Abstract
Background
Pulmonary embolism (PE) is not only a life-threatening disease but also a public health issue with significant economic burden. The aim of the study was to identify factors—including the role of primary care—that predict length of hospital stay (LOHS), mortality and re-hospitalization within 6 months of patients admitted for PE.
Method
A retrospective cohort study was conducted with patients presenting to a Swiss public hospital with PE diagnosed at the hospital between November 2018 and October 2020. Multivariable logistic and zero-truncated negative binomial regression analyses were performed to assess risk factors for mortality, re-hospitalization and LOHS. Primary care variables encompassed whether patients were sent by their general practitioner (GP) to the emergency department and whether a GP follow-up assessment after discharge was recommended. Further analyzed variables were pulmonary embolism severity index (PESI) score, laboratory values, comorbidities, and medical history.
Results
A total of 248 patients were analyzed (median 73 years and 51.6% females). On average patients were hospitalized for 5 days (IQR 3–8). Altogether, 5.6% of these patients died in hospital, and 1.6% died within 30 days (all-cause mortality), 21.8% were re-hospitalized within 6 months. In addition to high PESI scores, we detected that, patients with an elevated serum troponin, as well as with diabetes had a significantly longer hospital stay. Significant risk factors for mortality were elevated NT-proBNP and PESI scores. Further, high PESI score and LOHS were associated with re-hospitalization within 6 months. PE patients who were sent to the emergency department by their GPs did not show improved outcomes. Follow-up with GPs did not have a significant effect on re-hospitalization.
Conclusion
Defining the factors that are associated with LOHS in patients with PE has clinical implications and may help clinicians to allocate adequate resources in the management of these patients. Serum troponin and diabetes in addition to PESI score might be of prognostic use for LOHS. In this single-center cohort study, PESI score was not only a valid predictive tool for mortality but also for long-term outcomes such as re-hospitalization within 6 months.
Keywords: LOHS, pulmonary embolism, risk factors, length of hospital stay, multi morbidity, rehospitalization, in hospital death
1. Introduction
Pulmonary embolism (PE) is a serious, potentially life-threatening health condition that represents the third major cause of cardiovascular death behind myocardial infarction and cerebrovascular accidents (1, 2). PE can be considered a central public health issue since it is associated with a substantial economic burden (3–6).
The exact incidence rate for PE is not available but estimates range from 39 to 115 per 100,000 population. Additionally, as the incidence of PE rises with age, PE rates can be expected to continue increasing even further due to the rapidly ageing population in high-income countries, and therefore to significantly impact morbidity, mortality, and healthcare costs (6, 7). Most nations have an urgent dilemma in the realm of public health: how to address the difficulties brought on by the rise in the number of PE patients while utilizing the available medical resources to better fulfill their medical demand without impacting on cost and overtreatments.
Over the past decades, the incidence rate of PE has increased in Europe, whereas the mortality rate and the length of hospital stay (LOHS) have slightly decreased, due to advances in treatments and diagnostics (8–12). In a retrospective, Italian cohort study of 328 patients with PE, despite a trend in reduction in LOHS, the mean and median have not significantly decreased, due to a very small percentage (3%) of patients who received an ultra-early discharge and a large percentage of patients who were discharged within 6 days (31.5%) (13). LOHS is considered a crucial characteristic for health reports when it comes to the management and evaluation of inpatients and is a significant signal for the assessment of hospitals’ service quality (14). Several factors can influence LOHS in patients with PE such as sociodemographic, health-related characteristics and hospital care-related features (15–17). Due to the wide variability of influencing factors, there is no uniform approach to predict the length of stay for PE.
The primary aim of the study was to identify which factors may affect the length of stay of patients admitted for PE. The identification of patient characteristics influencing LOHS may allow decision-makers to plan hospital management accordingly.
Particularly we retrospectively explored if the primary outcome length of hospital stay for PE was influenced by commonly available sociodemographic and health-related variables measurable at entry time.
Although a reduced length of stay decreases hospital costs, it might negatively affect the quality of care. For this reason, as secondary outcomes, we analyzed factors associated with all-cause mortality (in hospital or 30 days mortality) and rehospitalization within 6 months.
2. Materials and methods
2.1. Design and setting
Our study was conducted in the cantonal hospital of Baselland (KSBL), a district general hospital covering a stable population of 280,000 in Northwest Switzerland. We undertook a retrospective cohort study with 378 consecutive patients hospitalized at the KSBL at the medical or surgical ward and who received the diagnosis of pulmonary embolism (according to the primary International Classification of Disease codes) during their hospital stay between November 2018 and October 2020. We were able to access the electronic case notes of 378 patients to retrieve presenting symptoms and clinical signs that have been associated with PE. Further, socio-demographics, vital signs, comorbidities and discharge variables (mortality and re-hospitalization) were assessed.
2.2. Inclusion and exclusion criteria
Data of these patients were individually reviewed. Patients were included in the study if a new PE was the main reason for their hospitalization and their diagnosis was confirmed by computer tomographic pulmonary angiogram (CTPA), scintigraphy or duplex ultra-sound by a specialist (deep vein thrombosis (DVT) combined with PE specific symptoms) within 12 h after presentation. Alternatively, they were also included if confirmatory, diagnostic imaging was performed later, but anticoagulant treatment was started within 12 h after presentation to the hospital due to high clinical suspicion of PE.
The following patients were excluded:
• Denied research consent.
• PE only as a suspected diagnosis and never confirmed with any imaging method.
• Primarily hospitalized for another reason, and PE was diagnosed after >12 h.
• Transferred from/to another hospital and therefore no complete case documentation.
After the application of the eligibility criteria, 248 patients were included in the analysis.
2.3. Statistical analyses
The outcome variables comprised LOHS (primary outcome), all-cause mortality in hospital and 30 days, and re-hospitalization within 6 months (secondary outcomes).
To minimize the risk of bias, optimism, and overfitting, no data-driven selection of variables was done. We selected potential predictors based on the literature and on clinical knowledge. Two researchers conducted a literature review and consulted clinical experts in the field. All variables are included in Table 1. Predictors included the PESI score based on age at entry, sex, history of cancer, history of chronic lung disease, history of heart failure, respiratory rate, hypothermia (below 36 degrees Celsius), systolic blood pressure (BP) <100 mmHg, heart rate (HR) ≥110 bpm, O2 saturation (SpO2) below 90%, altered mental status, and respiratory rate ≥ 30/min (18).
