Abstract
Introduction
Recently, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) has published an update on the Global Strategy for Prevention, Diagnosis and Management of COPD, introducing a new classification of chronic obstructive pulmonary disease (COPD). Our aim was to assess the prognostic value of the new GOLD classification system in comparison with the previous GOLD classification systems (GOLD stages I–IV and GOLD groups A-D) and the BODE index.
Methods
We used the data of 784 patients with COPD from the Czech Multicenter Research Database of COPD. Patient survival was analyzed with the use of Kaplan–Meier estimate and Cox model of proportional risks. ROC analysis and area under curve (AUC) were used for comparison of GOLD classifications and BODE index. The analyses were performed with the use of software R (version 4.2.0).
Results
We analyzed data of 782 patients with complete data on GOLD classifications. The study population comprised 72.9% of men, 89.1% current or former smokers, with a mean age of 66.6 years, a mean BMI of 27.4 and a mean FEV1 44.9% of predicted. Probability of 5-year survival differed by GOLD classification. Application of the 2023 GOLD classification showed increased risk of death in group B (HR 1.82, 95% CI 1.14–2.92; p = 0.013) and in group E (HR 2.48, 95% CI 1.54–3.99; p˂0.001). The ROC analysis showed that the overall prognostic value of the 2023 GOLD classification was similarly weak to previous A-D GOLD classification schemes (AUCs 0.557–0.576) and was lower compared to the GOLD 1–4 system (AUC 0.614) and even lower when compared to the BODE index (AUC 0.715).
Conclusion
We concluded that the new GOLD classification system has poor prognostic properties and that specific prediction tools (eg, the BODE index) should be used for mortality risk assessment.
Keywords: GOLD classification, COPD, mortality, prognosis
Introduction
By January 2023, the Global Initiative for Obstructive Lung Disease (GOLD) has published an update on its flagship document, the Global Strategy for Prevention, Diagnosis and Management of COPD, briefly the 2023 GOLD Report. Among other new conceptual elements of the document, a new classification of chronic obstructive pulmonary disease (COPD) has been proposed, limiting the case classification to only three groups/categories, based on symptoms degree and exacerbation history.1 The three categories include group A (oligo-/asymptomatic patients with an annual up to one moderate exacerbation), group B (symptomatic patients with an annual up to one moderate exacerbation) and group E (patients with an annual rate of ≥1 severe or ≥2 moderate exacerbations, regardless of symptoms degree).1
Despite the new GOLD classification of COPD is not intended for use as a prognostic tool, clinicians expect at least basic prognostic properties when using any disease classification system. Previous works reported that the prognostic properties of the latest GOLD classification were poor.2–4 Instead, composite multifactorial predictive tools should be used to assess the long-term mortality risk. Of these, the BODE index has gained the widest international use among clinicians, with well-documented excellent prognostic power.5
In this study, we aimed to assess the prognostic value of the new GOLD classification system in a head-to-head comparison with the previous GOLD classification systems (GOLD stages I–IV and GOLD groups A-D) and with the BODE index.
Methods
Study Population
For the purpose of this study, we used the data from the Czech Multicenter Research Database of COPD (CMRD) comprising individual data of 784 patients with forced expiratory volume in 1 second (FEV1) ˂60% of predicted value.
A detailed description of the CMRD cohort was published previously.6 Briefly, CMRD was a prospective multicenter study aiming to assess pattern of morbidity and mortality in patients with moderate to severe COPD.6 The CMRD project was registered at ClinicalTrials.gov (identifier number NCT01923051) and at the Czech Republic State Institute for Drug Control (identifier number 1301100001). Patient recruitment took place at fourteen centers providing respiratory care between February 2013 and December 2016. The patients were followed up for five years since recruitment or until death; the 5-year follow-up was completed in December 2021.
The assessed parameters included data on patient history, demographics, lung function tests, quality of life measures, symptoms assessment, details on treatment, and others.
