Abstract
In February 2018, the Melanoma Research Foundation and the Moffitt Cancer Center hosted the Second Summit on Melanoma Central Nervous System Metastases in Tampa, Florida. The meeting included investigators from multiple academic centers and disciplines. A consensus summary of the progress and challenges in melanoma parenchymal brain metastases was published (Eroglu et al., Pigment Cell & Melanoma Research, 2019, 32, 458). Here, we will describe the current state of basic, translational, clinical research, and therapeutic management, for melanoma patients with leptomeningeal disease. We also outline key challenges and barriers to be overcome to make progress in this deadly disease.
Keywords: intrathecal therapy, leptomeningeal carcinomatosis, leptomeningeal disease, melanoma, neoplastic meningitis
1 |. INTRODUCTION
The incidence of melanoma is rising, and, despite recent advances, treatment for metastatic melanoma patients remains a challenge (Franklin, Livingstone, Roesch, Schilling, & Schadendorf, 2017). A particular and common challenge is central nervous system (CNS) metastases, which are present in 50%–60% of metastatic melanoma patients during the course of their disease (Cohen et al., 2016).
Leptomeningeal disease (LMD) patients have a dismal prognosis (Davies et al., 2011) generally measured in weeks. Outcomes have been poor in part because treatment options and clinical trials in LMD have been extremely limited; NCCN guidelines recommend supportive care and/or palliative radiation. As the incidence of LMD is rising, since it is a site of treatment failure for otherwise effective systemic therapies, there is a critical need for research in LMD (Eroglu et al., 2019).
Here, we review preclinical and clinical research findings on LMD from melanoma and make recommendations for accelerating progress in this challenging disease.
2 |. EPIDEMIOLOGY
The most common solid tumors with the highest incidence of LMD are breast cancer, lung cancer, and melanoma (Le Rhun, Taillibert, & Chamberlain, 2013). It often occurs in the later stages of systemic disease and with concurrent CNS metastases (Le Rhun, Taillibert, et al., 2013). Risk factors for LMD include resection of brain metastases (Nahed et al., 2019). In contrast, stereotactic radiosurgery (SRS) to the resection bed does not appear to increase the risk of leptomeningeal carcinomatosis, and no significant difference in the risk for LMD between en bloc resection and use of SRS instead of craniotomy was found (Ojerholm et al., 2014).
3 |. THE BIOLOGY OF THE LEPTOMENINGES AND LMD
The leptomeningeal space consists of the pia mater and arachnoid mater and contains the circulating CSF (Figure 1). The pia mater (the pious mother) covers the surface of the brain and spinal cord and is comprised of one to two layers of fibroblastoid pial cells knitted together by tight junctions resting atop a basement membrane. This basement membrane, in contact with specialized astrocyte foot processes, is known as the glia limitans and limits entry of CSF components into the brain parenchyma. The pia mater and associated glia limitans reflect into sulci and the perivascular (Virchow–Robins) spaces. The arachnoid mater surrounds the pia mater, sealing the space from the dura. Web-like trabeculae span the arachnoid and pia mater. This subarachnoid space is filled with circulating CSF. In normal healthy state, the CSF is nearly acellular, with a limited number of lymphocytes and macrophages.
FIGURE 1.