Table 1.
Patient characteristics.
| Demographic | All (n = 248) | Missing n (%) |
|---|---|---|
| Age at diagnosis, median (IQR) in years | 73 (62–81.5) | -- |
| Gender (female) | 128 (51.6%) | -- |
| Insurance type | ||
| General | 199 (80.2%) | -- |
| Half-private | 30 (12.1%) | -- |
| Private | 19 (7.7%) | -- |
| Vital signs | ||
| Heart rate (bpm), mean (SD) | 92.1 (19.7) | -- |
| Tachycardia (>100 bpm) | 76 (30.6%) | -- |
| Blood pressure systolic (mmHg), mean (SD) | 140.7 (24.9) | -- |
| Blood pressure diastolic (mmHg), mean (SD) | 83.1 (14.1) | -- |
| Hypotension (<100/60 mmHg), n (%) | 11 (4.4%) | -- |
| Hypertension (>140/90 mmHg), n (%) | 124 (50%) | -- |
| Respiratory rate (/min) mean (SD) | 20.4 (5.7) | 35 (14.1%) |
| Hypothermia (<36°C), n (%) | 9 (3.6%) | -- |
| Oxygen saturation, mean (SD) | 93.8 (4.8) | -- |
| Oxygen requirement | 36 (14.5%) | -- |
| Comorbidities | 229 (92.3%) | -- |
| Dyslipidemia, n (%) | 44 (17.7%) | -- |
| Diabetes, n (%) | 37 (14.9%) | -- |
| Cardiovascular disease, n (%) | 86 (34.7%) | -- |
| Heart failure, n (%) | 5 (2%) | -- |
| Chronic lung disease, n (%) | 44 (17.7%) | -- |
| Rheumatic disease, n (%) | 30 (12.1%) | -- |
| Mental disease, n (%) | 55 (22.2%) | -- |
| Altered mental status, n (%) | 5 (2.2%) | -- |
| Active cancer, n (%) | 30 (12.1%) | -- |
| Medical history | ||
| Previous VTE, n (%) | 58 (23.4%) | -- |
| Previous PE, n (%) | 26 (10.5%) | -- |
| Previous DVT, n (%) | 44 (17.7%) | -- |
| History of cancer, n (%) | 50 (20.2%) | -- |
| History of hypertension, n (%) | 126 (50.8%) | -- |
| PESI | ||
| PESI score, mean (SD) | 96.8 (31.4) | -- |
| PESI retrospectively calculated, n (%) | 200 (81%) | |
| Laboratory values | ||
| NT-proBNP, (ng/L) median (IQR) * | 499 (125–2,479) | 70 (28.2%) |
| Troponin-T hs (ng/L), median (IQR) ** | 16.95 (7.22–44.38) | 94 (37.9%) |
| Entry and discharge circumstances | ||
| Housing situation before admission | ||
| Private home, n (%) | 211 (85.1%) | -- |
| Care facility, n (%) | 37 (14.9%) | -- |
| Sent by GP, n (%) | 122 (49.2%) | -- |
| Follow-up with GP, n (%) | 72 (29%) | Data of 14 patients missing (died) |
| Discharge destination | Data of 14 patients missing (died) | |
| Private home, n (%) | 200 (80.6%) | |
| Care facility, n (%) | 34 (13.7%) | |
| Rehabilitation unit, n (%) | 21 (8.5%) | Data of 14 patients missing (died) |
| Outcomes | ||
| Length of stay, in nights, median (IQR) | 5 (3–8) | -- |
| Median 5 (3–8) | ||
| Rehospitalization at KSBL within 6 months after discharge, n (%) | 51 (21.8%) | Data of 14 patients missing (died) |
| Death | 18 (7.3%) | -- |
| Death (in hospital death) | 14 (5.6%) | -- |
| Death (30 days mortality) | 4 (1.6%) | -- |
NT-proBNP normal range < 125 ng/L.
Troponin-T hs < 14 ng/L.
Other variables of interest were body mass index (BMI), a medical history of dyslipidemia, diabetes, or previous PE. Laboratory values of interest were serum N-terminal pro B-Type natriuretic peptide (NT-proBNP) and Troponin-T high-sensitive (hs). The analysis of LOHS was primarily on patients that were discharged alive, a sensitivity analysis was performed on the full data set.
Further, the housing situation before admission and admission via another doctor (usually the GP) were entered into the models. For re-hospitalization outcome, we further entered the variable if GP follow-up was suggested and the LOHS.
For descriptive statistics as measures of central tendency, we displayed mean and standard deviation (SD) in case of normal distribution and median with interquartile range in case of skewed distribution, which was assessed through histograms assessment. For categorical variables we reported absolute and relative frequencies.
Variables with missing values were imputed using the k-Nearest Neighbor algorithm [function knn.impute from the R package “bnstruct” (19)]. A zero-truncated negative binomial regression was conducted to estimate the LOHS and its association with potential risk factors using the R package “VGAM.” As a sensitivity analysis, all regression models were additionally performed on the original, non-imputed data set.
Logistic regression models were created to estimate the risk of death and rehospitalization, and its association with potential risk factors.
All statistical analyses were performed using R, version 4.0.3 statistical software (R Foundation for Statistical Computing). All p-values reported were 2-sided; statistical significance was defined as p < 0.05.
3. Results
3.1. Patient characteristics
A total of 378 patients were identified who received the diagnosis of pulmonary embolism during their hospital stay. After the exclusion of 24 patients who declined research consent, 15 patients in which the diagnosis was not confirmed with imaging methods, 67 patients who were diagnosed more than 12 h after admission, and a further 24 who had incomplete diagnostic documentation, 248 cases were analyzed. The patient characteristics are shown in Table 1.
The median age at admission was 73 years (range 19–96) and 51.6% were female. The majority of the patients had general insurance (80%). Vital signs measured at admission revealed that the mean HR was 92.1 but one third of the patients presented to the hospital with tachycardia (30.6%). The average blood pressure was 141/83 mmHg, while half of the patients had hypertension while only a minority of the patients had hypotension, (4.4%). Body temperature was usually in the normal range and oxygen saturation was on average 93.7%, but some of the patients needed oxygen supply at entry (14.5%).
