Abstract
Background
The triglyceride-glucose (TyG) index is regarded as an independent predictor of cardiovascular (CV) consequences and a reliable surrogate measure of insulin resistance (IR). However, the predictive significance of the TyG index in patients with type 2 diabetes mellitus (T2DM) and ischemic cardiomyopathy (ICM) remains unknown.
Methods
This study included 1514 consecutive subjects with ICM and T2DM. The tertile of the TyG index values was used to categorize these patients into three groups. Major adverse cardiac and cerebral events (MACCEs) were also noted. The TyG index was calculated using the [fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2] equation.
Results
After adjusting for age, BMI, and other potential confounders, the scores of multivariate Cox proportional hazards regression models for chest pain [9.056 (4.370 to 18.767), p<0.001], acute myocardial infarction [4.437 (1.420 to 13.869), p=0.010], heart failure [7.334 (3.424 to 15.708), p<0.001], cardiogenic shock [3.707 (1.207 to 11.384), p=0.022], malignant arrhythmia [5.309 (2.367 to 11.908), p<0.001], cerebral infarction [3.127 (1.596 to 6.128), p<0.001], gastrointestinal bleeding [4.326 (1.612 to 11.613), p=0.004], all-cause death [4.502 (3.478 to 5.827), p<0.001] and cumulative incidence of MACCEs [4.856 (3.842 to 6.136), p<0.001] increased significantly with an increase in TyG index levels (all p<0.05). Time-dependent ROC analysis revealed that the area under the TyG index curve (AUC) reached 0.653 in the 3rd year, 0.688 in the 5th year, and 0.764 in the 10th year. The predictive efficiency of this model on MACCEs improved [net reclassification improvement (NRI): 0.361 (0.253 to 0.454); C-index: 0.678 (0.658 to 0.698); integrated discrimination improvement (IDI): 0.138 (0.098 to 0.175), all p<0.05] following the incorporation of the TyG index into the base risk model.
Conclusion
TyG index could be useful in predicting MACCEs and initiating preventive measures in subjects with ICM and T2DM.
Keywords: insulin resistance, triglyceride‑glucose index, type 2 diabetes mellitus, ischemic cardiomyopathy, major adverse cardiac and cerebral events
Introduction
Coronary artery disease (CAD) is now recognized as a major cause of disability and mortality in modern society.1 It is also a major cause of heart failure (HF).2 Ischemic cardiomyopathy (ICM) is linked to myocardial insufficiency and inadequate perfusion caused by obstructive CAD.3 Furthermore, end-stage HF frequently stems from ICM, which can result in a significant decrease in the left ventricular ejection fraction (LVEF).4,5 HF refers to clinical syndromes characterized by pulmonary and/or body circulation stasis. Because of its high morbidity and mortality,6,7 HF has become a significant contributor to mortality worldwide and a pressing health concern.8 As a result, early prevention and diagnosis of HF are critical for mitigating relevant symptoms and improving the prognosis of these patients.
Studies have shown that type 2 diabetes mellitus (T2DM) influences the cardiovascular system in a variety of ways; for instance, it may increase the risk of CAD by affecting both major and minor blood vessels.9 An abnormal glucose metabolic status is common in patients with coronary syndromes, and about one-third of patients with coronary syndromes have DM. Moreover, research has consistently demonstrated that DM is a major contributor to cardiovascular diseases (CVDs).10 A subset of studies suggest that ICM patients who also have DM have a higher mortality rate than those without DM.11 Of note, type 2 diabetes mellitus (T2DM) patients are at higher risk of developing ICM, HF, stroke, CVDs, and peripheral arterial disease.12 Identifying other risk factors in ICM patients with T2DM has clinical implications for developing novel treatment targets and risk suppressor approaches based on individual risk levels.
Insulin resistance (IR), defined as a reduced response to insulin, is a significant contributor to CVDs and can be used to predict serious cardiovascular and cerebral incidents (MACCEs).13 IR plays a vital role in T2DM pathogenesis, and several studies have shown that IR is significantly associated with an increased risk of MACCEs.14–16 Although the hyperinsulin-hyperglycemic clamp is recognized as the best method for assessing IR, the complex testing procedures limit its application in clinical settings.17
The TyG index, which is calculated from fasting plasma glucose (FPG) and triglycerides (TG), has been proposed as a clinically reliable biomarker of IR.18 In addition, clinical investigations have confirmed the link between CVD prognosis and prevalence and the TyG index.19,20 The TyG index was found to be an accurate predictor of prognosis in a study of patients with T2DM and non-ST-segment elevation acute coronary syndrome.21 Another recent study found that the TyG index could be a significant warning sign of potential cardiovascular adverse events in patients with T2DM and acute myocardial infarction.22 Meanwhile, recent studies have confirmed that the TyG index can be used to assess the presence of IR and nonalcoholic fatty liver disease in patients with bladder cancer.23 However, the ability of the TyG index to predict MACCEs in subjects with ICM and T2DM has not been thoroughly examined. Therefore, this investigation aims to discover whether the TyG index can accurately predict MACCEs in patients with ICM and T2DM.
Materials and Methods
Study Population
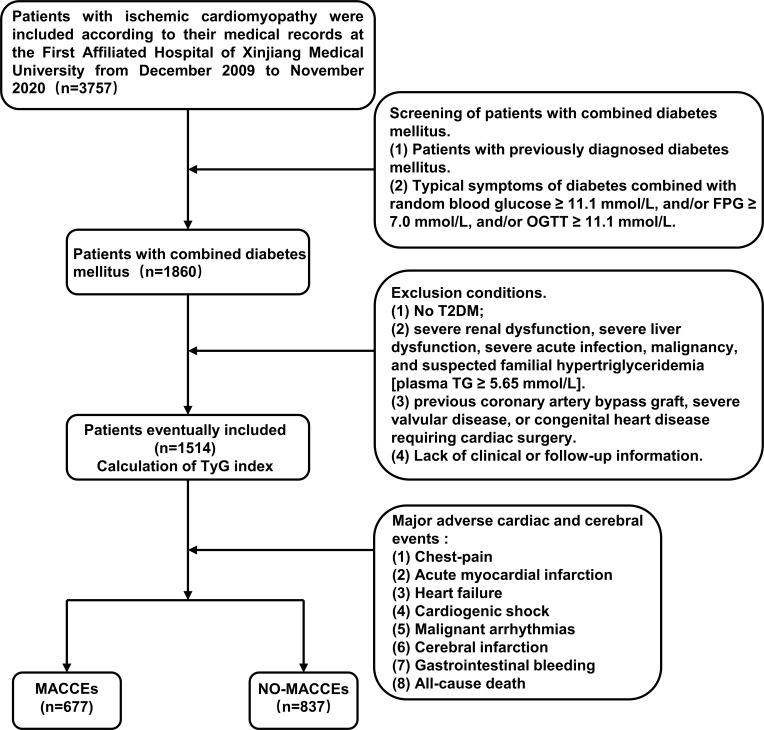
This study included 3757 patients with ischemic cardiomyopathy based on medical records and follow-up registrations at the First Affiliated Hospital of Xinjiang Medical University between December 2009 and November 2021. Exclusion criteria were as follows: (1) patients with no T2DM; (2) patients with severe acute infection, malignancy, severe liver dysfunction, severe renal dysfunction, and suspected familial hypertriglyceridemia [TG ≥ 5.65 mmol/L]; (3) patients with a history of coronary artery bypass, congenital heart disease or severe valvular requiring cardiac surgery. (4) Patients with incomplete clinical or follow-up data. The study excluded 2243 patients based on the exclusion criteria. Finally, 1514 patients were enrolled in this study. Medical records and/or a telephone interview with patients or their family members were used to determine the short or long-term prognosis of patients. Studies involving human participants were reviewed were approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (approval number: S220722-25) and followed the Declaration of Helsinki. Patients/participants provided written informed consent for their participation in this study. Individuals provided written informed consent for the publication of any potentially identifiable images or data included herein. Figure 1 shows the flow chart for participant inclusion and exclusion.
