Abstract
Introduction
Chronic low-grade inflammation (LGI) plays a role in the pathogenesis of gestational diabetes mellitus (GDM). LGI, on the one hand, promotes insulin resistance and at the same time, affects fetal development. The study aimed to use clinically feasible means to evaluate the association between maternal LGI and maternal insulin resistance and fetal growth indices by ultrasound in the third trimester.
Methods
A crossectional and descriptive study on 248 first-time diagnosed GDM in Vietnam.
Results
Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte (PLR) indices were significantly higher in GDM than in normal glucose-tolerant pregnancies (p = 0.048 and 0.016, respectively). GDM with LGI witnessed significantly higher systolic blood pressure, BMI, HbA1c, and significantly lower quantitative Insulin Sensitivity Check Index (QUICKI) than those without LGI. After adjusting for maternal BMI, fasting plasma glucose (FPG), age, and parity, C-reactive protein (CRP) was positively correlated with HOMA2-IR (B=0.13, p<0.01) and Mathews index (B=0.29, p<0.01). Regarding fetal characteristics, LGI was associated with fetal growth indices in the third trimester of GDM. NLR was negatively correlated with estimated fetal weight (EFW) (B=−64.4, p<0.05) after adjusting for maternal BMI and FPG. After adjusting for maternal BMI, FPG, age, and parity, PLR was negatively correlated with biparietal diameter (B=−0.02, p<0.01) and abdominal circumference (AC) (B=−0.16, p<0.05), and EFW (B=−1.1, p<0.01), and head circumference (HC) (B=−0.06, p<0.01); CRP was negatively correlated with AC (B=−0.16, p<0.001), EFW (B=−85.3, p<0.001), and HC (B=−5.0, p<0.001).
Conclusion
In the third trimester, LGI was associated with maternal glucose and insulin resistance in GDM. Moreover, LGI was associated with fetal characteristics in ultrasonic images. There were negative correlations between LGI and fetal developmental characteristics.
Keywords: gestational diabetes mellitus, low-grade inflammation, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, fetal ultrasound parameters
Introduction
Gestational diabetes mellitus (GDM) is a condition with hyperglycemia that develops during pregnancy, which affects approximately 18.4 million cases, accounting for 86.4% of all hyperglycemia in pregnant women worldwide.1 The complications of diabetes affecting the mother and fetus have been studied extensively. Some complications for pregnant women can include preterm birth, preeclampsia, birth trauma, and postoperative wound complications.2 Some fetal complications include stillbirth due to early miscarriage or congenital disabilities, macrosomia, and growth restriction.3 Additionally, GDM patients are at increased risk of metabolic syndrome and cardiovascular events2,4 related to obesity and inflammation.4
Chronic low-grade inflammation (LGI), clinically assessed via C-reactive protein (CRP), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR), shows an essential influence on systemic inflammation and plays an important role in GDM pathophysiology.5–8 NLR is an expression of the equilibrium between the number of neutrophils and lymphocytes. Elevated NLR indicates an inflammatory imbalance in which the pro-inflammatory effect (mainly due to neutrophil activation) predominates over the anti-inflammatory effect (specifically, T lymphocytes).9,10 Pathophysiologically, LGI activates platelets to increase blood cell adhesion and hepatic CRP production.11,12 Currently, there have been some studies evaluating the characteristics of the LGI (via CRP, NLR, and PLR indices) and the GDM predictive value of indicators.5–8
Although there have yet been many studies on the effect of LGI on insulin resistance and fetal development, most studies focus on fetal nervous system development and fetal changes postnatally.13 Inflammation can affect insulin signaling by impairing beta-cell function and viability, which leads to a higher risk of diabetes.14 The inflammatory process also has a particular effect on the development of the fetus. Abnormal expression of interferon (IFN)-γ, IFN-β, tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-17, and IL-1β can lead to dysfunction in multiple fetal organs such as lung, brain, placenta.15 Moreover, maternal effects due to metabolic disorders on the development of the fetus during the third trimester contribute to the negative impact on the fetal metabolic health postnatally.16 Therefore, this study aimed to investigate the association of LGI, assessed through clinically feasible indicators, including CRP, NLR, and PLR, with maternal insulin resistance and fetal growth indices by ultrasound in GDM the third trimester of pregnancy.