The majority of the patients had comorbidities (92.3%), the three most frequent disease types were: cardiovascular (34.7%), mental (22.2%) and chronic lung diseases (17.7%). Patients’ history revealed that previous VTE occurred in 23.4%, the most frequent one was DVT, followed by PE whereas 12 patients had both (4.8%).
Pulmonary embolism severity index (PESI) score was calculated at admission in 19% of the cases, in the remaining 81% of the cases the PESI score was calculated retrospectively. The mean of the PESI score was 96.8 (SD = 31.4). Regarding the laboratory values, NT-proBNP was measured in 71.8% of the patients and the values had a median of 499 ng/l (IQR 125–2,479), whereas troponin-T hs was measured in 62% of the patients with a median of 16.9 ng/l (6.84–46.2).
Before admission most of the patients lived independently, but 14.9% were admitted from a care facility. Almost half of the patients were sent to the hospital by a GP (49.2%). After the discharge, a follow-up with a GP was organized in 29% of all cases. The majority of the patients returned to their private homes (80.6%) while 13.7% were transferred to a care facility or a rehabilitation center (8.5%).
Out of 248 hospitalized patients with pulmonary embolism, 14 patients died during the hospital stay and were excluded from regression analyses with outcome LOHS and re-hospitalization. Patients with PE who did not die within the hospital stayed for a median of 5 days (IQR 3–8). Additionally, four patients died within 30 days (1.6% of the total patients) and rehospitalization at KSBL within 6 months after discharge occurred in 21.8% of the cases.
3.2. Prediction of LOHS
Our primary aim was to identify factors that predict LOHS. Table 2 provides coefficient estimates for predictors of LOHS in patients who did not die. Regression coefficients are shown as incident risk ratio (IRR). Patients with higher PESI scores (IRR = 1.068, 95%CI [1.034–1.104], value of p < 0.001), higher troponin values [IRR = 1.433, 95%CI (1.189–1.727), value of p < 0.001], and with diabetes [IRR = 1.293, 95%CI (1.007–1.66), value of p 0.044] had significantly longer LOHS.
Table 2.
Results of multivariable zero-truncated negative binomial regression model for length of hospital stay (LOHS) estimation in pulmonary embolism survivors (n = 234).
| LOHS prediction | IRR (95%CI) | Pr(>|z|) | |
|---|---|---|---|
| (Intercept):1 | 5.899 | 2.422 (1.742–5.722) | 0.004 |
| PESI score (per 10 points) | 6.273 | 1.068 (1.034–1.104) | <0.001 |
| NT-proBNP (per 1,000 units) | 6.012 | 1.021 (0.997–1.045) | 0.089 |
| Troponin-T hs (per 100 units) | 8.297 | 1.433 (1.189–1.727) | <0.001 |
| Pervious PE | 6.485 | 1.107 (0.829–1.478) | 0.492 |
| Previous DVT | 5.482 | 0.924 (0.724–1.178) | 0.522 |
| Diabetes | 7.517 | 1.293 (1.007–1.66) | 0.044 |
| Cardiovascular diseases | 6.904 | 1.183 (0.969–1.443) | 0.098 |
| Dyslipidemia | 5.041 | 0.842 (0.66–1.075) | 0.168 |
| BMI | 6.039 | 1.026 (0.87–1.209) | 0.763 |
| Housing situation before admission | 5.104 | 0.854 (0.656–1.112) | 0.241 |
| Sent by doctor | 5.98 | 1.015 (0.846–1.217) | 0.873 |
Statistically significant variables p < 0.05.
The LOHS prediction at the intercept (5.899 days) is the LOHS when all covariates are at 0 (for categorical covariates) or at their mean (for continuous covariates). The predicted LOHS of the model for each variable is presented for one unit increase. If the PESI score increases by one unit (on the original scale per 10 points), the predicted LOHS increases from 5.89 to 6.27 days. A higher increase occurs when the Troponin increases by one unit (on the original scale per 100 n/L) the predicted LOHS rise to 8.3 days. People with diabetes compared to those without tend to stay two nights longer, assuming all other variables are held constant.
Our secondary aims included the analyses of factors associated with mortality and rehospitalization rates. The results of the univariate logistic regression models for mortality, adjusted for PESI score, are displayed in Table 3. Higher PESI scores and NT-proBNP values were significantly associated with mortality in patients with pulmonary embolism [OR 1.617, 95%CI (1.359–1.981), value of p < 0.001 and OR 1.091, 95%CI (1.012–1.171) value of p 0.013, respectively]. No other variable was found to be statistically significant in the association with the mortality rate.
Table 3.
Results of univariate logistic regression model for mortality (in-hospital or within 30 days) in pulmonary embolism (n = 18).
| OR (95%CI) | Pr(>|z|) | |
|---|---|---|
| PESI score | 1.617 (1.359–1.981) | <0.001 |
| NT-proBNP | 1.091 (1.012–1.171) | 0.013 |
| Troponin-T hs | 0.139 (0.003–1.949) | 0.249 |
| Previous DVT | 0.41(0.022–2.322) | 0.408 |
| Diabetes | 0.782 (0.152–2.979) | 0.74 |
| Cardiovascular diseases | 1.628 (0.537–5.073) | 0.388 |
| Dyslipidemia | 0.341 (0.043–1.611) | 0.229 |
| BMI | 0.443 (0.117–1.399) | 0.199 |
| Housing situation before admission (care facility) | 1.443 (0.367–4.715) | 0.575 |
| Sent by doctor | 1.423 (0.454–4.715) | 0.548 |
| LOHS | 1.056 (0.967–1.137) | 0.172 |
All variables were adjusted for pulmonary embolism severity index (PESI) score. Statistically significant variables p < 0.05.
The results of our secondary multivariable analysis concerning rehospitalization rate are reported in Table 4. The odds for rehospitalization within 6 months in KSBL were also significantly higher for patients with a higher PESI score and for patients with a higher LOHS [OR 1.183, 95%CI (1.041–1.353) value of p 0.012 and OR 1.099, 95%CI (1.031–1.183) value of p 0.007, respectively]. No other variable was found to be statistically significant in the association with rehospitalization.
Table 4.
