Abstract
Metformin is the currently accepted first-line treatment for antipsychotic-associated weight gain (AAWG). However, not all patients benefit from metformin. Glucagon-like peptide-1 receptor agonists (GLP1-RA) have shown promise in the management of obesity in the general population, with preliminary evidence supporting efficacy in AAWG. Semaglutide is a weekly injectable GLP-1RA which received recent approval for obesity management and noted superiority over other GLP-1RAs. This study explored the efficacy and tolerability of semaglutide in AAWG among individuals with severe mental illness. A retrospective chart review of patients treated with semaglutide in the Metabolic Clinic at the Center for Addiction and Mental Health (CAMH) between 2019 and 2021 was conducted. Patients failing a trial of metformin (<5% weight loss or continuing to meet criteria for metabolic syndrome) after 3 months at the maximum tolerated dose (1500–2000 mg/day) were initiated on semaglutide up to 2 mg/week. The primary outcome measure was a change in weight at 3, 6, and 12 months. Twelve patients on weekly semaglutide injections of 0.71 ± 0.47 mg/week were included in the analysis. About 50% were female; the average age was 36.09 ± 13.32 years. At baseline, mean weight was 111.4 ± 31.7 kg, BMI was 36.7 ± 8.2 kg/m2, with a mean waist circumference of 118.1 ± 19.3 cm. A weight loss of 4.56 ± 3.15 kg (p < 0.001), 5.16 ± 6.27 kg (p = 0.04) and 8.67 ± 9 kg (p = 0.04) was seen at 3, 6, and 12 months, respectively, after initiation of semaglutide with relatively well-tolerated side-effects. Initial evidence from our real-world clinical setting suggests that semaglutide may be effective in reducing AAWG in patients not responding to metformin. Randomized control trials investigating semaglutide for AAWG are needed to corroborate these findings.
Keywords: antipsychotics, metformin, obesity, semaglutide, severe mental illness
Background
Antipsychotic (AP) drugs are the cornerstone treatment for schizophrenia and are commonly used for other psychiatric disorders. However, they are associated with severe metabolic adverse effects, including weight gain, dyslipidemia, and insulin resistance.1,2 Antipsychotic-associated weight gain (AAWG) has been reported as the most distressing medication-related side-effects; 3 it is associated with poor quality of life 4 and creates barriers to social engagement owing to associated stigma. 5 As a consequence, weight gain compromises adherence with treatment, 3 leading to relapse and poor mental health outcomes.
Conversely, interventions leading to even modest weight loss (i.e. 5% of body weight) has been shown to reduce the cardiometabolic risk in people with type 2 diabetes (T2D). 6 Even though obesity is highly prevalent and adds significantly to the burden of mental illness, there is a lack of effective treatment options, and metabolic comorbidity remains vastly undertreated.7–11 Lifestyle modifications form the first line of management for weight gain; however, their overall efficacy is diluted by the lack of adherence and motivation, which are challenges faced by the general population, 12 arguably even more so by patients with severe mental illness (SMI). 13 This accentuates the need for adjunctive pharmacological interventions for AAWG.
There are several pharmacological interventions currently available for adjunctive treatment of AAWG. These include topiramate, naltrexone, which is an opioid antagonist, bupropion, which is a combination of a norepinephrine–dopamine reuptake inhibitor and a nicotinic acetylcholine receptor antagonist, orlistat, and lorcaserin. However, evidence supporting their use for AAWG is underwhelming.14,15 Of the existing pharmacological interventions, metformin is recommended in several national guidelines to treat or prevent AAWG in patients with SMI. 6 It demonstrates a good safety profile and is not associated with increased dropout rates as compared to placebo. Despite safety profile, and established weight loss properties of metformin in AAWG, published data from a multisite randomized control trial (RCT) show that only approximately 17% of patients lose ⩾5% body weight with metformin, 16 leaving a large majority of patients with unclear subsequent options.
