Abstract
Carbon-supported Pt-based materials are highly promising electrocatalysts. The carbon support plays an important role in the Pt-based catalysts by remarkably influencing the growth, particle size, morphology, dispersion, electronic structure, physiochemical property and function of Pt. This review summarizes recent progress made in the development of carbon-supported Pt-based catalysts, with special emphasis being given to how activity and stability enhancements are related to Pt–C interactions in various carbon supports, including porous carbon, heteroatom doped carbon, carbon-based binary support, and their corresponding electrocatalytic applications. Finally, the current challenges and future prospects in the development of carbon-supported Pt-based catalysts are discussed.
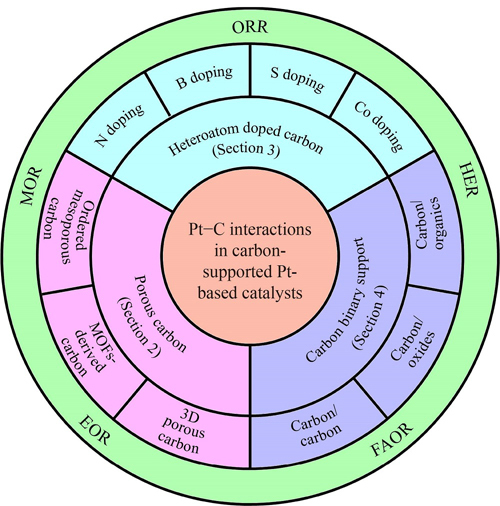
Keywords: Pt–C interactions, Pt-based materials, carbon support, electrocatalysis
Acknowledgements
Preparation of this review was financially supported by National Key Research and Development Program of China (Grant Nos. 2022YFB3805600 and 2022YFB3805604), National Natural Science Foundation of China (Grant No. 52201286), Sino-German Center COVID-19 Related Bilateral Collaborative Project (C-0046), FRFCU (2021qntd13), National 111 Project (B20002), Guangdong Basic and Applied Basic Research Foundation (Grant Nos. 2019A1515110436, 2021A1515111131, 2022A1515011905, and 2022A1515010137), Guangzhou Science and Technology Project (Grant No. 202102020463), Guangdong Province International Scientific and Technological Cooperation Projects (Grant No. 2020A0505100036), and Shenzhen Science and Technology Program (Grant Nos. GJHZ20210705143204014, JCYJ20210324142010029, and KCXFZ20211020170006010).
Contributor Information
Jie Ying, Email: yingj5@mail.sysu.edu.cn.
Xiao-Yu Yang, Email: xyyang@whut.edu.cn.
References
- 1.Zhou K L, Wang Z, Han C B, Ke X, Wang C, Jin Y, Zhang Q, Liu J, Wang H, Yan H. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction. Nature Communications. 2021;12(1):3783. doi: 10.1038/s41467-021-24079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li M, Zhao Z, Zhang W, Luo M, Tao L, Sun Y, Xia Z, Chao Y, Yin K, Zhang Q H, Gu L, Yang W, Yu Y, Lu G, Guo S. Submonolayer YOx/MoOx on ultrathin Pt nanowires boosts alcohol oxidation electrocatalysis. Advanced Materials. 2021;33(41):2103762. doi: 10.1002/adma.202103762. [DOI] [PubMed] [Google Scholar]
- 3.Wu S M, Čejka J, Yang X Y. Active sites in the right places. Nature Synthesis. 2022;1(10):757–758. doi: 10.1038/s44160-022-00168-4. [DOI] [Google Scholar]
- 4.Yu F Y, Lang Z L, Yin L Y, Feng K, Xia Y J, Tan H Q, Zhu H T, Zhong J, Kang Z H, Li Y G. Pt–O bond as an active site superior to Pt0 in hydrogen evolution reaction. Nature Communications. 2020;11(1):490. doi: 10.1038/s41467-019-14274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian X, Zhao X, Su Y Q, Wang L, Wang H, Dang D, Chi B, Liu H, Hensen E J, Lou X W, Xia B Y. Engineering bunched Pt–Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science. 2019;366(6467):850–856. doi: 10.1126/science.aaw7493. [DOI] [PubMed] [Google Scholar]
- 6.Ying J, Lenaerts S, Symes M D, Yang X Y. Hierarchical design in nanoporous metals. Advanced Science. 2022;9:2106117. doi: 10.1002/advs.202106117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ying J. Atomic-scale design of high-performance Pt-based electrocatalysts for oxygen reduction reaction. Frontiers in Chemistry. 2021;9:753604. doi: 10.3389/fchem.2021.753604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying J, Jiang G, Cano Z P, Ma Z, Chen Z. Spontaneous weaving: 3D porous Pt–Cu networks with ultrathin jagged nanowires for highly efficient oxygen reduction reaction. Applied Catalysis B: Environmental. 2018;236:359–367. doi: 10.1016/j.apcatb.2018.04.035. [DOI] [Google Scholar]
- 9.Xiao Y X, Ying J, Tian G, Zhang X Q, Janiak C, Ozoemena K I, Yang X Y. Pt–Pd hollow nanocubes with enhanced alloy effect and active facets for efficient methanol oxidation reaction. Chemical Communications. 2021;57(8):986–989. doi: 10.1039/D0CC06876D. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y X, Ying J, Tian G, Yang X, Zhang Y X, Chen J B, Wang Y, Symes M D, Ozoemena K I, Wu J, Yang X Y. Hierarchically fractal Pt–Pd–Cu sponges and their directed mass-and electron-transfer effects. Nano Letters. 2021;21(18):7870–7878. doi: 10.1021/acs.nanolett.1c02268. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Yu H Z, Ying J, Tian G, Liu Y, Geng W, Hu J, Lu Y, Chang G G, Ozoemena K I, Janiak C, Yang X Y. Ultimate corrosion to Pt–Cu electrocatalysts for enhancing methanol oxidation activity and stability in acidic media. Chemistry–A European Journal. 2021;27(35):9124–9128. doi: 10.1002/chem.202100754. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Zhang L, Ma W, Wan H, Zhang X, Zhang X, Jiang S, Zheng J Y, Zhou Z. In situ anchoring massive isolated Pt atoms at cationic vacancies of α-NixFe1−x(OH)2 to regulate the electronic structure for overall water splitting. Advanced Functional Materials. 2022;32(31):2203342. doi: 10.1002/adfm.202203342. [DOI] [Google Scholar]
- 13.Feng X, Bai Y, Liu M, Li Y, Yang H, Wang X, Wu C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy & Environmental Science. 2021;14(4):2036–2089. doi: 10.1039/D1EE00166C. [DOI] [Google Scholar]