Statistical Analyses
Categorical parameters were described by absolute and relative frequencies. The number of patients with available data is presented. Relative frequencies were calculated from valid data. Continuous parameters were described by valid N, mean (standard deviation = SD) and median (interquartile range = IQR). Differences of categorical parameters between groups were tested by Pearson chi-square test or Fisher exact test. Differences of continuous parameters between the two groups were tested by the Mann–Whitney U-test and between more groups by the Kruskal–Wallis test.
Patient survival was analyzed with the use of Kaplan–Meier estimate and Cox model of proportional risks. Estimates of survival probability, survival median and hazard ratios (HR) were supplemented with 95% confidence intervals (CI). ROC analysis and area under curve (AUC) were used for comparison of GOLD classifications and BODE index.
Finally, Kaplan–Meier estimates were calculated for GOLD stages and GOLD groups A-B-E.
The analyses were performed with the use of software R (version 4.2.0). All hypotheses were tested on 5% level of significance.
Results
We analyzed data of 782 patients with complete data on GOLD classifications. The study population comprised 72.9% of men, 89.1% current or former smokers, with a mean age of 66.6 years and a mean BMI of 27.4. Further characteristics of the cohort are summarized in Table 1.
Table 1.
Basic Characteristics of the Cohort
| Number of Patients | ||
|---|---|---|
| Sex, n (%) | ||
| Female | 212 (27.1) |
782 |
| Male | 570 (72.9) | |
| Age at inclusion | 66.6 (9.2) | |
| BMI | 27.4 (6.2) | |
| Smoking, n (%) | ||
| Current-smoker | 159 (20.3) | |
| Ex-smoker | 538 (68.8) | |
| Non-smoker | 85 (10.9) | |
| mMRC, n (%) | ||
| 0 | 37 (4.7) | |
| 1 | 144 (18.4) | |
| 2 | 310 (39.6) | |
| 3 | 165 (21.1) | |
| 4 | 126 (16.1) | |
| CAT | 16.0 (7.8) | 775 |
| Symptoms, n (%) |
782 |
|
| Fatigue | 369 (47.7) | |
| Cough | 562 (71.9) | |
| Expectoration | 453 (57.9) | |
| Sputum | 46 (5.9) | |
| Lung functions | 782 | |
| FEV1 (% predicted) | 44.9 (11.7) | |
| FVC (% predicted) | 68.6 (17.6) | |
| FEV1/FVC (%) | 0.53 (0.12) | |
| TLco (% predicted) | 52.4 (21.9) | 509 |
| 6MWD (m) | 331.3 (131.7) | 598 |
| 6MWD (% predicted) | 61.9 (24.4) | |
Notes: Categorical parameters are described by absolute (relative) frequencies. Relative frequencies are calculated from valid data. Continuous variables are described by valid N and mean (standard deviation).
Abbreviations: CAT, COPD assessment test; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV1/FVC, Tiffeneau index; m, meters; mMRC, modified Medical Research Council dyspnea scale; n, number of patients; TLco, diffusion capacity of the lungs for carbon monoxide; 6MWD, distance covered during 6-minute walk test.
Probability of long-term (5-year) survival differed by GOLD classification. Application of the new 2023 GOLD classification showed increased risk of death in group B (HR 1.82, 95% CI 1.14–2.92; p = 0.013) and in group E (HR 2.48, 95% CI 1.54–3.99; p˂0.001).