Multivariable logistic regression model for rehospitalization in pulmonary embolism (n = 51).
| OR (95%CI) | Pr(>|z|) | |
|---|---|---|
| PESI score | 1.183 (1.041–1.353) | 0.012 |
| NT-proBNP | 0.934 (0.831–1.03) | 0.546 |
| Troponin-T hs | 0.853 (0.265–1.926) | 0.957 |
| Previous PE | 0.522 (0.116–1.772) | 0.339 |
| Previous DVT | 1.346 (0.495–3.456) | 0.545 |
| Diabetes | 1.917 (0.733–4.893) | 0.175 |
| Cardiovascular diseases | 1.871 (0.873–4.013) | 0.106 |
| Dyslipidemia | 0.525 (0.181–1.372) | 0.208 |
| BMI | 0.747 (0.36–1.437) | 0.404 |
| Housing situation before admission (care facility) | 0.815 (0.279–2.139) | 0.69 |
| Sent by doctor | 1.019 (0.501–2.076) | 0.959 |
| Follow up with GP | 1.352 (0.626–2.869) | 0.435 |
| LOHS | 1.099 (1.031–1.183) | 0.007 |
Statistically significant variables p < 0.05.
4. Discussion
This retrospective observational cohort study of patients with PE showed that LOHS is influenced by PESI score, serum troponin values and diabetes. Other factors such as medical history, other types of comorbidities and whether the patients were sent to the hospital by a GP were not associated with longer LOHS.
Pulmonary embolism severity index score is a validated prognostic model for PE devised by Aujesky and colleagues (20). Originally it was developed to predict 30-day mortality using 11 clinical criteria [age at entry, sex, history of cancer, history of chronic lung disease, history of heart failure, respiratory rate, hypothermia (below 36 degrees Celsius), systolic blood Pressure <100 mmHg, heart rate ≥110 bpm, oxygen saturation below 90%, altered mental status, and respiratory rate ≥ 30 breaths per minute (20)]. The PESI score was also implemented to identify low-risk patients who might be treated outside the hospital and consequently be eligible for early discharge (18). In line with other studies, our results also confirm the prognostic validity of the PESI score in predicting the length of hospital stay (21, 22). The role of PESI in LOHS was confirmed by Rodriguez et al. (23), however the impact of the single items composing the score was unclear. For this reason, in our study, we have also analyzed the items of the PESI score separately and we found that age, sex heart rate over 110 bpm, oxygen saturation <90% and heart failure were significantly predictive for LOHS (for more details see Table A1 in the appendix).
A strong finding of our research is that serum troponin was statistically significant in association with LOHS, despite the model being controlled for cardiovascular disease. This trend was noted by Muktar et al. in 2018 where patients who had long LOHS had higher values of cardiac biomarkers compared to those with short LOHS, but no statistically significant difference was found in their study, possibly due to the small sample size (22). Elevated cardiac troponins are known to indicate subendothelial ischemia in the right ventricle (24) and to be associated with right ventricular dysfunction and pulmonary hypertension in acute PE (25, 26). These complications may explain the found association with extended LOHS.
Another point worth discussing is the fact that diabetes was the only health condition associated with longer LOHS. Previous studies have investigated the relationship between diabetes and PE incidence (27) or demonstrated that patients with diabetes have worse outcomes compared to patients without diabetes, especially in terms of mortality (28) or hospitalization rate (29). A recent study by Schmitt et al. found that PE patients with diabetes had prolonged LOHS (30), which is confirmed by our results. So, despite advances in treatments, diabetes is still associated with a higher risk of adverse outcomes and healthcare providers should take this finding into account. Although PE patients who also suffer from diabetes are at elevated risk for adverse events and a complicated clinical course (30, 31), further studies are required in order to clarify the underlying mechanisms and impact of disturbed glucose metabolism on the generation and clinical outcome of PE in light of LOHS.
The study followed up patients until late 2020 (the first year of the COVID-19 pandemic), however only one case out of 248 in our sample was tested positive for the SARS-CoV-2 virus. The reason why the proportion of patients with a positive test is low relies on our inclusion criteria, since we selected patients who were hospitalized with pulmonary embolism as their main diagnosis (reason for hospitalization). The majority of patients hospitalized with COVID-19 received a different ICD code as their main diagnosis and are not captured in our cohort.
Our secondary aim was to assess which factors were associated with morality rate. The overall in-hospital (5.6%) and the 30-days mortality rate (5.6 and 1.6%) observed in this study, were relatively low compared to that reported in recent studies by Matskiv et al. and by Jiménez et al. (32) (11.9 and 5.4%, respectively). Our results showed that mortality was associated with elevated values of NT-proBNP in addition to the PESI score. As previously stated, the PESI score is the most validated prognostic model for PE in predicting mortality and our study confirms this association and is in line with previous publications (17, 18, 20, 21, 33, 34).
The role of biomarkers in all-cause mortality of patients with pulmonary embolism has been debated. A meta-analysis by Lega et al. (35) showed that higher level of NT-proBNP was associated with higher risk of adverse outcomes, all-cause mortality among them. Interestingly in our research, we detected a difference between the role of NT-proBNP and troponin values. As previously discussed, our study revealed that higher serum troponin was significantly associated with LOHS whereas NT-proBNP was associated with mortality. The meta-analysis of Klok et al. (36) found that high concentrations of BNP in PE patients were associated with complicated in-hospital course and death, while LOHS was analyzed in particular. Another meta-analysis by Beccatini et al. (37) showed that elevated values of troponin were indicator of high risk for short-term death in patients with acute PE, but the study limited his research to troponin values only and did not include NT-proBNP values. In a recent Swiss study by Benmachiche et al. (38) results revealed that patients with high levels of NT-proBNP were at higher risk of in-hospital mortality and longer LOHS, regardless of their clinical characteristics. Although in other conditions like acute coronary syndrome, the level of NT-proBNP provided better predictive power than troponin (39), this difference in PE patients has still to be established. Ultimately, the precise role of biomarkers in early risk stratification is fundamental since PE may present with a wide spectrum of symptoms but in some cases with no evident symptoms. Biomarkers might be fundamental in order to detect a serious condition and allow consequently treatment adjustment with more aggressive therapy.
