Abstract
Background:
Circulating tumor cells (CTCs) are prognostic biomarker in non-small-cell lung cancer (NSCLC). CTCs could also be used as predictor of efficacy of systemic treatments in advanced NSCLC.
Objectives:
We described the dynamic changes of CTCs during first-line platinum-based chemotherapy in advanced NSCLC and clarified the correlation between CTC counts and efficacy of chemotherapy.
Design:
Chemotherapy is administered and blood specimens are collected at four time points from baseline to disease progression for CTC detection.
Methods:
This multicenter prospective study enrolled patients with previously untreated stage III or IV NSCLC fit for standard platinum-based chemotherapy. Bloods were sampled as per standard operating procedures at baseline, cycle 1 and cycle 4 of chemotherapy, and at disease progression for CTC analysis using the CellSearch system.
Results:
Among 150 patients enrolled, median overall survival (OS) was 13.8, 8.4, and 7.9 months in patients with CTC−, KIT−CTC, and KIT+CTC at baseline (p = 0.002). Patients with persistent negative CTC (46.0%) had longer progression-free survival [5.7 months, 95% confidence interval (CI): 5.0–6.5 versus 3.0 months, 0.6–5.4; hazard ratio (HR): 0.34, 95% CI: 0.18–0.67) and OS (13.1 months, 10.9–15.3 versus 5.6 months, 4.1–7.1; HR: 0.17, 0.08–0.36) compared with patients with persistent positive CTC (10.7%), which was not impacted by chemotherapy. Chemotherapy decreased CTC from 36.0% (54/150) to 13.7% (13/95).
Conclusions:
CTC persistent presence during treatment represents poor prognosis and resistance to chemotherapy in advanced NSCLC. Chemotherapy could effectively eliminate CTCs. Molecular characterization and the functionalization of CTC will be warranted for further intensive investigation.
Trial registration:
Keywords: chemotherapy, circulating tumor cell, KIT, non-small-cell lung cancer
Introduction
Lung cancer is the leading cause of cancer death worldwide. 1 The majority (85%) of patients with lung cancer are diagnosed with non-small-cell lung cancer (NSCLC). Approximately 40% newly diagnosed NSCLC present with advanced-stage disease. 2 Although platinum-based chemotherapy plus immune checkpoint inhibitors (ICIs) are the mainstay of first-line treatment in advanced NSCLC without targetable mutations, chemotherapy is a standard option for patients not suitable for ICIs. There are no convincing predictive biomarkers related to chemotherapy.
Circulating tumor cell (CTC) detection is a reliable and reproducible liquid biopsy, and is well established by the CellSearch system, which is an immunomagnetic purification method. 3 CTCs act as a potential diagnostic, prognostic, and predictive biomarker in metastatic lung, breast, colorectal, and prostate cancer.4–11 CTCs might also be a promising predictive biomarker of mortality and recurrence in early-stage NSCLC, and CTC detection can potentially serve as a tool for minimal residual disease detection in resectable lung cancer after curative surgery.12,13
CTC was used in molecular detection of human epidermal growth factor receptor 2 (HER2), KRAS, and EGFR mutation in breast, colon, and lung cancer. 14 CTC could be detected long before radiological diagnosis of lung cancer, 15 and therefore was applied in lung cancer screening in high-risk patients in a prospective study but failed to be used as biomarker. 16
KIT, also known as CD117 (cluster of differentiation 117) or mast/stem cell growth factor receptor, is the c-kit proto-oncogene product expressed in multiple organ cells and a type III receptor tyrosine kinase, which is activated by binding the stem cell factor (SCF) and regulates apoptosis, cell differentiation, proliferation, chemotaxis, and cell adhesion. 17 KIT serves as oncogenic role in gastrointestinal stromal tumor 18 and is associated with drug resistance, such as tyrosine kinase inhibitors imatinib in gastrointestinal tumors and pyrotinib in HER2-positive gastric cancers.19–22 The SCF and its receptor KIT play an important role in survival and proliferation of lung cancer stem cells (CSCs), which have high tumorigenic and metastatic properties. 19 KIT expression was found to be significantly higher in adenocarcinoma than in squamous cell carcinoma, and associated with lymph node metastasis and poor prognosis. 23
Serial CTC detection before and after one cycle of standard chemotherapy had already proved to be a prognostic factor for patients with stage IV NSCLC. 7 This prospective study aimed to determine prognostic and predictive significance of dynamic changes (baseline, after one cycle, and after four cycles of standard chemotherapy) of CTC and KIT+CTC using the CellSearch in advanced NSCLC treated with first-line platinum-based chemotherapy (CTONG1202, also named as POLICE).
Methods
Study design
This is a prospective, multicenter, observational study on dynamic CTC enumeration in advanced NSCLC undergoing first-line chemotherapy (POLICE), launched by China Thoracic Oncology Group, also known as CTONG1202, and registered at ClinicalTrial.gov, NCT01740804.
Patients
Eligible patients were chemotherapy-naïve, histologically diagnosed NSCLC with radiologically confirmed stage IV (7th Edition of TNM Staging Criteria). Patients with stage IIIB were also included if not amenable to definite local therapy on the basis of multidisciplinary team decision. Prior adjuvant chemotherapy or concurrent chemoradiation was permitted if >12 months before study entry. Other inclusion criteria included Eastern Cooperative Oncology Group performance status of 0 to 1 and life expectancy of at least 3 months, no history of prior malignancy within 5 years except for cured cervical carcinoma in situ, basal cell carcinoma, or superficial bladder cancer. Measurable lesions existed and response was assessed by response evaluation criteria in solid tumors, version 1.1 (RECIST 1.1). 24 Patients were excluded if they had brain metastasis and spinal cord compression except surgery or radiation was applied and relative conditions kept stable >4 weeks before study entry. Clinical data were collected for age, histological subtype, smoking status, treatment received, survival, and response.