Glucagon-like peptide 1-receptor agonists (GLP-1RA)s like liraglutide, exenatide, and the most recent addition, semaglutide, are a newer class of drugs with proven efficacy in the management of obesity in the general population. 17 Semaglutide’s efficacy and safety was compared to other antidiabetic agents in the SUSTAIN trials (Semaglutide Unabated Sustainability in Treatment of T2D).18–28 Across these trials, semaglutide demonstrated significant body weight reductions from baseline to end of treatment versus all comparators. A higher proportion of patients lost ⩾ 5% or ⩾10% of their baseline weight when treated with semaglutide. The Semaglutide Treatment Effect in People with Obesity (STEP) program included eight trials primarily focused on comparing 2.4 mg, once-weekly, subcutaneous (SC) semaglutide with placebo in the management of obesity in the general population with or without T2D.29–34 Results from STEP 1–4 showed that 2.4 mg SC semaglutide, when used in combination with lifestyle modifications, led to clinically significant weight loss in patients with obesity compared to placebo. A significant weight loss of 7.9–14.9 kg for 2.4 mg SC semaglutide and 7 kg in 1 mg SC semaglutide group was seen compared to 2.4–3.4 kg weight loss in the placebo-only group. STEP 8 trial compared SC semaglutide to SC liraglutide and found 15.8% change in baseline weight with semaglutide when compared to 6.4% reduction with liraglutide. Semaglutide also resulted in decrease in visceral adiposity in the STEP 6 trial. 34 Majority of the participants (68–83%) lost >5% of baseline bodyweight at the end of the study across the STEP trials. The safety profile of 2.4 mg semaglutide was similar to that of 1.0 mg SC semaglutide and oral semaglutide, with predominant gastrointestinal (GI) disease symptoms that were mild to moderate, with not many reports of hypoglycemia. The PIONEER (Peptide Innovation for Early Diabetes Treatment) clinical trial program35–44 not only showcased a significant decrease in both glycosylated hemoglobin (HbA1c) and body weight with oral semaglutide when compared with placebo but also proved superior to other oral antidiabetic medications like sitagliptin and empagliflozin along with agents from its own drug class like dulaglutide and liraglutide.
Semaglutide’s availability as a long-acting formulation offers advantages in SMI, where medication non-adherence is a larger issue compared to other medical conditions.45,46 Patients have also reported better quality of life and treatment satisfaction with once-weekly semaglutide in comparison to other drugs within the same drug class. 47 While semaglutide’s weight loss properties have been investigated extensively in the general population with obesity and weight-related comorbidities, no studies have investigated semaglutide’s efficacy in the mentally ill. In this case series, we retrospectively evaluate the effectiveness of semaglutide for the treatment of AAWG in a naturalistic clinical setting.
Methods
Participants
Charts of all patients attending the Mental health and Metabolism clinic (MHMC) at the Center for Addiction and Mental Health (CAMH), Toronto, between March 2019 and May 2021 were reviewed to identify patients initiated on semaglutide. Patients were initiated on semaglutide on failure to respond to a trial of metformin (defined as less than 5% weight loss in 3 months or continuing to meet criteria for metabolic syndrome at the end of 3 months).
Patients were on a stable dose of AP medication (>3 months) and met criteria for adjunctive pharmacological interventions [body mass index (BMI) > 27 kg/m2 with obesity-related comorbidities like high blood pressure, dyslipidemia, or dysglycemia, or BMI ⩾ 30 kg/m2, or meet criteria for metabolic syndrome].
Procedure
All demographic, metabolic, and anthropometric measures at baseline, 3, 6, and 12 months were retrieved. Anthropometric measures like weight, height, and waist circumference were done in the clinic when feasible or at home with the same scales, with one layer of light, loose-fitting clothing. Patients were trained by the clinician or the clinical staff to take these measurements and were guided through the process to ensure consistency to the maximum possible extent (same weighing scales, same tape measure at the level of iliac spine for waist circumference with one layer of light clothing).
The off-label use of semaglutide for obesity management and its potential adverse effects were explained in detail to the patients. Consent was then obtained to initiate the medication, and semaglutide was initiated at a starting dose of 0.25 mg weekly injections; dose was titrated up every 4 weeks, to a maximum dose of 1–2 mg/week based on patient response and tolerability of adverse effects. All included patients were continued on metformin and counselled regarding lifestyle interventions like dietary modifications and increased physical activity along with the pharmacological interventions similar to all other patients attending the MHMC. Patients were followed up at 4-week intervals to monitor adherence and adverse events either in person or virtually, owing to the ongoing COVID-19 pandemic.