Materials and Methods
Study Population and Design
Two-hundred and forty eight first-time diagnosed GDM and 40 normal glucose-tolerant (NGT) pregnant who had regular check-ups at the National Hospital of Endocrinology, Vietnam and Military Hospital 103 were enrolled in the study. The study was cross-sectional and descriptive. The enrollment duration was from January 2015 to December 2022.
In our study, GDM was first diagnosed between 24 and 28 weeks of gestation among participating women based on American Diabetes Association (ADA) 2014 criteria.17 Pregnant women established with a pre-pregnancy diabetes diagnosis or with medical conditions, treatments, or therapies that affect blood cells (infections, leukemia, blood cancer, ongoing glucocorticoids, and allergy) were not included in the study. NGT pregnancies were asssessed at week 24–28 and har normal glucose values at 3 time points before and during oral glucose tolerant test (OGT), ie, FPG, 1-hour, and 2-hour glucose values. Our study defined LGI as participants with GDM whose CRP levels ranged from 3 to 10 mg/L.18 NLR, PLR, and CRP were markers for LGI assessed in this study.
Clinical and Biochemical Assessments
All participants were clinically examined and checked for blood indicators according to research purposes. The clinical parameters included age, number of pregnancies, BMI, and blood pressure. Laboratory parameters measured from fasting venous blood samples on the same day of clinical examination included: fasting plasma glucose, HbA1c, insulin, lipid profiles (cholesterol, triglycerides), and total blood count. Insulin resistance indices, namely Homeostasis Model Assessment (HOMA)2-insulin resistance (IR), McAuley, Mathews, and quantitative Insulin Sensitivity Check Index (QUICKI) indices, were measured as previously described.19 All study participants were tested with OGTT with 75 grams of anhydrous glucose immediately after venipuncture on the examination day. Venous blood glucose levels were measured at 1 hour and 2 hours after OGTT. Particularly, CRP levels by high-sensitive assay were only performed in the GDM group due to restrictions of Vietnam health insurance.
Fetal ultrasounds were only performed in the GDM group. Fetal ultrasound technique and fetal ultrasound indicators (ie, BPD, biparietal diameter (mm); AC, abdominal circumference (mm); FL, femur length (mm); EFW, estimated fetal weight (g); AFI, amniotic fluid index (mm); HC, head circumference (mm)) were performed according to the recommendations of the American College of Radiology guidelines.20
Ethical Statement
Study participants agreed to join and provided written informed consent. The Institutional Review Board of Thai Binh University of Medicine and Pharmacy (Decision No. 1262/HĐĐĐ) approved the research proposal. Our study was conducted following the Declaration of Helsinki of 1964, as revised in 2013.
Statistical Analysis
Data were expressed as mean (standard deviation) and median (Q1-Q3). Pearson correlation was employed to measure correlations between CRP, NLR, and PLR and maternal biochemical parameters (BMI, FPG, 2-hour post-OGTT blood glucose, HbA1c, CRP, and insulin resistance indices) and fetal ultrasonography parameters, namely AC, HC, BDP, AFI, and EFW. Multivariate linear regression was employed to analyze the correlation between CRP, NLR, and PLR and maternal HbA1c and 2-hour post-OGTT blood glucose, insulin resistance indices, and fetal ultrasound characteristics. Significance was established at a P <0.05. All analyses were performed with SPSS 26.0.
