Summary
Background:
Harmonized population-based surveys with recent HIV-1 infection testing algorithms permit pooled cross-sectional HIV incidence estimation across multiple countries. The objective of this analysis is to estimate adult HIV-1 incidence rates and number of new infections by sex, age, and sub-region in sub-Saharan Africa (SSA).
Methods:
We analyzed data from 13 Population-based HIV Impact Assessment (PHIA) surveys and two additional population-based surveys conducted between 2015 and 2019 in 15 SSA countries. HIV-seropositive samples from adults 15-59 years-old were tested for recent HIV-1 infection using an algorithm consisting of the HIV-1 Limiting Antigen Avidity enzyme immunoassay, HIV-1 viral load, and qualitative detection of antiretrovirals. Data were pooled across countries; sampling weights were incorporated to represent all adults in the 15 national populations. Analyses accounted for the complex sample designs. HIV incidence rates (IRs), IR differences, and number of new annual infections were estimated.
Findings:
Among 445,979 adults sampled, 382 had recent HIV-1 infection. Estimated HIV-1 incidence was 3·3/1,000 PYs (95% CI: 2·6, 4·0) among females and 2·0/1,000 PYs (95% CI: 1·2, 2·7) among males (IR difference: 1.3/1,000 PYs (95% CI: 0.3, 2.3). Among 15-24 year-old adults, the IR was higher for females than males (3·5 versus. 1·2/1,000 PYs; IR difference: 2·3, 95% CI: 0·8, 3·8), but infection rates were comparable between sexes in all other age groups. HIV-1 incidence was 7·4/1,000 PYs (95% CI: 5·0, 9·7) in Southern Africa, 2·3/1,000 PYs (95% CI: 1.7, 2.9) in Eastern Africa, and 0·9/1,000 PYs (95% CI: 0·6, 1·2) in West/Central Africa. Overall, 689,000 (95% CI: 546,000-833,000) new HIV cases were estimated annually among the 265 million susceptible adults—61.6% in females.
Interpretation:
These findings identify differences in HIV-1 incidence and new infections by age, sex, and sub-region. Approaches for risk stratification are needed to guide comprehensive HIV-1 prevention.
Funding:
National Institutes of Health
Introduction
Sub-Saharan Africa (SSA) has long been the epicenter of the HIV epidemic. Although it has approximately 15% of the world’s population,1 it accounts for two-thirds of the 37·7 million people living with HIV globally.2 Despite the disproportionate burden of HIV-1 in SSA, national and regional estimates of HIV-1 incidence primarily have been based on mathematical models, which are sensitive to inputs and assumptions.3,4 Although there have been hundreds of HIV incidence estimates in the region, the vast majority have been conducted among select populations with elevated HIV incidence and few have been nationally representative.5 The absence of representative HIV-1 incidence estimates by age and sex is a critical knowledge gap.
Since 2014, the Population-based HIV Impact Assessment (PHIA) Project has conducted nationally representative household surveys in SSA to capture the state of the HIV epidemic in many high burden countries that are supported by the President’s Emergency Plan for AIDS Relief (PEPFAR).6 These cross-sectional surveys include an HIV-1 recent infection testing algorithm to estimate national HIV-1 incidence.7 South Africa and Nigeria have conducted similar HIV-focused population-based surveys, with comparable methods. For most countries, these surveys provide the first measured, empirical estimates of national HIV-1 incidence and their common methodology allows for pooled estimates across SSA. Although these surveys have been pooled together to answer many regional research questions, they have never been pooled to examine HIV-1 incidence rates and number of new infections. Pooling allows for such regional and sub-regional estimates of HIV-1 by age and sex, something that was not possible in the national surveys due to insufficient precision.
We report findings from the 15 PHIA and PHIA-like surveys conducted in SSA from 2015-2019. We provide pooled estimates of annual adult HIV-1 incidence and the number of new HIV-1 infections by age and sex overall and within three sub-regions (West/Central, Eastern, and Southern Africa). We also calculated incidence-to-prevalence ratios to assess epidemic growth trajectories. This is the first study to provide pooled, nationally representative HIV-1 incidence estimates across three sub-regions in SSA by age and sex and to formally compare them. Such estimates are essential for contextualizing current UNAIDS prevention targets.8
Methods
Data Sources
PHIA surveys are HIV-focused, cross-sectional, household surveys designed to estimate national HIV-1 incidence and, among those who are HIV-infected, national prevalence of viral load suppression.9,10 We analyzed all PHIA data that were publicly available as of September 30, 2022. Data came from 15 PHIA or PHIA-like surveys conducted in Cameroon (June, 2017-February, 2018), Côte d’Ivoire (August, 2017-March, 2018), and Nigeria (July-December, 2018) in West/Central Africa; Ethiopia (urban regions only, October, 2017-April, 2018), Kenya (May, 2018-March, 2019) Rwanda (October, 2018-March, 2019), Tanzania (October, 2016- June, 2017) and Uganda (August, 2016-March, 2017) in Eastern Africa; and Eswatini (August, 2016-March, 2017), Lesotho (November, 2016-May, 2017), Malawi (November, 2015-August, 2016), Namibia (June-December, 2017), South Africa (December, 2016-February, 2018), Zambia (March-August, 2016), and Zimbabwe (October, 2015-August, 2016) in Southern Africa. Throughout this manuscript, we refer to these 15 surveys as “national” surveys, even though Ethiopia’s survey only represented the adult populations in the urban regions. Thirteen PHIA surveys were led by the country’s Ministry of Health and funded by PEPFAR with technical assistance from ICAP at Columbia University and from the US Centers for Disease Control and Prevention (CDC). 9 The fifth South African National HIV Prevalence, Incidence, Behaviour, and Communication Survey, 2017 (SABSSM-V) was led by the Human Sciences Research Council with support from PEPFAR through the CDC.11 The Nigeria HIV/AIDS Indicator and Impact Survey was led by Federal Ministry of Health with funding from PEPFAR and the Global Fund to Fight AIDS, Tuberculosis and Malaria, and technical assistance from the University of Maryland, Baltimore.12 All protocols were approved by ethics committees in each country and the institutional review boards at their respective institutions and by the CDC.