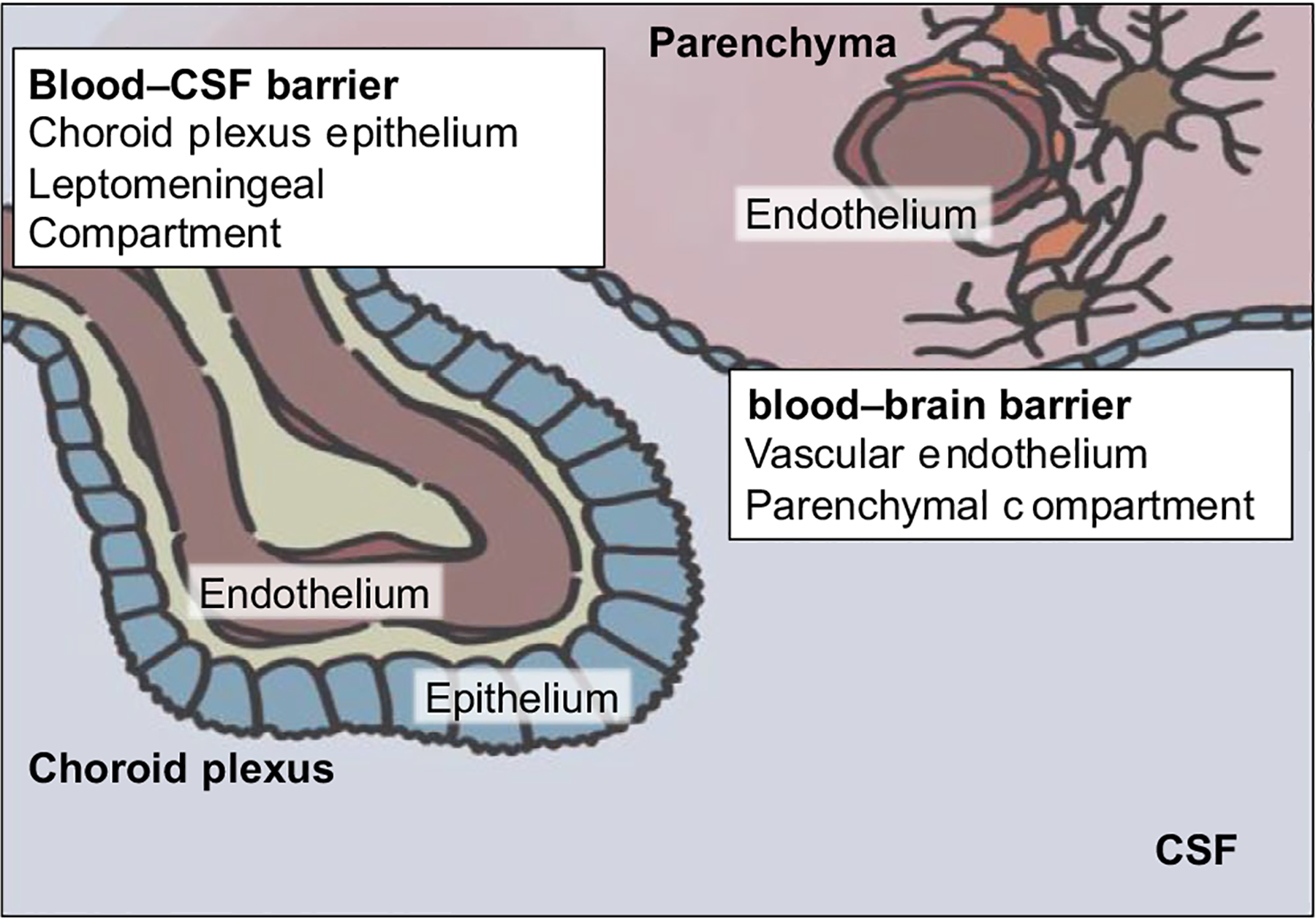
Schematic drawing of the blood–CSF and blood–brain barriers
Whereas the parenchyma is sequestered from the systemic circulation by the blood–brain barrier (Figure 1), the leptomeningeal space is isolated from the systemic circulation by the choroid plexi. These briskly perfused epithelial structures line the ventricular spaces and produce CSF. Tight junctions between choroid plexus epithelial cells constitute the blood–CSF barrier (B-CSF-B). Cancer cells may enter this privileged space through four main routes of entry: (a) via arterial circulation through the choroid plexus; (b) via venous circulation through Bateson’s plexus or bridging veins; (c) perineurally along the cranial nerves or spinal roots; and (d) from the brain parenchyma by broaching the glia limitans. Once within the CSF, cells can circulate over the entire CNS, settling onto the pia, and occasionally broaching the glia limitans and invading the brain or spinal cord parenchyma (Gleissner & Chamberlain, 2006). The perineural route of migration is thought to be rare in melanoma, but has been linked to desmoplastic melanoma (Chang et al., 2004).