Figure 1.
Flow chart of the study population enrollment.
Abbreviations: TyG, triglyceride glucose; FPG, fasting plasma glucose; TG, triglycerides; OGTT, Oral Glucose Tolerance Test; T2DM, type 2 diabetes mellitus; MACCEs, Major adverse cardiac and cerebral events.
Data Collection and Diagnostic Criteria
The data collection procedure was approved review committee of the First Affiliated Hospital of Xinjiang Medical University. Demographic and clinical information of patients, such as their age, gender, weight, height, medical history, family history, blood pressure, and the frequency of the medical visits were obtained from the medical data record system. The body mass index (BMI) was calculated using the following formula: weight (kg)/ [height (m)]2. The TyG index was calculated in the central lab of the First Affiliated Hospital of Xinjiang Medical University as ln [TG (mg/dL) FPG×(mg/dL)/2]18 based on the amounts of TG and FPG in the first fasting blood samples obtained at least 10 h during the hospitalization.
Patients with any of the following conditions were assessed for ICM: Prior myocardial infarction (MI) (recorded medical history, elevated series of cardiac markers, and ECG values along with MI); history of coronary revascularization; coronary angiographic flow-limiting stenosis (≥ 50% epicardial artery stenosis); reversible ischemia or MI detected on stress echocardiography or stress nuclear perfusion imaging. Individuals who did not meet any of the aforementioned criteria were excluded from ICM diagnosis.24 The diagnostic criteria for T2DM included (1) a previous diagnosis of T2DM on antidiabetic medication and (2) typical DM symptoms with FPG ≥ 7.0 mmol/L and/or random blood glucose (RBG) ≥ 11.1 mmol/L and/or oral glucose tolerance test (OGTT) ≥ 11.1 mmol/L.25
MACCEs included: chest pain, acute myocardial infarction, heart failure, cardiogenic shock, malignant arrhythmias, cerebral infarction, gastrointestinal bleeding, all-cause death.
Statistical Analysis
Continuous variables were represented using the median (IQR). The Mann–Whitney U-test was used to compare the two study groups. The Pearson chi-square analysis or Fisher’s exact test was employed to compare categorical variables, which were expressed as percentages and numbers. Additionally, the χ2 test was employed for comparison between the groups. The study participants were classified based on the tertile of TyG index values and the frequency of MACCEs during follow-up. The correlation of the TyG index with traditional cardiovascular risk factors was examined using Spearman correlation analysis. The Log rank test was used to determine the relevant significance after plotting the Kaplan-Meier survival curves. Multivariate models included baseline factors that are clinically relevant and significantly linked to MACCEs according to univariate analysis. Furthermore, the relationship between the variables was investigated using multivariate analysis. A priori assessment of clinicopathological variables associated with recurrence risk and survival was performed based on clinical significance, scientific understanding, and predictors discovered in previously published papers.
The independent prognostic function of the TyG index for each MACCE was verified using Cox proportional hazards regression models.
Additionally, receiver-operating characteristic (ROC) curve analysis was performed to determine the best cutoff value for the TyG index for predicting MACCEs. The net reclassification improvement (NRI) was estimated to determine the level at which the TyG index addition improved the original predictive ability of the risk model. IBM SPSS Statistics 24 and the R programming language (version 4.0; http://www.r-project.org) were employed for all statistical procedures. A two-tailed p-value < 0.05 denoted statistical significance.
Results
Baseline Characteristics
This study eventually included 1514 individuals with ICM and T2DM, 1119 of whom were men (73.9%), with an average age of 65.26 years and a standard deviation of 10.036. Table 1 depicts the baseline characteristics of the overall population, grouped based on MACCEs occurrence. Patients in the MACCE group had higher scores in terms of the TyG index, age, SBP, alkaline phosphatase (ALP), low-density lipoprotein-cholesterol (LDL-C), neutrophils, platelet (PLT), white blood cell (WBC), uric acid (UA), FPG, creatinine (Cr), TG, total cholesterol (TC) than the non-MACCE group (p < 0.05). In contrast, the MACCEs group had significantly lower scores in terms of the levels of LVEF, high-density lipoprotein-cholesterol (HDL-C), and total bilirubin (TBil) than the non-MACCEs group (p < 0.05). Furthermore, the MACCEs group had a substantially higher rate of smoking than the non-MACCEs group. (p < 0.05).
Table 1.
Baseline Characteristics of the Study Population According to the Occurrence of MACCEs
| Characteristic | Total (n=1514) | NO-MACCEs (n=837) | MACCEs (n=677) | P |
|---|---|---|---|---|
| Male, n (%) | 1119 (73.9%) | 633 (75.6%) | 486 (71.8%) | 0.091 |
| Ethnicity, n (%) | ||||
| Han | 791 (52.2%) | 453 (54.1%) | 338 (49.9%) | 0.262 |
| Uyghur | 537 (35.5%) | 292 (34.9%) | 245 (36.2%) | |