Results
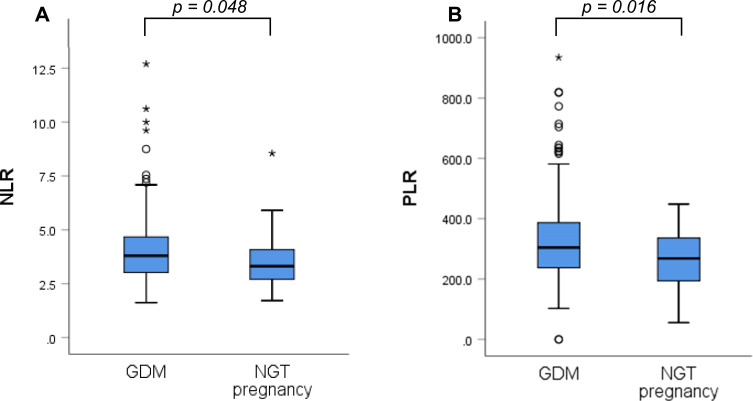
Regarding LGI, both NLR and PLR indices were significantly higher in GDM compared with NGT pregnancies (Figure 1).
Figure 1.
Comparison between gestational diabetes and normal glucose tolerant pregnancies regarding chronic low grade inflammation indices. (A) Neutrophil-to-lymphocyte index. (B) Platelet-to-lymphocyte index. (Mann–Whitney’s test).
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; GDM, gestational diabetes mellitus; NGT, normal glucose-tolerant.
In GDM, LGI was associated with systolic blood pressure (SBP), BMI, HbA1c, and QUICKI. Specifically, GDM women with LGI witnessed significantly higher SBP, BMI, HbA1c, and significantly lower QUICKI than those without LGI. No differences in age, parity, or lipid profiles (triglyceride and total cholesterol) were observed (Table 1).
Table 1.
Relationships Between Maternal Characteristics and Low-Grade Inflammation in Gestational Diabetes
| Low-Grade Inflammatory-GDM (n=96) | Non-Low-Grade Inflammatory-GDM (n=152) | p | |
|---|---|---|---|
| Age, years | 31 (28–35) | 30 (27–34) | 0.222 |
| SBP, mmHg | 110 (106–120) | 110 (100–120) | 0.005 |
| BMI, kg/m2 | 26.4±3.6 | 25.0±3.0 | 0.001 |
| Parity, times | 2 (1–3) | 2 (1–4) | 0.796 |
| HbA1c, % | 5.4 (5.1–5.6) | 5.3 (5.1–5.5) | 0.026 |
| QUICKI | 0.33±0.04 | 0.34±0.04 | 0.039 |
| Triglyceride, mmol/L | 2.7 (2.1–3.5) | 2.7 (2.1–3.6) | 0.912 |
| Cholesterol, mmol/L | 5.7±1.0 | 6.1±1.2 | 0.076 |
Notes: t-test for indepent variables and Mann–Whitney test were employed for comparing two means and two medians, respectively.
Abbreviations: GDM, gestational diabetes mellitus; SBP, systolic blood pressure; BMI, body mass index; QUICKI, Quantitative Insulin Sensitivity Check Index.
Regarding fetal characteristics in ultrasonic images, LGI was associated with fetal development in GDM in the third trimester of pregnancy. Specifically, BPD, AC, FL, EFW, and HC were significantly lower in the LGI group compared with the non-LGI group. No difference in AFI between the two groups was observed (Table 2).
Table 2.
Relationships Between Low-Grade Inflammatory Indices and Fetal Growth Indices in the Third Trimester in Gestational Diabetes
| Low-Grade Inflammatory-GDM (n=96) | Non-Low-Grade Inflammatory-GDM (n=152) | p | |
|---|---|---|---|
| BPD, mm | 79.5 (75.0–83.5) | 80.5 (74.0–85.0) | 0.018 |
| AC, mm | 261 (242.8–294.8) | 276.0 (242.8–305.3) | 0.008 |
| FL, mm | 57.5 (52.3–63.0) | 57.5 (51.0–63.0) | 0.016 |
| EFW, g | 1577.0 (1303.8–2191.0) | 1766.5 (1197.8–2306.3) | 0.007 |
| AFI, mm | 133.0 (96.8–168.3) | 146.0 (121.5–169.3) | 0.396 |
| HC, mm | 286.5 (265.3–310.5) | 294.5 (270.0–316.0) | 0.044 |
Note: Mann–Whitney test was employed for comparing two medians.
