ABSTRACT
More than 20 years ago, the survey of activities in medically assisted reproduction (MAR) was initiated in Europe and resulted in cross-sectional annual reports, as issued by the European IVF Monitoring (EIM) consortium of ESHRE. Over time, these reports mirror the continuous development of the technologies and contribute to increased transparency and surveillance of reproductive care. Meanwhile, progressive changes of existing treatment modalities and the introduction of new technologies resulted in the need of a cumulative approach in the assessment of treatment outcomes, which warrants a prospective cycle-by-cycle data registry on MAR activities, including fertility preservation. This change in the paradigm of data collection in Europe towards the construction of cumulative outcome results is expected to generate additional insights into cross-institutional but also cross-border movements of patients and reproductive material. This is essential to improve vigilance and surveillance. The European monitoring of Medically Assisted Reproduction (EuMAR) project, co-funded by the European Union, will establish a registry for the transnational collection of prospective cycle-by-cycle MAR and fertility preservation data on the basis of an individual reproductive care code (IRCC). The rationale for the project and the objectives are presented here.
Keywords: epidemiology, surveillance, fertility preservation, medically assisted reproduction, vigilance, registry, ART, Europe
Introduction
The impact of medically assisted reproduction (MAR) on various aspects of our society cannot be underestimated. In European countries, an average of 3% of children are born after prolonged periods of infertility and after the use of some form of ART (Wyns et al., 2022). Medical assistance in MAR is provided by using different treatment modalities, such as IUI, IVF, ICSI, and transfer of previously cryopreserved gametes and embryos. Pregnancies may also occur after preimplantation genetic testing (PGT) and fertility preservation strategies. The long-term effects of MAR on wellbeing but also possible harm have been recognized by both national and European health authorities. With the increasing impact of modern reproductive medicine on demography and society, the need for vigilance and surveillance has become ever more important for the safety and quality assurance of MAR. Surveillance refers to the continuous and systematic collection of health data needed for the analysis and interpretation of performance and evolution in medical care, including their safety, while vigilance refers to the detection and analysis of complications, errors, and adverse events (De Geyter, 2019; Kissin et al., 2019). Both surveillance and vigilance strive towards improved care and aim at making information transparent to all stakeholders.
The European IVF Monitoring (EIM) consortium of ESHRE was initiated in 2000, aimed at collecting annual cross-sectional data on ART. The first published report contained the data of 1997 retrieved from 18 European countries (Nygren and Andersen, 2001). The data set of 2001 was gradually extended to include information on IUI (Andersen et al., 2005) and fertility preservation (Wyns et al., 2020). Since then, the EIM consortium has progressed further and now collects the activities in MAR in 39 European countries, including 24 of the 27 European Union (EU) member states (Wyns et al., 2022). More than 90% of all European institutions offering MAR services now participate in EIM. At 15 and 20 years after its early beginnings, the steady progress of the EIM consortium was summarized and compared with similar large-scale registries (Ferraretti et al., 2017; De Geyter et al., 2020).
The annual reports of the EIM traditionally consist of amalgamated data sets, either provided by the representatives of national health authorities or by dedicated professional societies. Along with the introduction of novel technologies, the number of items that are being reported has increased over time, with details on data collection methods, number and size of the participating clinics, number of treatment cycles per technique (IVF, ICSI, frozen embryo replacement (FER), egg donation (ED) cycles, and IUI), availability of treatments in each country, patients’ age distribution, number of embryos transferred, pregnancies and deliveries, multiple pregnancies and deliveries, perinatal risks (e.g. prematurity), and complications in the new-borns. Over time, pooled data on innovative technologies were added, such as PGT (PGT for monogenic/single-gene defects (PGT-M); PGT for chromosomal structural rearrangements (PGT-SR); PGT for aneuploidies (PGT-A)) (since 2002). IVM has also been included since 2002, frozen oocyte replacements since 2006, and fertility preservation of oocytes, spermatozoa, embryos, and gonadal tissues since 2016. Through the adaptation of the data forms and the adoption of the definitions and terminology formulated by the International Committee for Monitoring Assisted Reproductive Technologies (ICMART)/World Health Organization (WHO) (Zegers-Hochschild et al., 2017), the EIM registry has been aligned with ICMART.