| Kazah | 48 (3.2%) | 24 (2.9%) | 24 (3.6%) | |
| Other | 138 (9.1%) | 68 (8.1%) | 70 (10.3%) | |
| Smoking, n (%) | 620 (40.9%) | 319 (38.1%) | 301 (44.5%) | 0.013 |
| Drinking, n (%) | 401 (26.5%) | 228 (27.2%) | 173 (25.6%) | 0.460 |
| HT his, n (%) | 1024 (67.6%) | 558 (66.7%) | 466 (68.8%) | 0.370 |
| TyG-Index | 7.53 (7.06–8.13) | 7.22 (6.76–7.79) | 8.04 (7.43–8.51) | <0.001 |
| Age, years | 66 (58–73) | 65 (57–73) | 67 (59–74) | <0.001 |
| BMI, kg/m2 | 25.71 (23.38–28.44) | 25.81 (23.44–28.53) | 25.61 (23.14–28.38) | 0.403 |
| SBP, mmHg | 122 (110–139) | 120 (110–138) | 125 (111–140) | 0.048 |
| DBP, mmHg | 74 (66–81) | 74 (66–82) | 75 (66–80) | 0.995 |
| WBC, 109/L | 7.27 (5.91–8.77) | 7.13 (5.81–8.61) | 7.45 (6.07–9.10) | <0.001 |
| Neutrophil, 109/L | 4.67 (3.68–5.95) | 4.56 (3.57–5.75) | 4.86 (3.80–6.29) | <0.001 |
| Lymphocyte, 109/L | 1.62 (1.20–2.09) | 1.64 (1.20–2.10) | 1.58 (1.17–2.07) | 0.114 |
| RBC, 109/L | 4.40 (3.93–4.81) | 4.40 (3.98–4.83) | 4.40 (3.90–4.81) | 0.427 |
| PLT, 109/L | 201.0 (162.0–249.0) | 195.0 (158.0–247.0) | 208.0 (166.5–252.0) | 0.010 |
| Cr, umol/L | 91.0 (73.0–118.3) | 87.0 (71.0–114.0) | 97.5 (77.0–126.7) | <0.001 |
| UA, umol/L | 379.20 (301.00–480.03) | 369.50 (297.76–465.00) | 393.00 (303.38–495.00) | 0.039 |
| FPG, mmol/L | 8.10 (5.98–11.97) | 6.67 (5.27–9.29) | 10.32 (7.57–14.38) | <0.001 |
| TG, mmol/L | 1.31 (0.96–1.83) | 1.16 (0.82–1.60) | 1.58 (1.15–2.27) | <0.001 |
| HDL-C, mmol/L | 0.88 (0.71–1.07) | 0.89 (0.72–1.09) | 0.87 (0.70–1.04) | <0.001 |
| LDL-C, mmol/L | 2.16 (1.65–2.76) | 2.09 (1.58–2.66) | 2.22 (1.73–2.84) | <0.001 |
| TC, mmol/L | 3.44 (2.78–4.11) | 3.28 (2.68–3.95) | 3.57 (2.98–4.34) | <0.001 |
| TBil, umol/L | 13.50 (9.50–19.61) | 14.3 (9.84–20.25) | 12.6 (9.31–18.68) | <0.001 |
| Albumin, g/L | 36.60 (32.70–40.20) | 36.82 (33.10–40.48) | 36.22 (32.30–40.00) | 0.051 |
| ALT, U/L | 19.70 (15.10–28.80) | 19.80 (15.00–28.10) | 19.60 (15.20–29.83) | 0.317 |
| AST, U/L | 19.50 (13.48–30.08) | 19.30 (13.40–29.75) | 19.80 (13.69–31.02) | 0.233 |
| LDH, U/L | 199.85 (167.00–242.7) | 198.00 (167.00–236.64) | 202.00 (166.86–254.12) | 0.288 |
| GGT, U/L | 32.1 (21.0–59.0) | 32.0 (20.2–58.06) | 32.7 (22.0–60.9) | 0.182 |
| ALP, U/L | 75.0 (60.0–97.0) | 72.0 (58.0–94.0) | 79.0 (62.0–102.1) | <0.001 |
| CK, U/L | 70.65 (46.00–107.00) | 71.00 (47.90–107.23) | 70.05 (44.91–106.95) | 0.717 |
| LVEF | 42.0 (36.8–47.0) | 43.0 (37.0–48.0) | 40.3 (36.0–46.0) | <0.001 |
| Medication, n (%) | ||||
| Antiplatelet agent | 493 (32.5%) | 290 (34.6%) | 203 (29.9%) | 0.054 |
| ACEI/ARB | 465 (30.7%) | 246 (29.3%) | 219 (32.3%) | 0.215 |
| Beta-blocker | 211 (13.9%) | 109 (13.0%) | 102 (15.0%) | 0.254 |
| Statins | 342 (22.6%) | 186 (22.2%) | 156 (23.0%) | 0.704 |
| Oral hypoglycemic agents | 1026 (67.7%) | 566 (67.6%) | 460 (67.9%) | 0.893 |
| Insulin | 509 (33.6%) | 277 (33.1%) | 232 (34.2%) | 0.631 |
Notes: Data are expressed as n (%) and median (25 and 75 interquartile). p values in bold are < 0.05.
Abbreviations: TyG index, triglyceride-glucose index; BMI, body mass index; HT his, history of hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; RBC, red blood cell; PLT, platelet; Cr, creatinine; UA, uric acid; FPG, fasting plasma glucose; TG, triglyceride; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; TC, total cholesterol; TBil, total bilirubin; ALT, alanine transferase; AST, aspartate transferase; LDH, lactate dehydrogenase; GGT, glutamyl transpeptidase; ALP, alkaline phosphatase; CK, creatine kinase; LVEF, left ventricle ejection fraction; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; MACCEs, Major adverse cardiac and cerebral events.
Participants were divided into three groups based on their TyG index tertile (Tertile 1: n=509, TyG index ≤7.21; Tertile 2: n=500, 7.21 < TyG index <7.89; Tertile 3: n=505, TyG index ≥7.89) (Table 2). The three groups had significant differences in terms of gender, ethnicity, TyG index, age, levels of BMI, WBC, neutrophils, TG, HDL-C, LDL-C, PLT, UA, TC, FPG, TBil, ALP, LVEF and Antiplatelet agents, Beta-blocker, oral hypoglycemic agents, and insulin use frequency but no other indexes were statistically different (p> 0.05).
Table 2.
Baseline Characteristics of the Study Population According to the Tertiles of the TyG Index
| Characteristic | Tertile1 (n=509) | Tertile 2 (n=500) | Tertile 3 (n=505) | P |
|---|---|---|---|---|
| Male, n (%) | 397 (78%) | 374 (74.8%) | 348 (68.9%) | 0.004 |
| Ethnicity, n (%) | ||||
| Han | 295 (57.9%) | 248 (49.6%) | 248 (49.1%) | 0.019 |
| Uyghur | 157 (30.8%) | 179 (35.8%) | 201 (39.8%) | |
| Kazah | 18 (3.5%) | 17 (3.4%) | 13 (2.6%) | |
| Other | 39 (7.7%) | 56 (11.2%) | 43 (8.5%) | |
| Smoking, n (%) | 196 (38.5%) | 204 (40.8%) | 220 (43.5%) | 0.261 |
| Drinking, n (%) | 138 (27.1%) | 141 (28.2%) | 122 (24.2%) | 0.323 |
| HT his, n (%) | 329 (64.6%) | 349 (69.8%) | 346 (68.5%) | 0.188 |
| TyG-Index | 6.83 (6.54–7.07) | 7.53 (7.37–7.72) | 8.39 (8.13–8.71) | < 0.001 |
| Age, years | 68 (61–74) | 65 (58–73) | 65 (57–73) | < 0.001 |
| BMI, kg/m2 | 25.21 (22.86–27.92) | 26.15 (23.56–28.74) | 25.78 (23.53–28.72) | < 0.001 |
| SBP, mmHg | 121 (110–137) | 121.5 (110–139) | 125 (111–140) | 0.079 |