Abbreviations: GDM, gestational diabetes mellitus; BPD, biparietal diameter; AC, abdominal circumference; FL, femur length; EFW, estimated fetal weight; AFI, amniotic fluid index; HC, head circumference.
In the univariate and multivariate linear regression, we found that maternal LGI correlated with maternal insulin resistance. Specifically, while no correlations between NLR and PLR and maternal glucose indices and insulin resistance, CRP was positively correlated with HbA1c, HOMA2-IR, and Mathews index; negatively correlated with QUICKI and McAuley indices. After adjusting with maternal FPG, age, and parity, CRP was positively correlated with HOMA2-IR and Mathew index (Table 3).
Table 3.
Correlations Between Low-Grade Inflammation Indices and Maternal and Fetal Growth Indices
| BMI | G0 | G2 | CRP | HOMA2-IR (Insulin) | McAuley | QUICKI | Mathews | |
|---|---|---|---|---|---|---|---|---|
| Maternal NLR | ||||||||
| M1 | −0.07 | −0.11 | −0.13* | 0.21** | 0.05 | −0.03 | −0.02 | 0.01 |
| M2 | −0.17 | 0.33¶ | ||||||
| Maternal PLR | ||||||||
| M1 | −0.06 | −0.01 | −0.08 | 0.24¶ | 0.12 | −0.09 | −0.08 | 0.11 |
| M2 | 0.004¶ | |||||||
| Maternal CRP | ||||||||
| M1 | 0.24 | 0.08 | −0.11 | 0.24¶ | −0.18** | −0.19** | 0.26¶ | |
| M2 | 0.13** | −0.03 | −0.001 | 0.29** | ||||
Notes: *p <0.05; **p <0.01; ¶p <0.001. NLR, PLR, and CRP: Model (M)1 = Pearson correlation without any adjustments. M2 = NLR adjusted with maternal BMI and maternal FPG; PLR adjusted with maternal BMI, maternal FPG, maternal age, and maternal parities, CRP adjusted with maternal BMI, maternal FPG, maternal age, and maternal parity.
Abbreviations: BMI, Body Mass index (kg/m2); G0, fasting plasma glucose (mmol/L); G2, 2-hour post oral glucose tolerant test plasma glucose (mmol/L); NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; CRP, C-reactive protein; HOMA2-IR, Homeostasis Model Assessment-2-Insulin resistance; QUICKI, Quantitative Insulin Sensitivity Check Index.
In the univariate and multivariate linear regression, we found that maternal LGI was negatively correlated with fetal characteristics in ultrasonic images in the third trimester. NLR was negatively correlated with fetal EFW. PLR negatively correlated with fetal BPD, AC, EFW, and HC. CRP was negatively correlated with AC, EFW, and HC (Table 4).
Table 4.
Correlations Between Low-Grade Inflammation Indices and Fetal Growth Indices
| BPD | AC | FL | EFW | AFI | HC | |
|---|---|---|---|---|---|---|
| Maternal NLR | ||||||
| M1 | 0.003 | −0.06 | 0.04 | −0.15* | 0.03 | −0.09 |
| M2 | −64.4* | |||||
| Maternal PLR | ||||||
| M1 | −0.17* | −0.16* | −0.10 | −0.22** | −0.05 | −0.22** |
| M2 | −0.02** | −0.06* | −1.1** | −0.06** | ||
| Maternal CRP | ||||||
| M1 | −0.13 | −0.16* | −0.09 | −0.15* | −0.08 | −0.16* |
| M2 | −5.5¶ | −85.3¶ | −5.0¶ | |||
Notes: *p <0.05; **p <0.01; ¶p <0.001. NLR, PLR, and CRP: Model (M)1 = Pearson correlation without any adjustments. M2 = NLR adjusted with maternal BMI and maternal FPG; PLR adjusted with maternal BMI, maternal FPG, maternal age, and maternal parity, CRP adjusted with maternal BMI, maternal FPG, maternal age, and maternal parities.