While EIM collects numerous data from an increasing number of participating MAR centres, the collection of aggregated data has several shortcomings including, but not limited to, the lack of linkage between subsequent treatments both within and between treating institutions, whether in the same country or not. As a result, cumulative outcome data sets cannot be constructed, while such data become even more relevant and challenging because current ART has developed from segmented to sequential treatment strategies (De Geyter et al., 2016). Thus, the most obvious next step is to move from the hitherto retrospective aggregated data collection to a prospective cycle-by-cycle registry. Considering the current opportunities offered by long-term storage of reproductive material and the ever-increasing mobility of European citizens, such a prospective data collection must also include information on interinstitutional and cross-border migration of infertile patients or patients seeking fertility preservation as well as transport of human reproductive biological material. However, for both financial and management reasons, the programming and the maintenance of IT software solutions for prospective cycle-by-cycle registration of treatments appears to be an unsurmountable obstacle in a majority of countries in Europe.
To fulfil these aims and address the current limitations of national or medical organizations’ registries, the European monitoring of Medically Assisted Reproduction (EuMAR) project has now been initiated. The EuMAR project has been granted funding from the EU4Health Framework of the EU and will be run from January 2023 to December 2025.
The project is in line with the proposal for a regulation of the European Health Data Space (EHDS) of the European Commission (EC) and the proposal for a regulation on standards of quality and safety for substances of human origin (SoHO) intended for human application. This new regulation is to replace the former European Tissue and Cell Directive of 2004 and corresponding implementing Directives.
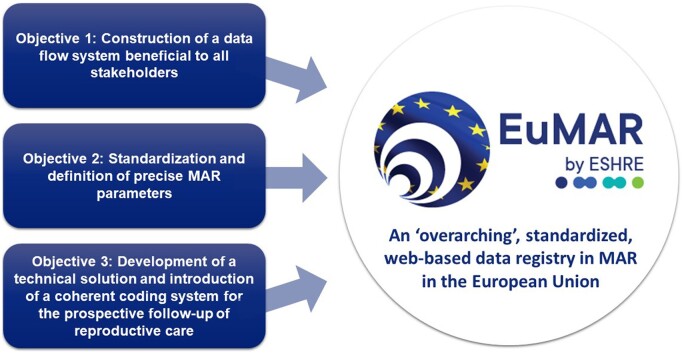
This opinion paper describes the three main objectives of the project (Fig. 1).
Figure 1.
Objectives of the EuMAR project. EuMAR: European monitoring of Medically Assisted Reproduction.
Objectives of EuMAR
EuMAR aims at establishing an ‘overarching’, standardized, web-based data registry on MAR activities in the EU. EuMAR will set a platform to obtain high-quality cycle-by-cycle data entries from medical professionals across the EU, facilitating data sharing for open science across institutes and allowing for longitudinal and cross-border data collection. Once implemented, this important project within the EU will also benefit European countries that are not part of the EU.
The data collection will cover all MAR treatments, including IVF, ICSI, and IUI, PGT treatments and fertility preservation through gamete/embryo/gonadal tissue cryopreservation. Whereas the definition of MAR also includes gynaecological surgery and ovulation induction, those activities will not be covered in the EuMAR registry as they do not involve the ex vivo handling of gametes or gonadal tissues. EuMAR will adhere to the FAIR Guiding Principles for scientific data management: Findable, Accessible, Interoperable, and Reusable (Wilkinson et al., 2016). To fulfil all legal requirements surrounding data protection and confidentiality, experts in the General Data Protection Regulations (GDPR) will provide advice and follow the entire project.
In recent decades, numerous novel procedures and techniques were introduced into MAR, often without an a priori analysis of benefits and potential risks. EuMAR intends to constitute a decisive step towards surveillance and vigilance in MAR, which, in turn, could allow for a better understanding of the overall effectiveness and potential risks related to established and newly introduced MAR treatments, both in the short and the longer term. These insights are not only of interest to patients seeking care and professionals pursuing medical excellence but also are of concern to the national and European health authorities.
The project provides an opportunity to resolve three urgent needs linked to the current MAR data collection with three specific project objectives.
Objective 1: construction of a data flow system beneficial to all stakeholders
The stakeholders of EuMAR are the national competent authorities (NCAs), the EC, the professionals of the institutions offering MAR services, infertile patients seeking medical support, and individuals requiring fertility preservation. Whereas the proper functioning of EuMAR requires the active participation and dedication of all stakeholders, EuMAR will be conceived such that all stakeholders will benefit from it.