Each survey used a two-stage stratified cluster sample design. Data collection was conducted once over a 6-14 month period in each country, with annual estimates generated from each country’s data collection period. Data were restricted to adults aged 15-59 years old. Some surveys collected data from persons ≥60 years old, but because these data were not collected across all countries, these observations were excluded from this analysis. Adults 18 years and older provided informed consent. Adolescents 15-17 years old provided assent after a parent or guardian granted permission for an interviewer to approach the adolescent.13 In 14 surveys, individuals who provided consent or assent completed an individual interview and provided venous blood for rapid HIV testing and measurement of HIV biomarkers.14 SABSSM-V collected dried blood spots for HIV-1 biomarkers.11
Outcomes
Samples from all consenting survey participants were tested for HIV-1 using each country’s national HIV testing algorithm, conducted with household-based rapid tests and confirmatory laboratory testing. 14, 15 HIV-1 positive individuals were classified as having recent infection according to the following algorithm: Limiting Antigen-Avidity EIA (LAg) normalized optical density values ≤ 1·5, viral load of ≥1,000 copies/ml, and no qualitative evidence of common antiretroviral agents in each country’s first- and second-line regimens.7 Other HIV-1 seropositive individuals whose plasma did not meet criteria for recent HIV-1 infection were classified as having long-term HIV-1 infection.
Pooled estimates of HIV-1 incidence and the number of new annual infections were estimated in the full population and also disaggregated by sex (male and female), age (15-24, 25-34, 35-44, 45-59 years), and sub-region (West/Central, Eastern, and Southern Africa). Pooling was valid because the methodologies that gave rise to the data were comparable and observations were appropriately weighted, such that each observation contributed appropriately to the overall estimates.
Incidence rates were estimated using a formula that includes the number of recent HIV-1 infections in the sample based on the LAg/VL/ARV algorithm, the number of susceptible individuals, the mean duration of recent infection (MDRI), the cutoff time for the assay, and the proportion of false positive recent cases (FRR).16 Consistent with published reports, the FRR was assumed to be zero and the cutoff time was 365 days.16-18 In fourteen countries and in pooled estimates, the MDRI was assumed to be 130 days (95% CI 118-142 days), and in Uganda, it was assumed to be 153 days (95% CI 127-178 days) due to a higher prevalence of HIV-1 subtype D, which has a higher MDRI value.19 Of note, the South African final report applied slightly different FRR and MDRIs, but we applied values used in the other PHIAs for consistency. Based on these assumptions, we estimated instantaneous HIV-1 incidence rates (IRs), expressed as cases per 1,000 person years (PYs), and IR differences with corresponding 95% confidence intervals (CIs).16 To facilitate estimation of the number of new infections, IR estimates were annualized to account for the changing susceptible population over time.
We also estimated the HIV-1 incidence to prevalence ratio (IPR), the ratio of the incidence rate to the prevalence in each country, expressed as a percentage. The IPR is a measure of epidemic growth which tracks the number of new infections relative to the number of people living with HIV. UNAIDS projects that at an IPR of approximately 3·0% or less, an epidemic is expected to contract, though this is not an absolute threshold.3
Statistical analysis
Within each country, sampling weights were computed to account for each individual’s probability of selection, with adjustments for non-participation in household, individual, and biomarker data collection, and post-stratification adjustment to account for under-coverage. During post-stratification, weights were benchmarked to the national population (in 14 countries) or urban population (in Ethiopia). PHIA data were pooled such that sampling weights summed to population totals by country, sex, and age group. To account for the complex two-stage sample design in each country, sampling weights for biomarker data were applied, and variances were estimated using Taylor Series Linearization. Except for the unweighted estimates presented in Table 1, all analyses applied the sampling weights.15
Table 1.
HIV-1 status in the unweighted and weighted PHIA samples by age, sex, and country
| Adults with recent HIV-1 n (%) |
Adults with long-term HIV-11 n (%) |
Adults without HIV-12 n (%) |
Total n (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| Unweighted | Weighted | Unweighted | Weighted | Unweighted | Weighted | Unweighted | Weighted | |
|
|
|
|
|
|||||
| Age (years) | ||||||||
| 15-24 | 129 (33.8%) | 82,749 (33.4%) | 3,154 (11.0%) | 1,860,271 (10.8%) | 152,705 (36.6%) | 100,848,640 (38.0%) | 155,988 (35.0%) | 102,791,659 (36.4%) |