CTC sampling and detection
Patients were required to fast for more than 8 h before blood collection. The first at least 1 mL of peripheral blood was discarded to avoid skin epithelial cell contamination. All blood samples were analyzed for CTC, which were captured using semiautomated epithelial cell adhesion molecule-based system CellSearch (Veridex LLC, Raritan, NJ, USA). Epithelial cells were identified with the presence of a nucleus, Epcam+CK+CD45− phenotype. 3 In brief, 7.5 mL of blood was drawn into evacuated CellSave Preservative Tube (Veridex LLC). Samples were maintained at room temperature and processed within 72 h of blood collection.
The CellSearch CTC detection kit contains an immunofluorescence reagent based on a ferromagnetic fluid capture kit. This ferromagnetic fluid reagent contains nanoparticles with magnetic cores. The polymer layer around the magnetic core is coated with antibodies against EpCAM antigen to capture CTC. After the capture and enrichment of the immune magnet, the samples were then stained with 4′,6-diamidino-2-phenylindole (DAPI) and anti-cytokeratin–phycoerythrin. White blood cells were negatively selected for CD45. Stained cells were analyzed on a florescent microscope using the Cell track Analyzer II (Veridex LLC). The criteria for defining a CTC include round to oval morphology, a visible nucleus (DAPI positive), positive staining for cytokeratin, and negative staining for CD45. CTC enumeration is expressed as the number of cells per 7.5 mL of blood. Labeling of KIT on the surface of CTC was integrated to CTC analysis and an optimal procedure was established. KIT marker was used to test the frequency of stem cell-like KIT+Epcam+CK+CD45− CTCs.
As per protocol, a 7.5-ml sample of peripheral blood was obtained at four time points, which included baseline, within 7 days prior to the second cycle of chemotherapy, 3 weeks after the fourth cycle of chemotherapy, and within 7 days at the diagnosis of progressive disease (PD) prior to subsequent anticancer regimen if any.
All samples were transported to the central laboratory of Guangdong Lung Cancer Institute for CTC analysis. The investigators, who performed CTC sampling and analysis, were blinded to the clinical status of the patients.
Clinical treatment and study endpoints
All patients were subjected to standard platinum doublet chemotherapy based on tumor histologic type every 3 weeks for up to four cycles. The primary endpoint was the efficacy of doublet chemotherapy based on dynamic CTC enumeration, which included overall response rate (ORR) and progression-free survival (PFS). The secondary endpoint was to investigate the correlation of CTCs and KIT+CTC to overall survival (OS).
Efficacy of chemotherapy was evaluated on the basis of investigators’ assessment per RECIST 1.1 24 every 6 weeks from the first cycle until PD. ORR was defined as the proportion of patients with a complete response or partial response (PR). PFS was defined as the time from the first day of chemotherapy to documented PD or death, whichever occurred first, or censored at last follow-up. Patient would withdraw and entered into survival follow-up every 3 months after PD. Subsequent anticancer treatments would be recommended by physicians’ decisions on the basis of each patient’s condition. OS was defined as the time from the first day of chemotherapy to death from any cause or censored at last follow-up.
Statistical analysis
On the basis of the reportedly 69% of CTC count (per 7.5 mL of peripheral blood) ⩾1 in NSCLC with clinical stage IV 9 and assumption of tolerance error 0.069, 134 patients were required with 90% confidence level and confidence interval (CI) width (two-sided) of 0.138. With an assumed dropout rate of 10%, a total of 150 patients were enrolled.
To determine the most appropriate CTC cutoff, CTC counts between 1 and 5 at baseline were tested for their estimate of the survival function using the Kaplan–Meier (KM) method. The association of CTC count with clinical characteristics, including stage, histology, smoking status, and driver genes mutation, was compared by Fisher’s exact test.
All patients were grouped into three cohorts: CTC−, KIT−CTC+, and KIT+CTC+. The intention to treat population included all patients who received at least one cycle of chemotherapy and had blood samples at baseline and at least one blood sample after chemotherapy. ORR was estimated based on CTCs and KIT+CTC levels and exact binomial 95% CIs were provided using the Clopper–Pearson method. Correlation between dynamic CTCs or KIT+CTC levels and OS was analyzed using Spearman’s correlation. PFS and OS were analyzed using the KM method. Hazard ratio for subgroup analysis was calculated by the Cox proportional hazards model.
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 27.0. (Armonk, NY: IBM Corp.) and R version 4.2.1 where p ⩽ 0.05 were considered significant.
Results
Patient demographics and clinical outcome
From August 2013 through October 2015, we screened a total of 180 patients and finally enrolled 150 patients, who underwent at least one cycle of chemotherapy and had blood samples at baseline for CTC analysis (Supplemental Figure S1). Patients’ demographics are listed in Table 1.
Table 1.
Patient demographic and clinical characteristics (ITT population).
| Characteristics | Total N = 150 |
|---|---|
| Age: median (min, max), years old | 58 (25–76) |
| Sex, n (%) | |
| Male | 111 (74.0) |
| Smoking status, n (%) | |
| Smoker | 94 (62.7) |
| Histology, n (%) | |
| Adenocarcinoma | 99 (66.0) |
| Squamous cell carcinoma | 35 (23.3) |
| Others | 16 (10.7) |
| Clinical stage, n (%) | |
| IIIB | 6 (4.0) |
| IV | 144 (96.0) |
| Driver mutations, n (%) | |
| EGFR a | 23 (15.3) |
| ALK | 6 (4.0) |
| Wild type | 102 (68.0) |
| Unknown | 19 (12.7) |
| Chemotherapy, n (%) | |
| Gemcitabine plus platinum | 95 (63.3) |
| Pemetrexed plus platinum | 40 (26.7) |
| Paclitaxol plus platinum b | 14 (9.3) |
| Pemetrexed | 1 (0.7) |
EGFR driver mutation: exon 19 deletion in 7, exon 20 insertion in 6, L858R in 3, L861Q in 2, and uncommon mutation in 5.