Data analysis
Identifying information was removed from the data which were then entered into an excel spreadsheet. Continuous data were represented as means ± standard deviation and range. Statistical analysis was performed using the student t-test for continuous variables and chi-square test for categorical variables. Mixed-model analysis was performed to evaluate the effect of time on weight in patients initiated on semaglutide. All analyses were done using IBM SPSS statistics software (version 28.0.0.0).
Results
A total of 399 patients were seen in the MHMC during the time period of March 2019 to May 2021; the final review sample consisted of 12 patients (3%) who met the inclusion criteria. Half were female; the average age was 36.09 ± 13.32 years. Baseline data were available for 12 patients at the pre-designated time points of 3 months, for 10 patients at 6 months and for 8 patients at 12 months. Major depressive disorder (MDD) was the primary psychiatric diagnosis in four patients, bipolar disorder in four patients, schizophrenia spectrum disorder (SSD) in three patients, and borderline personality disorder in one patient. All 12 patients were on second-generation AP medications. One patient (patient 9) had a diagnosis of diabetes and hypertension and was on empagliflozin and candesartan. Ten out of 12 patients were on psychotropic medication at the time of initiation of semaglutide (Table 1).
Table 1.
Clinical and demographic features of patients included in the case series at baseline visit.
| Patient | Age (y)/sex | Primary clinical diagnosis | Antipsychotic medication (APs) | Average dose of APs (mg) | Psychotropic medications | BMI (kg/m2) | WC (cm) | HbA1c (%) | TGL (mmol/L) | Metformin dose (mg) | AE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40/F | SAD | Clozapine | 450 | Sertraline, lorazepam, zopiclone, pregabalin | 26.6 | 99 | 5.5 | 0.38 | 2000 | Nil |
| 2 | 25/M | SSD | Paliperidone LAI | 100 | 28.5 | 114 | 5.5 | 1.32 | 2000 | Nil | |
| 3 | 23/M | BD | Aripiprazole | 22 | Lithium, pregabalin | 42.5 | 140 | 4.9 | 1.38 | 2500 | Nil |
| 4 | 35/F | BPD | Quetiapine, loxapine | 425; 12.5 | Citalopram, valproate, clonazepam | 63.7 | 120 | 5.2 | 3.16 | 1000 | Nil |
| 5 | 31/M | Schizophrenia | Aripiprazole, aripiprazole LAI | 15; 400 | 28.9 | 103.5 | 5 | 1.2 | 2000 | N, V | |
| 6 | 58/F | MDD | Risperidone | 0.25 | Lorazepam, venlafaxine | 31.4 | 94 | 6.1 | 1.28 | 2000 | N |
| 7 | 23/F | BD | Olanzapine | 15 | Lithium | 32.9 | 99 | 5.1 | 1.42 | 1500 | N, V |
| 8 | 36/M | BD | Brexiprazole | 3 | Valproate, zopiclone | 38.3 | 119 | 2500 | Nil | ||
| 9 | 44/F | MDD | Lurasidone, loxapine | 40; 25 | Sertraline, pregabalin, lorazepam, trazodone | 40.7 | 128 | 6.6 | 1.38 | 2000 | N, V |
| 10 | 22/M | MDD | Risperidone | 2 | Sertraline, trazodone | 52.7 | 156.2 | 6 | 1.23 | 2000 | Nil |
| 11 | 59/M | MDD | Quetiapine, aripiprazole | 25; 7 | Moclobemide | 37 | 117 | 5.2 | 0.67 | 1000 | Nil |
| 12 | 36/F | BD | Aripiprazole | 17.5 | Lithium | 45 | 130 | 5.8 | 0.96 | 2000 | Nil |
AE, adverse events; BD, bipolar disorder; BMI, body mass index; BPD, borderline personality disorder; D, diarrhea; HbA1c, glycosylated hemoglobin; Kg, kilograms; LAI, long-acting injectable; MDD, major depressive disorder; N, nausea; SAD, schizoaffective disorder; SSD, schizophrenia spectrum disorder; TGL, fasting triglyceride; V, vomiting; WC, waist circumference.
