Abstract
Objective
To examine whether treatment with the antiviral agent molnupiravir during the first five days of SARS-CoV-2 infection is associated with reduced risk of post-acute adverse health outcomes.
Design
Cohort study.
Setting
US Department of Veterans Affairs.
Participants
229 286 participants who tested positive for SARS-CoV-2 between 5 January 2022 and 15 January 2023, had at least one risk factor for progression to severe covid-19, and survived the first 30 days after testing positive were enrolled. 11 472 participants received a prescription for molnupiravir within five days of the positive test result and 217 814 received no covid-19 antiviral or antibody treatment (no treatment group).
Main outcome measures
Risks of post-acute sequelae of SARS-CoV-2 (PASC, defined based on a prespecified set of 13 post-acute sequelae), post-acute death, post-acute hospital admission, and each individual post-acute sequela between the molnupiravir group and no treatment group were examined after application of inverse probability weighting to balance the treatment and no treatment groups. Post-acute outcomes were ascertained from 30 days after the first SARS-CoV-2 positive test result until end of follow-up. Risks on the relative scale (relative risk or hazard ratio) and absolute scale (absolute risk reduction at 180 days) were estimated.
Results
Compared with no treatment, molnupiravir use within five days of a positive SARS-CoV-2 test result was associated with reduced risk of PASC (relative risk 0.86 (95% confidence interval 0.83 to 0.89); absolute risk reduction at 180 days 2.97% (95% confidence interval 2.31% to 3.60%)), post-acute death (hazard ratio 0.62 (0.52 to 0.74); 0.87% (0.62% to 1.13%)), and post-acute hospital admission (0.86 (0.80 to 0.93); 1.32% (0.72% to 1.92%)). Molnupiravir was associated with reduced risk of eight of the 13 post-acute sequelae: dysrhythmia, pulmonary embolism, deep vein thrombosis, fatigue and malaise, liver disease, acute kidney injury, muscle pain, and neurocognitive impairment. Molnupiravir was also associated with reduced risk of PASC in people who had not received a covid-19 vaccine, had received at one or two vaccine doses, and had received a booster dose, and in people with primary SARS-CoV-2 infection and reinfection.
Conclusions
In people with SARS-CoV-2 infection and at least one risk factor for progression to severe covid-19, compared with no treatment, molnupiravir use within five days of infection was associated with reduced risk of PASC in people who had not received a covid-19 vaccine, had received one or two vaccine doses, and had received a booster dose, and in those with primary SARS-CoV-2 infection and reinfection. Among people at high risk of progression to severe covid-19, molnupiravir use within five days of SARS-CoV-2 infection may be a viable approach to reduce the risk of PASC.
Introduction
Long covid represents the constellation of post-acute and chronic sequelae of SARS-CoV-2 infection.1 2 3 At least 65 million individuals globally are estimated to have long covid.4 Despite progress in characterizing long covid over the past three years, however, drug interventions for its prevention or treatment are not yet available.4
Nearly all the proposed mechanistic hypotheses to explain the myriad manifestations of long covid involve SARS-CoV-2 as the initiating agent.5 We previously reported that use of the antiviral nirmatrelvir (in combination with ritonavir) within five days of SARS-CoV-2 infection is associated with reduced risk of long covid.6 Collectively, these observations suggest that treatment of SARS-CoV-2 with antivirals during the early, acute phase may reduce the risk of post-acute adverse health outcomes.
Molnupiravir is an antiviral agent that impairs SARS-CoV-2 replication.7 Molnupiravir use within five days of testing positive for SARS-CoV-2 has been shown to reduce the risk of hospital admission or death in people at risk of progression to severe acute covid-19.8 9 In December 2021, the US Food and Drug Administration issued an emergency use authorization for molnupiravir in the treatment of people with acute SARS-CoV-2 infection at high risk of progression to severe covid-19 for whom alternative covid-19 treatment options were not accessible or were clinically inappropriate. Subsequently, millions of people in the United States have been treated with molnupiravir. The question of whether molnupiravir use during the acute phase of SARS-CoV-2 infection reduces the risk of long covid has been highlighted as a major knowledge gap that should be urgently addressed.10 Addressing this question will help inform treatment approaches for SARS-CoV-2 infections and guide the development and testing of additional drugs to prevent and treat long covid.
We used the electronic health record records of the US Department of Veterans Affairs to identify patients with a positive test result for SARS-CoV-2 between 5 January 2022 and 15 January 2023 and at least one risk factor for progression to severe covid-19. Weighting was then used to balance the personal and health characteristics of those who received a prescription for molnupiravir within five days of testing positive for SARS-CoV-2 (n=11 472) and those who did not receive outpatient covid-19 antiviral or antibody treatment within the first 30 days of infection (n=217 814). We evaluated whether treatment with molnupiravir was associated with reduced risk of post-acute sequelae of SARS-CoV-2 (PASC, based on 13 prespecified post-acute sequelae), post-acute death, post-acute hospital admission, and each individual post-acute sequela, where post-acute outcomes were ascertained 30 days after a first positive SARS-CoV-2 test result until end of follow-up.
Methods
Setting
Study data were collected from 1293 Veterans Affairs healthcare facilities (171 Veterans Affairs medical centers and 1112 outpatient sites) across the US. Veterans Affairs operates the largest integrated healthcare system in the US and provides healthcare services to veterans of the US armed forces. Healthcare provided includes preventive and health maintenance, outpatient care, inpatient hospital care, prescriptions, mental health care, home healthcare, primary care, specialty care, geriatric and extended care, medical equipment, and prosthetics.