Including three cases of polymeric micelle-formulated paclitaxel (Genexol-PM) plus cisplatin.
ALK, anaplastic lymphoma kinase; CTC, circulating tumor cells; EGFR, epidermal growth factor receptor; ITT, intention to treat.
At the cutoff date of 6 September 2021, the median follow-up was 94.8 months (interquartile range: 77.6–96.6 months) with 147 (98.0%) had a PFS event and 139 (92.7%) had died. Median PFS and OS were 5.6 (95% CI, 5.0–6.2) and 11.8 (95% CI, 9.7–13.9) months, respectively.
There were totally 120 patients with recorded disease progression per RECIST1.1 by investigators, 95 of them had blood samples available and 95 (79.2%) underwent subsequent anticancer treatment, which included chemotherapy (53, 44.2%), molecular targeted therapy (27, 22.5%), local treatment (palliative surgery or radiotherapy) (12, 10.0%), and ICIs (3, 2.5%).
CTC, KIT+CTC prevalence, and efficacy of chemotherapy
The prevalence was 36.0% for CTC and 13.3% for KIT+CTC at the cutoff of ⩾1 prior to chemotherapy. No significant association was found between detection rates of CTC and KIT+CTC at cutoff of 1–5 per 7.5 mL blood and clinicopathological characteristics, including stage, histology, smoking status, and driver genes mutation (Supplemental Table S1). Detection rates of CTC at cutoff of 1–5 and KIT+CTC at cutoff of 1–3 at baseline were showed to discriminate significantly in OS (supplemental Table S2).
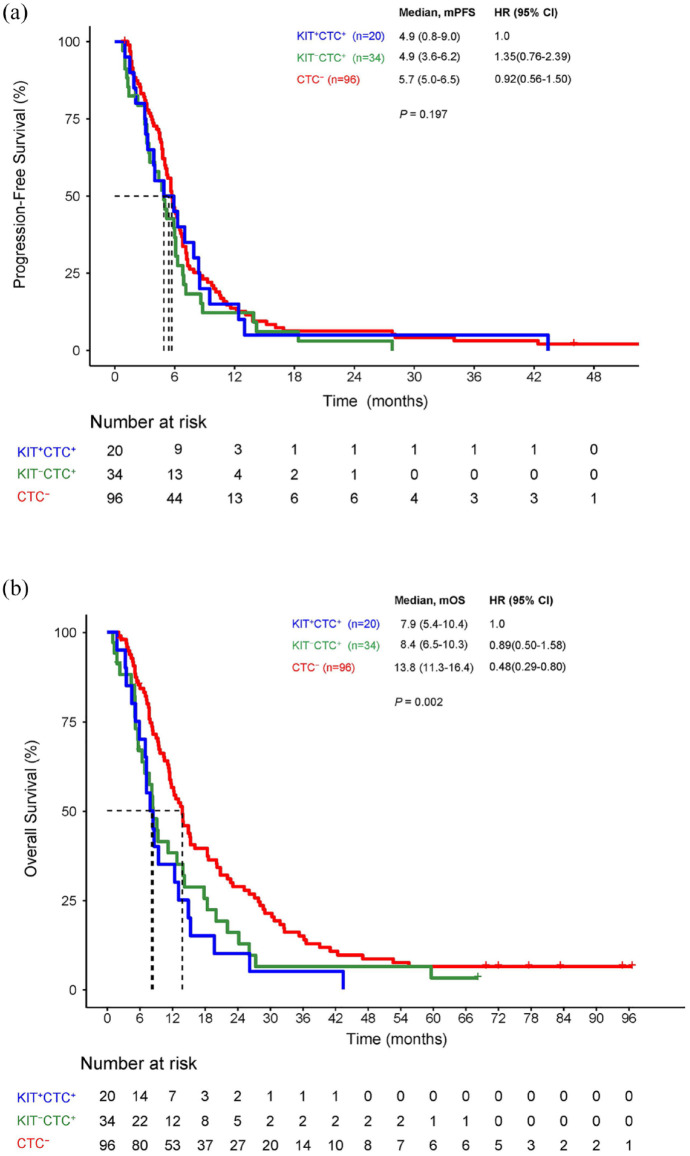
There were 96 patients in CTC− cohort, 34 in KIT−CTC+, and 20 in KIT+CTC+ cohort at baseline. CTC− cohort had better response rate than that of another two cohorts in number but not significant (Table 2). The OS rather than the median PFS (mPFS) has significant differences among cohorts of CTC−, KIT−CTC+, and KIT+CTC+ (Figure 1(a) and (b)).
Table 2.
The response rate of chemotherapy in CTC and KIT/CTC at baseline.
| Response, n (%) | KIT+/CTC+ (n = 20) | KIT−/CTC+ (n = 34) | CTC− (n = 96) | Total (n = 150) | p |
|---|---|---|---|---|---|
| ORR, % (95% CI) | 20.0 (5.7–43.7) | 23.5 (10.7–41.2) | 28.1 (19.4–38.2) | 26.0 (19.2–33.8) | 0.702 |
| Best overall response, n (%) | |||||
| Complete response | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Partial response | 4 (20.0) | 8 (23.5) | 27 (28.1) | 39 (26.0) | |
| Stable disease | 10 (50.0) | 20 (58.8) | 45 (46.9) | 75 (50.0) | |
| Progressive disease | 5 (25.0) | 6 (17.6) | 15 (15.6) | 26 (17.3) | |
| Non-evaluable* | 1 (5.0) | 0 (0) | 9 (9.4) | 10 (6.7) | |
One discontinued chemotherapy due to grade 3 hematological toxicity and nine switched to other regimens due to personal reasons.