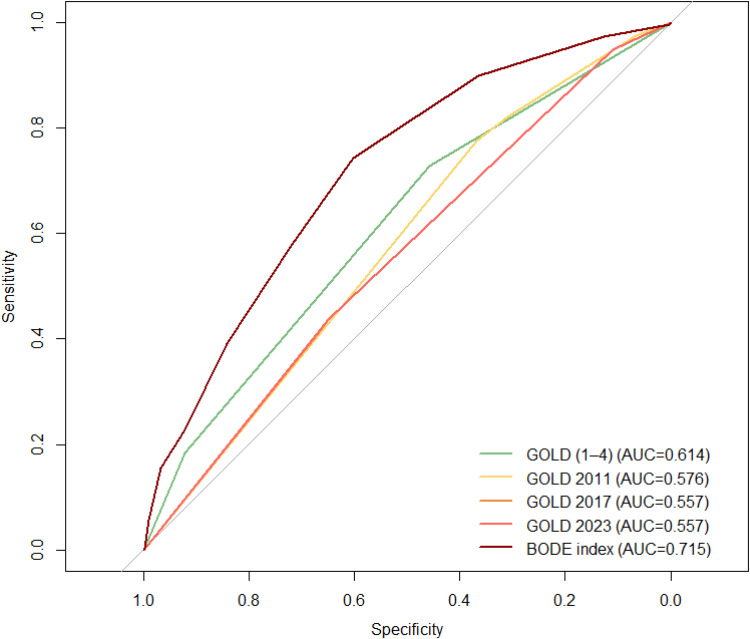
The ROC analysis showed that the overall prognostic value of the new 2023 GOLD classification was similarly weak to previous A-D GOLD classification schemes (AUCs 0.557–0.576) and was lower compared to the GOLD 1–4 system (AUC 0.614) and even lower when compared to BODE index (AUC 0.715) (Table 2 and Figure 1).
Table 2.
Results of the ROC Analysis Comparing Prognostic Properties of GOLD Classifications and BODE Index
| AUC (95% CI) | P | |
|---|---|---|
| GOLD (1–4) | 0.614 (0.577–0.651) | <0.001 |
| GOLD 2011 (A–D) | 0.576 (0.543–0.609) | <0.001 |
| GOLD 2017 (A–D) | 0.557 (0.521–0.592) | 0.006 |
| GOLD 2023 (A–E) | 0.557 (0.521–0.592) | 0.006 |
| BODE index | 0.715 (0.675–0.756) | <0.001 |
Note: Statistically significant changes are indicated in bold.
Abbreviations: AUC, area under the curve; BODE, acronym for Body mass index, Obstruction, Dyspnea and Exercise tolerance; GOLD, the Global Initiative for Chronic Obstructive Lung Disease; 95% CI, 95% confidence interval.
Figure 1.
ROC analysis with comparison of all GOLD classifications and the BODE index.
Survival analysis according to combinations of GOLD stages (I–IV) and 2023 GOLD groups revealed the highest probability of 5-year survival in group A patients (71–100%) while highest probability of death was noted in subgroups 3E, 4E and 4B (60%, 60% and 68%, respectively) (Supplementary Table and Supplementary Figure).
Discussion
The main finding of this study was that the new 2023 GOLD (A-B-E) classification system has comparably weak ability to predict risk of mortality in COPD patients as the previous GOLD classifications.
Prognosis assessment is an important issue in the management of chronic diseases as it identifies high-risk patients deserving of focused therapeutic effort. While in other chronic disorders, disease staging/classification is usually associated with prognosis, eg, the NYHA classes of CHF, the CKD classification of chronic renal failure or the TNM Classification of Malignant Tumors, the GOLD classification of COPD lost part of its prognostic power with shifting from GOLD stages (I–IV) to groups (A-D).2–4
Poor prognostic value of the GOLD A-D classification systems has been demonstrated previously.2–4 This finding relates to the determinants of the latest classification schemes, namely to symptoms degree and exacerbation history. Both exacerbations of COPD and symptoms are known to be associated with mortality risk.5,7 However, the COPD-specific complex/multidimensional instruments proved to be more accurate in terms of prognosis assessment, comprising various factors with variable strength of association with mortality risk. These include FEV1, distance walked during 6-minute walk test (for BODE index)5 or age, diffusion capacity of the lungs for carbon monoxide and chronic heart failure (CHF) in patient history (for the CADOT index).8 In our previous study, we demonstrated that the c-statistics (a parameter equal to the AUC in ROC analysis) of the BODE index and the CADOT index were significantly higher (0.677–0.799 and 0.701–0.842)8 when compared to the AUCs of GOLD classifications in the current study (0.557–0.576). In another Spanish work, the c-statistic of the original and recalculated BODE index was 0.61.9
Of the available pharmacological and non-pharmacological therapies, only a few of them decrease the risk of mortality, including smoking cessation, domiciliary long-term oxygen therapy, home non-invasive ventilation, alpha-1 antitrypsin augmentation therapy, lung-volume reduction surgery and lung transplant.10–15 While smoking cessation interventions can be performed in each patient and irrespective of disease severity, the other therapeutic options are limited to the small number of COPD patients with specific disease patterns and satisfying predefined indication criteria.16 These patients are more prevalent in high-risk subpopulation. It is therefore important to identify high-risk patients among the general COPD population.