- 14.Hu C, Dai L. Carbon-based metal-free catalysts for electrocatalysis beyond the ORR. Angewandte Chemie International Edition. 2016;55(39):11736–11758. doi: 10.1002/anie.201509982. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Shui J, Du L, Shao Y, Liu J, Dai L, Hu Z. Carbon-based metal-free ORR electrocatalysts for fuel cells: past, present, and future. Advanced Materials. 2019;31(13):1804799. doi: 10.1002/adma.201804799. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Jiang S, Ma W, Zhou Z. Oxygen reduction reaction on Pt-based electrocatalysts: four-electron vs. two-electron pathway. Chinese Journal of Catalysis. 2022;43(6):1433–1443. doi: 10.1016/S1872-2067(21)63961-X. [DOI] [Google Scholar]
- 17.Dong Y, Ying J, Xiao Y X, Chen J B, Yang X Y. Highly dispersed Pt nanoparticles embedded in N-doped porous carbon for efficient hydrogen evolution. Chemistry-an Asian Journal. 2021;16(14):1878–1881. doi: 10.1002/asia.202100438. [DOI] [PubMed] [Google Scholar]
- 18.Wei H, Hu Z Y, Xiao Y X, Tian G, Ying J, Van Tendeloo G, Janiak C, Yang X Y, Su B L. Control of the interfacial wettability to synthesize highly dispersed PtPd nanocrystals for efficient oxygen reduction reaction. Chemistry-an Asian Journal. 2018;13(9):1119–1123. doi: 10.1002/asia.201800191. [DOI] [PubMed] [Google Scholar]
- 19.Shen L, Ying J, Tian G, Jia M, Yang X Y. Ultralong PtPd alloyed nanowires anchored on graphene for efficient methanol oxidation reaction. Chemistry-an Asian Journal. 2021;16(9):1130–1137. doi: 10.1002/asia.202100156. [DOI] [PubMed] [Google Scholar]
- 20.Hammer B, Norskov J K. Why gold is the noblest of all the metals. Nature. 1995;376(6537):238–240. doi: 10.1038/376238a0. [DOI] [Google Scholar]
- 21.Singh J, Nelson R C, Vicente B C, Scott S L, van Bokhoven J A. Electronic structure of alumina-supported monometallic Pt and bimetallic Pt–Sn catalysts under hydrogen and carbon monoxide environment. Physical Chemistry Chemical Physics. 2010;12(21):5668–5677. doi: 10.1039/c000403k. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y J, Zhao N, Fang B, Li H, Bi X T, Wang H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: particle size, shape, and composition manipulation and their impact to activity. Chemical Reviews. 2015;115(9):3433–3467. doi: 10.1021/cr500519c. [DOI] [PubMed] [Google Scholar]
- 23.Ren X, Wang Y, Liu A, Zhang Z, Lv Q, Liu B. Current progress and performance improvement of Pt/C catalysts for fuel cells. Journal of Materials Chemistry A. 2020;8(46):24284–24306. doi: 10.1039/D0TA08312G. [DOI] [Google Scholar]
- 24.Zhang X Q, Xiao Y X, Tian G, Yang X, Dong Y, Zhang F, Yang X Y. Enhancing resistance to chloride corrosion by controlling the morphologies of PtNi electrocatalysts for alkaline seawater hydrogen evolution. Chemistry–A European Journal. 2022;29(5):e202202811. doi: 10.1002/chem.202202811. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Wang W, Qian Q, Zhu Y, Feng Y, Zhang Y, Zhang H, Cheng M, Zhang G. Magic hybrid structure as multifunctional electrocatalyst surpassing benchmark Pt/C enables practical hydrazine fuel cell integrated with energy-saving H2 production. eScience. 2022;2(4):416–427. doi: 10.1016/j.esci.2022.06.004. [DOI] [Google Scholar]
- 26.Gerber I C, Serp P. A theory/experience description of support effects in carbon-supported catalysts. Chemical Reviews. 2019;120(2):1250–1349. doi: 10.1021/acs.chemrev.9b00209. [DOI] [PubMed] [Google Scholar]
- 27.Kim J M, Lee Y J, Kim S, Chae K H, Yoon K R, Lee K A, Byeon A, Kang Y S, Park H Y, Cho M K, Ham H C, Kim J Y. High-performance corrosion-resistant fluorine-doped tin oxide as an alternative to carbon support in electrodes for PEM fuel cells. Nano Energy. 2019;65:104008. doi: 10.1016/j.nanoen.2019.104008. [DOI] [Google Scholar]
- 28.Yang F, Bao X, Zhao Y, Wang X, Cheng G, Luo W. Enhanced HOR catalytic activity of PGM-free catalysts in alkaline media: the electronic effect induced by different heteroatom doped carbon supports. Journal of Materials Chemistry A. 2019;7(18):10936–10941. doi: 10.1039/C9TA01916B. [DOI] [Google Scholar]
- 29.Geng W, Jiang N, Qing G Y, Liu X, Wang L, Busscher H J, Tian G, Sun T, Wang L Y, Montelongo Y, Janiak C, Zhang G, Yang X Y, Su B L. Click reaction for reversible encapsulation of single yeast cells. ACS Nano. 2019;13(12):14459–14467. doi: 10.1021/acsnano.9b08108. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Li Y, Yang X Y, Zhang B B, Ninane N, Busscher H J, Hu Z Y, Delneuville C, Jiang N, Xie H, Van Tendeloo G, Hasan T, Su B L. Single-cell yolk-shell nanoencapsulation for long-term viability with size-dependent permeability and molecular recognition. National Science Review. 2021;8(4):nwaa097. doi: 10.1093/nsr/nwaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doan H, Morais T, Borchtchoukova N, Wijsboom Y, Sharabi R, Chatenet M, Finkelshtain G. Bimetallic Pt or Pd-based carbon supported nanoparticles are more stable than their monometallic counterparts for application in membraneless alkaline fuel cell anodes. Applied Catalysis B: Environmental. 2022;301:120811. doi: 10.1016/j.apcatb.2021.120811. [DOI] [Google Scholar]
- 32.Đukić T, Moriau L J, Pavko L, Kostelec M, Prokop M, Ruiz-Zepeda F, Šala M, Dražić G, Gatalo M, Hodnik N. Understanding the crucial significance of the temperature and potential window on the stability of carbon supported Pt-alloy nanoparticles as oxygen reduction reaction electrocatalysts. ACS Catalysis. 2021;12(1):101–115. doi: 10.1021/acscatal.1c04205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao Y, Zhao X, Chang G, Yang X, Chen B. Hierarchically porous metal–organic frameworks: synthetic strategies and applications. Small Structures. 2023;4(1):2200187. doi: 10.1002/sstr.202200187. [DOI] [Google Scholar]