CTC, circulating tumor cell; ORR, overall response rate.
Figure 1.
PFS and OS between CTC and KIT+CTC at baseline.
CTC, circulating tumor cell; OS, overall survival; PFS, progression-free survival.
In all, 29 patients had activating mutations including EGFR and ALK mutations. ORR had no difference between mutant and wild-typed subgroups. Mutant subgroup had more favorable PFS than wild-typed subgroup (6.0 versus 5.2 months; log-rank test p = 0.039). The analysis demonstrated that the interaction effect between druggable genes and CTC, KIT+CTC on chemotherapy efficacy was nonsignificant (Supplemental Table S3).
CTC (r = 0.293, p = 0.001) and KIT+CTC (r = 0.183, p = 0.036) numbers examined after cycle one chemotherapy correlated with response (PR, SD, and PD), meaning that the higher level of CTC or KIT+CTC the lower efficacy of chemotherapy. CTC or KIT+CTC enumeration at other time points including baseline, cycle 4, and PD had no association with response.
CTC and KIT+CTC enumeration at baseline and survival
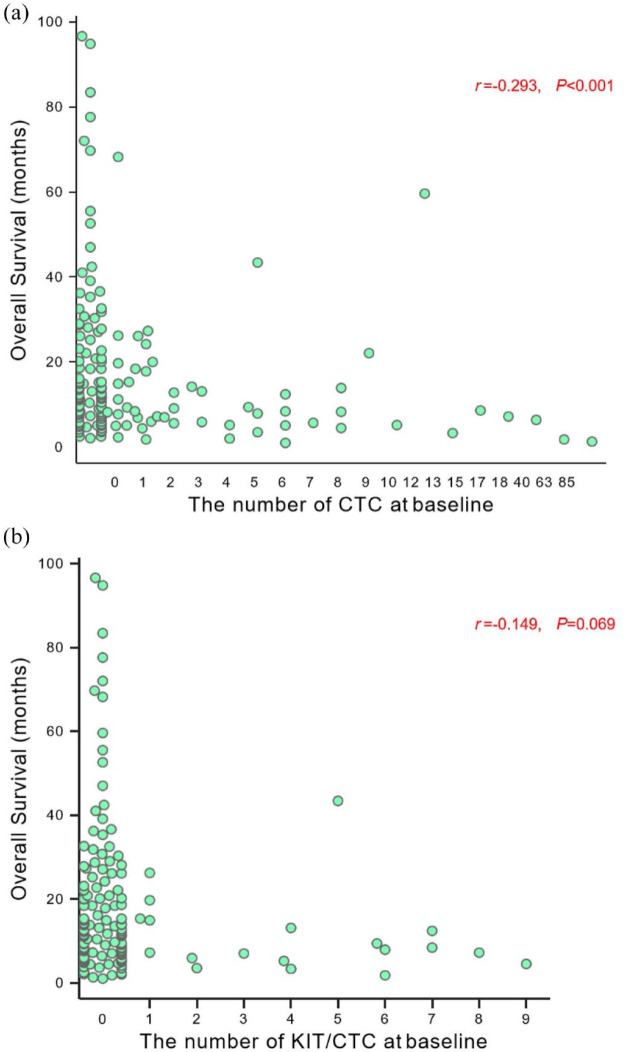
Spearman’s correlation analysis was performed to explore the associations of CTC and KIT+CTC level at baseline with OS. The survival superiority was related to CTC numbers from 0 to ⩾5 CTCs (r = −0.293, p < 0.001; Figure 2(a)) rather than that of KIT+CTC (r = −0.149, p = 0.069; Figure 2(b)).
Figure 2.
Association of (a) CTC and (b) KIT+CTC number at baseline with OS.
CTC, circulating tumor cell; OS, overall survival.
Longitudinal persistent negative CTC predict favorable prognosis
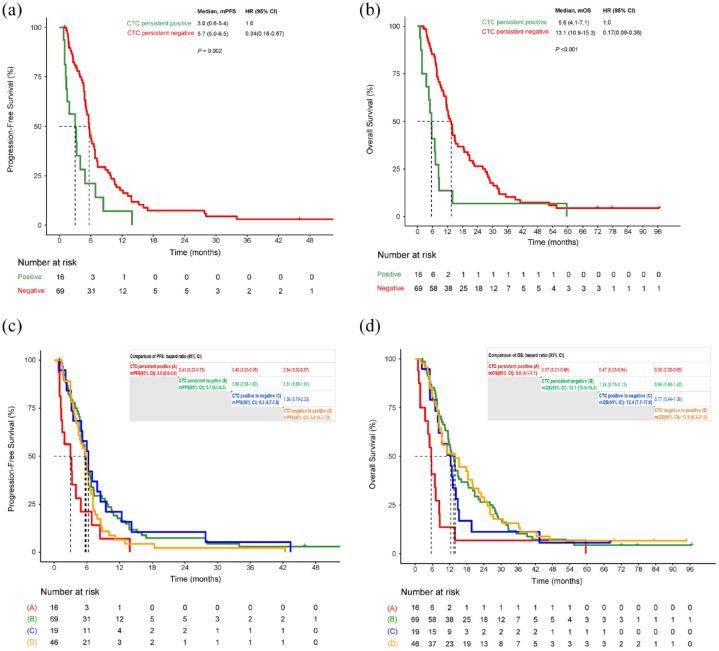
At four time points of blood sampling including baseline, cycle 1, cycle 4, and PD, patients with persistent negative CTC (46%, 69/150) had statically significantly longer PFS and OS than patients with persistent positive CTC (10.7%, 16/150) (Figure 3(a) and (b)). For patients with persistent negative KIT+CTC (74%, 111/150), the median PFS and OS were 5.8 (95% CI: 5.1–6.5) and 12.8 (95% CI: 10.9–14.7) months, respectively. There was only one patient with persistent positive KIT+CTC.