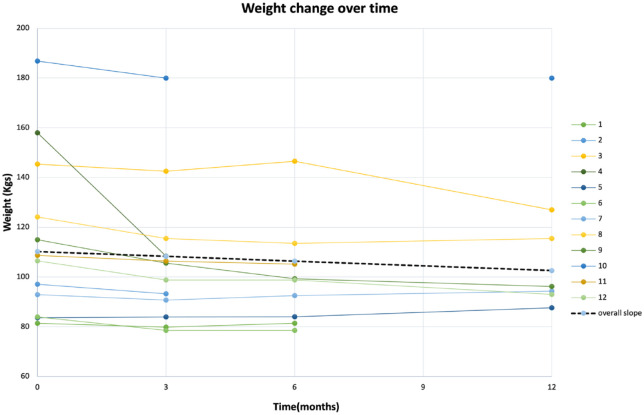
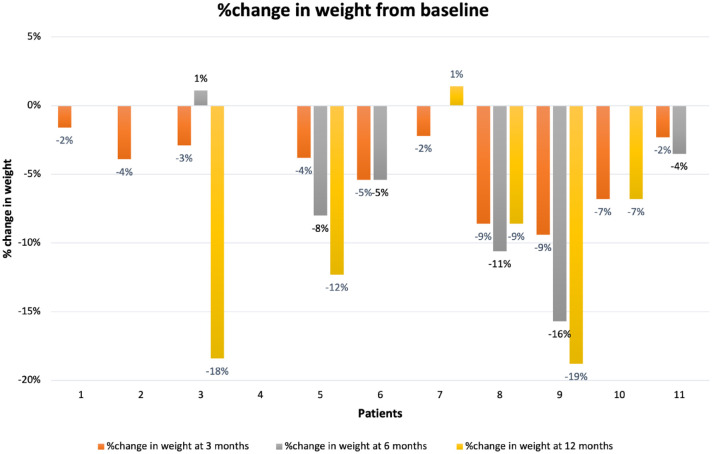
At baseline, the mean weight was 111.4 ± 31.7 kg, BMI was 36.7 ± 8.2 kg/m2, with a mean waist circumference of 118.1 ± 19.3 cms. Demographics and clinical characteristics are provided in detail in Table 1. Metformin was continued in 83% of the patients (10 out of 12). The data were visually inspected, and one outlier (patient 4, Figure 1) was identified and removed from the final analysis due to extreme weight loss (approximately 50 kg in 3 months). The mean dose of semaglutide was 0.71 ± 0.47 mg/week at the end of 12 months. A weight loss of 4.56 ± 3.15 kg (p < 0.001), 5.16 ± 6.27 kg (p = 0.04), and 8.67 ± 9 kg (p = 0.04) was seen at 3, 6, and 12 months, respectively, after initiation of semaglutide. This accounted for an average of 5% weight loss at 3 months, 6.6% weight loss at 6 months, and 8.7% weight loss at 12 months for the final sample of patients initiated on semaglutide. About 50% patients lost more than 5%, and 41.6% patients lost more than 7% of their baseline weight (Figure 2). Additional blood-based metabolic data were available for 6, 6, and 4 patients at 3, 6, and 12 months, respectively, as the current pandemic led to limited access to laboratory and blood work. Numerical improvements were noted in the fasting glucose, HbA1c, triglycerides, high-density lipoproteins, low-density lipoproteins (LDLs), total cholesterol, fasting insulin, homeostatic model assessment for insulin resistance (HOMA-IR); however, they did not reach statistical significance.
Figure 1.
Line graphs depicting change in body weight over time of the overall cohort and individual patients.
Figure 2.
Percentage change in body weight from baseline of patients included in the study.
Adverse effects
Four patients (33%) experienced gastrointestinal side-effects like nausea, vomiting, and diarrhea, which decreased over time. The remaining eight patients did not report any adverse effects. Metformin was continued in all except two patients who experienced intolerable nausea. It was restarted in one patient and slowly titrated to a dose of 2000 mg over a period of 3 months, once they were on a stable dose of semaglutide. No serious adverse events were reported in the treatment cohort.
Discussion
This case series reports the real-world effectiveness of semaglutide in the treatment of AAWG, in a naturalistic small subset of individuals who failed to respond to metformin. There was a statistically and clinically (i.e. >5% body weight) significant improvement in weight in the treated cohort. Even though a third of patients (4 of 12) reported minor GI side-effects on initiation of semaglutide, these improved over time, and semaglutide was generally well tolerated, which corroborated with the known GI adverse effects of semaglutide. 48 While the small convenient sample is acknowledged as a limitation, to the best of our knowledge, this is the first report demonstrating the real-world efficacy and tolerability of semaglutide in the context of AAWG in SMI.