Our secondary outcomes included rehospitalization rates. We detected that in our study population rehospitalization within 6 months was significantly influenced by PESI score and in addition by LOHS but not by other factors. PESI score has been validated in studies with a relatively short-term follow-up (30 and 90 days mortality), (17, 33, 40) one study showed its accuracy in predicting long-term prognosis (6 month and 1 year mortality) (33) but its accuracy in predicting long term outcome in terms of rehospitalization in 6 months has not been established. The role of LOHS and its association with the risk of rehospitalization has been debated in research (41–44). Literature suggests that both short length of stay and long length of stay can be associated with rehospitalization rates. On one hand, a shortening of the length of stay could point to the so colloquially called “bloody” discharges, where the patient is not yet in a sufficient state of health or still has open problems (45, 46). On the other hand, a longer length of stay could be associated with rehospitalizations because it could occur especially in critically ill people and multimorbid elderly patients, who are subsequently exposed to a higher risk of readmission (41, 43). In order to test the association of LOHS with rehospitalization we visualized the frequency of rehospitalization versus LOHS and detected rather a linear tendency, as the LOHS increased so did the percentage of rehospitalization rates (Figure 1). For the above-mentioned reasons, we included LOHS in our model as a continuous variable and did not dichotomize it in short versus long length of hospital stay. The explanation for such tendency is that patients with pulmonary embolism hospitalized in KSBL are on average old and multimorbid patients. The positive aspect of these results is that patients were usually not discharged too early and a short length of stay did not result in a higher risk of rehospitalization.
Figure 1.
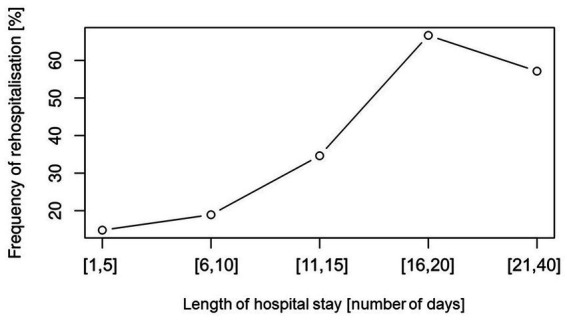
Frequency of rehospitalization versus length of hospital stay (LOHS). Rehospitalization rates increases with longer length of hospital stay in approximately lineal relationship.
In all our analyses the PESI score had significant influence on LOHS, mortality and rehospitalization. PESI score is an essential parameter for determining how to proceed with the patient (e.g., outpatient treatment, surveillance) (47). Therefore, whenever it is not calculated, the patient is at risk of being under- or overtreated. By definition, the PESI score is used to predict 30 day mortality and is a widely validated model that uses clinical parameters to stratify patients into five risk levels (20).Unfortunately, in our study PESI score was only calculated at the time of admission in 19% of the cases, in the remaining 81% of the cases the PESI score was calculated retrospectively.
Published audits on the management of PE patients only cover very particular aspects of the process, for instance, the use of clinical decision rules (48) or treatment strategies (49) or only look at single subsegmental PE (50) and do not focus on the frequency of the PESI score calculation. Therefore, do not know if the proportion is representative of usual practice. In our opinion, the fact that the PESI score is poorly reported can be due to two factors: either other indicators are given priority in the emergency department, or the PESI was actually calculated but not entered into the patients’ records.
The criteria that make up the score refer to easily accessible information and vital signs that can be measured by a GP. Moreover, the score is of high importance in predicting not only severity and mortality but also LOHS and the likelihood of re-hospitalization. Therefore, we believe that it may be appropriate for GPs who suspect pulmonary embolism to already calculate the PESI when sending a patient to the hospital.
4.1. Comparison between multivariable models and sensitivity analysis
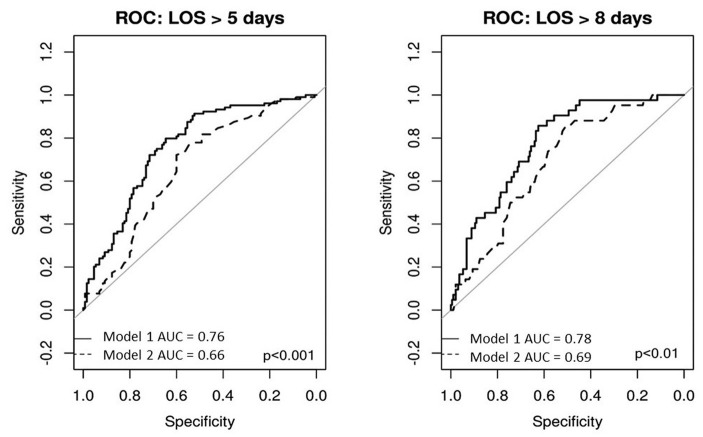
In our study we assessed the association between PESI score in addition to other risk factors and we also assessed the parameters composing the PESI score alone. As displayed in the Appendix Figure A1 LOHS prediction the receiver operating characteristic (ROC) curve shows a higher accuracy for the model with other parameters in addition to PESI score compared to the model with PESI score alone and this is valid both for 5 days prediction (median LOHS) and for 8 days prediction (the upper IQR).
The sensitivity analysis on the full data for the prediction of LOHS did not give any other predictor except for NT-proBNP which was predictive for in-hospital death. The sensitivity analysis on the full data for the prediction of the PESI items only on LOHS shows the same results as the analysis performed on the patients that were discharged alive.
4.2. Strengths, limitations, and further research
Despite the availability of a large amount of data regarding the prognosis of PE patients, only a few studies have investigated possible predictors of LOHS in these patients (15, 21, 51). The novelty of our study is that the analysis did not limit its focus just to the PESI score. Our analysis looked at a broader range of variables (demographic, health-related risk factors and the role of primary care) as well as the PESI score and determined their associations with outcomes of interest. The data collection involving manual extraction of information was conducted by a doctor alone but subsequently, the parameters forming the PESI score were reviewed independently by two researchers. The characteristics of the sample of this study are comparable with other cohorts with a bigger sample size, for example in our study the median age was 73 years 52% were female and 50% had hypertension as a comorbidity. In a German study from 2018 with almost 1 million PE patients, the median age was 72, 54% were female and 43% had hypertension (8).
All statistical analyses that were primarily performed on imputed data have also been applied to the non-imputed dataset, the statistical significance of the variables with missing data did not differ in the two models. The sensitivity analysis demonstrated that the internal validity of our research was not impacted by missing data.