Figure 3.
PFS and OS for dynamic changes of CTC. (a) PFS for patients with persistent positive CTC versus persistent negative CTC. (b) OS for patients with persistent negative CTC versus persistent positive CTC. (c) PFS for dynamic changes of CTC. (d) PFS for dynamic changes of CTC.
CTC, circulating tumor cell; OS, overall survival; PFS, progression-free survival.
Influence of chemotherapy on CTC and KIT+CTC
CTC-positive rates at three different time points in chemotherapy including baseline, after one cycle, and after four cycles were 36.0% (54/150), 21.8% (31/142), and 13.7% (13/95) (p < 0.001) while KIT+CTC-positive rates were 13.3% (20/150), 4.9% (7/142), and 5.3% (5/95) (p = 0.016).
PFS and OS had no significant difference among patients with CTC transferred from positive to negative, CTC from negative to positive, and CTC persistent negative. These three groups had longer PFS and OS compared with CTC persistent positive (Figure 3(c) and (d)).
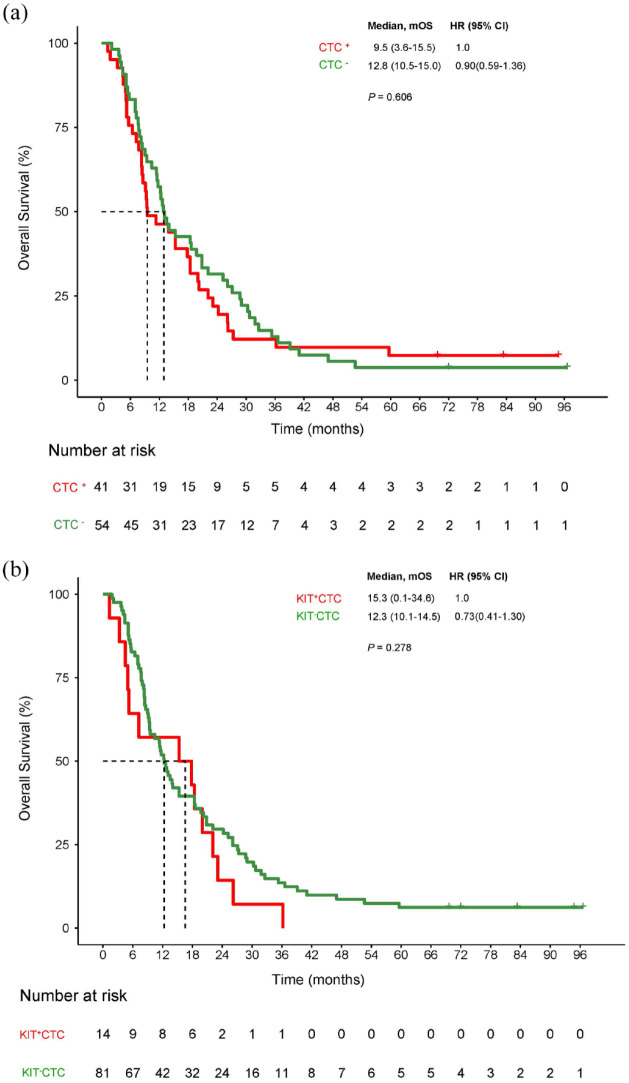
A total of 147 patients had PFS events with 120 disease progression and 95 patients had blood samples available at PD. Positive CTC was found in 41 and positive KIT+CTC in 14. The mOS had no difference between CTC positive and negative or between KIT+CTC positive and negative at PD (Figure 4(a) and (b)).
Figure 4.
Prognostics of CTC and KIT+CTC at disease progression. (a) OS for patients with positive CTC versus negative CTC. (b) OS for patients with positive KIT+CTC versus negative KIT+CTC.
CTC, circulating tumor cell; OS, overall survival.
Prognostic analysis revealed that positive CTC and KIT+CTC was associated with shorter PFS compared with negative CTC and KIT+CTC after cycle 1 and cycle 4 of chemotherapy. The presence of CTC and KIT+CTC was associated with shorter OS compared with negative CTC and KIT+CTC at baseline, after cycle 1, and cycle 4 of chemotherapy (Supplemental Figure S2).
Discussion
Detection of CTC has proved successful in predicting OS in various solid tumors and the cutoff value 5 was often used.4,7,11,25,26 However, no predefined CTC cutoff value was applied in metastatic NSCLC.27–29 In this study, we adopted CTC cutoff of ⩾1 in 7.5 mL of blood, same as previous report, 9 in which sensitivity and specificity were 71.0% and 83.0% at the CTC cutoff of ⩾1.
The detection rate of CTC using the CellSearch system in patients with advanced-stage NSCLC (IIIB-IV) varied from 24% to 75% with different settings including histology, sample size, CTC cutoff (1 or ⩾ 2), and blood volume (7.5, 10, or 20 mL).30,31
To our knowledge, CTONG1202 is the first study detecting longitudinal CTC combined with KIT-labeled CTC in monitoring chemotherapy in advanced NSCLC. Dynamic changes of CTC were more efficient in predicting efficacy and prognosis of chemotherapy than KIT+CTC. The presence of CTC represents the potential metastasis of cancer cells and therefore it is understandable that patient with persistent negative CTC presents with favorable prognosis.