| 25-34 | 139 (36.4%) | 96,854 (39.1%) | 8,328 (28.9%) | 5,376,349 (31.3%) | 115,864 (27.8%) | 73,907,383 (27.9%) | 124,331 (27.9%) | 79,380,586 (28.1%) |
| 35-44 | 73 (19.1%) | 39,617 (16.0%) | 9,521 (33.1%) | 5,678,793 (33.0%) | 79,054 (19.0%) | 49,038,308 (18.5%) | 88,648 (19.9%) | 54,756,718 (19.4%) |
| 45-59 | 41 (10.7%) | 28,424 (11.5%) | 7,787 (27.0%) | 4,286,921 (24.9%) | 69,184 (16.6%) | 41,342,526 (15.6%) | 77,012 (17.3%) | 45,657,871 (16.2%) |
| Sex | ||||||||
| Male | 116 (30.4%) | 94,300 (38.1%) | 8,847 (30.7%) | 6,170,607 (35.9%) | 185,251 (44.4%) | 134,400,623 (50.7%) | 194,214 (43.5%) | 140,665,530 (49.8%) |
| Female | 266 (69.6%) | 153,343 (61.9%) | 19,943 (69.3%) | 11,031,726 (64.1%) | 231,556 (55.6%) | 130,736,234 (49.3%) | 251,765 (56.5%) | 141,921,304 (50.2%) |
| Country | ||||||||
| West/Central | ||||||||
| Cameroon, 2017-2018 | 20 (5.2%) | 9,974 (4.0%) | 908 (3.2%) | 470,618 (2.7%) | 24,015 (5.8%) | 12,623,850 (4.8%) | 24,943 (5.6%) | 13,104,442 (4.6%) |
| Côte d'Ivoire, 2017-2018 | 5 (1.3%) | 1,437 (0.6%) | 412 (1.4%) | 358,480 (2.1%) | 16,902 (4.1%) | 12,964,412 (4.9%) | 17,319 (3.9%) | 13,324,329 (4.7%) |
| Nigeria, 2018 | 44 (11.5%) | 29,030 (11.7%) | 2,566 (8.9%) | 1,345,504 (7.8%) | 162,574 (39.0%) | 99,505,823 (37.5%) | 165,184 (37.0%) | 100,880,357 (35.7%) |
| Eastern | ||||||||
| Ethiopia, 2017-2018, urban | 4 (1.0%) | 1,447 (0.6%) | 589 (2.0%) | 371,389 (2.2%) | 17,970 (4.3%) | 12,003,099 (4.5%) | 18,563 (4.2%) | 12,375,934 (4.4%) |
| Kenya, 2018 | 11 (2.9%) | 12,803 (5.2%) | 1,448 (5.0%) | 1,245,452 (7.2%) | 25,050 (6.0%) | 24,653,016 (9.3%) | 26,509 (5.9%) | 25,911,271 (9.2%) |
| Rwanda, 2018-2019 | 8 (2.1%) | 1,931 (0.8%) | 880 (3.1%) | 198,797 (1.2%) | 28,588 (6.9%) | 6,570,922 (2.5%) | 29,476 (6.6%) | 6,771,649 (2.4%) |
| Tanzania, 2016-2017 | 33 (8.6%) | 25,721 (10.4%) | 1,676 (5.8%) | 1,423,103 (8.3%) | 26,643 (6.4%) | 27,457,147 (10.4%) | 28,352 (6.4%) | 28,905,971 (10.2%) |
| Uganda, 2016-2017 | 42 (11.0%) | 30,502 (12.3%) | 1,667 (5.8%) | 1,136,718 (6.6%) | 26,332 (6.3%) | 17,510,780 (6.6%) | 28,041 (6.3%) | 18,678,000 (6.6%) |
| Southern | ||||||||
| Eswatini, 2016-2017 | 27 (7.1%) | 2,047 (0.8%) | 2,764 (9.6%) | 185,074 (1.1%) | 6,768 (1.6%) | 483,023 (0.2%) | 9,559 (2.1%) | 670,144 (0.2%) |
| Lesotho, 2016-2017 | 35 (9.2%) | 3,520 (1.4%) | 3,164 (11.0%) | 302,333 (1.8%) | 8,483 (2.0%) | 890,843 (0.3%) | 11,682 (2.6%) | 1,196,696 (0.4%) |
| Malawi, 2015-2016 | 25 (6.5%) | 9,958 (4.0%) | 2,132 (7.4%) | 863,406 (5.0%) | 14,543 (3.5%) | 7,429,484 (2.8%) | 16,700 (3.7%) | 8,302,848 (2.9%) |
| Namibia, 2017 | 22 (5.8%) | 1,594 (0.6%) | 2,331 (8.1%) | 169,053 (1.0%) | 13,939 (3.3%) | 1,187,686 (0.4%) | 16,292 (3.7%) | 1,358,334 (0.5%) |
| South Africa, 2017 | 37 (9.7%) | 92,327 (37.3%) | 2,617 (9.1%) | 7,084,464 (41.2%) | 11,954 (2.9%) | 28,024,507 (10.6%) | 14,608 (3.3%) | 35,201,298 (12.5%) |
| Zambia, 2016 | 41 (10.7%) | 15,255 (6.2%) | 2,426 (8.4%) | 945,410 (5.5%) | 16,648 (4.0%) | 7,044,869 (2.7%) | 19,115 (4.3%) | 8,005,534 (2.8%) |
| Zimbabwe, 2015-2016 | 28 (7.3%) | 10,097 (4.1%) | 3,210 (11.1%) | 1,102,534 (6.4%) | 16,398 (3.9%) | 6,787,397 (2.6%) | 19,636 (4.4%) | 7,900,028 (2.8%) |
| Total 3 | 382 | 247,643 | 28,790 | 17,202,334 | 416,807 | 265,136,858 | 445,979 | 282,586,835 |
Adults with long-term HIV-1 infections are individuals who had positive HIV-1 test results, but not biomarkers for recent HIV-1.
Adults without HIV-1 include 13 unweighted participants (6,211 weighted) from Côte d'Ivoire who had positive HIV-2 test results and negative HIV-1 test results
Column percentages may not equal 100.0% due to rounding at the tenth decimal place.
Analyses were conducted in SAS version 9·4 (SAS Institute, Cary, NC), R version 4.2.0 (R Core Team, 2018), and SUDAAN Version 11 (RTI International, 2012).20 Country-level and pooled IR and IR difference estimates were computed using the inctools package in R.15 Population pyramids were created to visualize the population structure of 14 countries, the Ethiopia urban population, and the overall 15-country region. The number of new annual infections was estimated by multiplying the annualized IRs with the HIV-1 negative adult population estimated from the sample. Estimates of prevalence and IPRs were computed in SUDAAN. Variances for the estimated number of new infections and IPRs were approximated using methods described by Goodman for confidence interval construction.21
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The unweighted fifteen-country sample contained 445,979 adults aged 15-59 years and represented a weighted population of 283 million adults within this age range (Table 1). The cumulative weighted population was balanced between males (49·8%) and females (50·2%), with an expansive population pyramid shape (appendix 1) and younger age groups predominating: 36·4% were 15-24 years, 28·1% were 25-34 years, 19·4% were 35-44 years, and 16·2% were 45-59 years. Almost half (45·1%) lived in West/Central Africa, one third lived in Eastern Africa (32·8%), and the remaining fifth lived in Southern Africa (22·2%).