| DBP, mmHg | 73 (65–81) | 74.5 (67–82) | 75 (68–80) | 0.280 |
| WBC, 109/L | 7.05 (5.66–8.28) | 7.22 (5.95–8.8) | 7.57 (6.14–9.38) | < 0.001 |
| Neutrophil, 109/L | 4.43 (3.47–5.65) | 4.71 (3.73–6.01) | 4.93 (3.84–6.54) | < 0.001 |
| Lymphocyte, 109/L | 1.54 (1.16–1.99) | 1.64 (1.22–2.12) | 1.67 (1.22–2.13) | 0.084 |
| RBC, 109/L | 4.36 (3.87–4.78) | 4.4 (3.95–4.82) | 4.45 (3.96–4.84) | 0.177 |
| PLT, 109/L | 191 (155–238) | 201.5 (161–248) | 210 (170–257) | < 0.001 |
| Cr, umol/L | 90.0 (72.0–116.8) | 89.0 (72.1–117.3) | 94.8 (75.0–122.1) | 0.114 |
| UA, umol/L | 390.4 (315.1–491.0) | 384.5 (300.8–471.8) | 367.0 (291.5–474.0) | 0.013 |
| FPG, mmol/L | 5.74 (4.76–6.85) | 8.09 (6.64–10.13) | 13.03 (10.07–16.83) | < 0.001 |
| TG, mmol/L | 0.89 (0.71–1.13) | 1.32 (1.06–1.63) | 2.02 (1.58–2.94) | < 0.001 |
| HDL-C, mmol/L | 0.92 (0.72–1.09) | 0.87 (0.71–1.07) | 0.85 (0.7–1.05) | 0.015 |
| LDL-C, mmol/L | 1.91 (1.47–2.47) | 2.17 (1.76–2.68) | 2.38 (1.83–3.11) | < 0.001 |
| TC, mmol/L | 3.06 (2.51–3.71) | 3.38 (2.84–3.95) | 3.68 (2.91–4.70) | < 0.001 |
| TBil, umol/L | 14.9 (10.4–21) | 13.3 (9.4–19.3) | 12.4 (9.1–18.2) | < 0.001 |
| Albumin, g/L | 36.7 (33.1–39.7) | 36.6 (32.8–40.8) | 36.5 (32.2–40.3) | 0.657 |
| ALT, U/L | 20.3 (15.7–28.3) | 19.7 (14.9–28.9) | 19.1 (15.0–28.9) | 0.613 |
| AST, U/L | 18.1 (12.9–29.8) | 20.3 (13.5–29.6) | 19.6 (14.1–31.6) | 0.064 |
| LDH, U/L | 199.7 (172.9–241.0) | 202.6 (167.0–242.8) | 197.4 (160.0–243.7) | 0.444 |
| GGT, U/L | 35.6 (21.0–63.0) | 31.3 (20.6–55.3) | 31.0 (21.8–58.1) | 0.185 |
| ALP, U/L | 75.0 (59.0, 99.0) | 71.0 (58.2–90.9) | 80.0 (63.1–101.5) | < 0.001 |
| CK, U/L | 68.4 (46.0–102.0) | 72.6 (47.0–109.0) | 69.8 (45.0–109.1) | 0.493 |
| LVEF | 43.0 (37.3–48.9) | 41.0 (36.8–47.0) | 40.3 (35.0–46.0) | < 0.001 |
| Medication, n (%) | ||||
| Antiplatelet agent | 184 (36.1%) | 175 (35.0%) | 134 (26.5%) | 0.002 |
| ACEI/ARB | 152 (29.8%) | 154 (30.8%) | 159 (31.5%) | 0.854 |
| Beta-blocker | 92 (18.0%) | 60 (12.0%) | 59 (11.7%) | 0.004 |
| Statins | 104 (20.4%) | 120 (24.0%) | 118 (23.3%) | 0.351 |
| Oral hypoglycemic agents | 368 (72.2%) | 323 (64.6%) | 335 (66.3%) | 0.023 |
| Insulin | 189 (37.1%) | 149 (29.8%) | 171 (33.9%) | 0.048 |
Notes: Data are expressed as n (%) and median (25 and 75 interquartile). p values in bold are < 0.05.
Abbreviations: TyG index, triglyceride-glucose index; BMI, body mass index; HT his, history of hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; RBC, red blood cell; PLT, platelet; Cr, creatinine; UA, uric acid; FPG fasting plasma glucose; TG, triglyceride; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; TC, total cholesterol; TBil, total bilirubin; ALT, alanine transferase; AST, aspartate transferase; LDH, lactate dehydrogenase; GGT, glutamyl transpeptidase; ALP, alkaline phosphatase; CK, creatine kinase; LVEF, left ventricle ejection fraction; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; MACCEs, Major adverse cardiac and cerebral events.
Correlations Between the TyG Index and Cardiovascular Risk Factors
Spearman correlation was used to examine the correlation between cardiovascular risk factors and the TyG index. The TyG index was positively correlated with SBP, WBC, BMI, neutrophil, lymphocyte, red blood cell (RBC), PLT, LDL-C, TC, aspartate transferase (AST), and ALP (p < 0.05) (Table 3) but negatively correlated with age, UA, LVEF, TBil, and HDL-C.
Table 3.
Correlations Between the TyG Index and Cardiovascular Risk Factors
| Characteristic | Correlation Coefficient | p values |
|---|---|---|
| Age | −0.099 | <0.001 |
| BMI | 0.076 | 0.003 |
| SBP | 0.051 | 0.045 |
| DBP | 0.044 | 0.087 |
| WBC | 0.130 | <0.001 |
| Neutrophil | 0.123 | <0.001 |
| Lymphocyte | 0.059 | 0.021 |
| RBC | 0.059 | 0.022 |
| PLT | 0.119 | <0.001 |
| Cr | 0.045 | 0.079 |
| UA | −0.055 | 0.034 |
| HDL-C | −0.076 | 0.003 |
| LDL-C | 0.242 | <0.001 |
| TC | 0.338 | <0.001 |
| TBil | −0.111 | <0.001 |
| Albumin | 0.024 | 0.343 |
| ALT | −0.020 | 0.436 |
| AST | 0.063 | 0.014 |
| LDH | −0.037 | 0.151 |
| GGT | −0.001 | 0.961 |
| ALP | 0.055 | 0.033 |
| CK | 0.011 | 0.671 |
| LVEF | −0.100 | <0.001 |
Note: p values in bold are < 0.05.
Abbreviations: TyG index, triglyceride-glucose index; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; RBC, red blood cell; PLT, platelet; Cr, creatinine; UA, uric acid; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; TC, total cholesterol; TBil, total bilirubin; ALT, alanine transferase; AST, aspartate transferase; LDH, lactate dehydrogenase; GGT, glutamyl transpeptidase; ALP, alkaline phosphatase; CK, creatine kinase; LVEF, left ventricle ejection fraction.
TyG Index Predicts MACCEs Occurrence
Table 4 summarizes the results of the univariate and multivariate Cox proportional hazards regression analyses, as well as the MACCE predictors. The results of the univariate analysis revealed that the TyG index, age, neutrophils, PLT, UA, and ALP were all correlated with the occurrence of MACCEs. Notably, after controlling for confounders, including age and gender, Multivariate Cox regression analysis revealed that PLT, UA, age, TyG index, and ALP were independent predictors of the development of MACCEs in patients with ICM and T2DM.
Table 4.