There are some limitations to this study. As a retrospective study design, the data quality depends on precise documentation in the patient files. Particularly we assumed that if the PESI score was not reported, the clinician did not calculate it, so we could have underestimated the percentage of patients with a PESI calculation at entry to hospital. Additionally, information about rehospitalization within 6 months was only possible within KSBL: due to privacy policy, it was not possible to access information about rehospitalizations in other hospitals. However, in Switzerland readmissions usually occur within the same hospital; a Swiss study has shown that only 17% of unplanned readmissions occurred at a different hospital (41). Our results concerning mortality and rehospitalization rates must be interpreted carefully since their occurrence was relatively low (18 and 51 patients, respectively).
Further research to prospectively validate the statistical model’s accuracy in predicting LOHS—ideally in multicenter studies with a larger sample size—is needed.
5. Conclusion
Understanding the factors that are associated with LOHS in patients with PE has clinical implications and may help healthcare providers to deliver efficient care and to allocate adequate resources in the management of these patients. In summary, the results of this study showed that the PESI score is a major predictor of LOHS, mortality and rehospitalization in PE patients. Diabetes is an additional risk factor that healthcare providers should be aware of. Even though cardiac biomarkers and comorbidities are predictors of LOHS, their role in defining mortality and rehospitalization is yet to be established. Moreover, our study confirmed the essential role of PESI score calculation in the management of PE patients, clinicians and GPs should be aware of and perform the calculation as soon as PE is diagnosed.
Data availability statement
The dataset presented in this article is not readily available because it contains sensitive human personal data. All data generated were analyzed during this study and the results included in this article. Further inquiries can be directed to the corresponding author. Requests to access the datasets should be directed to JL, joerg.leuppi@ksbl.ch.
Ethics statement
The studies involving human participants were reviewed and approved by Nordwest- and Zentralschweiz ethic commission (Project-ID 2021–00964). The ethics committee waived the requirement of written informed consent for participation.
Author contributions
GL-C and CK were responsible of data collection. GL-C, SG, and MB were responsible for data analysis. GL-C, SG, and JL were responsible for designing the study. All authors contributed to the article and approved the submitted version.
Funding
The project was financed by the Swiss Personalized Health Network (SPHN Grant # 2018DRI08).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- BMI
Body mass index
- BP
Blood pressure
- BPM
Beats per minute
- CI
Confidence interval
- CTPA
Computed tomography pulmonary angiogram
- DVT
Deep vein thrombosis
- GP
General practitioner
- HR
Heart rate
- ICD
International Classification of Diseases
- IQR
Interquartile range
- IRR
Incident risk ratio
- KSBL
(Kantonsspital Baselland) cantonal hospital of Baselland
- LOHS
Length of hospital stay
- NT-proBNP
N-terminal pro b-type natriuretic peptide
- OR
Odds ratio
- PE
Pulmonary embolism
- PESI
Pulmonary embolism severity index
- ROC
Receiver operating characteristic
- SD
Standard deviation
- VTE
Venous thromboembolism
Appendix
Figure A1.
Receiver operating characteristic (ROC) curve accuracy of the models to predict 5 days and 8 days length of hospital stay. For both 5 days and 8 days prediction, model 1 showed higher accuracy than model 2. Model 1 includes the following variables: pulmonary embolism severity index (PESI) score, body mass index (BMI), a medical history of dyslipidemia, diabetes or previous PE, NT-proBNP and troponin-T hs, housing situation before admission and admission via GP. Model 2 only includes the PESI score. Pulmonary embolism severity index score is based age at entry, sex, history of cancer, history of chronic lung disease, history of heart failure, respiratory rate, hypothermia [below 36°C], systolic blood pressure (BP) <100 mmHg, heart rate (HR) ≥110 bpm, oxygen saturation below 90%, altered mental status, and respiratory rate ≥ 30 breaths per minute (18).
Table A1.
Multivariable zero-truncated negative binomial regression model for length of hospital stay (LOHS) estimation in pulmonary embolism survivors with pulmonary embolism severity index (PESI) items separately assessed (n = 234).
| LOHS prediction | IRR (95%CI) | Pr(>|z|) | |
|---|---|---|---|
| Intercept | 4.638 | 1.774(1.148–2.742) | 0.01 |
| Age | 5.207 | 1.138 (1.071–1.21) | <0.001 |
| Sex | 5.864 | 1.296 (1.083–1.55) | 0.005 |
| History or active cancer | 4.72 | 1.02 (0.829–1.255) | 0.851 |
| BP systolic < 100 mmHg | 5.452 | 1.197 (0.761–1.883) | 0.436 |
| Heart rate ≥ 110 bpm | 6.139 | 1.361 (1.075–1.724) | 0.01 |
| Respiratory rate ≥ 30 breaths/min | 4.876 | 1.058 (0.707–1.583) | 0.783 |
| Oxygen < 90% | 6.188 | 1.373 (1.109–1.699) | 0.004 |
| Chronic lung disease | 5.107 | 1.114 (0.878–1.414) | 0.375 |
| Altered mental status | 5.003 | 1.089 (0.51–2.324) | 0.826 |
| Hypothermia < 36°C | 4.821 | 1.045 (0.612–1.782) | 0.872 |
| Heart failure | 9.345 | 2.113 (1.165–3.832) | 0.014 |
The analysis of the items composing the PESI score showed that age, sex, heart rate over 110 bpm, oxygen saturation <9% and heart failure were significantly associated with LOHS. Statistically significant variables p < 0.05.