GLP1-RA acts by decreasing glucagon secretion and stimulating insulin secretion in a glucose-dependent manner. Thereby, GLP1-RAs delay gastric emptying and lower food intake by promoting satiety. Changes in satiety, alongside lipolytic effects in adipocytes, account for weight and visceral fat reduction properties of GLP-1RAs. Notably, as a class, GLP-1RAs are also associated with a lower risk of major adverse cardiovascular endpoints, including cardiovascular mortality and non-fatal strokes and myocardial infarctions. Five RCTs (exenatide and liraglutide) to date have examined the efficacy of GLP-1-RAs on weight loss among people on APs with schizophrenia spectrum disorders (SSDs).49–53 They found significant reductions in body weight, waist circumference, systolic blood pressure, visceral fat, and low-density lipoprotein (LDL) with liraglutide. 48
Even though this case series highlights the promise of semaglutide as an adjunctive pharmacological agent in the treatment of AAWG, the results should be interpreted in the presence of several limitations. The mean dose of semaglutide (0.7 mg weekly) was well below the approved dose for the management of obesity (2.4 mg weekly). This was reflective of the period during which these data were collected (March 2019–May 2021), prior to the approval of semaglutide at higher doses for obesity management (June 2021), with most individuals dosed in accordance with recommended dose for treatment of T2D (1.0 mg weekly)54. Given the current approval of semaglutide at higher dose (2.4 mg) for treatment of obesity, these preliminary results could translate to a greater weight loss that could be sustained over longer periods of time. 29 Furthermore, given that the report represents a case series, limitations inherent to the design, such as open-label treatment and lack of a placebo arm, are acknowledged. Furthermore, COVID-19 constraints limited adequate follow-up and restricted data in terms of anthropometric and laboratory measures.
Implications for clinical care
This case series demonstrates initial evidence of real-world effectiveness of semaglutide in reducing weight in patients with AAWG. Semaglutide was seen to improve body weight along with metabolic parameters. The once-weekly administration of the drug, along with its reasonably well-tolerated adverse effects, makes it a viable option in patients not responding to metformin.
Conclusion and future directions
In conclusion, results from this case series suggest that semaglutide may represent the next step in the treatment of AAWG, in individuals who fail to respond to metformin. Larger, well-powered, double-blind RCTs are needed to corroborate these findings.
Acknowledgments
Not applicable.
Footnotes
ORCID iDs: Femin Prasad  https://orcid.org/0000-0002-5445-2489
https://orcid.org/0000-0002-5445-2489
Dan Siskind  https://orcid.org/0000-0002-2072-9216
https://orcid.org/0000-0002-2072-9216
Sri Mahavir Agarwal  https://orcid.org/0000-0002-2705-5146
https://orcid.org/0000-0002-2705-5146
Contributor Information
Femin Prasad, Schizophrenia Division, Centre for Addiction and Mental Health (CAMH), Toronto, ON, Canada; Institute of Medical Science, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Riddhita De, Schizophrenia Division, Centre for Addiction and Mental Health (CAMH), Toronto, ON, Canada; Institute of Medical Science, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Vittal Korann, Schizophrenia Division, Centre for Addiction and Mental Health (CAMH), Toronto, ON, Canada; Institute of Medical Science, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Araba F. Chintoh, Schizophrenia Division, Centre for Addiction and Mental Health (CAMH), Toronto, ON, Canada Institute of Medical Science, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Department of Psychiatry, University of Toronto, Toronto, ON, Canada.
Gary Remington, Schizophrenia Division, Centre for Addiction and Mental Health (CAMH), Toronto, ON, Canada; Institute of Medical Science, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Department of Psychiatry, University of Toronto, Toronto, ON, Canada.
Bjørn H. Ebdrup, Center for Neuropsychiatric Schizophrenia Research, Mental Health Centre Glostrup, University of Copenhagen, Glostrup, Denmark Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, Copenhagen, Denmark; Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS) Glostrup, Denmark.