Independent Predictors of Composite MACCEs
| Univariate Cox Regression | Multivariate Cox Regression | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| TyG-Index | 2.043 | (1.871–2.230) | <0.001 | 2.204 | (1.997–2.443) | <0.001 |
| Male, gender | 0.946 | (0.800–1.119) | 0.518 | |||
| Age | 1.015 | (1.008–1.023) | <0.001 | 1.023 | (1.014–1.031) | <0.001 |
| BMI, kg/m2 | 0.990 | (0.973–1.007) | 0.254 | |||
| Neutrophil, 109/L | 1.059 | (1.033–1.087) | <0.001 | 1.008 | (0.979–1.038) | 0.602 |
| Lymphocyte, 109/L | 0.943 | (0.852–1.042) | 0.250 | |||
| PLT, 109/L | 1.002 | (1.001–1.003) | <0.001 | 1.002 | (1.001–1.003) | <0.001 |
| UA, umol/L | 1.001 | (1.000–1.001) | 0.002 | 1.001 | (1.000–1.001) | 0.003 |
| HDL-C, mmol/L | 0.842 | (0.682–1.039) | 0.109 | |||
| LDL-C, mmol/L | 1.069 | (0.991–1.154) | 0.084 | |||
| TC, mmol/L | 1.053 | (0.996–1.114) | 0.068 | |||
| TBil, umol/L | 0.997 | (0.990–1.004) | 0.404 | |||
| ALP, U/L | 1.002 | (1.000–1.003) | 0.013 | 1.002 | (1.001–1.003) | 0.048 |
| LVEF, % | 0.996 | (0.989–1.004) | 0.326 | |||
| Antiplatelet agent | 0.872 | (0.740–1.028) | 0.102 | |||
| ACEI/ARB | 1.042 | (0.887–1.224) | 0.617 | |||
| Beta-blocker | 1.185 | (0.960–1.463) | 0.114 | |||
| Statins | 1.022 | (0.854–1.222) | 0.813 | |||
| Oral hypoglycemic agents | 1.000 | (0.851–1.175) | 0.998 | |||
| Insulin | 1.022 | (0.872–1.198) | 0.788 | |||
Notes: Adjusted factors included TyG-Index, Age, neutrophil, PLT, UA, ALP, HDL-C, LDL-C, TC. p values in bold are < 0.05.
Abbreviations: TyG index, triglyceride-glucose index, BMI, body mass index; PLT, platelet; UA, uric acid; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; TC, total cholesterol; TBil total bilirubin; ALP, alkaline phosphatase; LVEF, left ventricle ejection fraction; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; MACCEs, Major adverse cardiac and cerebral events.
Over the course of a mean follow-up of 55.4 months, 677 (44.7%) patients had developed MACCEs [acute myocardial infarction: 34 (2.2%), heart failure: 86 (5.7%), cardiogenic shock: 25 (1.6%), chest pain: 91 (6%), malignant arrhythmia: 62 (4.1%), cerebral infarction: 64 (4.2%), gastrointestinal bleeding:33 (2.1%), and all-cause death: 529 (34.9%)].
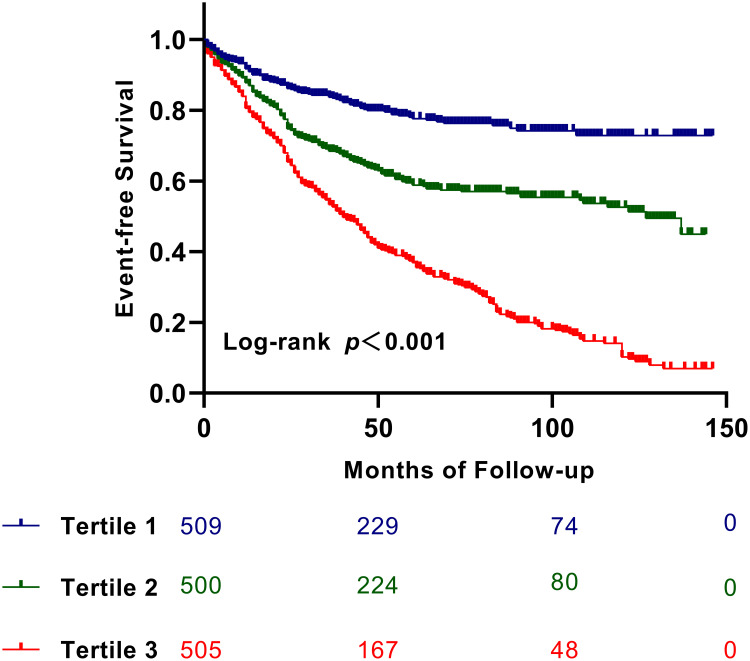
The Kaplan-Meier survival curve was used to visualize patient outcomes based on the TyG index levels (Figure 2). As depicted in Figure 2, the survival of subjects with ICM and T2DM decreased as the tertile of TyG index levels increased (Log rank test, p < 0.05).
Figure 2.
Kaplan–Meier survival curve for MACCEs across the TyG index tertiles.
Table 5 displays the Cox proportional hazards regression analysis results of MACCEs. In the uncorrected Cox proportional hazards regression models, the cumulative incidence of chest pain, heart failure, malignant arrhythmia, acute myocardial infarction, cerebral infarction, gastrointestinal bleeding, all-cause death, and MACCEs significantly increased with increasing TyG index levels (p<0.05). Furthermore, after controlling for potential confounders, the cumulative incidence and multivariate-adjusted hazard ratio (HR) of chest pain, acute myocardial infarction, heart failure, cardiogenic shock, malignant arrhythmias, cerebral infarction, gastrointestinal bleeding, all-cause death, and MACCEs increased significantly with increasing TyG index levels in the multivariate Cox proportional hazards regression models (p< 0.05).
Table 5.
Cox Proportional Hazards Regression Models of MACCEs
| Characteristic | n (%) | Univariate Cox Regression | Multivariate Cox Regression | ||
|---|---|---|---|---|---|
| HR (95% CI) | p | Adjusted HR (95% CI) | p | ||
| MACCEs | |||||
| TyG≤7.21 | 100 (19.6) | Ref | ‑/‑ | Ref | ‑/‑ |
| 7.21 < TyG <7.89 | 199 (39.8) | 1.996 (1.570–2.538) | <0.001 | 2.152 (1.684–2.751) | <0.001 |
| TyG≥7.89 | 378 (74.9) | 4.383 (3.515–5.465) | <0.001 | 4.856 (3.842–6.136) | <0.001 |
| Chest-pain | |||||
| TyG≤7.21 | 9 (1.8) | Ref | ‑/‑ | Ref | ‑/‑ |
| 7.21 < TyG <7.89 | 11 (2.2) | 1.218 (0.505–2.939) | 0.661 | 1.167 (0.477–2.854) | 0.735 |
| TyG≥7.89 | 71 (14.1) | 9.336 (4.661–18.699) | <0.001 | 9.056 (4.370–18.767) | <0.001 |
| Acute-myocardial infarction | |||||