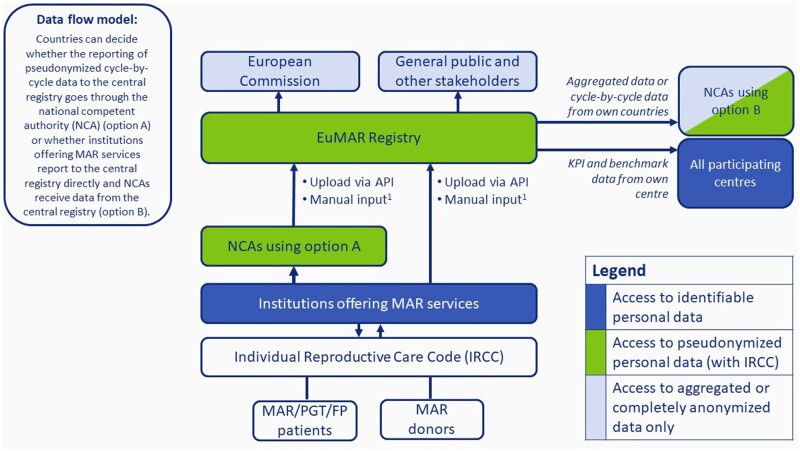
The first objective of EuMAR is to provide the national health authorities and European Health Data Space of the EC with high-quality data that allow surveillance and vigilance in all aspects of MAR. To address this need, the EuMAR project will manage and guide the flow of data between institutions that carry out MAR, the national competent authorities, the SoHO-X platform and European Health Data Space of the EC, and other stakeholders, wherever and whenever needed. The proposed data flow model depicted in Fig. 2 will serve as the starting point when setting up the organization. Bilateral consultations will be held with the national competent authorities of all EU member states to define a tailored data flow model adapted to the local contexts of each specific member state. Additional consultations will be held to ensure an optimal data flow from the EuMAR registry to stakeholders such as the EC, medical and laboratory professionals, and organizations representing patients. At the end of the project, the data flow model should allow the national and international health authorities insight into the required data in compliance with the specific legal frameworks.
Figure 2.
Theoretical (draft) model visualizing possible data flows between institutions offering MAR services, national competent authorities, the central EuMAR registry, the European Commission, and other stakeholders. In this theoretical model, countries can decide whether the reporting of pseudonymized cycle-by-cycle data to the central EuMAR registry goes through the national competent authority (NCA) (Option A) or whether institutions offering MAR services report to the central registry directly and NCAs receive data from the EuMAR registry (Option B). API, application processing interface (i.e. a software intermediary allowing two applications to exchange data); FP, fertility preservation; IRCC, individual reproductive care code; KPI, key performance indicator; MAR, medically assisted reproduction; NCA, national competent authority; EuMAR: European monitoring of Medically Assisted Reproduction; PGT: preimplantation genetic testing. 1If no data collection system is available.
The data flow, as envisaged in the concept of EuMAR, should also benefit the professionals in the institutions offering MAR services, whose primary task is to provide the data. EuMAR must be achieved without double entry of data by the local professionals, which is tedious and prone to error. By way of reward, EuMAR will provide them with a benchmark, which will provide each participating institution with an ongoing quality control of their performance for each of the key indices and at each step of the treatment processes. This benchmark will be directed towards single institutions and be strictly confidential, as guaranteed by GDPR regulations. Over time, we expect that this benchmark will become standard for quality assurance in MAR.
The next stakeholders in the process are the infertile patients and the donors of reproductive material. The infertile patients, individuals needing fertility preservation and donors of gametes will receive the benefit of tailored and harmonized reports with a unified design and content. These reports will make it easier for patients to transfer key data of their treatments from one professional to another when they decide to change the treating institution or when, in the case of successful outcome, they continue their journey with obstetricians and paediatricians. Standardized transfer of key data to the patients for prolonged usage will be crucial in cases of long-term storage of reproductive material, as is needed in fertility preservation.
Objective 2: standardization and definition of precise MAR parameters
Heterogeneity and inconsistencies between registries in different countries often render the comparison and interpretation of data at the international/EU level difficult. Many key elements of the MAR processes require updates of existing definitions, harmonization of outcome metrics, and a comprehensive evaluation of serious adverse occurrences (SAO, previously serious adverse reactions and events, or SARE).