Administration of chemotherapy could theoretically suppress potential metastasis of lung cancer cells, and four cycles of chemotherapy were more potent than one cycle. Actually, clearance of CTC at any time point in the whole process of chemotherapy led to more favorable prognosis. At this point, our results support the evidence that the addition of chemotherapy to ICIs enhanced their treatment efficacy as first-line treatment for advanced NSCLC patients. 32
The presence of CTC and KIT+CTC at baseline showed poor prognosis while their presence at disease progression had no influence on OS. Under high tumor burden in the context of resistance to chemotherapy, inability of CTC in predicting prognosis is diluted and in this situation radiological and clinical demonstration remain in the dominant position.
CSCs are a special subpopulation of cancer cells, which are responsible for the major capabilities of self-renewal and uncontrolled proliferation and differentiation, and increasing evidence suggests that CSCs contribute to cancer metastasis, a worse prognosis, and resistance to chemotherapy or radiation therapy. KIT or CD117 is one of embryonic stem cell surface markers and its expression in NSCLC tissue may be an independent prognostic indicator for worse OS in NSCLC. 23 In this study, prevalence of KIT-positive CTC was relatively low in the whole process of chemotherapy, probably owing to minority of stem cell in NSCLC. Detection rate of KIT-positive CTC at baseline was predictive of prognosis.
CTC presence before treatment was reportedly associated with low response rates and shorter PFS in chemotherapy or targeted therapy.7,28 Our study showed that CTC or KIT+CTC levels after the first cycle of chemotherapy rather than at other time points were associated with efficacy, suggesting that CTC might be an early indicator of response in chemotherapy.
Besides CTC, liquid biopsies in lung cancer include circulating tumor DNA (ctDNA), circulating microRNAs, circulating RNA, platelets, plasma/serum metabolites, or exosomes. Only CTCs and ctDNA could facilitate therapeutic decision-making due to high specificity. 33 Cancer personalized profiling by deep sequencing ctDNA analysis can reliably identify posttreatment molecular residual disease in patients with early-staged lung cancer and could allow personalized adjuvant treatment when disease burden is lowest.34–36 CTCs analysis has advantages such as evaluating cancer phenotype, 33 and single-cell sequencing of CTC avoids tumor heterogeneity by analyzing single-cell genomes, transcriptomes, and epigenetic groups in CTCs. 37
The limitation of this study lies in that the regimen adopted was platinum-based chemotherapy, which is not the best option for NSCLC without targetable driving mutations. It is due to that ICIs were not approved as first-line therapy for advanced NSCLC at the time of initiation. Furthermore, the technique of CTC capture needs to be improved for the sake of emergence of novel therapeutics and biomarker detection.
In summary, CTC persistent presence during treatment represents poor prognosis and resistance to therapy in advanced NSCLC. Chemotherapy could effectively eliminate CTCs. Molecular characteristics and the function of CTC will be warranted for further intensive investigation.
Supplemental Material
Supplemental material, sj-docx-3-tam-10.1177_17588359231167818 for Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients by Zhen Wang, Xu-Chao Zhang, Wei-Neng Feng, Li Zhang, Xiao-Qing Liu, Wei-Bang Guo, Yan-Ming Deng, Qing-Feng Zou, Jin-Ji Yang, Qing Zhou, Bin-Chao Wang, Hua-Jun Chen, Hai-Yan Tu, Hong-Hong Yan and Yi-Long Wu in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-4-tam-10.1177_17588359231167818 for Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients by Zhen Wang, Xu-Chao Zhang, Wei-Neng Feng, Li Zhang, Xiao-Qing Liu, Wei-Bang Guo, Yan-Ming Deng, Qing-Feng Zou, Jin-Ji Yang, Qing Zhou, Bin-Chao Wang, Hua-Jun Chen, Hai-Yan Tu, Hong-Hong Yan and Yi-Long Wu in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-5-tam-10.1177_17588359231167818 for Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients by Zhen Wang, Xu-Chao Zhang, Wei-Neng Feng, Li Zhang, Xiao-Qing Liu, Wei-Bang Guo, Yan-Ming Deng, Qing-Feng Zou, Jin-Ji Yang, Qing Zhou, Bin-Chao Wang, Hua-Jun Chen, Hai-Yan Tu, Hong-Hong Yan and Yi-Long Wu in Therapeutic Advances in Medical Oncology
Supplemental material, sj-jpg-1-tam-10.1177_17588359231167818 for Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients by Zhen Wang, Xu-Chao Zhang, Wei-Neng Feng, Li Zhang, Xiao-Qing Liu, Wei-Bang Guo, Yan-Ming Deng, Qing-Feng Zou, Jin-Ji Yang, Qing Zhou, Bin-Chao Wang, Hua-Jun Chen, Hai-Yan Tu, Hong-Hong Yan and Yi-Long Wu in Therapeutic Advances in Medical Oncology
Supplemental material, sj-jpg-2-tam-10.1177_17588359231167818 for Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients by Zhen Wang, Xu-Chao Zhang, Wei-Neng Feng, Li Zhang, Xiao-Qing Liu, Wei-Bang Guo, Yan-Ming Deng, Qing-Feng Zou, Jin-Ji Yang, Qing Zhou, Bin-Chao Wang, Hua-Jun Chen, Hai-Yan Tu, Hong-Hong Yan and Yi-Long Wu in Therapeutic Advances in Medical Oncology
Acknowledgments
We owe thanks to all patients and their families, all investigators and sites staff. All authors take full responsibility for this publication.
Footnotes
ORCID iD: Hong-Hong Yan  https://orcid.org/0000-0002-6228-2096
https://orcid.org/0000-0002-6228-2096
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Zhen Wang, Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China.
Xu-Chao Zhang, Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China.
Wei-Neng Feng, The First People’s Hospital of Foshan, Foshan, China.
Li Zhang, Cancer Center, Sun Yat-sen University, Guangzhou, China.