- 34.Huang J F, Zeng R H, Chen J L. Thermostable carbon-supported subnanometer-sized (< 1 nm) Pt clusters for the hydrogen evolution reaction. Journal of Materials Chemistry A. 2021;9(38):21972–21980. doi: 10.1039/D1TA06189E. [DOI] [Google Scholar]
- 35.Sun Y, Li X, Wang J, Ning W, Fu J, Lu X, Hou Z. Carbon film encapsulated Pt NPs for selective oxidation of alcohols in acidic aqueous solution. Applied Catalysis B: Environmental. 2017;218:538–544. doi: 10.1016/j.apcatb.2017.06.086. [DOI] [Google Scholar]
- 36.Shen L, Ying J, Ozoemena K I, Janiak C, Yang X Y. Confinement effects in individual carbon encapsulated nonprecious metal-based electrocatalysts. Advanced Functional Materials. 2022;32(15):2110851. doi: 10.1002/adfm.202110851. [DOI] [Google Scholar]
- 37.Geng W, Wang L, Yang X Y. Nanocell hybrids for green chemistry. Trends in Biotechnology. 2022;40(8):974–986. doi: 10.1016/j.tibtech.2022.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Shao Y, Mei S, Lu Y, Zhang M, Sun J K, Matyjaszewski K, Antonietti M, Yuan J. Polymer-derived heteroatom-doped porous carbon materials. Chemical Reviews. 2020;120(17):9363–9419. doi: 10.1021/acs.chemrev.0c00080. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Z, Liu T, Khan A U, Liu G. Block copolymer-based porous carbon fibers. Science Advances. 2019;5(2):eaau6852. doi: 10.1126/sciadv.aau6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G, Li X, Guan Z, Tong Y, Xu B, Wang X, Wang Z, Chen L. Insights into lithium and sodium storage in porous carbon. Nano Letters. 2020;20(5):3836–3843. doi: 10.1021/acs.nanolett.0c00943. [DOI] [PubMed] [Google Scholar]
- 41.Yin J, Zhang W, Alhebshi N A, Salah N, Alshareef H N. Synthesis strategies of porous carbon for supercapacitor applications. Small Methods. 2020;4(3):1900853. doi: 10.1002/smtd.201900853. [DOI] [Google Scholar]
- 42.Stadie N P, Wang S, Kravchyk K V, Kovalenko M V. Zeolite-templated carbon as an ordered microporous electrode for aluminum batteries. ACS Nano. 2017;11(2):1911–1919. doi: 10.1021/acsnano.6b07995. [DOI] [PubMed] [Google Scholar]
- 43.Sazama P, Pastvova J, Rizescu C, Tirsoaga A, Parvulescu V I, Garcia H, Kobera L, Seidel J, Rathousky J, Klein P, Jirka I, Moravkova J, Blechta V. Catalytic properties of 3D graphene-like microporous carbons synthesized in a zeolite template. ACS Catalysis. 2018;8(3):1779–1789. doi: 10.1021/acscatal.7b04086. [DOI] [Google Scholar]
- 44.Kim K, Lee T, Kwon Y, Seo Y, Song J, Park J K, Lee H, Park J Y, Ihee H, Cho S J, Ryoo R. Lanthanum-catalysed synthesis of microporous 3D graphene-like carbons in a zeolite template. Nature. 2016;535(7610):131–135. doi: 10.1038/nature18284. [DOI] [PubMed] [Google Scholar]
- 45.Xia Y, Mokaya R, Walker G S, Zhu Y. Superior CO2 asorption cpacity on N-doped, high-surface-area, microporous carbons templated from zeolite. Advanced Energy Materials. 2011;1(4):678–683. doi: 10.1002/aenm.201100061. [DOI] [Google Scholar]
- 46.Bae S E, Kim K J, Choi I H, Huh S. Preparation of N-doped microporous carbon nanospheres by direct carbonization of as-prepared mesoporous silica nanospheres containing cetylpyridinium bromide template. Carbon. 2016;99:8–16. doi: 10.1016/j.carbon.2015.11.069. [DOI] [Google Scholar]
- 47.Li K, Tian S, Jiang J, Wang J, Chen X, Yan F. Pine cone shell-based activated carbon used for CO2 adsorption. Journal of Materials Chemistry A. 2016;4(14):5223–5234. doi: 10.1039/C5TA09908K. [DOI] [Google Scholar]
- 48.Zhou J, Li Z, Xing W, Shen H, Bi X, Zhu T, Qiu Z, Zhuo S. A new approach to tuning carbon ultramicropore size at sub-angstrom level for maximizing specific capacitance and CO2 uptake. Advanced Functional Materials. 2016;26(44):7955–7964. doi: 10.1002/adfm.201601904. [DOI] [Google Scholar]
- 49.Blankenship L S, Balahmar N, Mokaya R. Oxygen-rich microporous carbons with exceptional hydrogen storage capacity. Nature Communications. 2017;8(1):1–12. doi: 10.1038/s41467-017-01633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Noonan O, Huang X, Yang Y, Xu C, Zhou L, Yu C. Surfactant-free assembly of mesoporous carbon hollow spheres with large tunable pore sizes. ACS Nano. 2016;10(4):4579–4586. doi: 10.1021/acsnano.6b00723. [DOI] [PubMed] [Google Scholar]
- 51.Phan T N, Gong M K, Thangavel R, Lee Y S, Ko C H. Enhanced electrochemical performance for EDLC using ordered mesoporous carbons (CMK-3 and CMK-8): role of mesopores and mesopore structures. Journal of Alloys and Compounds. 2019;780:90–97. doi: 10.1016/j.jallcom.2018.11.348. [DOI] [Google Scholar]
- 52.Zhou Y, Candelaria S L, Liu Q, Uchaker E, Cao G. Porous carbon with high capacitance and graphitization through controlled addition and removal of sulfur-containing compounds. Nano Energy. 2015;12:567–577. doi: 10.1016/j.nanoen.2015.01.026. [DOI] [Google Scholar]
- 53.Feng S, Li W, Wang J, Song Y, Elzatahry A A, Xia Y, Zhao D. Hydrothermal synthesis of ordered mesoporous carbons from a biomass-derived precursor for electrochemical capacitors. Nanoscale. 2014;6(24):14657–14661. doi: 10.1039/C4NR05629A. [DOI] [PubMed] [Google Scholar]
- 54.Wang J G, Liu H, Sun H, Hua W, Wang H, Liu X, Wei B. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors. Carbon. 2018;127:85–92. doi: 10.1016/j.carbon.2017.10.084. [DOI] [Google Scholar]
- 55.Peng L, Hung C T, Wang S, Zhang X, Zhu X, Zhao Z, Wang C, Tang Y, Li W, Zhao D. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. Journal of the American Chemical Society. 2019;141(17):7073–7080. doi: 10.1021/jacs.9b02091. [DOI] [PubMed] [Google Scholar]