The standardization and harmonization of MAR parameters will be addressed in a stepwise manner. In a first step, the project team will identify and list a set of relevant MAR parameters, including items that are already reported today as well as novel ones. In a second step, the updated parameters will be integrated into data collection sheets, taking into account the timely sequence of therapeutic events, the different potential sources of origin of the biological material (either third-party donation or within couple use), and the timely sequence of potential outcomes (complications during treatments, during early pregnancy and delivery, status of the new-born child).
Parameters will be defined in such a way that future developments can be integrated without disrupting the coherence of existing data sets. It goes without saying that relevant cross-sectional statistics on delivery rates by country and by treatment modality, as currently provided by the EIM, will remain possible. Finally, parameters will be defined such that they can be transferred to or linked with the SoHO-X platform and European Health Data Space of the EC.
All stakeholders, most notably the professionals in the institutions offering MAR services and the relevant national health authorities, will be asked to approve and endorse the semantics and parameters. Ultimately, they will form the blueprint for the EuMAR registry, as managed by ESHRE.
Objective 3: development of a technical solution and introduction of a coherent coding system for the prospective follow-up of reproductive care
The follow-up of patients requiring fertility treatments or fertility preservation methods, third-party donors and their reproductive material during subsequent treatment trials, across different institutions, across countries and over prolonged time periods will be the most important key requirement to collect the data in a cumulative fashion. To achieve such a prospective follow-up, an easy access reproductive care coding system is proposed. Each MAR donor/patient will be allocated a unique Individual Reproductive Care Code (IRCC). This IRCC will remain with the patients or donors (and all their reproductive material) during case-by-case and cycle-by-cycle data reporting to the EuMAR registry, even if patients or donors decide to change the treating institution within one country or across country borders. According to local requirements, the code may be linked to national health registration systems, but the identity of the IRCC will remain exclusively with the patients or donors and the treating physicians/institutions.
Beyond the immediate scope of EuMAR, the IRCC may also be used as a tool for patients to access standardized reports of sequential treatments, which they can use in discussions with referring physicians of other medical disciplines, or, in the case of successful treatment, with obstetricians for pregnancy care. The IRCC can also be used by the patients themselves to access their individual selected data stored in the EuMAR registry.
Discussion and conclusion
ESHRE has now obtained the funding in the frame of the EU4Health initiative of the EU for the organization of an entirely new register based on a prospective, cycle-by-cycle collection of harmonized MAR data: EuMAR.
The concept of EuMAR is to fulfil all requirements of both the national and the EU-health authorities in charge of surveillance and vigilance of MAR. The allocation of a unique IRCC to each infertile individual or donor of reproductive cells will guarantee their follow-up from one institution to another or from one country to another. Confidentiality of data concerning all processes in EuMAR will be secured through the aid of experts in GDPR. During the development of EuMAR, all care will be taken to respect existing and functional regional or national data registries by integrating them into the EuMAR data flow (Fig. 2). The allocation of a unique IRCC together with standardization of key parameters and semantics will be beneficial to the professionals by providing them with a tool for continuous benchmarks of all phases of the treatments.
The advantages resulting from participation in the EuMAR registry are expected to lead to a gradual improvement of quality assurance in all aspects of MAR. Although key data for MAR must be provided by the local professionals, EuMAR will be beneficial in streamlining both interprofessional and interdisciplinary reporting. Finally, the infertile patients are to benefit from carrying the unique IRCC because it provides them with an easy-to-use tool to manage all information on their treatments and their stored reproductive cells and tissues in a coherent and standardized format. The strengths, some weaknesses, the threats and the opportunities of a pan-European registry of harmonized data are summarized in a SWOT analysis (Fig. 3).
Figure 3.
SWOT analysis for building the EuMAR Registry, as a pan-European registry of harmonized MAR data. The SWOT analysis highlights the need to standardize and scale up the existing MAR data collection by the European IVF Monitoring (EIM) Consortium of ESHRE, but it also shows that the EIM data collection represents a strong basis on which EuMAR can build. SWOT: strengths, weaknesses, opportunities and threats; MAR: medically assisted reproduction; EU: European Union.
In conclusion, EuMAR provides a unique opportunity to install an affordable, Europe-wide, prospective, cycle-by-cycle platform for the collection of MAR data. EuMAR sets the stage not only for surveillance and vigilance but also for quality assurance in reproductive medicine.