Data sources
We extracted data from the US Department of Veterans Affairs healthcare databases. The databases are updated daily and include information collected during patients’ routine healthcare encounters. Data domains used were outpatient and inpatient diagnoses, outpatient and inpatient pharmacy records, and laboratory test results. Vaccination status was collected from the VA COVID-19 Shared Data Resource. Information about care outside of Veterans Affairs was collected from non-Veterans Affairs care program integrity tools data domain.11 The area deprivation index, a composite measure of income, education, employment, or housing, was used as a summary measure of contextual disadvantage at participants’ residential locations.12
Cohort
Supplementary figures S1 and S2 show the flowchart and timeline plots of cohort construction, respectively. We enrolled 339 719 participants with a positive SARS-CoV-2 test result between 5 January 2022 and 15 January 2023, with the date of a first positive result considered as T0. In accordance with the emergency use authorization issued by the FDA, which recommends initiation of molnupiravir within five days of SARS-CoV-2 infection, 13 007 of those who tested positive received a prescription for molnupiravir within five days of T0 and were selected for the molnupiravir group. We specified the inclusion and exclusion criteria in accordance with the MOVe-OUT (Efficacy and Safety of Molnupiravir in Non-Hospitalized Adult Participants With COVID-19) trial and the emergency use authorization issued by the FDA.8 13 Overall, we selected 12 684 participants with at least one of the following risk factors for progression to severe acute covid-19: age >60 years, body mass index (BMI) >30, cancer, cardiovascular disease, chronic kidney disease, chronic lung disease, diabetes, and immune dysfunction. We excluded participants with contraindications to treatment: end stage kidney disease or an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2 (remaining n=11 699). To examine the effect of molnupiravir on post-acute events, we further excluded those who used other outpatient covid-19 antiviral or antibody treatments within 30 days after T0, leaving 11 570 participants, and those who died within 30 days after the testing positive, resulting in a cohort comprising 11 472 participants.
We constructed the no treatment group using the same inclusion and exclusion criteria as for the molnupiravir group. The no treatment control group were selected from the 326 712 participants with a positive SARS-CoV-2 test result between 5 January 2022 and 15 January 2023 and who did not receive a prescription for molnupiravir within five days of testing positive. The specified inclusion and exclusion criteria enabled the selection of participants eligible for molnupiravir in accordance with the MOVE-OUT trial and the emergency use authorization issued by the FDA.8 13 Overall, we selected 292 408 of the participants with at least one risk factor for progression to severe acute covid-19 (see supplementary figure S1). We also excluded participants with a contraindication to molnupiravir—those with end stage kidney disease or an eGFR <30 mL/min/1.73m2 (remaining n=281 876). We then further excluded those who used outpatient covid-19 antiviral or antibody treatments within 30 days after T0, leaving 230 714 participants, and those who died within 30 days after testing positive, resulting in a cohort comprising 217 814 participants. The cohort was followed until 15 February 2023.
Outcomes
The outcomes measured included post-acute death and post-acute hospital admission and a composite of post-acute death or hospital admission. We also examined the risk of developing 13 prespecified post-acute sequelae of SARS-CoV-2: incident ischemic heart disease, dysrhythmia, deep vein thrombosis, pulmonary embolism, fatigue and malaise, liver disease, acute kidney injury, muscle pain, diabetes, neurocognitive impairment, dysautonomia, shortness of breath, and cough. Outcomes were ascertained as follows: deaths from vital status data, hospital admissions from inpatient encounter data, and individual sequelae from inpatient and outpatient ICD-10 (international classification of diseases, 10th revision) diagnosis codes, pharmacy records, and laboratory test results.1 2 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 Incident outcomes were assessed in those with no history of the related outcome within one year before testing positive.
We built the PASC score by assigning weights to each sequela (using the methods of the Global Burden of Disease Long COVID Collaborators32); this approach accounts for the number of sequelae experienced by an individual participant and the influence of each sequela (compared with other diseases) on health.33 34 35 The PASC score was then constructed for each participant as the sum of weights of all the incident sequelae in that participant (see https://github.com/yxie618/Molnupiravir_PASC for information on the weights and PASC score computation). To provide generalizable estimates for the burden of PASC on the absolute scale, we standardized the rate distribution of the PASC score to the rate distribution of the outcome, which was defined as having at least one sequela. All post-acute outcomes were ascertained 30 days after the first positive SARS-CoV-2 test result between 5 January 2022 and 15 January 2023 until 15 February 2023.
Covariates
Based on literature review and previous knowledge, we selected baseline covariates that might influence the treatment assignment and the occurrence or assessment of outcomes.1 27 36 37 38 Supplementary figure S3 shows the directed acyclic graph, which guided the selection of covariates. The ascertainment period for covariates was within three years before the baseline positive SARS-CoV-2 test result unless specified otherwise, and we used covariate values before and closest to the test date. Personal and health factors for age, race (white, black, and other), sex (men or women), area of deprivation index, state of residency, BMI, smoking status (never, former, and current), history of SARS-CoV-2 infection, and use of long term care were selected. We also controlled for clinical factors—use of steroids, eGFR, systolic and diastolic blood pressure, cancer, chronic lung disease, dementia, diabetes, hyperlipidemia, and immune dysfunction. Healthcare utilization variables were history of influenza vaccination, number of outpatient and inpatient encounters, number of laboratory tests, number of outpatient prescriptions, and number of Medicare related outpatient and inpatient encounters. We additionally specified pandemic related characteristics for week of the positive SARS-CoV-2 test result, hospital bed capacity, and hospital bed occupancy at the participants’ healthcare facility within the week of the positive test result. Hospital bed capacity was computed as the sum of number of beds occupied and number of beds available based on information reported from each healthcare facility. Hospital bed occupancy was computed as number of beds occupied divided by hospital bed capacity.36 Because the FDA’s emergency use authorization specified that molnupiravir may be considered when alternative options for covid-19 treatment (ie, nirmatrelvir-ritonavir) are not accessible or clinically appropriate, medicines that would have drug-drug interaction with and might preclude use of nirmatrelvir-ritonavir were classified at four levels (require an alternative covid-19 treatment to nirmatrelvir-ritonavir, require temporary suspension of concomitant drug, require adjustment of concomitant drug dosing, and require monitoring for adverse effects) and were adjusted for in the models. Within the study cohort, 8.0% of the eGFR, 9.1% of the BMI, and 3.9% of the blood pressure measurements were missing. We imputed missing data using the predictive mean matching method based on multivariate imputation by chained equations.39 To account for potential non-linear relationships, we transformed continuous variables into restricted cubic spline functions.