- 56.Ferrero G A, Fuertes A B, Sevilla M, Titirici M M. Efficient metal-free N-doped mesoporous carbon catalysts for ORR by a template-free approach. Carbon. 2016;106:179–187. doi: 10.1016/j.carbon.2016.04.080. [DOI] [Google Scholar]
- 57.Qian Y, Jiang S, Li Y, Yi Z, Zhou J, Tian J, Lin N, Qian Y. Understanding mesopore volume-enhanced extra-capacity: optimizing mesoporous carbon for high-rate and long-life potassium-storage. Energy Storage Materials. 2020;29:341–349. doi: 10.1016/j.ensm.2020.04.026. [DOI] [Google Scholar]
- 58.Hu X, Liu Y, Chen J, Jia J, Zhan H, Wen Z. FeS quantum dots embedded in 3D ordered macroporous carbon nanocomposite for high-performance sodium-ion hybrid capacitors. Journal of Materials Chemistry A. 2019;7(3):1138–1148. doi: 10.1039/C8TA10468A. [DOI] [Google Scholar]
- 59.Li X, Fan L, Xu B, Shang Y, Li M, Zhang L, Liu S, Kang Z, Liu Z, Lu X, Sun D. Single-atom-like B-N3 sites in ordered macroporous carbon for efficient oxygen reduction reaction. ACS Applied Materials & Interfaces. 2021;13(45):53892–53903. doi: 10.1021/acsami.1c15661. [DOI] [PubMed] [Google Scholar]
- 60.Fang Z, Fernandez D, Wang N, Bai Z, Yu G. Mo2C@3D ultrathin macroporous carbon realizing efficient and stable nitrogen fixation. Science China Chemistry. 2020;63(11):1570–1577. doi: 10.1007/s11426-020-9740-8. [DOI] [Google Scholar]
- 61.Wang J, Yao Y, Zhang C, Sun Q, Cheng D, Huang X, Feng J, Wan J, Zou J, Liu C, Yu C. Superstructured macroporous carbon rods composed of defective graphitic nanosheets for efficient oxygen reduction reaction. Advanced Science. 2021;8(18):2100120. doi: 10.1002/advs.202100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balgis R, Widiyastuti W, Ogi T, Okuyama K. Enhanced electrocatalytic activity of Pt/3D hierarchical bimodal macroporous carbon nanospheres. ACS Applied Materials & Interfaces. 2017;9(28):23792–23799. doi: 10.1021/acsami.7b05873. [DOI] [PubMed] [Google Scholar]
- 63.Li J, Zhang N, Zhao H, Li Z, Tian B, Du Y. Cornstalk-derived macroporous carbon materials with enhanced microwave absorption. Journal of Materials Science Materials in Electronics. 2021;32(21):25758–25768. doi: 10.1007/s10854-020-04571-5. [DOI] [Google Scholar]
- 64.Meng T, Shang N, Zhao J, Su M, Wang C, Zhang Y. Facile one-pot synthesis of Co coordination polymer spheres doped macroporous carbon and its application for electrocatalytic oxidation of glucose. Journal of Colloid and Interface Science. 2021;589:135–146. doi: 10.1016/j.jcis.2020.12.119. [DOI] [PubMed] [Google Scholar]
- 65.Qu Y, Zan G, Wang J, Wu Q. Preparation of eggplant-derived macroporous carbon tubes and composites of EDMCT/Co (OH)(CO3)0.5 nano-cone-arrays for high-performance supercapacitors. Journal of Materials Chemistry A. 2016;4(11):4296–4304. doi: 10.1039/C5TA09948J. [DOI] [Google Scholar]
- 66.Dong S, Yang Z, Liu B, Zhang J, Xu P, Xiang M, Lu T. (Pd, Au, Ag) nanoparticles decorated well-ordered macroporous carbon for electrochemical sensing applications. Journal of Electroanalytical Chemistry. 2021;897:115562. doi: 10.1016/j.jelechem.2021.115562. [DOI] [Google Scholar]
- 67.Wang H, Yang D, Liu S, Yin S, Yu H, Xu Y, Li X, Wang Z, Wang L. Cage-bell structured Pt@N-doped hollow carbon sphere for oxygen reduction electrocatalysis. Chemical Engineering Journal. 2021;409:128101. doi: 10.1016/j.cej.2020.128101. [DOI] [Google Scholar]
- 68.Hu D, Fan W, Liu Z, Li L. Three-dimensionally hierarchical Pt/C nanocomposite with ultra-high dispersion of Pt nanoparticles as a highly efficient catalyst for chemoselective cinnamaldehyde hydrogenation. ChemCatChem. 2018;10(4):779–788. doi: 10.1002/cctc.201701301. [DOI] [Google Scholar]
- 69.Eftekhari A, Fan Z. Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion. Materials Chemistry Frontiers. 2017;1(6):1001–1027. doi: 10.1039/C6QM00298F. [DOI] [Google Scholar]
- 70.Wu S M, Beller M, Yang X Y. A clear view of zeolite-catalyzed processes. Matter. 2022;5(10):3104–3107. doi: 10.1016/j.matt.2022.09.006. [DOI] [Google Scholar]
- 71.Zhou X L, Zhang H, Shao L M, Lü F, He P J. Preparation and application of hierarchical porous carbon materials from waste and biomass: a review. Waste and Biomass Valorization. 2021;12(4):1699–1724. doi: 10.1007/s12649-020-01109-y. [DOI] [Google Scholar]
- 72.Wan X K, Wu H B, Guan B Y, Luan D, Lou X W. Confining sub-nanometer Pt clusters in hollow mesoporous carbon spheres for boosting hydrogen evolution activity. Advanced Materials. 2020;32(7):1901349. doi: 10.1002/adma.201901349. [DOI] [PubMed] [Google Scholar]
- 73.Kuang P, Wang Y, Zhu B, Xia F, Tung C W, Wu J, Chen H M, Yu J. Pt single atoms supported on N-doped mesoporous hollow carbon spheres with enhanced electrocatalytic H2-evolution activity. Advanced Materials. 2021;33(18):2008599. doi: 10.1002/adma.202008599. [DOI] [PubMed] [Google Scholar]
- 74.Ying J, Yang X Y, Hu Z Y, Mu S C, Janiak C, Geng W, Pan M, Ke X, Van Tendeloo G, Su B L. One particle@one cell: highly monodispersed PtPd bimetallic nanoparticles for enhanced oxygen reduction reaction. Nano Energy. 2014;8:214–222. doi: 10.1016/j.nanoen.2014.06.010. [DOI] [Google Scholar]
- 75.Ying J, Hu Z Y, Yang X Y, Wei H, Xiao Y X, Janiak C, Mu S C, Tian G, Pan M, Van Tendeloo G, Su B L. High viscosity to highly dispersed PtPd bimetallic nanocrystals for enhanced catalytic activity and stability. Chemical Communications. 2016;52(53):8219–8222. doi: 10.1039/C6CC00912C. [DOI] [PubMed] [Google Scholar]