Among the 283 million adults represented through these surveys, an estimated 93·8% (95% CI: 93.6%, 94.1%) were not living with HIV-1, 6·1% (95% CI: 5.8%, 6.3%) had long-term HIV-1, and 0·1% (95% CI: 0.1%, 0.1%) had recent HIV-1. Females accounted for a large majority of the recent HIV-1 infections (61·9%) as compared to males (38·1%). Cumulatively, the two younger age groups (15-34 years) accounted for nearly three quarters of the recent HIV-1 infections (72·5%), as compared to the two older age groups (35-59 years, 27·5%). The majority of recent infections occurred in Southern Africa (54·4%), followed by Eastern Africa (29·2%), and West/Central Africa (16·3%).
In the pooled target population of 283 million adults 15-59 years old, HIV-1 incidence was estimated to be 2·6/1,000 PYs (95% CI: 2·1, 3·2). Results were similar for those 15-49 years: 2.7/1,000 PYs (95% CI: 2.1, 3.3). There were differences in HIV incidence by sub-region: 0.9 (95% CI: 0.6, 1.2) in West/Central, 2.3 (95% CI: 1.7, 2.9) in the East, and 7.4 (95% CI: 5.0, 9.7) in the South.
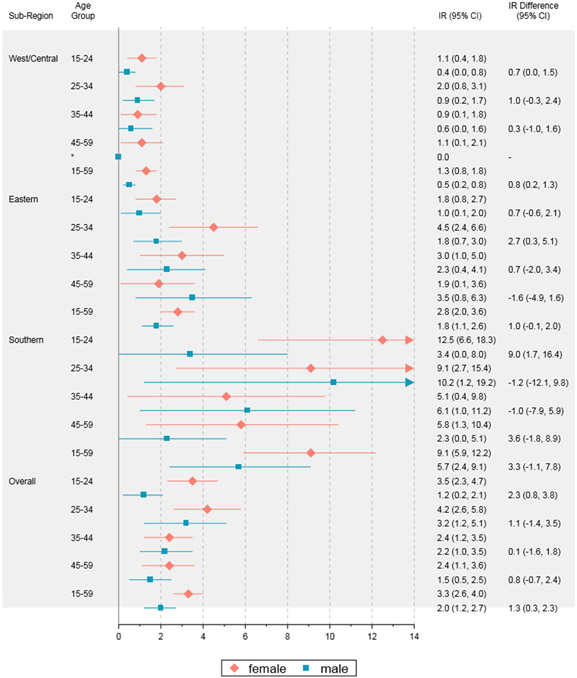
Pooled HIV-1 incidence was estimated to be 3·3/1,000 PYs (95% CI: 2·6, 4·0) among adult females and 2·0/1,000 PYs (95% CI: 1·2, 2·7) among adult males (Figure 2). Among females, the HIV-1 incidence point estimate was highest in the 25-34 year-old age group (IR: 4·2, 95% CI: 2·6, 5·8), though confidence intervals overlapped with the other three female age groups. Among males, HIV-1 incidence was also highest in the 25-34 year-old age group (IR: 3·2, 95% CI: 1·2, 5·1); confidence intervals overlapped with the other three male age groups.
Figure 2. Pooled HIV-1 incidence rates and 95% confidence intervals by age, sex, and sub-region.
*No recent cases in this group
In the region overall, HIV-1 incidence was significantly higher among females than males (IR difference: 1·3, 95% CI: 0·3, 2·3) (Figure 2). It was higher among females than males in the 15-24 year-old age group, but not in any of the other three age groups (IR difference: 2·3, 95% CI: 0·8, 3·8). There was sub-regional variability in these point estimates. In the Southern and West/Central sub-regions, females experienced significantly higher HIV incidence than males in the 15-24 year-old age group, whereas in the Eastern sub-region, females had significantly higher HIV-1 incidence than males in the 25-34 year-old age group. IRs were comparable between females and males in the 35-44 and 45-59 year-old age groups in all three sub-regions.
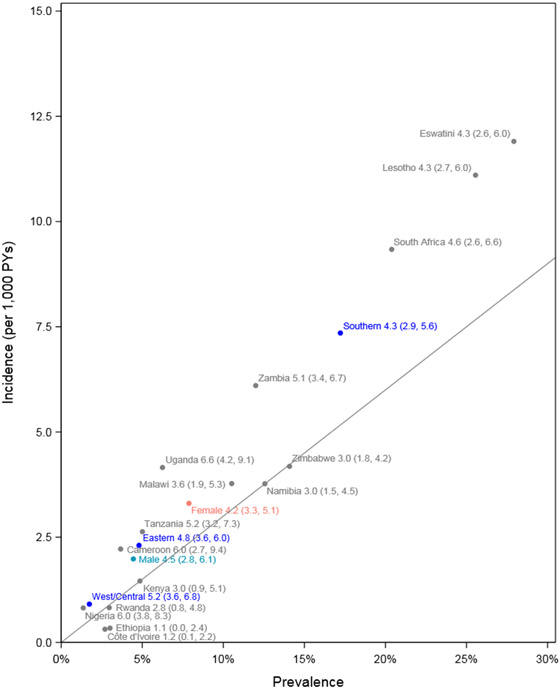
The incidence: prevalence ratio was 4·3 overall (95% CI: 3·4, 5·1): 4·2 (CI: 3·3, 5·1) among females and 4·5 (CI: 2·8, 6·1) among males. Regional estimates were comparable: 4·3 (95% CI: 2·9, 5·6) in Southern Africa, 4·8, (95% CI: 3·6, 6·0) in Eastern Africa, and 5·2 (95% CI: 3·6, 6·8) in West/Central Africa.