References
- 1.Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. (2014) 34:2363–71. doi: 10.1161/ATVBAHA.114.304488 [DOI] [PubMed] [Google Scholar]
- 2.Tarbox AK, Swaroop M. Pulmonary embolism. Int J Crit Illn Inj Sci. (2013) 3:69–72. doi: 10.4103/2229-5151.109427, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willich SN, Chuang L-H, van Hout B, Gumbs P, Jimenez D, Kroep S, et al. Pulmonary embolism in Europe—burden of illness in relationship to healthcare resource utilization and return to work. Thromb Res. (2018) 170:181–91. doi: 10.1016/j.thromres.2018.02.009, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. (2016) 118:1340–7. doi: 10.1161/CIRCRESAHA.115.306841 [DOI] [PubMed] [Google Scholar]
- 5.Barco S, Woersching AL, Spyropoulos AC, Piovella F, Mahan CE. European Union-28: an annualised cost-of-illness model for venous thromboembolism. Thromb Haemost. (2016) 115:800–8. doi: 10.1160/TH15-08-0670, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. (2010) 38:S495–501. doi: 10.1016/j.amepre.2009.12.017 [DOI] [PubMed] [Google Scholar]
- 7.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. (1998) 158:585–93. doi: 10.1001/archinte.158.6.585 [DOI] [PubMed] [Google Scholar]
- 8.Keller K, Hobohm L, Ebner M, Kresoja K-P, Münzel T, Konstantinides SV, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J. (2020) 41:522–9. doi: 10.1093/eurheartj/ehz236, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Miguel-Díez J de, Jiménez-García R, Jiménez D, Monreal M, Guijarro R, Otero R, et al. Trends in hospital admissions for pulmonary embolism in Spain from 2002 to 2011. Eur Respir J (2014); 44:942–950, doi: 10.1183/09031936.00194213 [DOI] [PubMed] [Google Scholar]
- 10.Dentali F, Ageno W, Pomero F, Fenoglio L, Squizzato A, Bonzini M. Time trends and case fatality rate of in-hospital treated pulmonary embolism during 11 years of observation in northwestern Italy. Thromb Haemost. (2016) 115:399–405. doi: 10.1160/TH15-02-0172, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Lehnert P, Lange T, Møller CH, Olsen PS, Carlsen J. Acute pulmonary embolism in a National Danish Cohort: increasing incidence and decreasing mortality. Thromb Haemost. (2018) 118:539–46. doi: 10.1160/TH17-08-0531, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. (2007) 98:756–64. [DOI] [PubMed] [Google Scholar]
- 13.Masotti L, Vannucchi V, Poggi M, Landini G. Trends in length of hospital stay in acute pulmonary embolism over the years. What is changing in the era of direct oral anticoagulants? J Cardiovasc Med (Hagerstown). (2017) 18:556–9. doi: 10.2459/JCM.0000000000000356, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Mainz J. Defining and classifying clinical indicators for quality improvement. Int J Qual Health Care. (2003) 15:523–30. doi: 10.1093/intqhc/mzg081 [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Baser O, Wells P, Peacock WF, Coleman CI, Fermann GJ, et al. Predictors of hospital length of stay among patients with low-risk pulmonary embolism. J Health Econ Outcomes Res. (2019) 6:84–94. doi: 10.36469/9744, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aujesky D, Jiménez D, Mor MK, Geng M, Fine MJ, Ibrahim SA. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. (2009) 119:962–8. doi: 10.1161/CIRCULATIONAHA.108.824292, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aujesky D, Stone RA, Kim S, Crick EJ, Fine MJ. Length of hospital stay and postdischarge mortality in patients with pulmonary embolism: a statewide perspective. Arch Intern Med. (2008) 168:706–12. doi: 10.1001/archinte.168.7.706, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Donzé J, Le Gal G, Fine MJ, Roy P-M, Sanchez O, Verschuren F, et al. Prospective validation of the pulmonary embolism severity index. A clinical prognostic model for pulmonary embolism. Thromb Haemost. (2008) 100:943–8. doi: 10.1160/TH08-05-0285, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Mucherino A, Papajorgji PJ, Pardalos PM. K-nearest neighbor classification In: Mucherino A, Papajorgji PJ, Pardalos PM, editors. Data Mining in Agriculture. New York, NY: Springer New York; (2009). 83–106. (Springer Optimization and Its Applications) [Google Scholar]
- 20.Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. (2005) 172:1041–6. doi: 10.1164/rccm.200506-862OC, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donadini MP, Dentali F, Castellaneta M, Gnerre P, La Regina M, Masotti L, et al. Pulmonary embolism prognostic factors and length of hospital stay: a cohort study. Thromb Res. (2017) 156:155–9. doi: 10.1016/j.thromres.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 22.Mukhtar O, Alhafidh O, Khalid M, Kaler J, Rahman E, Shrestha B, et al. Predictors of hospital stay in normotensive acute pulmonary embolism: a retrospective pilot study. J Commun Hosp Intern Med Perspect. (2018) 8:95–100. doi: 10.1080/20009666.2018.1466602, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Núñez N, Ruano-Raviña A, Abelleira R, Ferreiro L, Lama A, González-Barcala FJ, et al. Factors influencing hospital stay for pulmonary embolism. A Cohort Study Arch Bronconeumol. (2017) 53:432–6. doi: 10.1016/j.arbres.2017.01.003, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Binder L, Pieske B, Olschewski M, Geibel A, Klostermann B, Reiner C, et al. N-terminal pro-brain natriuretic peptide or troponin testing followed by echocardiography for risk stratification of acute pulmonary embolism. Circulation. (2005) 112:1573–9. doi: 10.1161/CIRCULATIONAHA.105.552216 [DOI] [PubMed] [Google Scholar]
- 25.Choi HS, Kim KH, Yoon HJ, Hong YJ, Kim JH, Ahn Y, et al. Usefulness of cardiac biomarkers in the prediction of right ventricular dysfunction before echocardiography in acute pulmonary embolism. J Cardiol. (2012) 60:508–13. doi: 10.1016/j.jjcc.2012.07.006, PMID: [DOI] [PubMed] [Google Scholar]
- 26.Kilinc G, Dogan OT, Berk S, Epozturk K, Ozsahin SL, Akkurt I. Significance of serum cardiac troponin I levels in pulmonary embolism. J Thorac Dis. (2012) 4:588–93. doi: 10.3978/j.issn.2072-1439.2012.10.13, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai J, Ding X, Du X, Zhao X, Wang Z, Ma Z. Diabetes is associated with increased risk of venous thromboembolism: a systematic review and meta-analysis. Thromb Res. (2015) 135:90–5. doi: 10.1016/j.thromres.2014.11.003, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Faller N, Limacher A, Méan M, Righini M, Aschwanden M, Beer JH, et al. Predictors and causes of long-term mortality in elderly patients with acute venous thromboembolism: a prospective cohort study. Am J Med. (2017) 130:198–206. doi: 10.1016/j.amjmed.2016.09.008, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Miguel-Díez J de, Muñoz-Rivas N, Jiménez-García R, Hernández-Barrera V, Carrasco-Garrido P, Monreal M, et al. Type 2 diabetes is associated with a higher incidence of hospitalization for pulmonary embolism in Spain: analysis of hospital discharge data during 2004-2013. Respirology (2016); 21:1277–1284, doi: 10.1111/resp.12847 [DOI] [PubMed] [Google Scholar]