- 76.Yu F, Bai X, Liang M, Ma J. Recent progress on metal-organic framework-derived porous carbon and its composite for pollutant adsorption from liquid phase. Chemical Engineering Journal. 2021;405:126960. doi: 10.1016/j.cej.2020.126960. [DOI] [Google Scholar]
- 77.Liu B, Shioyama H, Akita T, Xu Q. Metal-organic framework as a template for porous carbon synthesis. Journal of the American Chemical Society. 2008;130(16):5390–5391. doi: 10.1021/ja7106146. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L, Fischer J M T A, Jia Y, Yan X, Xu W, Wang X, Chen J, Yang D, Liu H, Zhuang L, Hankel M, Searles D J, Huang K, Feng S, Brown C L, Yao X. Coordination of atomic Co–Pt coupling species at carbon defects as active sites for oxygen reduction reaction. Journal of the American Chemical Society. 2018;140(34):10757–10763. doi: 10.1021/jacs.8b04647. [DOI] [PubMed] [Google Scholar]
- 79.Wu X Q, Zhao J, Wu Y P, Dong W W, Li D S, Li J R, Zhang Q. Ultrafine Pt nanoparticles and amorphous nickel supported on 3D mesoporous carbon derived from Cu-metal–organic framework for efficient methanol oxidation and nitrophenol reduction. ACS Applied Materials & Interfaces. 2018;10(15):12740–12749. doi: 10.1021/acsami.8b01970. [DOI] [PubMed] [Google Scholar]
- 80.Ying J, Jiang G, Cano Z P, Han L, Yang X Y, Chen Z. Nitrogen-doped hollow porous carbon polyhedrons embedded with highly dispersed Pt nanoparticles as a highly efficient and stable hydrogen evolution electrocatalyst. Nano Energy. 2017;40:88–94. doi: 10.1016/j.nanoen.2017.07.032. [DOI] [Google Scholar]
- 81.Ying J, Li J, Jiang G, Cano Z P, Ma Z, Zhong C, Su D, Chen Z. Metal-organic frameworks derived platinum-cobalt bimetallic nanoparticles in nitrogen-doped hollow porous carbon capsules as a highly active and durable catalyst for oxygen reduction reaction. Applied Catalysis B: Environmental. 2018;225:496–503. doi: 10.1016/j.apcatb.2017.11.077. [DOI] [Google Scholar]
- 82.Liu H, Wu S, Tian N, Yan F, You C, Yang Y. Carbon foams: 3D porous carbon materials holding immense potential. Journal of Materials Chemistry A. 2020;8(45):23699–23723. doi: 10.1039/D0TA08749A. [DOI] [Google Scholar]
- 83.Zhang W, Minett A I, Gao M, Zhao J, Razal J M, Wallace G G, Romeo T, Chen J. Integrated high-efficiency Pt/carbon nanotube arrays for PEM fuel cells. Advanced Energy Materials. 2011;1(4):671–677. doi: 10.1002/aenm.201100092. [DOI] [Google Scholar]
- 84.Chen H, Liu T, Ren J, He H, Cao Y, Wang N, Guo Z. Synergistic carbon nanotube aerogel-Pt nanocomposites toward enhanced energy conversion in dye-sensitized solar cells. Journal of Materials Chemistry A. 2016;4(9):3238–3244. doi: 10.1039/C5TA10185A. [DOI] [Google Scholar]
- 85.Ye J, Zhou M, Le Y, Cheng B, Yu J. Three-dimensional carbon foam supported MnO2/Pt for rapid capture and catalytic oxidation of formaldehyde at room temperature. Applied Catalysis B: Environmental. 2020;267:118689. doi: 10.1016/j.apcatb.2020.118689. [DOI] [Google Scholar]
- 86.Atwa M, Li X, Wang Z, Dull S, Xu S, Tong X, Tang R, Nishihara H, Prinz F, Birss V. Scalable nanoporous carbon films allow line-of-sight 3D atomic layer deposition of Pt: towards a new generation catalyst layer for PEM fuel cells. Materials Horizons. 2021;8(9):2451–2462. doi: 10.1039/D1MH00268F. [DOI] [PubMed] [Google Scholar]
- 87.Cherevko S, Kulyk N, Mayrhofer K J. Durability of platinum-based fuel cell electrocatalysts: dissolution of bulk and nanoscale platinum. Nano Energy. 2016;29:275–298. doi: 10.1016/j.nanoen.2016.03.005. [DOI] [Google Scholar]
- 88.Perini L, Durante C, Favaro M, Perazzolo V, Agnoli S, Schneider O, Granozzi G, Gennaro A. Metal-support interaction in platinum and palladium nanoparticles loaded on nitrogen-doped mesoporous carbon for oxygen reduction reaction. ACS Applied Materials & Interfaces. 2015;7(2):1170–1179. doi: 10.1021/am506916y. [DOI] [PubMed] [Google Scholar]
- 89.Lai Q, Zheng J, Tang Z, Bi D, Zhao J, Liang Y. Optimal configuration of N-doped carbon defects in 2D turbostratic carbon nanomesh for advanced oxygen reduction electrocatalysis. Angewandte Chemie International Edition. 2020;59(29):11999–12006. doi: 10.1002/anie.202000936. [DOI] [PubMed] [Google Scholar]
- 90.Ning X, Li Y, Ming J, Wang Q, Wang H, Cao Y, Peng F, Yang Y, Yu H. Electronic synergism of pyridinic- and graphitic-nitrogen on N-doped carbons for the oxygen reduction reaction. Chemical Science. 2019;10(6):1589–1596. doi: 10.1039/C8SC04596H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li J, Yang X Y. Membrane catalysts eliminate trace pollutants. Chem. 2022;8(6):1551–1553. doi: 10.1016/j.chempr.2022.05.007. [DOI] [Google Scholar]
- 92.Ning X, Yu H, Peng F, Wang H. Pt nanoparticles interacting with graphitic nitrogen of N-doped carbon nanotubes: effect of electronic properties on activity for aerobic oxidation of glycerol and electro-oxidation of CO. Journal of Catalysis. 2015;325:136–144. doi: 10.1016/j.jcat.2015.02.010. [DOI] [Google Scholar]
- 93.Xiao Y X, Ying J, Chen J B, Dong Y, Yang X, Tian G, Wu J, Janiak C, Ozoemena K I, Yang X Y. Confined ultrafine Pt in porous carbon fibers and their N-enhanced heavy d−π effect. Chemistry of Materials. 2022;34(8):3705–3714. doi: 10.1021/acs.chemmater.1c04400. [DOI] [Google Scholar]
- 94.Bulushev D A, Zacharska M, Lisitsyn A S, Podyacheva O Y, Hage F S, Ramasse Q M, Bangert U, Bulusheva L G. Single atoms of Pt-group metals stabilized by N-doped carbon nanofibers for efficient hydrogen production from formic acid. ACS Catalysis. 2016;6(6):3442–3451. doi: 10.1021/acscatal.6b00476. [DOI] [Google Scholar]