| TyG≤7.21 | 4 (0.8) | Ref | ‑/‑ | Ref | ‑/‑ |
| 7.21 < TyG <7.89 | 15 (3) | 3.737 (1.240–11.262) | 0.019 | 4.236 (1.368–13.179) | 0.012 |
| TyG≥7.89 | 15 (3) | 4.313 (1.430–13.008) | <0.009 | 4.437 (1.420–13.869) | 0.010 |
| Heart failure | |||||
| TyG≤7.21 | 8 (1.6) | Ref | ‑/‑ | Ref | ‑/‑ |
| 7.21 < TyG <7.89 | 22 (4.4) | 2.758 (1.228–6.195) | 0.014 | 2.657 (1.162–6.072) | 0.021 |
| TyG≥7.89 | 56 (11.1) | 8.025 (3.824–16.843) | <0.001 | 7.334 (3.424–15.708) | <0.001 |
| Cardiogenic-shock | |||||
| TyG≤7.21 | 5 (0.9) | Ref | ‑/‑ | Ref | ‑/‑ |
| 7.21 < TyG <7.89 | 8 (1.6) | 1.563 (0.511–4.780) | 0.433 | 1.957 (0.617–6.325) | 0.252 |
| TyG≥7.89 | 12 (2.3) | 2.779 (0.977–7.905) | 0.055 | 3.707 (1.207–11.384) | 0.022 |
| Malignant-arrhythmia | |||||
| TyG≤7.21 | 8 (1.5) | Ref | ‑/‑ | Ref | ‑/‑ |
| 7.21 < TyG <7.89 | 19 (3.8) | 2.353 (1.030–5.376) | 0.042 | 2.554 (1.100–5.931) | 0.029 |
| TyG≥7.89 | 35 (6.9) | 5.128 (2.376–11.065) | <0.001 | 5.309 (2.367–11.908) | <0.001 |
| Cerebral-infarction | |||||
| TyG≤7.21 | 14 (2.7) | Ref | ‑/‑ | Ref | ‑/‑ |
| 7.21 < TyG <7.89 | 17 (3.4) | 1.223 (0.603–2.481) | 0.577 | 1.269 (0.614–2.624) | 0.520 |
| TyG≥7.89 | 33 (6.5) | 2.857 (1.526–5.348) | <0.001 | 3.127 (1.596–6.128) | <0.001 |
| Gastrointestinal bleeding | |||||
| TyG≤7.21 | 6 (1.1) | Ref | ‑/‑ | Ref | ‑/‑ |
| 7.21 < TyG <7.89 | 10 (2) | 1.688 (0.614–4.646) | 0.310 | 2.117 (0.748–5.996) | 0.158 |
| TyG≥7.89 | 17 (3.3) | 3.351 (1.318–8.517) | 0.011 | 4.326 (1.612–11.613) | 0.004 |
| All-cause death | |||||
| TyG≤7.21 | 85 (16.7) | Ref | ‑/‑ | Ref | ‑/‑ |
| 7.21 < TyG <7.89 | 157 (31.4) | 1.854 (1.424–2.414) | <0.001 | 2.022 (1.543–2.649) | <0.001 |
| TyG≥7.89 | 287 (56.8) | 3.945 (3.096–5.027) | <0.001 | 4.502 (3.478–5.827) | <0.001 |
Notes: Adjusted factors included Age, BMI, neutrophil, lymphocyte, PLT, UA, HDL-C, LDL-C, ALP, LVEF, gender, TC, HT his, Ethnicity, Medication (Antiplatelet agent, ACEI/ARB, Beta-blocker, Statins, Oral hypoglycemic agents, Insulin). p values in bold are < 0.05.
Abbreviations: TyG index, triglyceride-glucose index; BMI, body mass index; PLT, platelet; UA, uric acid; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; ALP, alkaline phosphatase; LVEF, left ventricle ejection fraction; TC, total cholesterol; HT his, history of hypertension; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker, MACCEs Major adverse cardiac and cerebral events; HR, hazard ratio; CI, confidence interval; Ref, reference.
Assessment of the Predictive Effectiveness of the TyG Index for MACCEs
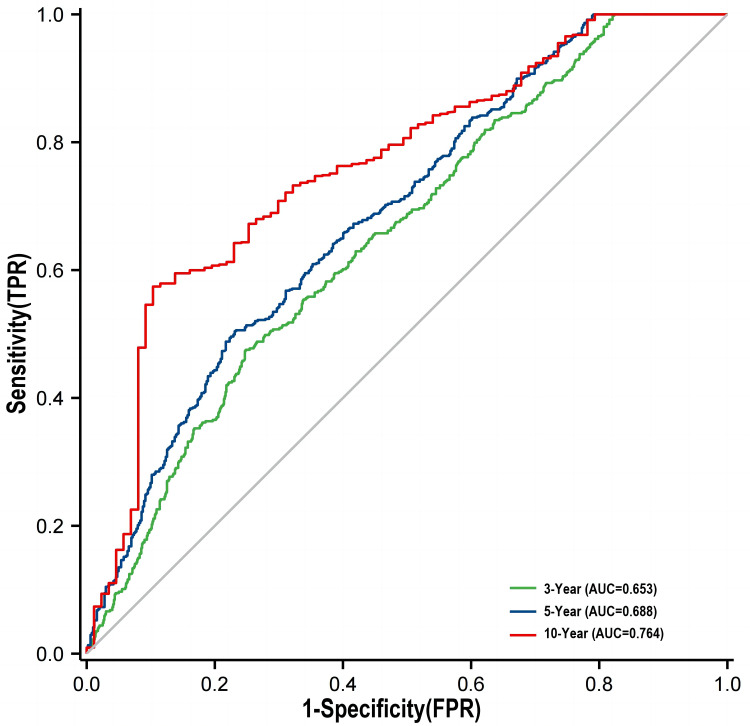
A time-dependent ROC analysis was performed to assess the predictive significance and prediction efficacy of the TyG index. The area under the curve (AUC) increased to 0.653 in the 3rd year, 0.688 in the 5th year, and 0.764 in the 10th year (Figure 3). As depicted in Table 6, incorporating the TyG index into the original risk model demonstrated a marked improvement in reclassification and discrimination compared with the FPG and TG, with an NRI of 0.361 and an IDI of 0.138 (P < 0.05). Furthermore, the C-index increased from [0.588 (0.565 to 0.610), P<0.001] to [0.678 (0.658 to 0.698), P<0.001] following the inclusion of the TyG index.
Figure 3.
Time-dependent ROC curves of the TyG index for the prediction of MACCEs.
Table 6.
Evaluate the Incremental Predictive Value and Predictive Power of Various Models with NRI, IDI and C-Index
| Characteristic | C-Index | p | Continuous NRI | p | IDI | p |
|---|---|---|---|---|---|---|
| Original model | 0.588 (0.565–0.610) | <0.001 | Ref | Ref | ||
| +TG | 0.622 (0.600–0.644) | <0.001 | 0.173 (0.057–0.282) | 0.004 | 0.027 (0.010–0.043) | 0.004 |
| +FPG | 0.662 (0.641–0.683) | <0.001 | 0.278 (0.171–0.384) | 0.004 | 0.089 (0.061–0.116) | <0.001 |
| +TyG-Index | 0.678 (0.658–0.698) | <0.001 | 0.361 (0.253–0.454) | 0.002 | 0.138 (0.098–0.175) | 0.002 |
Notes: Baseline risk model including age, PLT, UA, ALP. p values in bold are < 0.05.
Abbreviations: NRI, net reclassification improvement; IDI, integrated discrimination improvement; FPG, fasting plasma glucose; TG, triglyceride; TyG, triglyceride-glucose index; UA, uric acid; ALP, alkaline phosphatase; PLT, platelet; Ref, reference.