Abbreviations: BPD, biparietal diameter (mm); AC, abdominal circumference (mm); FL, femur length (mm); EFW, estimated fetal weight (gram); AFI, amniotic fluid index (mm); HC, head circumference (mm); NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; CRP, C-reactive protein (mg/L).
Discussion
Our results show that NLR and PLR, markers for LGI, in GDM were significantly higher than in NGT pregnant women. LGI was positively correlated with insulin resistance indices in GDM. With regard to the fetus, LGI was negatively correlated with BPD, AC, EFW, and HC.
Our study shows that, compared with NGT pregnant women, PLR and NLR in GDM were significantly higher in GDM. However, the change in immune response in GDM is unclear. Several studies have shown different results for changes in neutrophil, lymphocyte, and platelet components in GDM compared with NGT pregnancy. Most studies showed an increase in the above components in the GDM group compared with the NGT pregnancy.21–23 The study by Sahin et al found that NLR was positively correlated with FPG (r = 0.285, p = 0.001) in GDM in the third trimester of pregnancy.21 In contrast, the study of Hassan et al did not find any difference in the above components between pregnant women with GDM or without GDM.24 This difference is probably due to the difference in the timing of the study (the first or third trimester). Most studies that reported increased blood counts were done in the third trimester. Therefore, our study contributes to further strengthening the association of LGI in GDM in the third trimester of pregnancy.
Many studies have shown that pregnant women with GDM have increased inflammatory factors such as interleukin 6, human chitinase-3-like protein 1, leptin, and resistin.25,26 Clinically, quantifying cytokines and chemokines indicative of LGI is not routinely performed. Instead, CRP levels, NLR, and PLR are feasibly implemented. Many studies have shown that CRP, NLR, and PLR are predictive markers of the risk of developing GMD. A meta-analysis of 1271 participants with GDM and 1504 NGT pregnancies found that NLR was higher in the former group (SMD = 0.58, p < 0.001).5 LGI contributes to insulin resistance through various mechanisms.27 First, LGI affects adipose, liver, and skeletal muscle tissues through macrophage penetration and the production of inflammatory cytokines within these tissues (eg, TNF-α) that decrease peripheral tissue sensitivity to insulin through inhibition of the insulin receptor substrate (IRS)-protein kinase B (Akt)-glucose transport (GLUT) pathway.28 In addition, chronic inflammation promotes inflammation in the pancreas leading to increased beta-cell apoptosis. Interestingly, among LGI markers, CRP is consistently associated with insulin resistance. In our study, although LGI was associated with insulin resistance, with insulin resistance and HbA1c in GDM with LGI being significantly higher than in the group without LGI, only was CRP negatively correlated with insulin resistance in GDM.29,30
Our results indicated that LGI was related to fetal development in the third trimester. In the study of Fink et al, maternal CRP levels in the third trimester were associated with fetal CRP levels one week after birth, and the correlation persisted until newborn babies aged 6-month-old, with β of 0.39 and 0.11 and p of <0.01 and 0.03, respectively. The impact of LGI on the fetus was independent of maternal BMI.31 In terms of fetal development, LGI is negatively correlated with indicators of fetal development. LGI is associated with decreased levels of Insulin-like growth factor (IGF)-1 and IGF binding protein (IGFBP)-3, contributing to the decline in linear fetal growth.32 Additionally, LGI is also associated with an increase in proinflammatory cytokines.33 These cytokines also contribute to fetal growth retardation and preterm birth; however, the mechanism is unclear.34,35 In GDM, cytokines such as IL-6, IL-2, and TNF-alpha increased compared to ordinary pregnant women.36 However, there are few studies on the effects of LGI on fetal development in the prenatal period. A recent study by author Shafiq et al on 218 Indian pregnant women found that the chronic inflammatory status was related to lower birth weight with adjusted β = −0.07; 95% CI, −0.14 to 0.001 and lower length for gestational age with adjusted β = −0.10; 95% CI, −0.18 to −0.01.34 However, the effects of insulin resistance and related factors in GDM on fetal development are complex.37 Insulin resistance and maternal obesity during pregnancy also affect the development of the fetus.38 In our study, when analyzing multivariate regression, the LGI evaluation indices were negatively correlated with some fetal development indicators after adjusting with maternal BMI and FPG, especially the EFW. The above result highlights the influence of LGI on fetal development.