One of the possible strategies is to combine various factors with relevance to mortality risk. As demonstrated in our previous studies, a combination of multiple factors may increase the prognostic value.5,8,17 In this recent study, we combined GOLD stages and groups A-B-E, identifying subgroups 3E, 4E and 4B as clearly high-risk, while oligosymptomatic patients (letter A subgroups - 2A, 3A and 4A) were at relatively low risk of 5-year mortality. Alongside the multidimensional instruments, new prognostic factors emerged recently, including physical capacity (PC).18 It was found that patients with COPD with a decreased PC had a significantly higher long-term mortality risk, regardless of the level of physical activity.18 Presumably, combining GOLD groups and PC could increase the prognostic ability, similarly to our previous work where we combined GOLD groups and arterial blood gases, clearly increasing prognostic value at least in GOLD group B.17
Limitations of this study include: a potential selection bias (the CMRD cohort is composed of patients with moderate-to-severe COPD with FEV1< 60% predicted), and a potential gender bias (the cohort included 73% of men).
Conclusion
We concluded that the new GOLD classification system has poor prognostic properties and that specific prediction tools (eg, the BODE) should be used for mortality risk assessment.
Acknowledgments
The authors thank to all physicians for their help with data collection and input, as well as to all patients that participated in the CMRD study and agreed to share their data with the scientific community.
Funding Statement
Supported by the Czech Pneumological and Phthisiological Society (open access publication fee grant). The CMRD research project was funded by the Ministry of Health of the Czech Republic (MH CZ‐DRO FNBr 65269705), and a consortium of pharmaceutical companies (Sandoz, Novartis, GSK, CSL Behring, Cipla, Boehringer Ingelheim, AstraZeneca, and Angelini). The companies supported the CMRD project via unrestricted research grants. The supporters had no role in the study design, data analysis or in preparation of the manuscript. All opinions, results, and conclusions reported in this paper are independent from the sponsors.
Abbreviations
AUC, area under the curve; BMI, body mass index; BODE, acronym for Body mass index, Obstruction, Dyspnea and Exercise tolerance; CADOT, Chronic heart failure, Age, Dyspnea, Obstruction and Diffusing capacity of the lungs for carbon monoxide; CI, confidence interval; CKD, chronic kidney disease; CMRD, Czech Multicenter Research Database of COPD; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; CHF, chronic heart failure; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IQR, interquartile range; NYHA, New York Heart Association; PC, physical capacity; SD, standard deviation; TNM, TNM Classification of Malignant Tumors.
Study Registration
The CMRD project has been registered at ClinicalTrials.gov with the identifier NCT01923051. Website: https://chopn.registry.cz/index-en.php.
Data Sharing Statement
The datasets analyzed during the study are available from the corresponding author on a reasonable request.
Ethics
The CMRD project was conducted in accordance with the Declaration of Helsinki and the Czech and European Union legislation. The CMRD study was approved by the Multicenter Ethics Committee of Masaryk University, Brno, Czech Republic (date of approval: Jan 16th, 2013, protocol code: CHOPN) and by regional review boards of all fourteen participating centers.6 All patients signed a written informed consent at study enrolment and agreed to use their anonymized data for scientific purpose.
Author Contributions
Vladimir Koblizek is the senior author. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Jaromir Zatloukal reports personal fees from AstraZeneca, personal fees from Boehringer-Ingelheim, personal fees from Chiesi, outside the submitted work. Dr Vladimir Koblizek reports grants, personal fees from Boehringer Ingelheim CZ, grants from Boehringer Ingelheim RCV, personal fees from AstraZeneca CZ, grants, personal fees from Angelini CZ, personal fees from Chiesi CZ, personal fees from Berlin Chemie CZ, during the conduct of the study; personal fees from MSD CZ, personal fees from Gilead CZ, outside the submitted work. The authors of this article report no other conflicts of interest with relevance to this study.