Statistical analysis
Baseline characteristics of the overall cohort and the molnupiravir and no treatment groups were reported, when appropriate, as mean and standard deviation or frequency and percentage. Balance of baseline characteristics between the two study groups was evaluated using absolute standardized differences, with a value <0.1 considered evidence of good balance.
To evaluate the risk of outcomes in the two study groups, we used inverse probability weighting to balance baseline characteristics between the groups. Multivariate logistic regression was applied to estimate the probability of receiving molnupiravir (the propensity score) given the covariates. The inverse probability weights to estimate treatment effect within treated participants was then constructed as 1 for those in the molnupiravir group and as propensity score/(1−propensity score) for those in the no treatment group. Although we prespecified our analytic approach to truncate weights that were larger than 10 to reduce the influence of extreme weights, no weights were that large and hence no truncation was required. To estimate the effect of molnupiravir on PASC score, we applied the inverse probability weights to a zero inflated Poisson regression, where the follow-up time was set to be the offset of the model. From the Poisson regression we estimated the relative risk (incident rate ratio) and absolute risk reduction at 180 days. The inverse probability weights were also applied to Cox survival models to estimate the effect of molnupiravir on risk of post-acute death, post-acute hospital admission, post-acute death or hospital admission, and individual post-acute sequela. Hazard ratio and absolute risk reduction were estimated from the models, where risk reduction was estimated as the difference in survival probability between the molnupiravir group and no treatment group at 180 days.
The effect of molnupiravir on risk of PASC was also examined in those who had received no covid-19 vaccine, received one or two vaccine doses, and received at least one booster dose. We also examined the effect in those with primary SARS-CoV2 infection and reinfection. We then further examined the effect within prespecified subgroups of age (≤65 and >65 years), race (white and black), sex (women and men), smoking status (never, former, and current), and presence or absence of risk factors for progression to severe acute covid-19: cancer, cardiovascular disease, kidney disease, chronic lung disease, diabetes, and immune dysfunction. Interaction analyses were conducted to examine whether subgroup status (eg, being in different age subgroups) modified the association between molnupiravir and PASC. Inverse probability weighting and outcome model were constructed independently within each subgroup examined. The effect of molnupiravir on risk of PASC across ages was also examined, where age was treated as a cubic spline function.
We conducted multiple sensitivity analyses for the outcome of PASC and the composite outcome of post-acute death or hospital admission (when applicable) to examine the robustness of findings by: balancing baseline characteristics through overlap weighting, instead of inverse probability weighting used in the primary approach; additionally adjusting for covariates in the weighted outcome model using a doubly robust approach, compared with the primary approach, which only applied inverse probability weighting to the outcome model; utilizing additional 100 covariates selected from a high dimensional variable selection algorithm to construct the propensity score, compared with the primary approach which used only prespecified covariates; using a negative binomial model to estimate the effect, compared with the primary approach, which used a Poisson model; enrolling those who died during the acute phase of infection (within 30 days of a SARS-CoV-2 positive test result) and applying inverse probability of censoring weight to account for the death, compared with the primary approach, which removed those who died during the acute phase from the analyses; examining effects within those who received treatment on the same day as the positive SARS-CoV-2 test result and, separately, within those who received treatment 2-5 days after testing positive, compared with the primary approach, which estimated the effect in people who received treatment within five days of testing positive; examining the effect in those who were not admitted to hospital during the acute phase of infection, compared with the primary approach, which included participants who were and were not admitted to hospital during the acute phase of infection; additionally adjusting for hospital admission, intensive care unit admission, and ventilator use during the acute phase of infection, compared with the primary approach, which used covariate information at T0; additionally adjusting for healthcare utilization, including outpatient encounters, hospital admissions, laboratory tests, and medicines during follow-up in the outcome model, compared with the primary approach, which used covariate information at T0; defining outcomes based on events that occurred 90 days after infection, compared with the primary approach, which defined outcomes based on events that occurred 30 days after infection; defining outcomes based on events that occurred any time after the infection, compared with the primary approach, which defined outcomes based on events that occurred 30 days after infection; and assigning the same weight to each individual sequela, compared with the primary approach, in which each individual sequela was weighted based on its health burden.
We considered a relative risk or hazard ratio value (and 95% confidence interval) that did not cross 1 and an absolute risk reduction value (and 95% confidence interval) that did not cross 0 to be statistically significant. Data management and analyses were performed with SAS Enterprise Guide, version 8.2 (SAS Institute, Cary, NC). Data visualizations were performed in R 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
No patients or members of the public were directly involved in developing the research question, study design, and implementation. We did, however share drafts of the manuscript with members of the public to obtain feedback, including informing the selection of outcomes, which were incorporated in the revisions.
Results
The overall cohort comprised 229 286 participants with a positive SARS-CoV-2 test result between 5 January 2022 and 15 January 2023 and at least one risk factor for progression to severe covid-19. Of these participants, 11 472 received a prescription for molnupiravir within five days after testing positive and 217 814 received no covid-19 outpatient treatment within the first 30 days after testing positive. Supplementary table S1 presents the baseline personal and health characteristics of the molnupiravir and no treatment groups before inverse probability weighting. Table 1 presents the characteristics of the study groups after inverse probability weighting. Values for the absolute standardized mean differences of personal and health characteristics between the molnupiravir and no treatment groups after weighting were <0.1, indicating good balance between the groups (table 1).
Table 1.