However, when comparing the associations of specific components of LGI and maternal insulin resistance and fetal characteristics, CRP was positively and negatively correlated with maternal insulin resistance indices and fetal development indices, respectively, but NLR and PLR were not. This difference was probably due to a number of reasons. First, CRP has a long half-life (~19 hour)39 and is relatively stable compared with blood cells, for example, the half-life of neutrophils is 7 hour,40 and platelet turnover is increased in GDM.41 Furthermore, CRP is measured by highly sensitive assays and increases nonspecifically in inflammatory settings.
Our study has some limitations. First, the study was designed to be a cross-sectional description, so causality cannot be inferred from this cross-sectional study. Second, fetal variability has not been longitudinally monitored until postpartum to confirm clear fetal consequences of LGI. Third, the maternal characteristics such as the supplements of nutrition, education, ethnic, previous data of glucose tests have not been fully exploited, so it is difficult to analyze all the factors affecting the development of the fetus.
Conclusion
In the third trimester, LGI increase leads to increases in maternal glucose and insulin resistance in GDM. Moreover, LGI was associated with fetal characteristics in ultrasonic images. There were negative correlations between LGI and fetal developmental characteristics, which implies that LGI contributes to the retards in fetal development in GDM. Therefore, the evaluation of LGI by CRP, NLR, and PLR should be included in the examination of pregnant women with GDM at weeks 24 to 28. Future studies of LGI-preventing interventions should be conducted to evaluate the benefit of LGI reduction on fetal development as well as insulin resistance in GDM.
Acknowledgments
We thank the staff in the Outpatients Department of the Vietnam Endocrinology Hospital and Department of Endocrinology and Rheumatology, Military Hospital 103, for collecting the samples and supporting the study.
Funding Statement
The author(s) received no financial support for this article’s research, authorship, and/or publication.
Abbreviations
GDM, Gestational Diabetes mellitus; LGI, low-grade inflammation; FPG, fasting plasma glucose; PPG, postprandial glucose; OGTT, oral glucose tolerance test; BMI, Body Mass index; G2, 2-hour post OGTT; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; CRP, C-reactive protein; HOMA2-IR, Homeostasis Model Assessment-2-Insulin resistance; QUICKI, Quantitative Insulin Sensitivity Check Index; BPD, Biparietal diameter; HC, Head circumference; AC, Abdominal circumference; FL, Femur length; AFI, Amniotic fluid index; HR, fetal Heart rate; EFW, Estimated fetal weight.
Author Contributions
Kien Xuan Nguyen and Tien Bui Minh share first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no potential conflicts of interest to this article’s research, authorship, and/or publication.