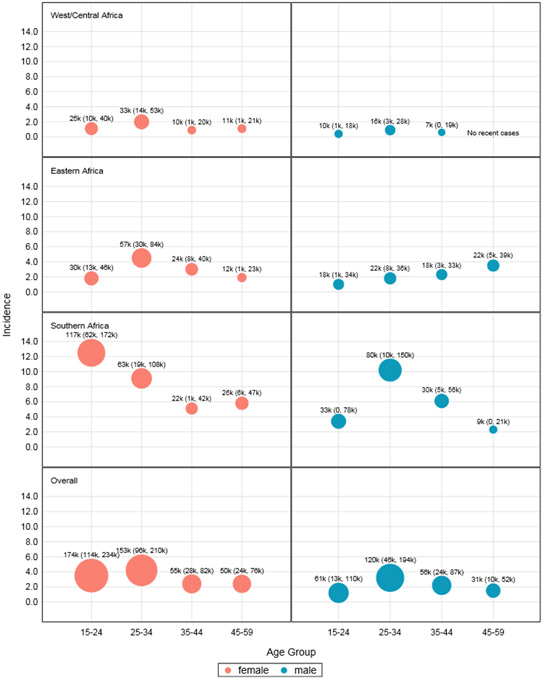
Among the 265 million HIV-1 negative adults in the target population, approximately 689 thousand new HIV-1 infections were estimated to have occurred annually. A large majority (61·6%) of these infections were estimated among females and 38·4% among males (Figure 4). Among females, three quarters (75·7%) of new infections were concentrated in the two youngest age groups: 15-24 years (40·3%) and 25-34 years (35·5%), as compared to the two older age groups: 35-44 (12·7%), and 45-59 years (11·6%). The large number of infections among these younger age groups was due to a combination of high incidence rates in these two age groups coupled with the young age structure. Among males, infections were highest in the 25-34 year age group: 15-24 years (22·9%), 25-34 years (44·8%), 35-44 years (20·8%), and 45-59 years (11·5%).
Figure 4. Pooled HIV Incidence (per 1,000 PYs) and estimated number of new infections in thousands (k) and 95% confidence intervals by sub-region, age, and sex.
The West/Central region accounted for nearly half of the target population, but only 16.2% of the infections (113,000, 95% CI: 77,000-148,000). The Southern region accounted for approximately one fifth of the population, but 54.5% of the new infections (378,000, 95% CI: 259,000, 498,000). The Eastern region contained approximately one third of the population and a similar share (29.2%) of the new infections (203,000, 95% CI: 151,000, 255,000).
Between the sub-regions, there was considerable heterogeneity in the estimated number of infections by age and sex, though all estimates were imprecise (Figure 4). In the West/Central and Eastern sub-regions, females 25-34 years-old had the highest number of new infections (33,000 and 57,000, respectively). In the Southern sub-region, females 15-24 years-old had the highest number of new infections (117,000).
Discussion
In 15 high HIV-1 burden countries in SSA, approximately 3 out of every 1,000 susceptible adults 15-59 years old acquired HIV-1 annually during the study period. Incidence was lowest in West/Central Africa, higher in Eastern Africa, and highest in Southern Africa. Both HIV-1 incidence and the absolute number of new infections were considerably higher for females than males, trends observed in all three sub-regions. For both sexes, incidence peaked in the 25-34 year-old age group in the region overall, but there was considerable sub-regional heterogeneity. Females in their prime childbearing years (15-34 years) accounted for nearly half of the 689 thousand adult HIV-1 infections that were estimated annually in these 15 countries during the study period.
Our study provides the largest empirical estimate of adult HIV-1 incidence by age, sex, and sub-region to date, a noteworthy contribution. These findings are relevant to 265 million HIV-negative adults living in 15 of the highest HIV-1 burden countries, approximately half of the adults in SSA in this age range. Numerous empirical estimates of HIV-1 incidence have been conducted, but none have been representative at such a large scale.5
Overall and within each sub-region, HIV-1 incidence and the absolute number of new infections were considerably higher among females than males. These sex differences stem, in part, from age-disparate relationship structures which are prevalent in SSA22 and lower rates of viral suppression among men living with HIV.23 Although females experienced higher HIV-1 incidence and number of new infections in all three sub-regions, there were differences with respect to timing of female infection. In Southern Africa, females 15-24 accounted for the largest number of new infections and the highest incidence rate, whereas in Eastern and West/Central Africa, females 25-34 accounted for the largest number of new infections and the highest incidence rates.
Women 15-34 years-old in their prime childbearing years accounted for nearly half of the new infections in these 15 countries. This observation—coupled with high fertility rates24 and elevated risk of vertical transmission during acute HIV-1 infection25—contributes to the pediatric HIV-1 burden in these settings. Focusing primary prevention efforts throughout this age group is critical, not only for women’s long-term health but also for their infants. Such efforts should include primary HIV prevention during pregnancy and breastfeeding, periods associated with elevated maternal HIV-1 incidence 25,26 and a growing proportion of new infant HIV-1 infections, particularly in settings with high ART coverage.27
The nature of HIV incidence in sub-Saharan Africa is paradoxical. On the one hand, HIV-1 incidence rates are higher than on any other continent. The overall HIV-1 incidence-prevalence ratio point estimates exceed the 3·0% epidemic transition guidepost among both sexes and in all three sub-regions, suggesting slow epidemic growth, rather than contraction.3 The estimated HIV-1 incidence rate in both males and females exceeds the 1/1,000 PYs HIV epidemic transition benchmark which guided the UNAIDS 90-90-90 targets.28 Additionally, the absolute number of infections is staggering with hundreds of thousands of infections each year and concerns about decelerating progress.29 Nonetheless, new infection remains a relatively rare event, with only 3 of every 1000 adults acquiring HIV annually.