Association of the TyG Index with MACCEs in Different Subgroups
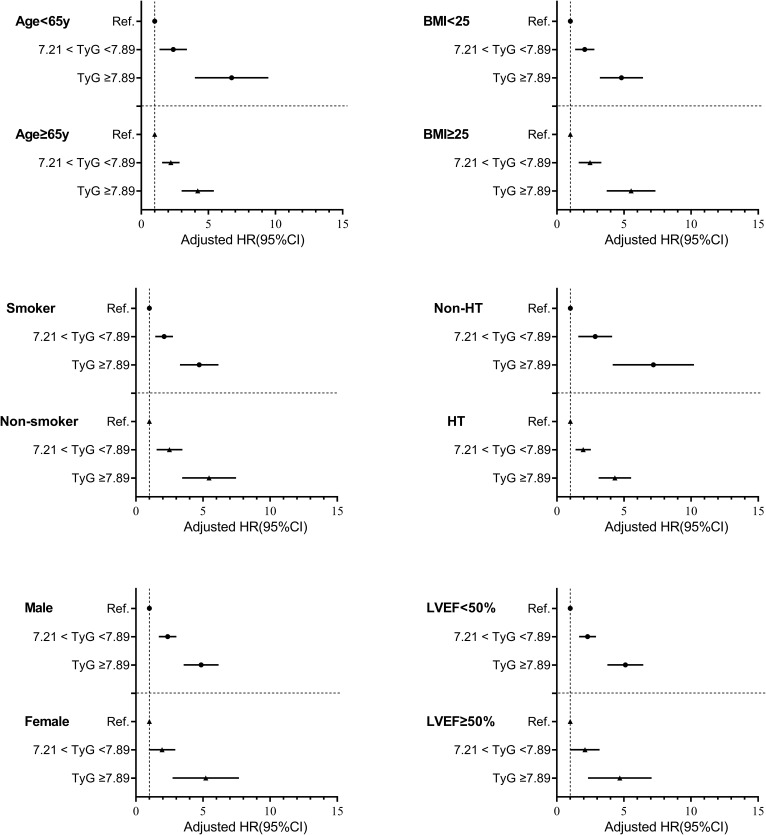
The ability of the TyG index to predict endpoint events was evaluated in various subgroups of the study population, classified based on age, BMI, sex, LVEF, smoking status, and a history of hypertension. The findings revealed that an increase in TyG index levels was related to MACCE in various subgroups, indicating that the TyG index was a reliable predictor in the majority of subgroups (Figure 4).
Figure 4.
Forest plot of composite MACCEs according to different subgroups.
Notes: Adjusted model included Age, gender, smoking, HT his, BMI, Neutrophil, lymphocyte, HDL-C, LDL-C, TC, UA, PLT, ALP, LVEF, Medication (Anti platelet agent, ACEI/ARB, Beta blocker, Statins, Oral hypoglycemic agents, Insulin).
Abbreviations: MACCEs, Major adverse cardiac and cerebral events; TyG, triglyceride‑glucose index; BMI, body mass index; HT his, history of hypertension; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; TC, total cholesterol; UA, uric acid; PLT, platelet; ALP, alkaline phosphatase; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; HR, hazard ratio; CI, confidence interval; Ref., reference (TyG ≤ 7.21 group).
Discussion
To the best of our knowledge, this is the first study to examine the correlation of MACCEs with the TyG index in individuals with ICM and T2DM. The following are the main findings of this study: (1) A rise in TyG index levels increases the incidence of MACCEs; (2) TyG index may be employed to independently predict the occurrence of MACCEs (chest pain, acute myocardial infarction, heart failure, cardiogenic shock, malignant arrhythmia, cerebral infarction, gastrointestinal bleeding, and all-cause death) in subjects with ICM combined with T2DM; (3) TyG index can predicts the occurrence of MACCEs, with an AUC of 0.653 in the 3rd year, 0.688 in the 5th year, and 0.764 in the 10th year, with best cutoff values of 8.01, 7.98, and 7.94, respectively; (4) Incorporating TyG index into the risk model can improve MACCE prediction accuracy of the model; In the present investigation, a simple IR valuation method was employed for potential optimization of the combined recurrent cardiovascular risk prediction method for ICM patients with T2DM.
IR is a symptom of glucose metabolism dysfunction and refers to decreased ability of insulin to promote glucose uptake and utilization. IR may hasten the development of CVDs by inducing vascular endothelial dysfunction, changes in systemic lipid metabolism, and abnormalities in glucose metabolism.26
It has been established that IR is a risk factor for CVDs and MACCEs. The hyperinsulin-normal glucose clamp method and the HOMA-IR method are the two main traditional IR identification approaches. Nevertheless, due to the difficulty and high cost of the test procedure, neither of these two approaches can be used widely in clinical practice. Several studies on the TyG index have resolved this clinical issue, and the results show that the TyG index is a reliable alternative to IR.27 Furthermore, TyG has been proposed as a better predictor of IR and atherosclerosis than HOMA-IR.28,29
A growing number of studies have confirmed the close association between the TyG index and CVD prognosis. Laura Sánchez-Íñigo et al confirmed that the TyG index is an indicator of IR and can be utilized to identify individuals at increased risk of developing cardiovascular events.19 Zhenguo Wu et al concluded that the TyG index is significantly associated with premature CAD adverse prognostic events, confirming the early intervention effect of the TyG index.30 Yue Zhang et al demonstrated for the first time that a high TyG index level is a reliable predictor of poor prognosis in subjects with acute myocardial infarction and T2DM. The present study demonstrated that individuals with T2DM and acute myocardial infarction can be classified and their risk predicted using the TyG index.22 Wang et al conducted a 3-year investigation of 2531 patients with acute coronary syndrome and T2DM and found an increased incidence of MACCEs at a higher TyG index, with the best cutoff value for predicting major adverse prognostic events of 9.323.18
In addition, an investigation of 798 individuals with T2DM and non-ST-segment elevation acute coronary syndrome who underwent PCI revealed a significant link between a higher TyG index and poor prognosis post-procedure. Incorporating the TyG index into the fundamental risk model potentially increased the accuracy of the model in predicting poor prognosis.21 Moreover, Thiago Hueb et al stated that subjects with ischemic cardiomyopathy and diabetes had a significantly lower survival rate than those without diabetes. This finding confirmed that diabetes negatively impacts patients with ischemic cardiomyopathy.11 However, the predictive significance of the TyG index in patients with T2DM and ICM remains unknown.
This is the first study to look into the prognostic value of the TyG index on the prognosis of subjects with ICM and T2DM, and the findings are expected to improve clinical treatment plans. The relationship between various types of MACCEs (including chest pain, acute myocardial infarction, heart failure, cardiogenic shock, malignant arrhythmias, cerebral infarction, gastrointestinal bleeding, and all-cause death) and the TyG index was examined in this study. The TyG index was also shown to positively impact a more comprehensive risk assessment and classification of this population based on conventional risk factors, confirming that it is an effective medical predictor for individuals with ICM combined with T2DM. Additionally, according to the findings of Zhao et al, incorporating the TyG index into the original risk model significantly improved the ability of the model to predict MACCEs.21
The Spearman correlation analysis revealed that the TyG index is positively associated with several CVD traditional risk factors, including LDL-C, BMI, and SBP. An earlier study found that high blood pressure, particularly high SBP, can trigger reduced myocardial compliance, high left ventricular filling pressures, left atrial enlargement, and pulmonary venous congestion, all of which can predispose these individuals to heart failure.31 High BMI and LDL-C are known as risk factors for CVD worldwide.32,33 As a result, early detection of high-risk individuals and timely implementation of treatment plans based on the TyG index improve the prognosis of these patients. In addition, it aids in the screening of high-risk patients for early intervention.
While findings from the present study demonstrate that the TyG index is effective in predicting adverse prognostic events in patients with ICM and T2DM, some limitations should be considered. (1) A strictly screened cohort with a relatively small sample size was used for this retrospective and observational study; therefore, the findings of this study must be confirmed in prospective and multicenter with a larger sample size and a larger population. (2) Changes in the TyG index during the follow-up period were not taken into account in this study. (3) Some patients may have undiagnosed systemic illnesses, such as occult malignancies, which may influence prognosis. (4) This study focused on the predictive ability of the TyG index; therefore it cannot be compared to HOMA-IR because HOMA-IR estimation was not performed. (5) The present study did not look into the primary role of visceral adiposity due to the lack of waist circumference data and could not adjust TyG data for this variable.