References
- 1.Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Prac. 2018;138:271–281. [DOI] [PubMed] [Google Scholar]
- 2.Yogev Y, Visser GHA. Obesity, gestational diabetes and pregnancy outcome. Semin Fetal Neonatal Med. 2009;14:77–84. doi: 10.1016/j.siny.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 3.Kondracki AJ, Valente MJ, Ibrahimou B, Bursac Z. Risk of large for gestational age births at early, full and late term in relation to pre‐pregnancy body mass index: mediation by gestational diabetes status. Paediatr Perinat Epidemiol. 2022;36:566–576. doi: 10.1111/ppe.12809 [DOI] [PubMed] [Google Scholar]
- 4.Al-Ofi E, Alrafiah A, Maidi S, Almaghrabi S, Hakami N. Altered expression of angiogenic biomarkers in pregnancy associated with gestational diabetes. Int J Gen Med. 2021;14:3367–3375. doi: 10.2147/IJGM.S316670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pace NP, Vassallo J. Association between neutrophil-lymphocyte ratio and gestational diabetes—a systematic review and meta-analysis. J Endocrinol Soc. 2021;5(7). doi: 10.1210/jendso/bvab051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessami K, Tabrizi R, Homayoon N, et al. Gestational diabetes mellitus and inflammatory biomarkers of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio: a systematic review and meta-analysis. Biomarkers. 2021;26(6):491–498. doi: 10.1080/1354750X.2021.1926542 [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Lou X, Zhang Z, Chai Y, Yu Q. Association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, mean platelet volume with the risk of gestational diabetes mellitus. Gynecol Endocrinol. 2021;37(2):105–107. doi: 10.1080/09513590.2020.1780579 [DOI] [PubMed] [Google Scholar]
- 8.Amirian A, Rahnemaei FA, Abdi F. Role of C-reactive Protein (CRP) or high-sensitivity CRP in predicting gestational diabetes mellitus: systematic review. Diabetes Metab Syndr. 2020;14(3):229–236. doi: 10.1016/j.dsx.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Chávez Valencia V, Orizaga de la Cruz C, Mejía Rodríguez O, et al. Inflammation in hemodialysis and their correlation with neutrophil-lymphocyte ratio and platelet- lymphocyte ratio. Nefrología. 2017;37:554–556. doi: 10.1016/j.nefro.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Wang W, Zeng L, et al. Association between neutrophil-lymphocyte ratio and all-cause mortality and cause-specific mortality in US adults, 1999–2014. Int J Gen Med. 2021;14:10203–10211. doi: 10.2147/IJGM.S339378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balta S, Ozturk C. The platelet-lymphocyte ratio: a simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets. 2015;26(7):680–681. doi: 10.3109/09537104.2014.979340 [DOI] [PubMed] [Google Scholar]
- 12.Stanimirovic J, Radovanovic J, Banjac K, et al. Role of C-reactive protein in diabetic inflammation. Mediators Inflamm. 2022;2022:1–15. doi: 10.1155/2022/3706508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goeden N, Velasquez J, Arnold KA, et al. Maternal inflammation disrupts fetal neurodevelopment via increased placental output of serotonin to the fetal brain. J Neurosci. 2016;36(22):6041–6049. doi: 10.1523/JNEUROSCI.2534-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- 15.Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018;49(3):397–412. doi: 10.1016/j.immuni.2018.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogt MC, Paeger L, Hess S, et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell. 2014;156(3):495–509. doi: 10.1016/j.cell.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant RW, Donner TW, Fradkin JE, et al. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14–S80. doi: 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 18.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49(12):1958–1970. doi: 10.1017/S0033291719001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinh LT, Minh Bui T, Hien Vu T, et al. Insulin resistance in gestational diabetes mellitus and its association with anthropometric fetal indices. Clin Med Insights Endocrinol Diabetes. 2022;15:117955142210984. doi: 10.1177/11795514221098403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Radiology. Practice guideline for the performance of obstetrical ultrasound. In: Practical Guideline and Technical Standards. Reston. VA: American College of Radiology; 2007:1025–1033. [Google Scholar]
