritional status (CONUT) as a prognostic immunonutritional biomarker for gastric cancer after curative gastrectomy: a propensity score-matched analysis. Surg Endosc. (2019) 33:4143–52. 10.1007/s00464-019-06723-z [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Huang Y, Lu M, Sun W, Sun X, Chen X, et al. Controlling nutritional status (CONUT) score is a predictor of post-operative outcomes in elderly gastric cancer patients undergoing curative gastrectomy: a prospective study. Cancer Manag Res. (2019) 11:9793–800. 10.2147/CMAR.S233872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon C, Park K, Jung Y, Seo H, Park C, Song K, et al. Modified controlling nutritional status score: a refined prognostic indicator depending on the stage of gastric cancer. Surg Oncol. (2020) 34:261–9. 10.1016/j.suronc.2020.05.008 [DOI] [PubMed] [Google Scholar]
- 31.Jin H, Zhu K, Wang W. The predictive values of pretreatment controlling nutritional status (CONUT) score in estimating short- and long-term outcomes for patients with gastric cancer treated with neoadjuvant chemotherapy and curative gastrectomy. J Gastric Cancer. (2021) 21:155–68. 10.5230/jgc.2021.21.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin J, Lin L, Tang Y, Wang J, Lu J, Chen Q, et al. Which nutritional scoring system is more suitable for evaluating the short- or long-term prognosis of patients with gastric cancer who underwent radical gastrectomy? J Gastrointest Surg. (2020) 24:1969–77. 10.1007/s11605-019-04360-4 [DOI] [PubMed] [Google Scholar]
- 33.Qian Y, Liu H, Pan J, Yu W, Lv J, Yan J, et al. Preoperative controlling nutritional status (CONUT) score predicts short-term outcomes of patients with gastric cancer after laparoscopy-assisted radical gastrectomy. World J Surg Oncol. (2021) 19:25. 10.1186/s12957-021-02132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun F, Zhang C, Liu Z, Ai S, Guan W, Liu S. Controlling nutritional status (CONUT) score as a predictive marker for short-term complications following gastrectomy of gastric cancer: a retrospective study. BMC Gastroenterol. (2021) 21:107. 10.1186/s12876-021-01682-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao Q, Li X, Duan B, Li X, Liu S, Xu B, et al. Clinical significance of controlling nutritional status score (CONUT) in evaluating outcome of postoperative patients with gastric cancer. Sci Rep. (2022) 12:93. 10.1038/s41598-021-04128-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X, Zhao Y, Ma F, Wu S. Controlling nutritional status score predict the individualized survival of patients with gastric cancer. Asia Pac J Clin Nutr. (2021) 30:51–9. 10.6133/apjcn.202103_30(1).0007 [DOI] [PubMed] [Google Scholar]
- 37.Benoist S, Brouquet A. Nutritional assessment and screening for malnutrition. J Visc Surg. (2015) 152(Suppl. 1):S3–7. 10.1016/S1878-7886(15)30003-5 [DOI] [PubMed] [Google Scholar]
- 38.Xu L, Shi M, Huang Z, Zhang W, Zhang H, Shen X, et al. Impact of malnutrition diagnosed using Global leadership initiative on malnutrition criteria on clinical outcomes of patients with gastric cancer. JPEN J Parenter Enteral Nutr. (2022) 46:385–94. 10.1002/jpen.2127 [DOI] [PubMed] [Google Scholar]
- 39.Salas S, Cottet V, Dossus L, Fassier P, Ginhac J, Latino-Martel P, et al. Nutritional factors during and after cancer: impacts on survival and quality of life. Nutrients. (2022) 14:2958. 10.3390/nu14142958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian M, Fu H, Du J. Application value of NRS2002 and PG-SGA in nutritional assessment for patients with cervical cancer surgery. Am J Transl Res. (2021) 13:7186–92. [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Wan Z, Zhu Y, Zhang L, Zhang L, Wan H. Prevalence of malnutrition comparing NRS2002, MUST, and PG-SGA with the GLIM criteria in adults with cancer: a multi-center study. Nutrition. (2021) 83:111072. 10.1016/j.nut.2020.111072 [DOI] [PubMed] [Google Scholar]