- 95.Luo H, Liu Y, Dimitrov S D, Steier L, Guo S, Li X, Feng J, Xie F, Fang Y, Sapelkin A, Wang X, Titirici M M. Pt single-atoms supported on nitrogen-doped carbon dots for highly efficient photocatalytic hydrogen generation. Journal of Materials Chemistry A. 2020;8(29):14690–14696. doi: 10.1039/D0TA04431H. [DOI] [Google Scholar]
- 96.Schmies H, Hornberger E, Anke B, Jurzinsky T, Nong H N, Dionigi F, Kühl S, Drnec J, Lerch M, Cremers C, Strasser P. Impact of carbon support functionalization on the electrochemical stability of Pt fuel cell catalysts. Chemistry of Materials. 2018;30(20):7287–7295. doi: 10.1021/acs.chemmater.8b03612. [DOI] [Google Scholar]
- 97.Hornberger E, Merzdorf T, Schmies H, Hübner J, Klingenhof M, Gernert U, Kroschel M, Anke B, Lerch M, Schmidt J, Thomas A, Chattot R, Martens I, Drnec J, Strasser P. Impact of carbon N-doping and pyridinic-N content on the fuel cell performance and durability of carbon-supported Pt nanoparticle catalysts. ACS Applied Materials & Interfaces. 2022;14(16):18420–18430. doi: 10.1021/acsami.2c00762. [DOI] [PubMed] [Google Scholar]
- 98.Duan J, Chen S, Jaroniec M, Qiao S Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catalysis. 2015;5(9):5207–5234. doi: 10.1021/acscatal.5b00991. [DOI] [Google Scholar]
- 99.Sawant S V, Patwardhan A W, Joshi J B, Dasgupta K. Boron doped carbon nanotubes: synthesis, characterization and emerging applications—a review. Chemical Engineering Journal. 2022;427:131616. doi: 10.1016/j.cej.2021.131616. [DOI] [Google Scholar]
- 100.Kang Y, Tang Y, Zhu L, Jiang B, Xu X, Guselnikova O, Li H, Asahi T, Yamauchi Y. Porous nanoarchitectures of nonprecious metal borides: from controlled synthesis to heterogeneous catalyst applications. ACS Catalysis. 2022;12(23):14773–14793. doi: 10.1021/acscatal.2c03480. [DOI] [Google Scholar]
- 101.Hu M, Yao Z, Li L, Tsou Y H, Kuang L, Xu X, Zhang W, Wang X. Boron-doped graphene nanosheet-supported Pt: a highly active and selective catalyst for low temperature H2-SCR. Nanoscale. 2018;10(21):10203–10212. doi: 10.1039/C8NR01807C. [DOI] [PubMed] [Google Scholar]
- 102.Yao R, Gu J, He H, Yu T. Improved electrocatalytic activity and durability of Pt nanoparticles supported on boron-doped carbon black. Catalysts. 2020;10(8):862. doi: 10.3390/catal10080862. [DOI] [Google Scholar]
- 103.Samad S, Loh K S, Wong W Y, Lee T K, Sunarso J, Chong S T, Daud W R W. Carbon and non-carbon support materials for platinum-based catalysts in fuel cells. International Journal of Hydrogen Energy. 2018;43(16):7823–7854. doi: 10.1016/j.ijhydene.2018.02.154. [DOI] [Google Scholar]
- 104.Kwon K, Jin S A, Pak C, Chang H, Joo S H, Lee H I, Kim J H, Kim J M. Enhancement of electrochemical stability and catalytic activity of Pt nanoparticles via strong metal-support interaction with sulfur-containing ordered mesoporous carbon. Catalysis Today. 2011;164(1):186–189. doi: 10.1016/j.cattod.2010.10.030. [DOI] [Google Scholar]
- 105.Guo Y, Park T, Yi J W, Henzie J, Kim J, Wang Z, Jiang B, Bando Y, Sugahara Y, Tang J, Yamauchi Y. Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting. Advanced Materials. 2019;31(17):1807134. doi: 10.1002/adma.201807134. [DOI] [PubMed] [Google Scholar]
- 106.Higgins D, Hoque M A, Seo M H, Wang R, Hassan F, Choi J Y, Pritzker M Y A, Zhang J, Chen Z. Development and simulation of sulfur-doped graphene supported platinum with exemplary stability and activity towards oxygen reduction. Advanced Functional Materials. 2014;24(27):4325–4336. doi: 10.1002/adfm.201400161. [DOI] [Google Scholar]
- 107.Hoque M A, Hassan F M, Higgins D, Choi J Y, Pritzker M, Knights S, Ye S, Chen Z. Multigrain platinum nanowires consisting of oriented nanoparticles anchored on sulfur-doped graphene as a highly active and durable oxygen reduction electrocatalyst. Advanced Materials. 2015;27(7):1229–1234. doi: 10.1002/adma.201404426. [DOI] [PubMed] [Google Scholar]
- 108.Hoque M A, Hassan F M, Seo M H, Choi J Y, Pritzker M, Knights S, Ye S, Chen Z. Optimization of sulfur-doped graphene as an emerging platinum nanowires support for oxygen reduction reaction. Nano Energy. 2016;19:27–38. doi: 10.1016/j.nanoen.2015.11.004. [DOI] [Google Scholar]
- 109.Hoque M A, Hassan F M, Jauhar A M, Jiang G, Pritzker M, Choi J Y, Knights S, Ye S, Chen Z. Web-like 3D architecture of Pt nanowires and sulfur-doped carbon nanotube with superior electrocatalytic performance. ACS Sustainable Chemistry & Engineering. 2018;6(1):93–98. doi: 10.1021/acssuschemeng.7b03580. [DOI] [Google Scholar]
- 110.Xu C, Hoque M A, Chiu G, Sung T, Chen Z. Stabilization of platinum-nickel alloy nanoparticles with a sulfur-doped graphene support in polymer electrolyte membrane fuel cells. RSC Advances. 2016;6(113):112226–112231. doi: 10.1039/C6RA19924K. [DOI] [Google Scholar]
- 111.Fan J J, Fan Y J, Wang R X, Xiang S, Tang H G, Sun S G. A novel strategy for the synthesis of sulfur-doped carbon nanotubes as a highly efficient Pt catalyst support toward the methanol oxidation reaction. Journal of Materials Chemistry A. 2017;5(36):19467–19475. doi: 10.1039/C7TA05102F. [DOI] [Google Scholar]
- 112.Kiciński W, Szala M, Bystrzejewski M. Sulfur-doped porous carbons: synthesis and applications. Carbon. 2014;68:1–32. doi: 10.1016/j.carbon.2013.11.004. [DOI] [Google Scholar]