- 21.Sahin M, Oguz A, Tüzün D, et al. A new marker predicting gestational diabetes mellitus: first trimester neutrophil/lymphocyte ratio. Medicine. 2022;101(36):e30514. doi: 10.1097/MD.0000000000030511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yılmaz ZV, Yılmaz E, Içer B, Küçüközkan T. Association of complete blood count parameters with gestational diabetes mellitus. GORM. 2017;23:65–69. doi: 10.21613/GORM.2016.649 [DOI] [Google Scholar]
- 23.Yang H, Zhu C, Ma Q, Long Y, Cheng Z. Variations of blood cells in prediction of gestational diabetes mellitus. J Perinat Med. 2014;43:89. doi: 10.1515/jpm-2014-0007 [DOI] [PubMed] [Google Scholar]
- 24.Hassan B, Rayis DA, Musa IR, et al. Blood groups and hematological parameters do not associate with first trimester gestational diabetes mellitus (institutional experience). Ann Clin Lab Sci. 2021;51(1):97–101. [PubMed] [Google Scholar]
- 25.Aktulay A, Engin-Ustun Y, Ozkan MS, et al. Gestational diabetes mellitus seems to be associated with inflammation. Acta Clin Croat. 2015;54(4):475–478. [PubMed] [Google Scholar]
- 26.Vrachnis N, Belitsos P, Sifakis S, et al. Role of adipokines and other inflammatory mediators in gestational diabetes mellitus and previous gestational diabetes mellitus. Int J Endocrinol. 2012;2012:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.León-Pedroza JI, González-Tapia LA, Del Olmo-Gil E, et al. Low-grade systemic inflammation and the development of metabolic diseases: from the molecular evidence to the clinical practice. Cir Cir. 2015;83:543–551. doi: 10.1016/j.circir.2015.05.041 [DOI] [PubMed] [Google Scholar]
- 28.Li H, Meng Y, He S, et al. Macrophages, chronic inflammation, and insulin resistance. Cells. 2022;11(19):3001. doi: 10.3390/cells11193001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin T, Abbasi F, Lamendola C, et al. Differentiation between obesity and insulin resistance in the association with C-reactive protein. Circulation. 2002;106(23):2908–2912. doi: 10.1161/01.CIR.0000041046.32962.86 [DOI] [PubMed] [Google Scholar]
- 30.Festa A, Hanley AJG, Tracy RP, D’Agostino R, Haffner SM. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003;108:1822–1830. doi: 10.1161/01.CIR.0000091339.70120.53 [DOI] [PubMed] [Google Scholar]
- 31.Fink NR, Chawes B, Bønnelykke K, et al. Levels of systemic low-grade inflammation in pregnant mothers and their offspring are correlated. Sci Rep. 2019;9(1):3043. doi: 10.1038/s41598-019-39620-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Sun H, Ma B, et al. Insulin-like growth factor 1 related to chronic low-grade inflammation in patients with obesity and early change of its levels after laparoscopic sleeve gastrectomy. Obes Surg. 2020;30(9):3326–3332. doi: 10.1007/s11695-020-04473-9 [DOI] [PubMed] [Google Scholar]
- 33.Koelman L, Pivovarova-Ramich O, Pfeiffer AFH, Grune T, Aleksandrova K. Cytokines for evaluation of chronic inflammatory status in ageing research: reliability and phenotypic characterisation. Immun Ageing. 2019;16(1):11. doi: 10.1186/s12979-019-0151-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafiq M, Mathad JS, Naik S, et al. Association of maternal inflammation during pregnancy with birth outcomes and infant growth among women with or without HIV in India. JAMA Netw Open. 2021;4(12):e2140584. doi: 10.1001/jamanetworkopen.2021.40584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero R, Espinoza J, Gonçalves LF, et al. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–326. doi: 10.1016/j.siny.2006.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, et al. Cytokine levels in gestational diabetes mellitus: a systematic review of the literature. Am J Reprod Immunol. 2013;69(6):545–557. doi: 10.1111/aji.12088 [DOI] [PubMed] [Google Scholar]
- 37.Mirabelli M, Chiefari E, Tocci V, et al. Gestational diabetes: implications for fetal growth, intervention timing, and treatment options. Curr Opin Pharmacol. 2021;60:1–10. doi: 10.1016/j.coph.2021.06.003 [DOI] [PubMed] [Google Scholar]
- 38.Page KA, Luo S, Wang X, et al. Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain. Diabetes Care. 2019;42(8):1473–1480. doi: 10.2337/dc18-2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI200318921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter CM. Alterations in blood components. In: Comprehensive Toxicology. Elsevier; 2018:249–293. doi: 10.1016/B978-0-12-801238-3.64251-4 [DOI] [Google Scholar]
- 41.Baldane S, Ipekci SH, Kebapcilar A. Relationship between insulin resistance and mean platelet volume in gestational diabetes mellitus. J Lab Physicians. 2015;7(02):112–115. doi: 10.4103/0974-2727.163134 [DOI] [PMC free article] [PubMed] [Google Scholar]