This paradox poses a challenge for reaching those in greatest need of pre-exposure prophylaxis (PrEP). UNAIDS has historically recommended PrEP to most adolescents and adults when HIV-1 incidence is 3 per 100 person-years (30 per 1,000 PYs), a level that we did not observe in any sub-population. It has also recommended PrEP to individuals with sexually transmitted infections or more than one sexual partner in settings with HIV-1 incidence between 1 and 3 per 100 person-years.8 We identified only two countries (Eswatini, Lesotho) and two age-sex groups in the Southern region (females 15-24 and males 25-34 years) that had HIV-1 incidence rates above 1 per 100 person-years, though estimates were imprecise. However, the majority of infections occurred outside of these groups. The World Health Organization has recently challenged these guidelines as too restrictive, a development supported by our findings.30
Deliberate approaches are still needed to identify adults across all three sub-regions at highest risk for HIV-1 acquisition who would benefit most from PrEP. Although risk assessment tools have been developed to assist with HIV-1 risk stratification, none have been created using nationally representative data, and most have been focused on select populations in a limited number of countries.31 Building upon the current analysis, we plan to develop a set of risk assessment tools that can be used to identify clinics and individuals in greatest need of PrEP.
Our analysis has strong internal and external validity. Methodological strengths include rigorous and comparable survey sampling methodology implemented across countries, excellent participation at each survey stage, appropriate weighting techniques in the pooled sample, and a validated recent HIV-1 testing algorithm to measure HIV-1 incidence.7,18 Furthermore, it contains one of the largest numbers of new infections to date.
Longitudinal cohort studies are considered the gold standard for HIV incidence estimation. Incidence estimation based on recent infection testing algorithms has important cost and feasibility advantages over cohort studies,32 but also some limitations. This algorithm hinges on several assumptions—the LAg cut-point is appropriate, the MDRI is accurate, and the FRR is zero when viral load and antiretrovirals are included.7 This method will over- or under-estimate HIV incidence if these parameters are inaccurate. However, LAg-based HIV incidence algorithms have been validated against longitudinal measures,18 enhanced with the antiretroviral drug detection,7 and recommended by the World Health Organization.33
Although pooled surveys represent one of the largest samples of recent or incident cases in a single dataset, imprecision remains a limitation. Though the pooled dataset included 446,000 individuals, only 382 individuals had recent HIV-1 infection (less than 0·1%). Each survey was designed to provide national estimates of HIV-1 incidence, rather than estimates by age, sex, or sub-national areas. However, when pooled across sub-Saharan African sub-regions, estimates of HIV-1 incidence by age and sex were possible. This ability to examine trends by age and sex across sub-regions is an important contribution of our pooled analysis.
This analysis included data from surveys conducted in 15 PEPAR-supported countries that had publicly available datasets on or before September 30, 2022. As such, these results do not generalize to the entire SSA region. Specifically, they do not represent many lower-burden countries that lack PEPFAR support or PEPFAR-supported countries that did not conduct PHIA surveys. Our results should not be generalized to the entire continent, but rather to the 15-59 year-old adults in the 15 countries in our analysis. Based on UNAIDS estimates, these countries account for more than two thirds of the HIV infections in SSA during this period and approximately half of the population in this age group.
For practical reasons, data were collected only once in each country between 2015 and 2019, with most data collected in 2016-2018. These datasets were pooled together despite minor differences in the timing of data collection. Across the 2015-2019 period, slight annual variations in incidence and number of new infections likely occurred, but such estimates were beyond the scope of this analysis. Although this analysis did not include such trends, PHIA is designed to track temporal trends, with second wave data collection completed in Eswatini, Lesotho, Malawi, Uganda, and Zimbabwe, and a subsequent wave of SABSSM now underway in South Africa. Comparing trends over time is a key next step.
Despite formidable progress in testing and treatment, the HIV-1 epidemic in these high-burden countries continues to smolder, infecting hundreds of thousands of adults each year.29 Clarity around the patterns of HIV-1 incidence by sub-region, age, and sex are key for identifying adults at greatest risk for HIV-1 acquisition and linking them to well-designed comprehensive HIV prevention programs. Such identification and linkage are essential for reaching the Sustainable Development Goal of ending the HIV epidemic as a public health threat by 2030.
Supplementary Material
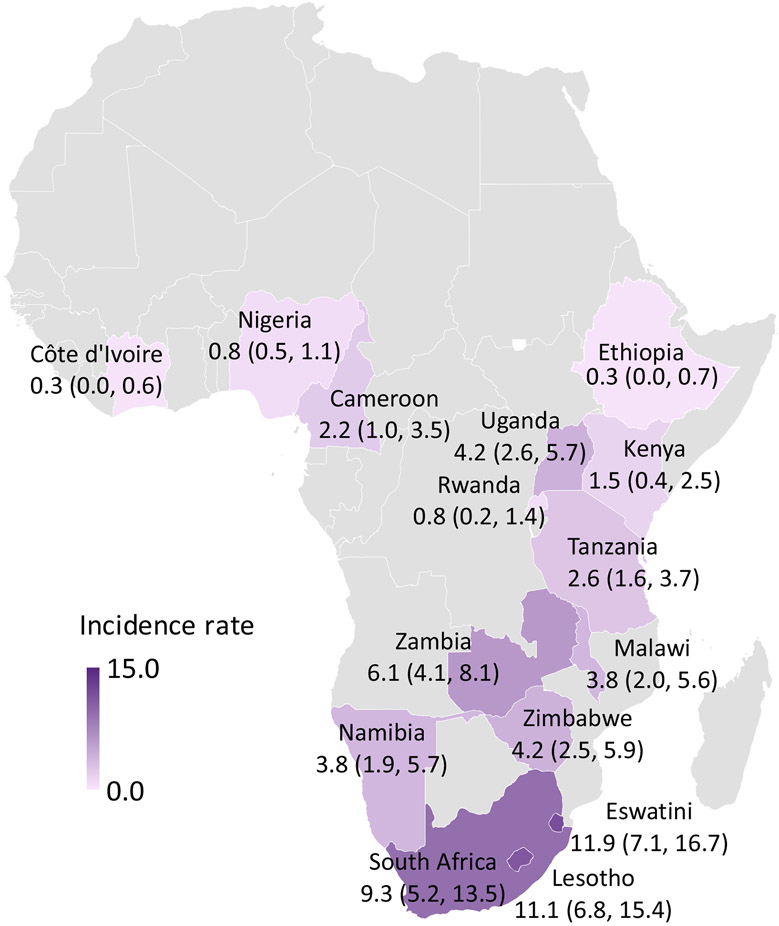
Figure 1. HIV Incidence (cases per 1,000 PYs) by country.