- 113.Kang Y, Guo Y, Zhao J, Jiang B, Guo J, Tang Y, Li H, Malgras V, Amin M A, Nara H, Sugahara Y, Yamauchi Y, Asahi T. Soft template-based synthesis of mesoporous phosphorus-and boron-codoped NiFe-based alloys for efficient oxygen evolution reaction. Small. 2022;18(31):2203411. doi: 10.1002/smll.202203411. [DOI] [PubMed] [Google Scholar]
- 114.Li Z, Lin J, Li B, Yu C, Wang H, Li Q. Construction of heteroatom-doped and three-dimensional graphene materials for the applications in supercapacitors: a review. Journal of Energy Storage. 2021;44:103437. doi: 10.1016/j.est.2021.103437. [DOI] [Google Scholar]
- 115.Liang J, Jiao Y, Jaroniec M, Qiao S Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angewandte Chemie International Edition. 2012;51(46):11496–11500. doi: 10.1002/anie.201206720. [DOI] [PubMed] [Google Scholar]
- 116.Shin D Y, Sung K W, Ahn H J. Synergistic effect of heteroatomdoped activated carbon for ultrafast charge storage kinetics. Applied Surface Science. 2019;478:499–504. doi: 10.1016/j.apsusc.2019.01.186. [DOI] [Google Scholar]
- 117.Chang Y, Hong F, Liu J, Xie M, Zhang Q, He C, Niu H, Liu J. Nitrogen/sulfur dual-doped mesoporous carbon with controllable morphology as a catalyst support for the methanol oxidation reaction. Carbon. 2015;87:424–433. doi: 10.1016/j.carbon.2015.02.063. [DOI] [Google Scholar]
- 118.Zhu J, He G, Tian Z, Liang L, Shen P K. Facile synthesis of boron and nitrogen-dual-doped graphene sheets anchored platinum nanoparticles for oxygen reduction reaction. Electrochimica Acta. 2016;194:276–282. doi: 10.1016/j.electacta.2016.01.222. [DOI] [Google Scholar]
- 119.Paul R, Du F, Dai L, Ding Y, Wang Z L, Wei F, Roy A. 3D heteroatom-doped carbon nanomaterials as multifunctional metal-free catalysts for integrated energy devices. Advanced Materials. 2019;31(13):1805598. doi: 10.1002/adma.201805598. [DOI] [PubMed] [Google Scholar]
- 120.Yang Z, Tian J, Yin Z, Cui C, Qian W, Wei F. Carbon nanotube-and graphene-based nanomaterials and applications in high-voltage supercapacitor: a review. Carbon. 2019;141:467–480. doi: 10.1016/j.carbon.2018.10.010. [DOI] [Google Scholar]
- 121.Fan S S, Shen L, Dong Y, Tian G, Wu S M, Chang G G, Janiak C, Wei P, Wu J S, Yang X Y. sp3-like defect structure of hetero graphene-carbon nanotubes for promoting carrier transfer and stability. Journal of Energy Chemistry. 2021;57:189–197. doi: 10.1016/j.jechem.2020.09.020. [DOI] [Google Scholar]
- 122.Ji S G, Kwon H C, Kim T H, Sim U, Choi C H. Does the encapsulation strategy of Pt nanoparticles with carbon layers really ensure both highly active and durable electrocatalysis in fuel cells? ACS Catalysis. 2022;12(12):7317–7325. doi: 10.1021/acscatal.2c01618. [DOI] [Google Scholar]
- 123.Tong X, Zhang J, Zhang G, Wei Q, Chenitz R, Claverie J P, Sun S. Ultrathin carbon-coated Pt/carbon nanotubes: a highly durable electrocatalyst for oxygen reduction. Chemistry of Materials. 2017;29(21):9579–9587. doi: 10.1021/acs.chemmater.7b04221. [DOI] [Google Scholar]
- 124.Karuppannan M, Kim Y, Gok S, Lee E, Hwang J Y, Jang J H, Cho Y H, Lim T, Sung Y E, Kwon O J. A highly durable carbon-nanofiber-supported Pt–C core-shell cathode catalyst for ultra-low Pt loading proton exchange membrane fuel cells: facile carbon encapsulation. Energy & Environmental Science. 2019;12(9):2820–2829. doi: 10.1039/C9EE01000A. [DOI] [Google Scholar]
- 125.Xiao H, Xue S, Zhang J, Zhao M, Ma J, Chen S, Zheng Z, Jia J, Wu H. Facile electrolytic synthesis of Pt and carbon quantum dots coloaded multiwall carbon nanotube as highly efficient electrocatalyst for hydrogen evolution and ethanol oxidation. Chemical Engineering Journal. 2021;408:127271. doi: 10.1016/j.cej.2020.127271. [DOI] [Google Scholar]
- 126.Dang Q, Sun Y, Wang X, Zhu W, Chen Y, Liao F, Huang H, Shao M. Carbon dots-Pt modified polyaniline nanosheet grown on carbon cloth as stable and high-efficient electrocatalyst for hydrogen evolution in pH-universal electrolyte. Applied Catalysis B: Environmental. 2019;257:117905. doi: 10.1016/j.apcatb.2019.117905. [DOI] [Google Scholar]
- 127.Xiao H, Zhang J, Zhao M, Ma J, Li Y, Hu T, Zheng Z, Jia J, Wu H. Electric field-assisted synthesis of Pt, carbon quantum dots-coloaded graphene hybrid for hydrogen evolution reaction. Journal of Power Sources. 2020;451:227770. doi: 10.1016/j.jpowsour.2020.227770. [DOI] [Google Scholar]
- 128.Yan M, Jiang Q, Zhang T, Wang J, Yang L, Lu Z, He H, Fu Y, Wang X, Huang H. Three-dimensional low-defect carbon nanotube/nitrogen-doped graphene hybrid aerogel-supported Pt nanoparticles as efficient electrocatalysts toward the methanol oxidation reaction. Journal of Materials Chemistry A. 2018;6(37):18165–18172. doi: 10.1039/C8TA05124K. [DOI] [Google Scholar]
- 129.Sun Y, Li M, Qu X, Zheng S, Alvarez P J, Fu H. Efficient reduction of selenite to elemental selenium by liquid-phase catalytic hydrogenation using a highly stable multiwalled carbon nanotube-supported Pt catalyst coated by N-doped carbon. ACS Applied Materials & Interfaces. 2021;13(25):29541–29550. doi: 10.1021/acsami.1c05101. [DOI] [PubMed] [Google Scholar]
- 130.Sharma S, Pollet B G. Support materials for PEMFC and DMFC electrocatalysts—a review. Journal of Power Sources. 2012;208:96–119. doi: 10.1016/j.jpowsour.2012.02.011. [DOI] [Google Scholar]