Personal and health characteristics of overall cohort and study groups after inverse probability weighting. Values are number (percentage) unless stated otherwise
| Characteristics | Overall cohort (n=229 286) | No treatment group (n=217 814) | Molnupiravir group (n=11 472) | Absolute SMD between molnupiravir and no treatment group |
|---|---|---|---|---|
| Mean (SD) age (years) | 69.78 (11.66) | 69.83 (11.64) | 69.73 (11.68) | 0.01 |
| Race: | ||||
| White | 181 457 (79.14) | 172 581 (79.23) | 9068 (79.05) | 0.005 |
| Black | 37 328 (16.28) | 35 325 (16.22) | 1875 (16.34) | 0.003 |
| Other | 10 501 (4.58) | 9908 (4.55) | 529 (4.61) | 0.003 |
| Ethnicity: | ||||
| Hispanic | 14 743 (6.43) | 13 934 (6.40) | 742 (6.47) | 0.003 |
| Non-Hispanic | 214 543 (93.57) | 203 880 (93.60) | 10 730 (93.53) | 0.003 |
| Sex: | ||||
| Men | 210 072 (91.62) | 199 718 (91.69) | 10 503 (91.55) | 0.005 |
| Women | 19 214 (8.38) | 18 096 (8.31) | 969 (8.45) | 0.005 |
| Smoking status: | ||||
| Never | 88 160 (38.45) | 83 569 (38.37) | 4420 (38.53) | 0.003 |
| Former | 104 715 (45.67) | 99 450 (45.66) | 5241 (45.69) | 0.001 |
| Current | 36 411 (15.88) | 34 796 (15.98) | 1811 (15.79) | 0.005 |
| Mean (SD) area deprivation index* | 54.99 (18.95) | 55.07 (18.94) | 54.91 (18.96) | 0.01 |
| Long term care | 4448 (1.94) | 4208 (1.93) | 223 (1.94) | 0.001 |
| Covid-19 vaccination status: | ||||
| None | 29 119 (12.70) | 27 625 (12.68) | 1460 (12.73) | 0.001 |
| 1 vaccine dose | 6443 (2.81) | 6114 (2.81) | 322 (2.81) | <0.001 |
| 2 vaccine doses | 48 586 (21.19) | 46 079 (21.16) | 2434 (21.22) | 0.002 |
| Booster dose | 145 138 (63.30) | 137 996 (63.36) | 7256 (63.25) | 0.002 |
| Mean (SD) BMI | 30.89 (6.52) | 30.89 (6.59) | 30.89 (6.44) | <0.001 |
| Mean (SD) eGFR (mL/min/1.73m2) | 74.34 (19.15) | 74.27 (19.18) | 74.41 (19.11) | 0.01 |
| Mean (SD) systolic blood pressure (mm Hg) | 134.28 (11.47) | 134.28 (11.46) | 134.27 (11.48) | <0.001 |
| Mean (SD) diastolic blood pressure (mm Hg) | 77.10 (6.89) | 77.10 (6.89) | 77.11 (6.89) | <0.001 |
| History of SARS-CoV-2 infection | 41 088 (17.92) | 39 150 (17.97) | 2049 (17.86) | 0.003 |
| Use of steroid | 31 733 (13.84) | 30 514 (14.01) | 1569 (13.68) | <0.001 |
| Medicines that would have drug-drug interaction with nirmatrelvir-ritonavir: | ||||
| Require an alternative covid-19 treatment | 25 520 (11.13) | 24 785 (11.38) | 1249 (10.89) | 0.02 |
| Require temporary suspension of concomitant drug | 150 778 (65.76) | 143 762 (66.00) | 7517 (65.53) | 0.01 |
| Require adjustment of concomitant drug dosing | 128 859 (56.20) | 123032 (56.49) | 6414 (55.91) | 0.01 |
| Require monitoring for adverse effects | 121 063 (52.80) | 115 559 (53.05) | 6027 (52.54) | 0.01 |
| Comorbidities: | ||||
| Cancer | 53 515 (23.34) | 50 997 (23.41) | 2668 (23.26) | 0.004 |
| Chronic lung disease | 77 911 (33.98) | 74 336 (34.13) | 3882 (33.84) | 0.006 |
| Dementia | 26 987 (11.77) | 25 761 (11.83) | 1344 (11.72) | 0.003 |
| Type 2 diabetes mellitus | 103 958 (45.34) | 99 051 (45.48) | 5186 (45.21) | 0.005 |
| Cardiovascular disease | 111 754 (48.74) | 106 799 (49.03) | 5557 (48.44) | 0.01 |
| Hyperlipidemia | 100 427 (43.80) | 95 498 (43.84) | 5019 (43.75) | 0.002 |
| Liver disease | 871 (0.38) | 828 (0.38) | 44 (0.38) | 0.001 |
| Immune dysfunction | 11 304 (4.93) | 10 767 (4.94) | 563 (4.91) | 0.002 |
| Mean (SD) No of hospital admissions† | 0.32 (0.90) | 0.32 (0.87) | 0.32 (0.92) | 0.01 |
| Mean (SD) No of outpatient visits† | 3.68 (1.45) | 3.68 (1.45) | 3.67 (1.44) | 0.01 |
| Mean (SD) No of blood panel tests† | 8.55 (9.71) | 8.61 (9.53) | 8.49 (9.89) | 0.01 |
| Mean (SD) No of drugs† | 14.02 (8.46) | 14.08 (8.48) | 13.97 (8.44) | 0.01 |
| Mean (SD) No of hospital admissions from Medicare | 0.06 (0.32) | 0.06 (0.34) | 0.06 (0.31) | <0.001 |
| Mean (SD) No of outpatient visits from Medicare | 0.22 (0.75) | 0.23 (0.76) | 0.22 (0.74) | 0.01 |
| Influenza vaccine | 173 019 (75.46) | 164 552 (75.55) | 8646 (75.37) | 0.004 |
| Mean (SD) calendar week of SARS-CoV-2 infection | 30.49 (14.18) | 30.58 (14.15) | 30.39 (14.2) | 0.01 |
BMI=body mass index; eGFR=estimated glomerular filtration rate; SMD=standardized mean difference; SD=standard deviation.
Measure of socioeconomic disadvantage from low to high disadvantage (range 0-100).
Data collected within one year before study enrollment.