Figure 3. HIV Incidence and prevalence by country and sex relative to 3.0% transition benchmark (diagonal line).
Research in Context.
Evidence before this study
Regional and sub-regional estimates of HIV incidence, disaggregated by age and sex, are crucial to our ongoing understanding of the HIV epidemic in sub-Saharan Africa. However, until recently, such data have been limited to sub-national populations, and have not been pooled at a regional or sub-regional level. We identified a published systematic review synthesizing empirical estimates of HIV-1 incidence in SSA from 2010-2019, which coincided with our current report. Authors of the review used MESH terms for “HIV,” “incidence” and “Africa,” and included peer-reviewed articles in PubMed, Embase, Scopus and OVID global health, as well as non-peer-reviewed PHIA country reports. The search was completed in July 2020 and identified 236 studies. In most studies, HIV-1 incidence was higher among females than males, but overall incidence rates, female-male incidence rate differences, and absolute number of infections were not estimated. In addition, most of these studies came from highly selected populations, such as adults from key populations or those enrolled in HIV-1 prevention trials, and thus over-represented high incidence populations, and lacked broad external validity. The absence of representative empirical estimates of HIV-1 incidence and incidence rate differences by age and sex is a notable gap.
Added value of this study
This is the first study to estimate HIV-1 incidence by age and sex across three sub-regions using nationally representative data from adults 15-59 years old. Based on a large probability-based sample (N=445,979) from 15 countries representing 283 million adults from the general population, pooled annual HIV-1 incidence was higher among females (3·3 per 1,000 PYs) than males (2·0 per 1,000 PYs) (incidence rate difference: 1·3/ 1,000 PYs, 95% CI: 0·3, 2·3). This difference was driven by the substantial difference in incidence by sex among 15-24 year-olds. The overall estimated number of annual infections was substantial and notably higher among females (431,000) than among males (269,000). Nearly half of all adult infections occurred among females 15-34 years-old, women in their prime childbearing years.
Implications of all the available evidence.
This analysis, which accounts for more than two thirds of adult HIV-1 infections in SSA, reinforces the gendered nature of the HIV-1 epidemic, with women in their prime childbearing years bearing the brunt of the HIV-1 epidemic. Though consistent with smaller studies and modeled estimates, the external validity of our results vastly exceeds any prior empirical work.
Funding and Acknowledgments
This analysis and NER, ML, BES, MGH, and BHC were funded by the National Institutes of Health (R21-MH125705) with investigator support (P30-AI050410, K24-AI120796, 5U2G-GH001226). LS-B received funding from ViiV Healthcare to support travel to an Adolescent and HIV Workshop in 2022. MY received grant funding and an honoraria payment from NIH. NAS-A received funding from NIH and a grant from ViiV Healthcare. JJ served on the Roche Diagnostics Scientific Advisory Board in April 2022. BHC received a consulting fee from UNICEF. NAIIS was supported by PEPFAR through the CDC under the Cooperative Agreement #U2GGH002108 to the University of Maryland, Baltimore and by the Global Fund to Fight AIDS, Tuberculosis and Malaria through the National Agency for the Control of AIDS, Nigeria, under the contract #NGA-H-NACA to the University of Maryland, Baltimore. SABSSM was supported by PEPFAR through the CDC Cooperative Agreement #NU2GGH001629. The PHIAs were supported by PEPFAR through the CDC under the terms of cooperative agreement #U2GGH001226 and U2GGH000994 to ICAP Columbia. We extend our gratitude to the study teams and participants across all surveys.
Footnotes
Declarations of interest
We declare no competing interests.
Data availability
The Nigeria data were publicly available from the NAIIS Data Archive portal https://nadanaiis.nascp.gov.ng. The data for the SABSSMV were requested from the Human Sciences Research Council: https://hsrc.ac.za. The data for the remaining 13 PHIA surveys were publicly available upon request from the PHIA website, housed at ICAP, Columbia University: https://phia.icap.columbia.edu/
References
- 1.World Population Review 2021. Available from: https://worldpopulationreview.com/. Accessed August 3, 2022.
- 2.UNAIDS. Global Data on HIV Epidemiology and Responses 2021. Available from: https://aidsinfo.unaids.org/. Accessed August 3, 2022.