Xiao-Qing Liu, No. 307 Hospital of PLA, Beijing, China.
Wei-Bang Guo, Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China.
Yan-Ming Deng, The First People’s Hospital of Foshan, Foshan, China.
Qing-Feng Zou, Affiliated Cancer Hospital of Guangzhou Medical University, Guangzhou, China.
Jin-Ji Yang, Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China.
Qing Zhou, Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China.
Bin-Chao Wang, Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China.
Hua-Jun Chen, Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China.
Hai-Yan Tu, Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China.
Hong-Hong Yan, Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, 106 Zhongshan Er Rd, Guangzhou 510080, China.
Yi-Long Wu, Guangdong Lung Cancer Institute, Guangdong Provincial Key Laboratory of Translational Medicine in Lung Cancer, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, 106 Zhongshan Er Rd, Guangzhou 510080, China.
Declarations
Ethics approval and consent to participate: This study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (Ethics Code: 2012127H) on November 17, 2012. All participants provided written informed consent prior to enrolment in the study. This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Consent for publication: Not applicable.
Author contribution(s): Zhen Wang: Conceptualization; Formal analysis; Investigation; Methodology; Resources; Writing – original draft; Writing – review & editing.
Xu-Chao Zhang: Conceptualization; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Wei-Neng Feng: Formal analysis; Investigation; Resources; Writing – original draft; Writing – review & editing.
Li Zhang: Investigation; Resources; Writing – review & editing.
Xiao-Qing Liu: Investigation; Resources; Writing – review & editing.
Wei-Bang Guo: Formal analysis; Investigation; Resources; Writing – review & editing.
Yan-Ming Deng: Investigation; Resources; Writing – review & editing.
Qing-Feng Zou: Investigation; Resources; Writing – review & editing.
Jin-Ji Yang: Investigation; Resources; Writing – review & editing.
Qing Zhou: Investigation; Resources; Writing – review & editing.
Bin-Chao Wang: Investigation; Resources; Writing – review & editing.
Hua-Jun Chen: Investigation; Resources; Writing – review & editing.
Hai-Yan Tu: Investigation; Resources; Writing – review & editing.
Hong-Hong Yan: Conceptualization; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Yi-Long Wu: Conceptualization; Formal analysis; Investigation; Methodology; Resources; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by J&J and following grants: Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (No. 2017B030314120 to Yi-Long Wu); General Research Project of Guangzhou S&T Bureau (No. 201607010391 to Xu-Chao Zhang); Public Welfare and Capacity Establishment Program of Guangdong S&T Department (No. 2014A020212225 to Xu-Chao Zhang); Guangdong Provincial Applied S&T R&D Program (No. 2016B020237006 to Xu-Chao Zhang), Guangzhou Science and Technology Project (grant 201804010477 to Zhen Wang), CSCO Research Fund (grant Y-2019Roche-022 to Zhen Wang), and CSCO Research Fund (Y-2019AZMS-0034 to Zhen Wang).
Yi-Long Wu reports receiving speaker bureau fees from AstraZeneca, Bristol-Myers Squibb, Pfizer Inc., Roche AG, Boehringer Ingelheim, Eli Lilly & Co., Merck Sharp & Dohme, and Sanofi and research grants from AstraZeneca, Bristol-Myers Squibb, Pfizer Inc., and Roche AG. Other authors declare that there is no conflict of interest.
Availability of data and materials: The data generated in this study are available upon reasonable request from the corresponding author Dr. Yi-Long Wu (syylwu@live.cn).
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2.Socinski MA, Crowell R, Hensing TE, et al. Treatment of non-small cell lung cancer, stage IV: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132: 277S–289S. [DOI] [PubMed] [Google Scholar]
- 3.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004; 10: 6897–6904. [DOI] [PubMed] [Google Scholar]
- 4.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351: 781–791. [DOI] [PubMed] [Google Scholar]
- 5.Cohen SJ, Punt CJA, Iannotti N, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol 2009; 20: 1223–1229. [DOI] [PubMed] [Google Scholar]
- 6.Hou JM, Greystoke A, Lancashire L, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 2009; 175: 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011; 29: 1556–1563. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal C, Wang XM, Ranganathan A, et al. Circulating tumor cells as a predictive biomarker in patients with small cell lung cancer undergoing chemotherapy. Lung Cancer 2017; 112: 118–125. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 2009; 15: 6980–6986. [DOI] [PubMed] [Google Scholar]
- 10.Alix-Panabières C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res 2008; 14: 5013–5021. [DOI] [PubMed] [Google Scholar]
- 11.De Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14: 6302–6309. [DOI] [PubMed] [Google Scholar]
- 12.Wu CY, Lee CL, Wu CF, et al. Circulating tumor cells as a tool of minimal residual disease can predict lung cancer recurrence: a longitudinal, prospective trial. Diagnostics 2020; 10: 144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wankhede D, Grover S, Hofman P. Circulating tumor cells as a predictive biomarker in resectable lung cancer: a systematic review and meta-analysis. Cancers 2022; 14: 6112–6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Flaherty JD, Gray S, Richard D, et al. Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer 2012; 76: 19–25. [DOI] [PubMed] [Google Scholar]
- 15.Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res 2011; 17: 827–835. [DOI] [PubMed] [Google Scholar]