Risk of PASC, post-acute death, post-acute hospital admission, and post-acute sequalae
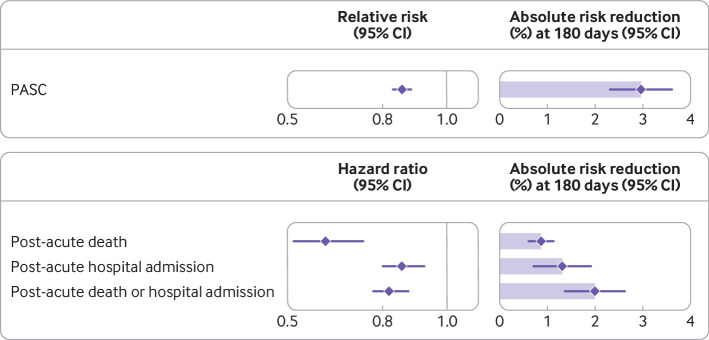
Molnupiravir use was associated with a reduced risk of PASC (relative risk 0.86 (95% confidence interval 0.83 to 0.89) and absolute risk reduction at 180 days 2.97% (95% confidence interval 2.31% to 3.60%)) (fig 1, fig 2, table 2, and supplementary table S2) compared with no treatment. The corresponding event rates of PASC at 180 days were 18.58% (17.94% to 19.24%) in the molnupiravir group and 21.55% (21.04% to 22.07%) in the no treatment group.
Fig 1.
Relative and absolute risk reduction of molnupiravir on post-acute sequelae of SARS-CoV-2 (PASC), post-acute death, post-acute hospital admission, and composite outcome of post-acute death or hospital admission compared with no treatment. Post-acute outcomes were ascertained 30 days after a first SARS-CoV-2 positive test result between 5 January 2022 and 15 January 2023 until end of follow-up. Adjusted relative risk or hazard ratios and 95% confidence intervals are presented. Length of shaded area represents risk reduction per 100 people at 180 days. Whiskers represent 95% confidence intervals (CIs)
Fig 2.
Event rates of post-acute sequelae in molnupiravir group (n=11 472) and no treatment group (n=217 814). Post-acute outcomes were ascertained 30 days after a first SARS-CoV-2 positive test result between 5 January 2022 and 15 January 2023 until end of follow-up. Event rates were estimated based on inverse probability weighting method. Shading represents 95% confidence intervals (CIs)
Table 2.
Relative risk, hazard ratio, and absolute risk reduction of molnupiravir use on post-acute sequelae of covid-19, post-acute death, post-acute hospital admission, and composite outcome of post-acute death or hospital admission compared with no treatment
| Outcome | Estimate (95% CI) | Event rate (%) at 180 days (95% CI) | Absolute risk reduction (%) at 180 days (95% CI) | |
|---|---|---|---|---|
| Molnupiravir group* | No treatment group† | |||
| Post-acute sequalae of covid-19 | RR 0.86 (0.83 to 0.89) | 18.58 (17.94 to 19.24) | 21.55 (21.04 to 22.07) | 2.97 (2.31 to 3.60) |
| Post-acute death | HR 0.62 (0.52 to 0.74) | 1.44 (1.20 to 1.69) | 2.32 (2.24 to 2.39) | 0.87 (0.62 to 1.13) |
| Post-acute hospital admission | HR 0.86 (0.80 to 0.93) | 8.82 (8.24 to 9.40) | 10.15 (10.00 to 10.29) | 1.32 (0.72 to 1.92) |
| Post-acute death or hospital admission | HR 0.82 (0.77 to 0.88) | 9.65 (9.05 to 10.26) | 11.65 (11.50 to 11.80) | 2.00 (1.37 to 2.62) |
CI=confidence interval; HR=hazard ratio; RR=relative risk.
Post-acute outcomes were ascertained 30 days after the first SARS-CoV-2 positive test result between 5 January 2022 and 15 January 2023 until end of follow-up.
Received prescription for molnupiravir within five days after testing positive for SARS-CoV-2 and did not use any other outpatient antiviral or antibody during first 30 days after testing positive.
Did not use any outpatient antiviral or antibody during first 30 days after testing positive for SARS-CoV-2 and served as reference group in analyses.
Compared with no treatment, molnupiravir use was associated with reduced risk of post-acute death (hazard ratio 0.62 (95% confidence interval 0.52 to 0.74) and absolute risk reduction 0.87% (0.62% to 1.13%)), post-acute hospital admission (0.86 (0.80 to 0.93) and 1.32% (0.72% to 1.92%)), and composite outcome of post-acute death or hospital admission (0.82 (0.77 to 0.88) and 2.00% (1.37% to 2.62%) (fig 1, fig 2, and table 2).
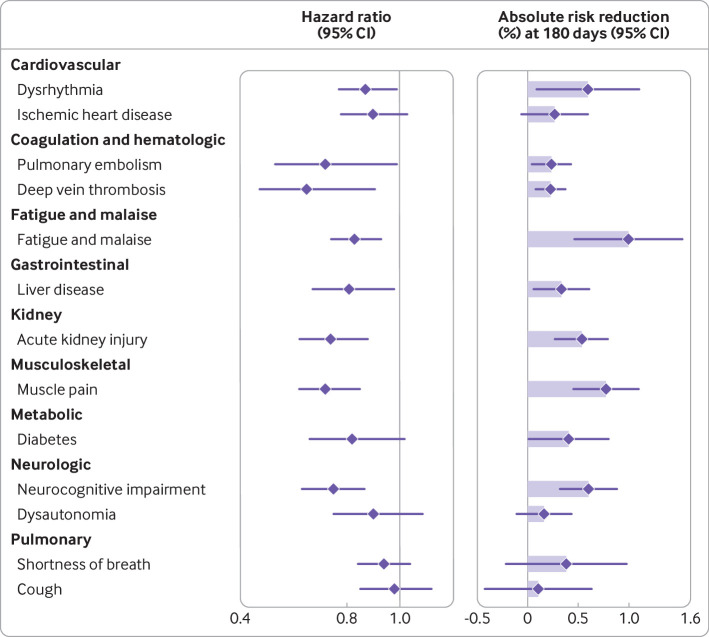
Molnupiravir use was associated with reduced risk of eight of the 13 individual post-acute sequela examined compared with no treatment—dysrhythmia, pulmonary embolism, deep vein thrombosis, fatigue and malaise, liver disease, acute kidney injury, muscle pain, and neurocognitive impairment. We did not observe statistically significant associations between molnupiravir and ischemic heart disease, diabetes, dysautonomia, shortness of breath, and cough (fig 3 and supplementary table S3).
Fig 3.