Footnotes
ESHRE pages content is not externally peer reviewed. The manuscript has been approved by the Executive Committee of ESHRE.
Contributor Information
Christian De Geyter, Reproductive Medicine and Gynecological Endocrinology (RME), University Hospital, University of Basel, Basel, Switzerland.
Carlos Calhaz-Jorge, Faculdade de Medicina da Universidade de Lisboa, Lisbon, Portugal.
Veerle Goossens, European Society of Human Reproduction and Embryology, Central Office, Strombeek-Bever, Belgium.
Cristina M Magli, SISMER Reproductive Medicine Unit, Bologna, Italy.
Jesper Smeenk, Elisabeth Twee Steden Hospital, Tilburg, The Netherlands.
Kristina Vesela, European Society of Human Reproduction and Embryology, Central Office, Strombeek-Bever, Belgium.
Nathalie Vermeulen, European Society of Human Reproduction and Embryology, Central Office, Strombeek-Bever, Belgium.
Christine Wyns, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium.
Data availability
All data are incorporated into the article.
Authors’ roles
C.D.G. and N.V. prepared the first draft of the article. All authors participated in revising and correcting the text. All authors approved the final article.
Funding
The European monitoring of Medically Assisted Reproduction (EuMAR) project is co-funded by the European Union (Call EU4H-2021-PJ2, project number 101079865) and ESHRE. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HaDEA). Neither the European Union nor the granting authority can be held responsible for them.
Conflict of interest
J.S. reports speaker’s fees from Ferring and Merck, support to attend conferences from Ferring, Merck and Good Life, and participation in an advisory board for Merck, outside the scope of the current work. The other authors have no conflicts of interest to declare.
References
- Andersen AN, Gianaroli L, Felberbaum R, de Mouzon J, Nygren KG; European IVF-monitoring programme (EIM), European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2001. Results generated from European registers by ESHRE. Hum Reprod 2005;20:1158–1176. [DOI] [PubMed] [Google Scholar]
- De Geyter C. Assisted reproductive technology: impact on society and need for surveillance. Best Pract Res Clin Endocrinol Metab 2019;33:3–8. [DOI] [PubMed] [Google Scholar]
- De Geyter C, Wyns C, Calhaz-Jorge C, de Mouzon J, Ferraretti AP, Kupka M, Nyboe Andersen A, Nygren KG, Goossens V.. 20 years of the European IVF-monitoring Consortium registry: what have we learned? A comparison with registries from two other regions. Hum Reprod 2020;35:2832–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geyter C, Wyns C, Mocanu E, de Mouzon J, Calhaz-Jorge C.. Data collection systems in ART must follow the pace of change in clinical practice. Hum Reprod 2016;31:2160–2163. [DOI] [PubMed] [Google Scholar]
- Ferraretti AP, Nygren K, Andersen AN, de Mouzon J, Kupka M, Calhaz-Jorge C, Wyns C, Gianaroli L, Goossens V. ; European IVF-Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE). Trends over 15 years in ART in Europe: an analysis of 6 million cycles. Hum Reprod Open 2017;2017:hox012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin DM, Adamson GD, Chambers G, De Geyter C.. Assisted Reproductive Technology Surveillance. Cambridge University Press, Cambridge, UK, 2019. [Google Scholar]
- Nygren KG, Andersen AN.. Assisted reproductive technology in Europe, 1997. Results generated from European registers by ESHRE. European IVF-Monitoring Programme (EIM), for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod 2001;16:384–391. [DOI] [PubMed] [Google Scholar]
- Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, Blomberg N, Boiten JW, da Silva Santos LB, Bourne PE. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data 2016;3:160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyns C, Bergh C, Calhaz-Jorge C, De Geyter C, Kupka MS, Motrenko T, Rugescu I, Smeenk J, Tandler-Schneider A, Vidakovic S. et al. ; European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open 2020;2020:hoaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyns C, De Geyter C, Calhaz-Jorge C, Kupka MS, Motrenko T, Smeenk J, Bergh C, Tandler-Schneider A, Rugescu IA, Goossens V; for the European IVF Monitoring Consortium (EIM), for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2018: results generated from European registries by ESHRE. Hum Reprod Open 2022;2022:hoac022.35795850 [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID. et al. The international glossary on infertility and fertility care, 2017. Hum Reprod 2017;32:1786–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are incorporated into the article.