Dan Siskind, Faculty of Medicine, The University of Queensland, Brisbane, QLD, Australia; Metro South Addiction and Mental Health Service, Brisbane, QLD, Australia.
Filip Krag Knop, Center for Neuropsychiatric Schizophrenia Research, Mental Health Centre Glostrup, University of Copenhagen, Glostrup, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Steno Diabetes Center Copenhagen, Copenhagen, Denmark; Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Tina Vilsbøll, Center for Neuropsychiatric Schizophrenia Research, Mental Health Centre Glostrup, University of Copenhagen, Glostrup, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; Steno Diabetes Center Copenhagen, Copenhagen, Denmark; Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Anders Fink-Jensen, Center for Neuropsychiatric Schizophrenia Research, Mental Health Centre Glostrup, University of Copenhagen, Glostrup, Denmark; Psychiatric Centre Copenhagen, Rigshospitalet, Mental Health Services – Capital Region of Denmark, Copenhagen, Denmark.
Margaret K. Hahn, Clinician-Scientist, Schizophrenia Division, Centre for Addiction and Mental Health, 1051 Queen St W, Toronto, ON M6J 1H3, Canada; Institute of Medical Science, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Department of Psychiatry, University of Toronto, Toronto, ON, Canada Banting and Best Diabetes Centre (BBDC), University of Toronto, Toronto, ON, Canada.
Sri Mahavir Agarwal, Clinician-Scientist, Schizophrenia Division, Centre for Addiction and Mental Health, 1051 Queen St W, Toronto, ON M6J 1H3, Canada; Institute of Medical Science, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Department of Psychiatry, University of Toronto, Toronto, ON, Canada Banting and Best Diabetes Centre (BBDC), University of Toronto, Toronto, ON, Canada.
Declarations
Ethics approval and consent to participate: The Center for Addiction and Mental Health Research Ethics Board (REB) approved this study (approval number: 072/2021) and waived of need for specific informed consent for participation.
Consent for publication: Not applicable.
Author contributions: Femin Prasad: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – original draft.
Riddhita De: Data curation; Writing – review & editing.
Vittal Korann: Writing – review & editing.
Araba F. Chintoh: Writing – review & editing.
Gary Remington: Writing – review & editing.
Bjørn H. Ebdrup: Writing – review & editing.
Dan Siskind: Writing – review & editing.
Filip Krag Knop: Writing – review & editing.
Tina Vilsbøll: Writing – review & editing.
Anders Fink-Jensen: Writing – review & editing.
Margaret K. Hahn: Conceptualization; Methodology; Resources; Supervision; Validation; Visualization; Writing – review & editing.
Sri Mahavir Agarwal: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RD is supported by the Banting and Best Diabetes Center (BBDC) Novo-Nordisk Graduate Studentship. GR has received research support from the Canadian Institutes of Health Research (CIHR), University of Toronto, and HLS Therapeutics Inc. DS is supported in part by an NHMRC EL2 Fellowship GNT1194635. MKH is supported in part by an Academic Scholars Award from the Department of Psychiatry, University of Toronto and has grant support from the Banting and Best Diabetes Center (BBDC), the Canadian Institutes of Health Research (PJT-153262) (CIHR), PSI foundation, Ontario, holds the Kelly and Michael Meighen Chair in Psychosis Prevention, and the Cardy Schizophrenia Research Chair. SMA is supported in part by an Academic Scholars Award from the Department of Psychiatry, University of Toronto and has grant support from the Canadian Institutes of Health Research, PSI foundation, Ontario, and the CAMH Discovery Fund.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BE received lecture fees and/or is part of the advisory board at Bristol-Myers Squibb, Eli Lilly and Company, Janssen-Cilag, Otsuka Pharma Scandinavia AB, Takeda Pharmaceutical Company, Boehringer Ingelheim, and Lundbeck Pharma A/STV has served on scientific advisory panels and/or speakers’ bureaus or has served as a consultant to and/or received research support from Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, Gilead, MSD/Merck, Mundipharma, Novo Nordisk, Sanofi, and Sun Pharmaceuticals. FKK has served on scientific advisory panels and/or been part of speaker’s bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi, and Zealand Pharma; and is a co-founder of and minority share-holder in Antag Therapeutics Aps. TV has served on scientific advisory panels for, been part of speaker’s bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, MSD/ Merck, Novo Nordisk, and Sun Pharmaceutical Industries.