Hazard ratio and absolute risk reduction of molnupiravir compared with no treatment on individual post-acute sequelae. Post-acute outcomes were ascertained 30 days after a first SARS-CoV-2 positive test result between 5 January 2022 and 15 January 2023 until end of follow-up. Length of shaded area represents risk reduction per 100 people at 180 days, Whiskers represent 95% confidence intervals (CIs)
Risk of PASC in subgroups
Compared with no treatment, molnupiravir use was associated with reduced risk of PASC in those who had not received a covid-19 vaccine, received one or two vaccine doses, and received a booster dose. Molnupiravir use was also associated with reduced risk of PASC in those with primary SARS-CoV-2 infection and reinfection (fig 4 and supplementary table S4).
Fig 4.
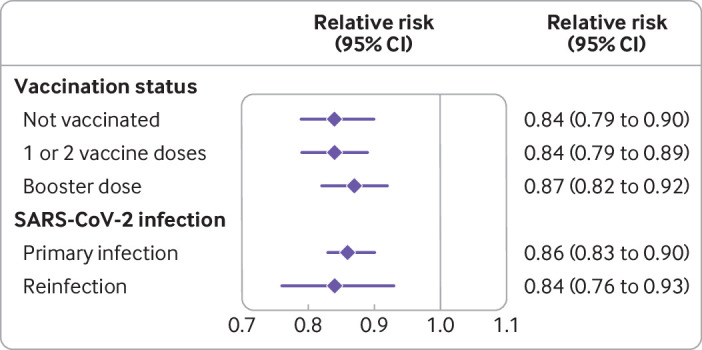
Relative risk of molnupiravir compared with no treatment on post-acute sequelae of covid-19 according to vaccination status and primary SARS-CoV-2 infection or reinfection. Outcomes were ascertained 30 days after a first SARS-CoV-2 positive test result between 5 January 2022 and 15 January 2023 until end of follow-up. CI=confidence interval
In subgroup analyses based on age (≤65 and >65 years), race (white and black), sex (men and women), smoking status (never, former, and current), and disease status (cancer, cardiovascular disease, chronic kidney disease, chronic lung disease, diabetes, and immune dysfunction), molnupiravir use was associated with reduced risk of PASC compared with no treatment (fig 5, supplementary figure S4, supplementary table S4). No statistically significant interaction was observed in any of the subgroups.
Fig 5.

Relative risk of molnupiravir on post-acute sequelae of covid-19 compared with no treatment in subgroups by personal characteristics and comorbidities. Post-acute outcomes were ascertained 30 days after a first positive SARS-CoV-2 test result between 5 January 2022 and 15 January 2023 until end of follow-up. CI=confidence interval
Sensitivity analyses
Multiple sensitivity analyses were conducted to assess the robustness of our findings. First, we balanced baseline characteristics through overlap weighting, instead of inverse probability weighting used in the primary approach. Second, we additionally adjusted for covariates in the weighted outcome model using a doubly robust methodology, instead of inverse probability weighting to the outcome model used in the primary approach. Third, we utilized additional 100 covariates selected using a high dimensional variable selection algorithm to construct the propensity score, instead of using only prespecified covariates as in the primary approach. Fourth, we used a negative binomial model to estimate the effect, instead of a Poisson model as used in the primary approach. Fifth, we enrolled those who died during the acute phase of infection (within 30 days of a positive SARS-CoV-2 test result) and applied inverse probability of censoring weight to account for the death, instead of excluding those who died during the acute phase from analyses as was used in the primary approach. Sixth, we examined effect within those who received treatment on the same day as a positive SARS-CoV-2 test result, and separately within those who received treatment 2-5 days after the date of testing positive, compared with the primary approach, which estimated the effect in people who received treatment within five days of testing positive. Seventh, we examined the effect in those who were not admitted to hospital during the acute phase of infection, compared with the primary approach, which included participants who were and were not admitted to hospital during the acute phase. Eighth, we additionally adjusted for hospital admission, intensive care unit admission, and ventilator use during the acute phase of infection, instead of using covariate information at T0 as was applied in the primary approach. Ninth, we additionally adjusted for healthcare utilization during follow-up (outpatient encounters, hospital admissions, laboratory tests, and drugs) in the outcome model, compared with the primary approach, in which we used covariate information at T0. Tenth, we defined outcomes based on events that occurred 90 days after infection, compared with the primary approach, which defined outcomes based on events that occurred 30 days after infection Eleventh, we defined outcomes based on events that occurred any time after the infection, instead of defining outcomes based on events that occurred 30 days after infection as in the primary approach. Finally, we assigned the same weight to each individual sequela, compared with the primary approach, in which we weighted each individual sequela based on its health burden. We obtained consistent results in all sensitivity analyses, suggesting that, compared with no treatment, molnupiravir use was associated with reduced risk of PASC and post-acute death or hospital admission (see supplementary table S5).
Discussion
Among 229 286 people included in this study with SARS-CoV-2 infection and at least one risk factor for progression to severe covid-19, use of the antiviral molnupiravir within five days of testing positive for SARS-CoV-2 compared with no treatment was associated with reduced risk of PASC, post-acute death, and post-acute hospital admission. Molnupiravir use was associated with reduced risk of PASC in those who had not received a covid-19 vaccine, received one or two vaccine doses, and received a booster dose, and in people with primary SARS-CoV-2 infection and reinfection. Molnupiravir use was also associated with reduced risk of PASC in various subgroups based on age, race, sex, smoking status, cancer, cardiovascular disease, chronic kidney disease, chronic lung disease, diabetes, and immune dysfunction. Compared with no treatment, molnupiravir use was also associated with reduced risk of eight out of the 13 prespecified sequelae—dysrhythmia, pulmonary embolism, deep vein thrombosis, fatigue and malaise, liver disease, acute kidney injury, muscle pain, and neurocognitive impairment. Overall, the findings suggest that among people at high risk of progression to severe covid-19, molnupiravir use within five days of a positive SARS-CoV-2 test result was associated with reduced risk of post-acute adverse health outcomes.