Conclusions
A high TyG index level is associated with the development of MACCEs in patients with ICM and T2DM. As such, the TyG index could be a valuable biomarker in the early detection and prevention of MACCE in these patients. Furthermore, early intervention in IR may improve the prognosis of patients with ICM and T2DM.
Acknowledgments
The authors would like to express their gratitude to all the healthcare workers and patients who participated in the study.
Funding Statement
This work was supported by the National Natural Science Foundation of China (U1903212) and the key project of Xinjiang Natural Science Foundation.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Statement
Studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (approval number: S220722-25) and followed the Declaration of Helsinki. Patients/participants provided written informed consent for their participation in this study as well as for the publication of any potentially identifiable images or data included herein.
Author Contributions
All authors contributed significantly to the work reported, whether s in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; participated in drafting, revising, or critically reviewing the article; gave final approval of the publishable version; agreed on the journal for the submission of the manuscript; and agreed to be accountable for all aspects of the work.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
- 1.Ardeshna DR, Bob-Manuel T, Nanda A, et al. Asian-Indians: a review of coronary artery disease in this understudied cohort in the United States. Ann Transl Med. 2018;6(1):12. doi: 10.21037/atm.2017.10.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Zhang J, Butler J, et al. Contemporary epidemiology, management, and outcomes of patients hospitalized for heart failure in China: results from the China Heart Failure (China-HF) Registry. J Card Fail. 2017;23(12):868–875. doi: 10.1016/j.cardfail.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 3.Shipulin VM, Pryakhin AS, Andreev SL, Shipulin VV, Kozlov BN. Results of reconstructive interventions in patients with ischemic cardiomyopathy. Asian Cardiovasc Thorac Ann. 2021;29(6):508–517. doi: 10.1177/0218492320983464 [DOI] [PubMed] [Google Scholar]
- 4.Gheorghiade M, Sopko G, De Luca L, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114(11):1202–1213. doi: 10.1161/CIRCULATIONAHA.106.623199 [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39(2):210–218. doi: 10.1016/S0735-1097(01)01738-7 [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Jin R, Li B. Serum NT-proBNP and TUG1 as novel biomarkers for elderly hypertensive patients with heart failure with preserved ejection fraction. Exp Ther Med. 2021;21(5):446. doi: 10.3892/etm.2021.9874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metra M, Carubelli V, Ravera A, Stewart Coats AJ. Heart failure 2016: still more questions than answers. Int J Cardiol. 2017;227:766–777. doi: 10.1016/j.ijcard.2016.10.060 [DOI] [PubMed] [Google Scholar]
- 8.Filippatos G, Khan SS, Ambrosy AP, et al. International Registry to assess medical practice with longitudinal Observation for Treatment of Heart Failure (REPORT-HF): rationale for and design of a global registry. Eur J Heart Fail. 2015;17(5):527–533. doi: 10.1002/ejhf.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. Front Endocrinol. 2018;9:2. doi: 10.3389/fendo.2018.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray KK, Colhoun HM, Szarek M, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(8):618–628. doi: 10.1016/S2213-8587(19)30158-5 [DOI] [PubMed] [Google Scholar]
- 11.Hueb T, Rocha MS, Siqueira SF, et al. Impact of diabetes mellitus on ischemic cardiomyopathy. Five-year follow-up. REVISION-DM trial. Diabetol Metab Syndr. 2018;10:19. doi: 10.1186/s13098-018-0320-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Ark J, Hammes HP, van Dijk MC, et al. Circulating alpha-klotho levels are not disturbed in patients with type 2 diabetes with and without macrovascular disease in the absence of nephropathy. Cardiovasc Diabetol. 2013;12:116. doi: 10.1186/1475-2840-12-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma CX, Ma XN, Guan CH, Li YD, Mauricio D, Fu SB. Cardiovascular disease in type 2 diabetes mellitus: progress toward personalized management. Cardiovasc Diabetol. 2022;21(1):74. doi: 10.1186/s12933-022-01516-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adeva-Andany MM, Martínez-Rodríguez J, González-Lucán M, Fernández-Fernández C, Castro-Quintela E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab Syndr. 2019;13(2):1449–1455. doi: 10.1016/j.dsx.2019.02.023 [DOI] [PubMed] [Google Scholar]
- 15.Tenenbaum A, Adler Y, Boyko V, et al. Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am Heart J. 2007;153(4):559–565. doi: 10.1016/j.ahj.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 16.Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM, Hernandez AV. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One. 2012;7(12):e52036. doi: 10.1371/journal.pone.0052036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Consensus development conference on insulin resistance. 5–6 November 1997. Diabetes Care. 1998;21(2):310–314. doi: 10.2337/diacare.21.2.310 [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Cong HL, Zhang JX, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80. doi: 10.1186/s12933-020-01054-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–197. doi: 10.1111/eci.12583 [DOI] [PubMed] [Google Scholar]
- 20.Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18(1):361. doi: 10.1186/s12916-020-01824-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Q, Zhang TY, Cheng YJ, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19(1):108. doi: 10.1186/s12933-020-01086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Ding X, Hua B, et al. Predictive effect of triglyceride‑glucose index on clinical events in patients with type 2 diabetes mellitus and acute myocardial infarction: results from an observational cohort study in China. Cardiovasc Diabetol. 2021;20(1):43. doi: 10.1186/s12933-021-01236-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarantino G, Crocetto F, Di Vito C, et al. Association of NAFLD and insulin resistance with non metastatic bladder cancer patients: a cross-sectional retrospective study. J Clin Med. 2021;10(2):346. doi: 10.3390/jcm10020346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng AC, Sindone AP, Wong HS, Freedman SB. Differences in management and outcome of ischemic and non-ischemic cardiomyopathy. Int J Cardiol. 2008;129(2):198–204. doi: 10.1016/j.ijcard.2007.07.014 [DOI] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: [DOI] [PubMed] [Google Scholar]
- 26.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 28.Vasques AC, Novaes FS, de Oliveira Mda S, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100. doi: 10.1016/j.diabres.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 29.Irace C, Carallo C, Scavelli FB, et al. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–672. doi: 10.1111/ijcp.12124 [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Liu L, Wang W, et al. Triglyceride-glucose index in the prediction of adverse cardiovascular events in patients with premature coronary artery disease: a retrospective cohort study. Cardiovasc Diabetol. 2022;21(1):142. doi: 10.1186/s12933-022-01576-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorrentino MJ. The evolution from hypertension to heart failure. Heart Fail Clin. 2019;15(4):447–453. doi: 10.1016/j.hfc.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 32.Li J, Yu S, Zhou W, et al. U-shaped association of body mass index with the risk of peripheral arterial disease in Chinese hypertensive population. Int J Gen Med. 2021;14:3627–3634. doi: 10.2147/IJGM.S323769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu L, Wu G, Yang X, et al. Low density lipoprotein mimics insulin action on autophagy and glucose uptake in endothelial cells. Sci Rep. 2019;9(1):3020. doi: 10.1038/s41598-019-39559-7 [DOI] [PMC free article] [PubMed] [Google Scholar]