Availability of data and materials: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.De Hert M, Detraux J, Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2011; 8: 114–126. [DOI] [PubMed] [Google Scholar]
- 2.Ballon JS, Pajvani U, Freyberg Z, et al. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol Metab 2014; 25: 593–600. [DOI] [PubMed] [Google Scholar]
- 3.Cooper SJ, Reynolds GP, Barnes T, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 2016; 30: 717–748. [DOI] [PubMed] [Google Scholar]
- 4.Faulkner G, Cohn T, Remington G, et al. Body mass index, waist circumference and quality of life in individuals with schizophrenia. Schizophr Res 2007; 90: 174–178. [DOI] [PubMed] [Google Scholar]
- 5.Young SJ, Praskova A, Hayward N, et al. Attending to physical health in mental health services in Australia: a qualitative study of service users’ experiences and expectations. Health Soc Care Community 2017; 25: 602–611. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen SD, Manjoo P, Wharton S. Pharmacotherapy in obesity management, http://obesitycanada.ca/wp-content/uploads/2020/08/Pharmacotherapy-v3-with-links_FINAL_NP.pdf
- 7.Correll CU.Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry 2014; 71: 1350–1363. [DOI] [PubMed] [Google Scholar]
- 8.Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006; 86: 15–22. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RW, Kreyenbuhl JA, Medoff DR, et al. Quality of diabetes care among adults with serious mental illness. Psychiatr Serv 2007; 58: 536–543. [DOI] [PubMed] [Google Scholar]
- 10.Kreyenbuhl J, Dickerson FB, Medoff DR, et al. Extent and management of cardiovascular risk factors in patients with type 2 diabetes and serious mental illness. J Nerv Ment Dis 2006; 194: 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frayne SM.Disparities in diabetes care: impact of mental illness. Arch Intern Med 2005; 165: 2631–2638. [DOI] [PubMed] [Google Scholar]
- 12.Leung AWY, Chan RSM, Sea MMM, et al. An overview of factors associated with adherence to lifestyle modification programs for weight management in adults. Int J Environ Res Public Health 2017; 14: 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts SH, Bailey JE.Bailey incentives and barriers to lifestyle interventions for people with severe mental illness: a narrative synthesis of quantitative, qualitative and mixed methods studies. J Adv Nurs, https://click.endnote.com/viewer?doi=10.1111%2Fj.1365-2648.2010.05546.x&token=WzMxNjUyMTUsIjEwLjExMTEvai4xMzY1LTI2NDguMjAxMC4wNTU0Ni54Il0.Fu8zH1VY3SZz0w3mz6ExDt0vrMk (accessed 6 June 2022). [DOI] [PubMed]
- 14.Goh KK, Chen CH, Lu ML.Topiramate mitigates weight gain in antipsychotic- treated patients with schizophrenia: meta-analysis of randomised controlled trials. Int J Psychiatry Clin Pract 2019; 23: 14–32. [DOI] [PubMed] [Google Scholar]
- 15.Marteene W, Winckel K, Hollingworth S, et al. Strategies to counter antipsychotic-associated weight gain in patients with schizophrenia. Expert Opin Drug Saf 2019; 18: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 16.Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry 2013; 170: 1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensterle M, Rizzo M, Haluzík M, et al. Efficacy of GLP-1 RA approved for weight management in patients with or without diabetes: a narrative review. Adv Ther 2022; 39: 2452–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 251–260. [DOI] [PubMed] [Google Scholar]
- 19.Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care 2018; 41: 258–266. [DOI] [PubMed] [Google Scholar]
- 20.Rodbard HW.Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab 2018; 103: 2291–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 355–366. [DOI] [PubMed] [Google Scholar]
- 22.Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol 2018; 6: 275–286. [DOI] [PubMed] [Google Scholar]
- 23.Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 2017; 5: 341–354. [DOI] [PubMed] [Google Scholar]
- 24.Ji L, Dong X, Li Y, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: a 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes Metab 2021; 23: 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 26.Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2019; 7: 356–367. [DOI] [PubMed] [Google Scholar]
- 27.Lingvay I, Catarig AM, Frias JP, et al. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol 2019; 7: 834–844. [DOI] [PubMed] [Google Scholar]
- 28.Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab 2020; 46: 100–109. [DOI] [PubMed] [Google Scholar]