Findings in relation to other studies
Our study findings provide evidence that use of molnupiravir within the first five days of a positive SARS-CoV-2 test result is associated with reduced risk of PASC. Molnupiravir use was associated with 14% less risk of PASC, 38% lower risk of post-acute death, and 14% lower risk of post-acute hospital admission; the magnitude of risk reduction on the absolute scale was also substantial—a 2.97%, 0.87%, and 1.32% lower absolute risk of PASC, post-acute death, and post-acute hospital admission for every 100 treated people between 30 and 180 days of infection. These findings align with previous observations that use of combined antivirals nirmatrelvir-ritonavir is associated with reduced risk of PASC, post-acute death, and post-acute hospital admission.6 Together, these findings suggest that use of antivirals in the acute phase of infection may not only help reduce the risk of progression to severe covid-19 but may be a viable strategy to reduce the risk of post-acute adverse health outcomes, including PASC. The clinical decision to use antivirals during acute SARS-CoV-2 infection should consider the effectiveness of antivirals in reducing the burden of death and disease in both the acute and the post-acute phases of covid-19.
We specified our enrollment criteria to follow the FDA’s emergency use authorization for molnupiravir—which is generally used in people with at least one risk factor for progression to severe covid-19 and for whom the alternative treatment nirmatrelvir is not accessible or clinically appropriate. Evidence points to the possibility that although molnupiravir might be effective in the acute phase of SARS-CoV-2 in adults at high risk of progression to severe covid-19, these beneficial effects may not translate to effectiveness in the acute phase among populations with relatively lower risks.8 9 40 Whether the reduced risk of PASC (and the other post-acute health outcomes) observed in this report (in people with at least one risk factor for progression to severe covid-19) extends to other populations (eg, those without risk factors for progression to severe disease) is not known. Furthermore, whether higher dose or more prolonged duration of treatment results in higher risk reduction is also not clear. Also, for the millions of people already experiencing long covid, the question of whether initiation of antiviral treatment in the post-acute phase of covid-19 is effective in treating long covid demands urgent attention.
Our results show risk reduction in eight out of 13 prespecified post-acute sequelae. It is possible that these sequelae may be driven by various mechanistic pathways with different sensitivity to antiviral treatment during the acute disease phase; it is also possible that the putative effect of molnupiravir on these sequelae is variable and that a higher level of power and precision is required for the observation of statistically significant results.
The mechanisms underpinning the association between molnupiravir and PASC are not entirely clear. Because PASC is a consequence of SARS-CoV-2 infection, and because the incidence of PASC—although evident in those with mild infection—is generally associated with severity of acute infection, it is reasonable to hypothesize that impairing viral replication and reducing severity of acute infection by early use of antivirals may lead to reduced risk of PASC.6 9 This hypothesis is consistent with the putative mechanisms that are being proposed to explain the occurrence of PASC, including viral persistence, immune dysfunction, occult organ injury during the acute phase that may manifest in the post-acute phase, and microbiome dysbiosis. All these putative mechanisms of long covid originate with SARS-CoV-2 as the initiating agent, suggesting the hypothesis that reduced viral replication (through use of antivirals) may ameliorate these manifestations.
Strengths and limitations of this study
This study has several strengths. We conducted this study using real world data from the Veterans Affairs, which operates the largest nationally integrated healthcare system in the US. We comprehensively leveraged the breadth and depth of Veterans Affairs data sources to incorporate information in multiple domains, such as personal characteristics, diagnoses, laboratory tests, drugs, vital signs, and healthcare utilization as well as contextual factors from the previous three years before the start of follow-up. For this cohort study, we enrolled a larger number of participants than are typically included in randomized trials and evaluated a prespecified set of 13 post-acute sequelae informed by our extensive research on long covid.1 2 4 13 19 23 27 28 36 38 41 We conceptualized the PASC score by assigning weights to each individual sequela not only to reflect its occurrence but also to reflect the number of sequelae experienced by an individual, and the influence of each sequela (compared with other diseases) on health. We assessed robustness of our approach in multiple sensitivity analyses, and results were consistent.
This study has several limitations. Our cohort comprised veterans, who are predominately white men, which may limit generalizability of study findings. We conducted an observational cohort study using data from the Veterans Affairs electronic record system; although we carefully designed our enrollment criteria to reduce differences between the study groups and subsequently used a large set of covariates from multiple data domains to balance these groups, biases, such as misclassification and residual confounding, may still exist. We relied on Veterans Affairs pharmacy records to define use of molnupiravir and did not capture drug use outside the Veterans Affairs system. If a large proportion of participants in the no treatment group received molnupiravir or other covid-19 treatment outside of the Veterans Affairs healthcare system, and if association in those participants was directionally similar to that reported here, this could have biased the results toward the null. Our analyses were restricted to a set of 13 sequelae and did not comprehensibly include all possible post-acute sequelae of SARS-CoV-2 infection. Finally, the pandemic remains highly dynamic, SARS-CoV-2 continues to mutate and new variants and subvariants are constantly emerging, and immunity levels in populations are also changing as a function of infection, reinfection, and vaccine uptake. Several factors may lead to the emergence of antiviral resistance; PASC itself may change both in terms of clinical features and in magnitude of risk. As a result, the real world effectiveness of molnupiravir on post-acute outcomes may also change dynamically over time.
Conclusions
Among people with SARS-CoV-2 infection and at least one risk factor for progression to severe covid-19, molnupiravir use during the first five days of infection was associated with reduced risk of PASC, regardless of an individual’s vaccination status and history of infection. These findings suggest that treatment with molnupiravir in the acute phase of SARS-CoV-2 infection may be a viable strategy to reduce the risk of post-acute adverse health outcomes.
What is already known on this topic
Among people with SARS-CoV-2 infection, molnupiravir use within five days of symptom onset has been shown to reduce the risk of hospital admission or death in people at risk of progression to severe covid-19
What this study adds
In people with SARS-CoV-2 infection and at least one risk factor for progression to severe covid-19, compared with no treatment, molnupiravir use during the first five days after a positive SARS-CoV-2 test result was associated with reduced risk of post-acute sequelae of SARS-CoV-2
The reduced risk in the molnupiravir group was evident in those who had not received a covid-19 vaccine, received at least one vaccine dose, and had received a booster dose, and in those with primary SARS-CoV-2 infection and reinfection
Acknowledgments
This study used data from the Veterans Affairs COVID-19 Shared Data Resource.