- 16.Marquette CH, Boutros J, Benzaquen J, et al. Circulating tumour cells as a potential biomarker for lung cancer screening: a prospective cohort study. Lancet Respir Med 2020; 8: 709–716. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol 2005; 13: 205–220. [DOI] [PubMed] [Google Scholar]
- 18.Wu CE, Tzen CY, Wang SY, et al. Clinical diagnosis of gastrointestinal stromal tumor (GIST): from the molecular genetic point of view. Cancers 2019; 11: 679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorelik E, Lokshin A, Levina V. Lung cancer stem cells as a target for therapy. Anticancer Agents Med Chem 2010; 10: 164–171. [DOI] [PubMed] [Google Scholar]
- 20.Waskow C, Paul S, Haller C, et al. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity 2002; 17: 277–288. [DOI] [PubMed] [Google Scholar]
- 21.Foster BM, Zaidi D, Young TR, et al. CD117/c-kit in cancer stem cell-mediated progression and therapeutic resistance. Biomedicines 2018; 6: 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su BB, Huang TT, Jin Y, et al. Apatinib exhibits synergistic effect with pyrotinib and reverses acquired pyrotinib resistance in HER2-positive gastric cancer via stem cell factor/c-kit signaling and its downstream pathways. Gastric Cancer 2021; 24: 352–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su Y, Chen R, Han Z, et al. Clinical and prognostic significance of CD117 in non-small cell lung cancer: a systemic meta-analysis. Pathobiology 2021; 88: 267–276. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 25.Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol 2010; 2010: 617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muinelo-Romay L, Vieito M, Abalo A, et al. Evaluation of circulating tumor cells and related events as prognostic factors and surrogate biomarkers in advanced NSCLC patients receiving first-line systemic treatment. Cancers (Basel) 2014; 6: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch assay and the isolation by size of epithelial tumor cell method. Int J Cancer 2011; 129: 1651–1660. [DOI] [PubMed] [Google Scholar]
- 28.Tamminga M, Wit SD, Schuuring E, et al. Circulating tumor cells in lung cancer are prognostic and predictive for worse tumor response in both targeted- and chemotherapy. Transl Lung Cancer Res 2019; 8: 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punnoose EA, Atwal S, Liu WQ, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012; 18: 2391–2401. [DOI] [PubMed] [Google Scholar]
- 30.Yu N, Zhou J, Cui F, et al. Circulating tumor cells in lung cancer: detection methods and clinical applications. Lung 2015; 193: 157–171. [DOI] [PubMed] [Google Scholar]
- 31.Yousefi M, Ghaffari P, Nosrati R, et al. Prognostic and therapeutic significance of circulating tumor cells in patients with lung cancer. Cell Oncol 2020; 43: 31–49. [DOI] [PubMed] [Google Scholar]
- 32.Dafni U, Tsourti Z, Vervita K, et al. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 2019; 134: 127–140. [DOI] [PubMed] [Google Scholar]
- 33.Calabuig-Fariñas S, Jantus-Lewintre E, Herreros-Pomares A, et al. Circulating tumor cells versus circulating tumor DNA in lung cancer-which one will win? Transl Lung Cancer Res 2016; 5: 466–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhuri AA, Chabon JJ, Lovejoy AF, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 2017; 7: 1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang JT, Liu SY, Gao W, et al. Longitudinal undetectable molecular residual disease defines potentially cured population in localized non–small cell lung cancer. Cancer Discov 2022; 12: 1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu SM, Jie GL, Wu YL. Toward a cure for lung cancer: important advances in operable non-small cell lung cancer. Sci Bull 2002; 67: 1402–1405. [DOI] [PubMed] [Google Scholar]
- 37.Xu JS, Liao KL, Yang X, et al. Using single-cell sequencing technology to detect circulating tumor cells in solid tumors. Mol Cancer 2021; 20: 104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-3-tam-10.1177_17588359231167818 for Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients by Zhen Wang, Xu-Chao Zhang, Wei-Neng Feng, Li Zhang, Xiao-Qing Liu, Wei-Bang Guo, Yan-Ming Deng, Qing-Feng Zou, Jin-Ji Yang, Qing Zhou, Bin-Chao Wang, Hua-Jun Chen, Hai-Yan Tu, Hong-Hong Yan and Yi-Long Wu in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-4-tam-10.1177_17588359231167818 for Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients by Zhen Wang, Xu-Chao Zhang, Wei-Neng Feng, Li Zhang, Xiao-Qing Liu, Wei-Bang Guo, Yan-Ming Deng, Qing-Feng Zou, Jin-Ji Yang, Qing Zhou, Bin-Chao Wang, Hua-Jun Chen, Hai-Yan Tu, Hong-Hong Yan and Yi-Long Wu in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-5-tam-10.1177_17588359231167818 for Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients by Zhen Wang, Xu-Chao Zhang, Wei-Neng Feng, Li Zhang, Xiao-Qing Liu, Wei-Bang Guo, Yan-Ming Deng, Qing-Feng Zou, Jin-Ji Yang, Qing Zhou, Bin-Chao Wang, Hua-Jun Chen, Hai-Yan Tu, Hong-Hong Yan and Yi-Long Wu in Therapeutic Advances in Medical Oncology
Supplemental material, sj-jpg-1-tam-10.1177_17588359231167818 for Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients by Zhen Wang, Xu-Chao Zhang, Wei-Neng Feng, Li Zhang, Xiao-Qing Liu, Wei-Bang Guo, Yan-Ming Deng, Qing-Feng Zou, Jin-Ji Yang, Qing Zhou, Bin-Chao Wang, Hua-Jun Chen, Hai-Yan Tu, Hong-Hong Yan and Yi-Long Wu in Therapeutic Advances in Medical Oncology
Supplemental material, sj-jpg-2-tam-10.1177_17588359231167818 for Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients by Zhen Wang, Xu-Chao Zhang, Wei-Neng Feng, Li Zhang, Xiao-Qing Liu, Wei-Bang Guo, Yan-Ming Deng, Qing-Feng Zou, Jin-Ji Yang, Qing Zhou, Bin-Chao Wang, Hua-Jun Chen, Hai-Yan Tu, Hong-Hong Yan and Yi-Long Wu in Therapeutic Advances in Medical Oncology