- 29.Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA 2021; 325: 1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA 2022; 327: 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021; 384: 989. [DOI] [PubMed] [Google Scholar]
- 32.Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021; 397: 971–984. [DOI] [PubMed] [Google Scholar]
- 33.Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA 2021; 325: 1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadowaki T, Isendahl J, Khalid U, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol 2022; 10: 193–206. [DOI] [PubMed] [Google Scholar]
- 35.Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA 2019; 321: 1466–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care 2019; 42: 2272–2281. [DOI] [PubMed] [Google Scholar]
- 37.Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol 2019; 7: 515–527. [DOI] [PubMed] [Google Scholar]
- 38.Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol 2019; 7: 528–539. [DOI] [PubMed] [Google Scholar]
- 39.Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet 2019; 394: 39–50. [DOI] [PubMed] [Google Scholar]
- 40.Thethi TK, Pratley R, Meier JJ.Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: the PIONEER programme. Diabetes Obes Metab 2020; 22: 1263–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishii H, Hansen BB, Langer J, et al. Effect of orally administered semaglutide versus dulaglutide on diabetes-related quality of life in japanese patients with type 2 diabetes: the PIONEER 10 randomized, active-controlled trial. Diabetes Ther 2021; 12: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yabe D, Nakamura J, Kaneto H, et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): an open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocrinol 2020; 8: 392–406. [DOI] [PubMed] [Google Scholar]
- 43.Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care 2019; 42: 2262–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada Y, Katagiri H, Hamamoto Y, et al. Dose-response, efficacy, and safety of oral semaglutide monotherapy in Japanese patients with type 2 diabetes (PIONEER 9): a 52-week, phase 2/3a, randomised, controlled trial. Lancet Diabetes Endocrinol 2020; 8: 377–391. [DOI] [PubMed] [Google Scholar]
- 45.Oehl M, Hummer M, Fleischhacker WW.Compliance with antipsychotic treatment. Acta Psychiatr Scand Suppl 2000; 407: 83–86. [DOI] [PubMed] [Google Scholar]
- 46.Cramer JA, Rosenheck R.Compliance with medication regimens for mental and physical disorders. Psychiatr Serv 1998; 49: 196–201. [DOI] [PubMed] [Google Scholar]
- 47.Jendle J, Birkenfeld AL, Polonsky WH, et al. Improved treatment satisfaction in patients with type 2 diabetes treated with once-weekly semaglutide in the SUSTAIN trials. Diabetes Obes Metab 2019; 21: 2315–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siskind D, Hahn M, Correll CU, et al. Glucagon-like peptide-1 receptor agonists for antipsychotic- associated cardio-metabolic risk factors: a systematic review and individual participant data meta-analysis. Diabetes Obes Metab 2019; 21: 293–302. [DOI] [PubMed] [Google Scholar]
- 49.Ishøy PL, Knop FK, Broberg BV, et al. Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial byline. Diabetes Obes Metab 2017; 19: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsen JR, Vedtofte L, Jakobsen MSL, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine-or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry 2017; 74: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patino LR, Rummelhoff ER, Blom T, et al. A double-blind placebo-controlled study of exenatide for the treatment of weight gain associated with olanzapine in overweight or obese adults with bipolar disorder, major depressive disorder, schizophrenia or schizoaffective disorder. Biol Psychiatry 2015; 77: 132S. [Google Scholar]
- 52.Whicher CA, Price HC, Phiri P, et al. The use of liraglutide 3.0 mg daily in the management of overweight and obesity in people with schizophrenia, schizoaffective disorder and first episode psychosis: results of a pilot randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2021; 23: 1262–1271. [DOI] [PubMed] [Google Scholar]
- 53.Siskind D, Russell AW, Gamble C, et al. Treatment of clozapine-associated obesity and diabetes with exenatide in adults with schizophrenia: a randomised controlled trial (CODEX). Diabetes Obes Metab 2018; 20: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 54.US Food and Drug Administration. FDA approves new drug treatment for chronic weight management, first since 2014, https://www.fda.Gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014 (2021, accessed 18 April 2022).