- 131.Hu G, Xiao Y, Ying J. Nano-SiO2 and silane coupling agent co-decorated graphene oxides with enhanced anti-corrosion performance of epoxy composite coatings. International Journal of Molecular Sciences. 2021;22(20):11087. doi: 10.3390/ijms222011087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen J, Ying J, Xiao Y, Dong Y, Ozoemena K I, Lenaerts S, Yang X. Stoichiometry design in hierarchical CoNiFe phosphide for highly efficient water oxidation. Science China Materials. 2022;65(10):1–9. doi: 10.1007/s40843-021-1809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park K, Ohnishi T, Goto M, So M, Takenaka S, Tsuge Y, Inoue G. Improvement of cell performance in catalyst layers with silica-coated Pt/carbon catalysts for polymer electrolyte fuel cells. International Journal of Hydrogen Energy. 2020;45(3):1867–1877. doi: 10.1016/j.ijhydene.2019.11.097. [DOI] [Google Scholar]
- 134.Islam J, Kim S K, Kim K H, Lee E, Park G G. Enhanced durability of Pt/C catalyst by coating carbon black with silica for oxygen reduction reaction. International Journal of Hydrogen Energy. 2021;46(1):1133–1143. doi: 10.1016/j.ijhydene.2020.09.194. [DOI] [Google Scholar]
- 135.Barbosa E C, Parreira L S, de Freitas I C, Aveiro L R, de Oliveira D C, dos Santos M C, Camargo P H C. Pt-decorated TiO2 materials supported on carbon: increasing activities and stabilities toward the ORR by tuning the Pt loading. ACS Applied Energy Materials. 2019;2(8):5759–5768. doi: 10.1021/acsaem.9b00879. [DOI] [Google Scholar]
- 136.Lee W J, Bera S, Woo H, Kim H G, Baek J H, Hong W, Park J Y, Oh S J, Kwon S H. In situ engineering of a metal oxide protective layer into Pt/carbon fuel-cell catalysts by atomic layer deposition. Chemistry of Materials. 2022;34(13):5949–5959. doi: 10.1021/acs.chemmater.2c00928. [DOI] [Google Scholar]
- 137.de Oliveira D C, Silva W O, Chatenet M, Lima F H B. Pt/C nanocomposites: highly active electrocatalysts for the electrochemical oxidation of hydrazine. Applied Catalysis B: Environmental. 2017;201:22–28. doi: 10.1016/j.apcatb.2016.08.007. [DOI] [Google Scholar]
- 138.Gu B, Sun T, Wang Y, Long Y, Fu J, Fan G. Maximizing hydrogen production by AB hydrolysis with Pt@cobalt oxide/N, O-rich carbon and alkaline ultrasonic irradiation. Inorganic Chemistry Frontiers. 2022;9(10):2204–2212. doi: 10.1039/D1QI01629F. [DOI] [Google Scholar]
- 139.Song Z, Wang B, Cheng N, Yang L, Banham D, Li R, Ye S, Sun X. Atomic layer deposited tantalum oxide to anchor Pt/C for a highly stable catalyst in PEMFCs. Journal of Materials Chemistry A. 2017;5(20):9760–9767. doi: 10.1039/C7TA01926B. [DOI] [Google Scholar]
- 140.Ma Z, Li S, Wu L, Song L, Jiang G, Liang Z, Su D, Zhu Y, Adzic R R, Wang J X, Chen Z. NbOx nano-nail with a Pt head embedded in carbon as a highly active and durable oxygen reduction catalyst. Nano Energy. 2020;69:104455. doi: 10.1016/j.nanoen.2020.104455. [DOI] [Google Scholar]
- 141.He D, Zeng C, Xu C, Cheng N, Li H, Mu S, Pan M. Polyaniline-functionalized carbon nanotube supported platinum catalysts. Langmuir. 2011;27(9):5582–5588. doi: 10.1021/la2003589. [DOI] [PubMed] [Google Scholar]
- 142.Wei L, Fan Y J, Ma J H, Tao L H, Wang R X, Zhong J P, Wang H. Highly dispersed Pt nanoparticles supported on manganese oxide-poly(3,4-ethylenedioxythiophene)-carbon nanotubes composite for enhanced methanol electrooxidation. Journal of Power Sources. 2013;238:157–164. doi: 10.1016/j.jpowsour.2013.03.051. [DOI] [Google Scholar]
- 143.Wang R X, Fan J J, Fan Y J, Zhong J P, Wang L, Sun S G, Shen X C. Platinum nanoparticles on porphyrin functionalized graphene nanosheets as a superior catalyst for methanol electrooxidation. Nanoscale. 2014;6(24):14999–15007. doi: 10.1039/C4NR04140B. [DOI] [PubMed] [Google Scholar]
- 144.Wang R X, Fan Y J, Wang L, Wu L N, Sun S N, Sun S G. Pt nanocatalysts on a polyindole-functionalized carbon nanotube composite with high performance for methanol electrooxidation. Journal of Power Sources. 2015;287:341–348. doi: 10.1016/j.jpowsour.2015.03.181. [DOI] [Google Scholar]
- 145.Eßbach C, Senkovska I, Unmüssig T, Fischer A, Kaskel S. Selective alcohol electrooxidation by ZIF-8 functionalized Pt/carbon catalyst. ACS Applied Materials & Interfaces. 2019;11(23):20915–20922. doi: 10.1021/acsami.9b06122. [DOI] [PubMed] [Google Scholar]
- 146.Choi J, Lee Y J, Park D, Jeong H, Shin S, Yun H, Lim J, Han J, Kim E J, Jeon S S, Jung Y, Lee H, Kim B J. Highly durable fuel cell catalysts using crosslinkable block copolymer-based carbon supports with ultralow Pt loadings. Energy & Environmental Science. 2020;13(12):4921–4929. doi: 10.1039/D0EE01095B. [DOI] [Google Scholar]
- 147.Xiao Y X, Ying J, Tian G, Tao Y, Wei H, Fan S Y, Sun Z H, Zou W J, Hu J, Chang G G, Li W, Yang X Y, Janiak C. Highly dispersed PtPd on graphitic nanofibers and its heavy d-π effect. Applied Catalysis B: Environmental. 2019;259:118080. doi: 10.1016/j.apcatb.2019.118080. [DOI] [Google Scholar]
- 148.Dong Y, Li J, Yang X Y. Cu catalysts detour hydrogen evolution reaction. Matter. 2022;5(8):2537–2540. doi: 10.1016/j.matt.2022.06.057. [DOI] [Google Scholar]
- 149.Bai G, Liu C, Gao Z, Lu B, Tong X, Guo X, Yang N. Atomic carbon layers supported Pt nanoparticles for minimized CO poisoning and maximized methanol oxidation. Small. 2019;15(38):1902951. doi: 10.1002/smll.201902951. [DOI] [PubMed] [Google Scholar]
- 150.Dong Y, Chen J B, Ying J, Xiao Y X, Tian G, Symes M D, Yang X Y. Efficient water dissociation on confined ultrafine Pt via pyridinic N-enhanced heavy d−π interaction. Chemistry of Materials. 2022;34(18):8271–8279. doi: 10.1021/acs.chemmater.2c01738. [DOI] [Google Scholar]
- 151.Fan H, Cheng M, Wang L, Song Y, Cui Y, Wang R. Extraordinary electrocatalytic performance for formic acid oxidation by the synergistic effect of Pt and Au on carbon black. Nano Energy. 2018;48:1–9. doi: 10.1016/j.nanoen.2018.03.018. [DOI] [Google Scholar]