- 3.Ghys PD, Williams BG, Over M, Hallett TB, Godfrey-Faussett P. Epidemiological metrics and benchmarks for a transition in the HIV epidemic. PLoS medicine. 2018;15(10):e1002678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eaton JW, Brown T, Puckett R, Glaubius R, Mutai K, Bao L, et al. The Estimation and Projection Package Age-Sex Model and the r-hybrid model: new tools for estimating HIV incidence trends in sub-Saharan Africa. AIDS. 2019;33 Suppl 3:S235–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi K, Lessler J, Olawore O, Loevinsohn G, Bushey S, Tobian AAR, et al. Declining HIV incidence in sub-Saharan Africa: a systematic review and meta-analysis of empiric data. Journal of the International AIDS Society. 2021;24(10):e25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Justman JE, Mugurungi O, El-Sadr WM. HIV Population Surveys - Bringing Precision to the Global Response. The New England journal of medicine. 2018;378(20):1859–61. [DOI] [PubMed] [Google Scholar]
- 7.Voetsch AC, Duong YT, Stupp P, Saito S, McCracken S, Dobbs T, et al. HIV-1 Recent Infection Testing Algorithm With Antiretroviral Drug Detection to Improve Accuracy of Incidence Estimates. Journal of acquired immune deficiency syndromes. 2021;87(Suppl 1):S73–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNAIDS. Global AIDS Strategy 2021-2026: End Inequalities. End AIDS. Geneva, Swizerland: 2021. [Google Scholar]
- 9.Sachathep K, Radin E, Hladik W, Hakim A, Saito S, Burnett J, et al. Population-Based HIV Impact Assessments Survey Methods, Response, and Quality in Zimbabwe, Malawi, and Zambia. Journal of acquired immune deficiency syndromes. 2021;87(Suppl 1):S6–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PHIA Project: Population-based HIV Impact Assessment: Guiding the global HIV response New York, NY: ICAP at Columbia University; [Available from: https://phia.icap.columbia.edu/. [Google Scholar]
- 11.Human Sciences Resarch Council. South African National HIV Prevalence, HIV Incidence, Behaviour and Communication Survey (SABSSM) 2017: Combined—All provinces [data set]. Version 1. Pretoria, South Africa: Human Sciences Research Council [producer] 2017, Human Sicences Research Council [distributor] 2020. [Google Scholar]
- 12.Federal Ministry of Health Nigeria. Nigeria HIV/AIDS Indicator and Impact Survey (NAIIS) 2018: Technical Report. Abuja, Nigeria: 2019. p. 297. [Google Scholar]
- 13.Low A, Teasdale C, Brown K, Barradas DT, Mugurungi O, Sachathep K, et al. Human Immunodeficiency Virus Infection in Adolescents and Mode of Transmission in Southern Africa: A Multinational Analysis of Population-Based Survey Data. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021;73(4):594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel HK, Duong YT, Birhanu S, Dobbs T, Lupoli K, Moore C, et al. A Comprehensive Approach to Assuring Quality of Laboratory Testing in HIV Surveys: Lessons Learned From the Population-Based HIV Impact Assessment Project. Journal of acquired immune deficiency syndromes. 2021;87(Suppl 1):S17–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Population-based HIV Impact Assessment (PHIA) Data Use Manual. New York, NY: 2021. [Google Scholar]
- 16.Kassanjee R, McWalter TA, Barnighausen T, Welte A. A new general biomarker-based incidence estimator. Epidemiology. 2012;23(5):721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duong YT, Qiu M, De AK, Jackson K, Dobbs T, Kim AA, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PloS one. 2012;7(3):e33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duong YT, Dobbs T, Mavengere Y, Manjengwa J, Rottinghaus E, Saito S, et al. Field Validation of Limiting-Antigen Avidity Enzyme Immunoassay to Estimate HIV-1 Incidence in Cross-Sectional Survey in Swaziland. AIDS research and human retroviruses. 2019;35(10):896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laeyendecker O, Gray RH, Grabowski MK, Reynolds SJ, Ndyanabo A, Ssekasanvu J, et al. Validation of the Limiting Antigen Avidity Assay to Estimate Level and Trends in HIV Incidence in an A/D Epidemic in Rakai, Uganda. AIDS research and human retroviruses. 2019;35(4):364–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R--.Core.Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 21.Goodman LA. On the Exact Variance of Products. Journal of the American Statistical Association. 2012;55(292):8. [Google Scholar]
- 22.Bajunirwe F, Semakula D, Izudi J. Risk of HIV infection among adolescent girls and young women in age-disparate relationships in sub-Saharan Africa. AIDS. 2020;34(10):1539–48. [DOI] [PubMed] [Google Scholar]
- 23.Cohn J, Ake J, Moorhouse M, Godfrey C. Sex Differences in the Treatment of HIV. Current HIV/AIDS reports. 2020;17(4):373–84. [DOI] [PubMed] [Google Scholar]
- 24.Bongaarts J. Trends in fertility and fertility preferences in sub-Saharan Africa: the roles of education and family planning programs Genus Journal of Population Sciences. 2020;76(32). [Google Scholar]
- 25.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS medicine. 2014;11(2):e1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graybill LA, Kasaro M, Freeborn K, Walker JS, Poole C, Powers KA, et al. Incident HIV among pregnant and breast-feeding women in sub-Saharan Africa: a systematic review and meta-analysis. AIDS. 2020;34(5):761–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chi BH, Mbori-Ngacha D, Essajee S, Mofenson LM, Tsiouris F, Mahy M, et al. Accelerating progress towards the elimination of mother-to-child transmission of HIV: a narrative review. Journal of the International AIDS Society. 2020;23(8):e25571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. [DOI] [PubMed] [Google Scholar]
- 29.UNAIDS. In Danger: UNAIDS Global AIDS Update 2022. 2022.
- 30.World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach Geneva: World Health Organization. 2021 [PubMed] [Google Scholar]
- 31.Jia KM, Eilerts H, Edun O, Lam K, Howes A, Thomas ML, et al. Risk scores for predicting HIV incidence among adult heterosexual populations in sub-Saharan Africa: a systematic review and meta-analysis. Journal of the International AIDS Society. 2022;25(1):e25861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg NE, Pilcher CD, Busch MP, Cohen MS. How can we better identify early HIV infections? Current opinion in HIV and AIDS. 2015;10(1):61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO, UNAIDS. UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance. When and how to use assays for recent infection to estimate HIV incidence at a population level. 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Nigeria data were publicly available from the NAIIS Data Archive portal https://nadanaiis.nascp.gov.ng. The data for the SABSSMV were requested from the Human Sciences Research Council: https://hsrc.ac.za. The data for the remaining 13 PHIA surveys were publicly available upon request from the PHIA website, housed at ICAP, Columbia University: https://phia.icap.columbia.edu/