- 30.Schmitt VH, Hobohm L, Sivanathan V, Brochhausen C, Gori T, Münzel T, et al. Diabetes mellitus and its impact on mortality rate and outcome in pulmonary embolism. J Diabetes Investig. (2022) 13:725–37. doi: 10.1111/jdi.13710, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piazza G, Goldhaber SZ, Kroll A, Goldberg RJ, Emery C, Spencer FA. Venous thromboembolism in patients with diabetes mellitus. Am J Med. (2012) 125:709–16. doi: 10.1016/j.amjmed.2011.12.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiménez D, Bikdeli B, Quezada A, Muriel A, Lobo JL, De M-DJ, et al. Hospital volume and outcomes for acute pulmonary embolism: multinational population based cohort study. BMJ. (2019) 366:l4416. doi: 10.1136/bmj.l4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dentali F, Riva N, Turato S, Grazioli S, Squizzato A, Steidl L, et al. Pulmonary embolism severity index accurately predicts long-term mortality rate in patients hospitalized for acute pulmonary embolism. J Thromb Haemost. (2013) 11:2103–10. doi: 10.1111/jth.12420, PMID: [DOI] [PubMed] [Google Scholar]
- 34.El Ghoul J, Bendayekh A, Fki W, Yengui I, Ferjani S, Milouchi S, et al. Risk factors for hospital mortality during pulmonary embolism. Ann Cardiol Angeiol (Paris). (2020) 69:7–11. doi: 10.1016/j.ancard.2020.01.001, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Lega J-C, Lacasse Y, Lakhal L, Provencher S. Natriuretic peptides and troponins in pulmonary embolism: a meta-analysis. Thorax. (2009) 64:869–75. doi: 10.1136/thx.2008.110965, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Klok FA, Mos ICM, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med. (2008) 178:425–30. doi: 10.1164/rccm.200803-459OC, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. (2007) 116:427–33. doi: 10.1161/CIRCULATIONAHA.106.680421 [DOI] [PubMed] [Google Scholar]
- 38.Benmachiche M, Marques-Vidal P, Waeber G, Méan M. In-hospital mortality is associated with high NT-proBNP level. PLoS One. (2018) 13:e0207118. doi: 10.1371/journal.pone.0207118, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogiatzis I, Dapcevic I, Datsios A, Koutsambasopoulos K, Gontopoulos A, Grigoriadis S. A comparison of prognostic value of the levels of ProBNP and troponin T in patients with acute coronary syndrome (ACS). Mediev Archaeol. (2016) 70:269–73. doi: 10.5455/medarh.2016.70.269-273, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan CM, Woods C, Shorr AF. The validation and reproducibility of the pulmonary embolism severity index. J Thromb Haemost. (2010) 8:1509–14. doi: 10.1111/j.1538-7836.2010.03888.x [DOI] [PubMed] [Google Scholar]
- 41.Uhlmann M, Lécureux E, Griesser A-C, Duong HD, Lamy O. Prediction of potentially avoidable readmission risk in a division of general internal medicine. Swiss Med Wkly. (2017) 147:w14470. [DOI] [PubMed] [Google Scholar]
- 42.Donzé JD, Williams MV, Robinson EJ, Zimlichman E, Aujesky D, Vasilevskis EE, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. (2016) 176:496–502. doi: 10.1001/jamainternmed.2015.8462, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanc A-L, Fumeaux T, Stirnemann J, Dupuis Lozeron E, Ourhamoune A, Desmeules J, et al. Development of a predictive score for potentially avoidable hospital readmissions for general internal medicine patients. PLoS One. (2019) 14:e0219348. doi: 10.1371/journal.pone.0219348, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang VL, Halm EA, Fine MJ, Johnson CS, Anzueto A, Mortensen EM. Predictors of rehospitalization after admission for pneumonia in the veterans affairs healthcare system. J Hosp Med. (2014) 9:379–83. doi: 10.1002/jhm.2184, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kutz A, Gut L, Ebrahimi F, Wagner U, Schuetz P, Mueller B. Association of the Swiss Diagnosis-Related Group Reimbursement System with Length of stay, mortality, and readmission rates in hospitalized adult patients. JAMA Netw Open. (2019) 2:e188332. doi: 10.1001/jamanetworkopen.2018.8332, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers WH. Quality of care before and after implementation of the DRG-based prospective payment system. JAMA. (1990) 264:1989–94. doi: 10.1001/jama.1990.03450150089037, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G-J, Harjola V-P, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. (2020) 41:543–603. doi: 10.1093/eurheartj/ehz405, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Al Dandan O, Hassan A, Alnasr A, Al Gadeeb M, AbuAlola H, Alshahwan S, et al. The use of clinical decision rules for pulmonary embolism in the emergency department: a retrospective study. Int J Emerg Med. (2020) 13:23. doi: 10.1186/s12245-020-00281-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caprini JA, Tapson VF, Hyers TM, Waldo AL, Wittkowsky AK, Friedman R, et al. Treatment of venous thromboembolism: adherence to guidelines and impact of physician knowledge, attitudes, and beliefs. J Vasc Surg. (2005) 42:726–33. doi: 10.1016/j.jvs.2005.05.053, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Mehta D, Barnett M, Zhou L, Woulfe T, Rolfe-Vyson V, Rowland V, et al. Management and outcomes of single subsegmental pulmonary embolus: a retrospective audit at north shore hospital. New Zealand Intern Med J. (2014) 44:872–6. doi: 10.1111/imj.12507, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Sheikh AR, Mantoo S, Shah S, Hamid Wani RU, Wani SN. Predictors of prolonged hospital stay in patients with acute pulmonary thromboembolism-a hospital based cohort study. J Assoc Physicians India. (2022) 70:11–2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset presented in this article is not readily available because it contains sensitive human personal data. All data generated were analyzed during this study and the results included in this article. Further inquiries can be directed to the corresponding author. Requests to access the datasets should be directed to JL, joerg.leuppi@ksbl.ch.