Web extra.
Extra material supplied by authors
Supplementary information: Figures S1-S4 and tables S1-S5
Contributors: ZAA and YX contributed to the development of the study concept and design. ZAA, YX, and TC contributed to data analysis and interpretation of results. ZAA and YX drafted the manuscript. ZAA and YX contributed to critical revision of the manuscript. ZAA provided administrative, technical, and material support, as well as supervision and mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors approved the final version of the report. ZAA is the guarantor. The contents do not represent the views of the US Department of Veterans Affairs or the US government. The corresponding author attests that all the listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research was funded by the US Department of Veterans Affairs (ZAA). The funders had no role in considering the study design, or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the US Department of Veterans Affairs for the submitted work. ZAA reports receiving consultation fees from Gilead Sciences and receipt of funding (unrelated to this work) from Tonix pharmaceuticals. ZAA and YX reports consulting (uncompensated) for Pfizer; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (ZAA) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: The authors have no specific plans to disseminate the findings to study participants. However, study results will be disseminated via a press release with mainstream media and will also be promoted on Twitter (via the corresponding senior author’s account @zalaly). We will also share the findings with public health agencies and our contacts in the US government (the US covid-19 task force), British government, and the Canadian government (the Office of the Chief Science Advisor of Canada). The results will be shared with advocacy groups and will be presented in scientific meetings and at academic institutions.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved, and waiver of consent granted, by the institutional review board of the VA Saint Louis Health Care System (protocol No 1606333).
Data availability statement
The data that support the findings of this study are available from the US Department of Veterans Affairs (VA). VA data are made freely available to researchers behind the VA firewall with an approved VA study protocol (see https://www.virec.research.va.gov or contact the VA Information Resource Center at VIReC@va.gov for more information).
References
- 1. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021;594:259-64. 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 2. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 2022;28:1461-7. 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023;21:133-46. 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Aly Z, Agarwal A, Alwan N, Luyckx VA. Long COVID: long-term health outcomes and implications for policy and research. Nat Rev Nephrol 2023;19:1-2. 10.1038/s41581-022-00652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science 2022;375:1122-7. 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 6. Xie Y, Choi T, Al-Aly Z. Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition. JAMA Intern Med 2023;e230743. 10.1001/jamainternmed.2023.0743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kabinger F, Stiller C, Schmitzová J, et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol 2021;28:740-6. 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. MOVe-OUT Study Group . Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med 2022;386:509-20. 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xie Y, Bowe B, Al-Aly Z. Molnupiravir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records. BMJ 2023;380:e072705. 10.1136/bmj-2022-072705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ledford H. Long-COVID treatments: why the world is still waiting. Nature 2022;608:258-60. 10.1038/d41586-022-02140-w. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Veterans Affairs. Community Care Data – Program Integrity Tool (PIT). 2022. https://www.herc.research.va.gov/include/page.asp?id=choice-pit
- 12. Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible - The Neighborhood Atlas. N Engl J Med 2018;378:2456-8. 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al-Aly Z. Diabetes after SARS-CoV-2 infection. Lancet Diabetes Endocrinol 2023;11:11-3. 10.1016/S2213-8587(22)00324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen K, Ren S, Heath K, et al. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2022;376:e068414. 10.1136/bmj-2021-068414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bull-Otterson L, Baca S, Saydah S, et al. Post–COVID Conditions Among Adult COVID-19 Survivors Aged 18-64 and ≥65 Years — United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep 2022;71:713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2021;373:n1098. 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ 2021;372:n693. 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carfì A, Bernabei R, Landi F, Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020;324:603-5. 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun 2021;12:6571. 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021;18:e1003773. 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022;28:583-90. 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney Outcomes in Long COVID. J Am Soc Nephrol 2021;32:2851-62. 10.1681/ASN.2021060734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with covid-19: cohort study. BMJ 2022;376:e068993. 10.1136/bmj-2021-068993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021;18:e1003773. 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021;38:101019. 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021;8:416-27. 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol 2022;10:311-21. 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med 2022;28:2406-15. 10.1038/s41591-022-02001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu E, Xie Y, Al-Aly Z. Risks and burdens of incident dyslipidaemia in long COVID: a cohort study. Lancet Diabetes Endocrinol 2023;11:120-8. 10.1016/S2213-8587(22)00355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al-Aly Z. Diabetes after SARS-CoV-2 infection. Lancet Diabetes Endocrinol 2023;11:11-3. 10.1016/S2213-8587(22)00324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu E, Xie Y, Al-Aly Z. Long-term gastrointestinal outcomes of COVID-19. Nat Commun 2023;14:983. 10.1038/s41467-023-36223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wulf Hanson S, Abbafati C, Aerts JG, et al. Global Burden of Disease Long COVID Collaborators . Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022;328:1604-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wulf Hanson S, Abbafati C, Aerts JG, et al. Global Burden of Disease Long COVID Collaborators . Estimated Global Proportions of Individuals With Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022;328:1604-15. 10.1001/jama.2022.18931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vos T, Lim SS, Abbafati C, et al. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1204-22. 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute for Health Metrics and Evaluation (IHME). GBD results. Seattle, WA: IHME, University of Washington, 2021. https://vizhub.healthdata.org/gbd-results/.
- 36. Xie Y, Bowe B, Maddukuri G, Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ 2020;371:m4677. 10.1136/bmj.m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute Kidney Injury in a National Cohort of Hospitalized US Veterans with COVID-19. Clin J Am Soc Nephrol 2020;16:14-25. 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 2022;28:583-90. 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219-42. 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 40. Butler CC, Hobbs FDR, Gbinigie OA, et al. PANORAMIC Trial Collaborative Group . Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 2023;401:281-93. 10.1016/S0140-6736(22)02597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med 2022;28:2398-405. 10.1038/s41591-022-02051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Figures S1-S4 and tables S1-S5
Data Availability Statement
The data that support the findings of this study are available from the US Department of Veterans Affairs (VA). VA data are made freely available to researchers behind the VA firewall with an approved VA study protocol (see https://www.virec.research.va.gov or contact the VA Information Resource Center at VIReC@va.gov for more information).