References
- 1.Global strategy for the diagnosis, management and prevention of COPD; 2023. Available from: https://goldcopd.org/2023-gold-report-2/. Accessed February 27, 2023.
- 2.Plutinsky M, Brat K, Svoboda M, Zatloukal J, Popelkova P, Koblizek V. Prognostic accuracy of three COPD classification systems in relation to long-term mortality of COPD patients: a prospective multicenter study. Lung. 2019;197(2):173–179. doi: 10.1007/s00408-019-00196-6 [DOI] [PubMed] [Google Scholar]
- 3.Han MZ, Hsiue TR, Tsai SH, Huang TH, Liao XM, Chen CZ. Validation of the GOLD 2017 and new 16 subgroups (1A-4D) classifications in predicting exacerbation and mortality in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:3425–3433. doi: 10.2147/COPD.S179048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le LAK, Johannessen A, Hardie JA, et al. Prevalence and prognostic ability of the GOLD 2017 classification compared to the GOLD 2011 classification in a Norwegian COPD cohort. Int J Chron Obstruct Pulmon Dis. 2019;14:1639–1655. doi: 10.2147/COPD.S194019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 6.Novotna B, Koblizek V, Zatloukal J, et al. Czech multicenter research database of severe COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:1265–1274. doi: 10.2147/COPD.S71828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brat K, Svoboda M, Hejduk K, et al. Introducing a new prognostic instrument for long-term mortality prediction in COPD patients: the CADOT index. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2021;165(2):139–145. doi: 10.5507/bp.2020.035 [DOI] [PubMed] [Google Scholar]
- 9.Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374(9691):704–711. doi: 10.1016/S0140-6736(09)61301-5 [DOI] [PubMed] [Google Scholar]
- 10.Tashkin DP, Murray RP. Smoking cessation in chronic obstructive pulmonary disease. Respir Med. 2009;103(7):963–974. doi: 10.1016/j.rmed.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 11.The Alpha-1-Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha1-antitrypsin. Am J Respir Crit Care Med. 1998;158(1):49–59. doi: 10.1164/ajrccm.158.1.9712017 [DOI] [PubMed] [Google Scholar]
- 12.Thabut G, Ravaud P, Christie JD, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(10):1156–1163. doi: 10.1164/rccm.200708-1283OC [DOI] [PubMed] [Google Scholar]
- 13.Chandra D, Lipson DA, Hoffman EA, et al; National Emphysema Treatment Trial Research Group. Perfusion scintigraphy and patient selection for lung volume reduction surgery. Am J Respir Crit Care Med. 2010;182(7):937–946. doi: 10.1164/rccm.201001-0043OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93(3):391–398. doi: 10.7326/0003-4819-93-3-391 [DOI] [PubMed] [Google Scholar]
- 15.Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1(8222):681–686. [PubMed] [Google Scholar]
- 16.Zatloukal J, Brat K, Neumannova K, et al. Chronic obstructive pulmonary disease - diagnosis and management of stable disease; a personalized approach to care, using the treatable traits concept based on clinical phenotypes. Position paper of the Czech Pneumological and Phthisiological Society. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020;164(4):325–356. doi: 10.5507/bp.2020.056 [DOI] [PubMed] [Google Scholar]
- 17.Brat K, Plutinsky M, Hejduk K, et al. Respiratory parameters predict poor outcome in COPD patients, category GOLD 2017 B. Int J Chron Obstruct Pulmon Dis. 2018;13:1037–1052. doi: 10.2147/COPD.S147262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaes AW, Spruit MA, Koolen EH, et al. ”Can Do, Do Do” quadrants and 6-year all-cause mortality in patients with COPD. Chest. 2022;161(6):1494–1504. doi: 10.1016/j.chest.2021.12.657 [DOI] [PubMed] [Google Scholar]