- 42.González-Madroño A, Mancha A, Rodríguez F, Culebras J, de Ulibarri J. Confirming the validity of the CONUT system for early detection and monitoring of clinical undernutrition: comparison with two logistic regression models developed using SGA as the gold standard. Nutr Hosp. (2012) 27:564–71. 10.1590/S0212-16112012000200033 [DOI] [PubMed] [Google Scholar]
- 43.Lu C, Chen Q, Fei L, Wang J, Wang C, Yu L. Prognostic impact of the controlling nutritional status score in patients with hematologic malignancies: a systematic review and meta-analysis. Front Immunol. (2022) 13:952802. 10.3389/fimmu.2022.952802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng L, Meng C, Li J, You C, Du Y, Xiong W, et al. The prognostic significance of controlling nutritional status (CONUT) score for surgically treated renal cell cancer and upper urinary tract urothelial cancer: a systematic review and meta-analysis. Eur J Clin Nutr. (2022) 76:801–10. 10.1038/s41430-021-01014-0 [DOI] [PubMed] [Google Scholar]
- 45.Takagi K, Buettner S, Ijzermans J. Prognostic significance of the controlling nutritional status (CONUT) score in patients with colorectal cancer: a systematic review and meta-analysis. Int J Surg. (2020) 78:91–6. 10.1016/j.ijsu.2020.04.046 [DOI] [PubMed] [Google Scholar]
- 46.Niu X, Zhu Z, Bao J. Prognostic significance of pretreatment controlling nutritional status score in urological cancers: a systematic review and meta-analysis. Cancer Cell Int. (2021) 21:126. 10.1186/s12935-021-01813-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Li X, Cong Z, Zheng C, Luo C, Xie K, et al. Controlling nutritional status is a prognostic factor for patients with lung cancer: a systematic review and meta-analysis. Ann Palliat Med. (2021) 10:3896–905. 10.21037/apm-20-2328 [DOI] [PubMed] [Google Scholar]
- 48.Takagi K, Domagala P, Polak W, Buettner S, Ijzermans J. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. (2019) 19:211. 10.1186/s12876-019-1126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta D, Lis C. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. (2010) 9:69. 10.1186/1475-2891-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reis T, Silva R, Nascimento EDS, Bessa J, Oliveira M, Fava A, et al. Early postoperative serum albumin levels as predictors of surgical outcomes in head and neck squamous cell carcinoma. Braz J Otorhinolaryngol. (2022) 88 (Suppl. 1):S48–56. 10.1016/j.bjorl.2021.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu X, Duan W, Su C, Mao F, Lv Y, Teng Y, et al. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol Immunother. (2017) 66:1597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strasak A, Pfeiffer R, Brant L, Rapp K, Hilbe W, Oberaigner W, et al. Time-dependent association of total serum cholesterol and cancer incidence in a cohort of 172,210 men and women: a prospective 19-year follow-up study. Ann Oncol. (2009) 20:1113–20. 10.1093/annonc/mdn736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou P, Li B, Liu B, Chen T, Xiao J. Prognostic role of serum total cholesterol and high-density lipoprotein cholesterol in cancer survivors: a systematic review and meta-analysis. Clin Chim Acta. (2018) 477:94–104. 10.1016/j.cca.2017.11.039 [DOI] [PubMed] [Google Scholar]
- 54.Wang Q, Lau W, Zhang B, Zhang Z, Huang Z, Luo H, et al. Preoperative total cholesterol predicts postoperative outcomes after partial hepatectomy in patients with chronic hepatitis B- or C-related hepatocellular carcinoma. Surgery. (2014) 155:263–70. 10.1016/j.surg.2013.08.017 [DOI] [PubMed] [Google Scholar]
- 55.Qin W, Yang Z, Li M, Chen Y, Zhao X, Qin Y, et al. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology. (2020) 158:1713–27. 10.1053/j.gastro.2020.01.028 [DOI] [PubMed] [Google Scholar]
- 56.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 57.Saroha S, Uzzo R, Plimack E, Ruth K, Al-Saleem T. Lymphopenia is an independent predictor of inferior outcome in clear cell renal carcinoma. J Urol. (2013) 189:454–61. 10.1016/j.juro.2012.09.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.