At this time, relatively little is known about the molecular and genetic mechanisms that underlie LMD development. One of the few preclinical studies to have addressed this utilized gene expression profiling of breast and lung cancer clones that metastasized to the brain parenchyma and leptomeninges to identify genes that marked metastases to each site. This work identified increased expression of the innate immunity mediator complement 3 (C3) in cancer cells that metastasize to the leptomeninges (Boire et al., 2017). Functional studies showed that tumor associated C3 to interact with the C3a receptor (C3aR) on the cells of the choroid plexus to decrease barrier function, allowing the free passage of growth factors, such as amphiregulin, to enter the CSF space and drive tumor growth in the leptomeninges. There was evidence that the formation of LMD could be abrogated following the inhibition of C3 signaling (Boire et al., 2017). Further mechanistic questions to be addressed include mechanisms by which cancer cells gain entry into the leptomeninges, as well as how these cells interact with immune cells within the CSF.
4 |. DIAGNOSIS
The diagnosis of LMD is notoriously challenging. The current “gold standard” for the diagnosis of LMD is positive CSF cytology. However, the sensitivity of CSF cytology is only 50% and increases with up to three LPs/CSF samplings (Le Rhun, Taillibert, et al., 2013). The diagnosis of LMD can also be made in the setting of characteristic clinical and MRI findings in the absence of positive CSF cytology (Le Rhun, Taillibert, et al., 2013). While MRI findings may be diagnostic (see below), caution should be exercised when they appear in the absence of symptoms or +cytology or CSF studies that support the diagnosis, such as increased opening pressure and elevated WBC and protein levels (Le Rhun, Taillibert, et al., 2013; Taillibert & Chamberlain, 2018; Wang, Bertalan, & Brastianos, 2018).
4.1 |. Clinical criteria
Leptomeningeal disease can result in a wide range of clinical signs and symptoms, reflecting the location of involvement of the nervous system and could be entirely cranial, spinal, or a combination of the above. Typical cranial symptoms include headache from raised intracranial pressure, cranial neuropathies, confusion, hearing loss, double vision, drowsiness, and a stiff neck. Spinal symptoms can include radicular pain, dermatomal sensory loss, and bowel or bladder dysfunction or extremity weakness (Wang et al., 2018). None of these are specific, and other differential diagnoses must be excluded, including infectious meningo-encephalitis, inflammatory meningitis/encephalitis (particularly for patients receiving immunotherapies such as checkpoint inhibitors, adoptive cell therapy or CAR-T therapies etc.), systemic metabolic or toxic syndromes or, albeit rare, a paraneoplastic syndrome.
4.2 |. Imaging criteria
MRI of both the brain and spinal axis should be performed in patients suspected of having LMD. A negative MRI does not exclude the diagnosis of LMD. Imaging should be performed prior to lumbar puncture to avoid potential iatrogenic dural and leptomeningeal enhancement from transient cerebral hypotension or meningeal irritation from blood products. Contrast enhancement seen with iatrogenic etiologies in the spine and brain is typically smooth, non-focal, and dural based but can involve the pial surface or the spinal cord and brain. In the setting of negative CSF cytology, diagnosing LMD by imaging alone should be made with caution and is best done in a multidisciplinary setting in collaboration with neuroradiology, neuro-oncology, and neurosurgery. There are many benign mimics of leptomeningeal disease that can result in both smooth and nodular leptomeningeal enhancement making the imaging diagnosis of LMD by imaging alone challenging.
Leptomeningeal disease can result in both nodular and curvilinear enhancement over the cortical sulci of the cerebral hemispheres as well as the folia of the cerebellum (Figure 2a). LMD can involve the cranial nerves and basal cisterns (Figure 2b). In the spine, it can be seen as both smooth and nodular enhancement along the pial surface of the spinal cord and involving the nerve roots of the cauda equina (Figure 3). LMD can also involve arachnoid granulations and impair CSF resorption resulting in communicating hydrocephalus.
FIGURE 2.
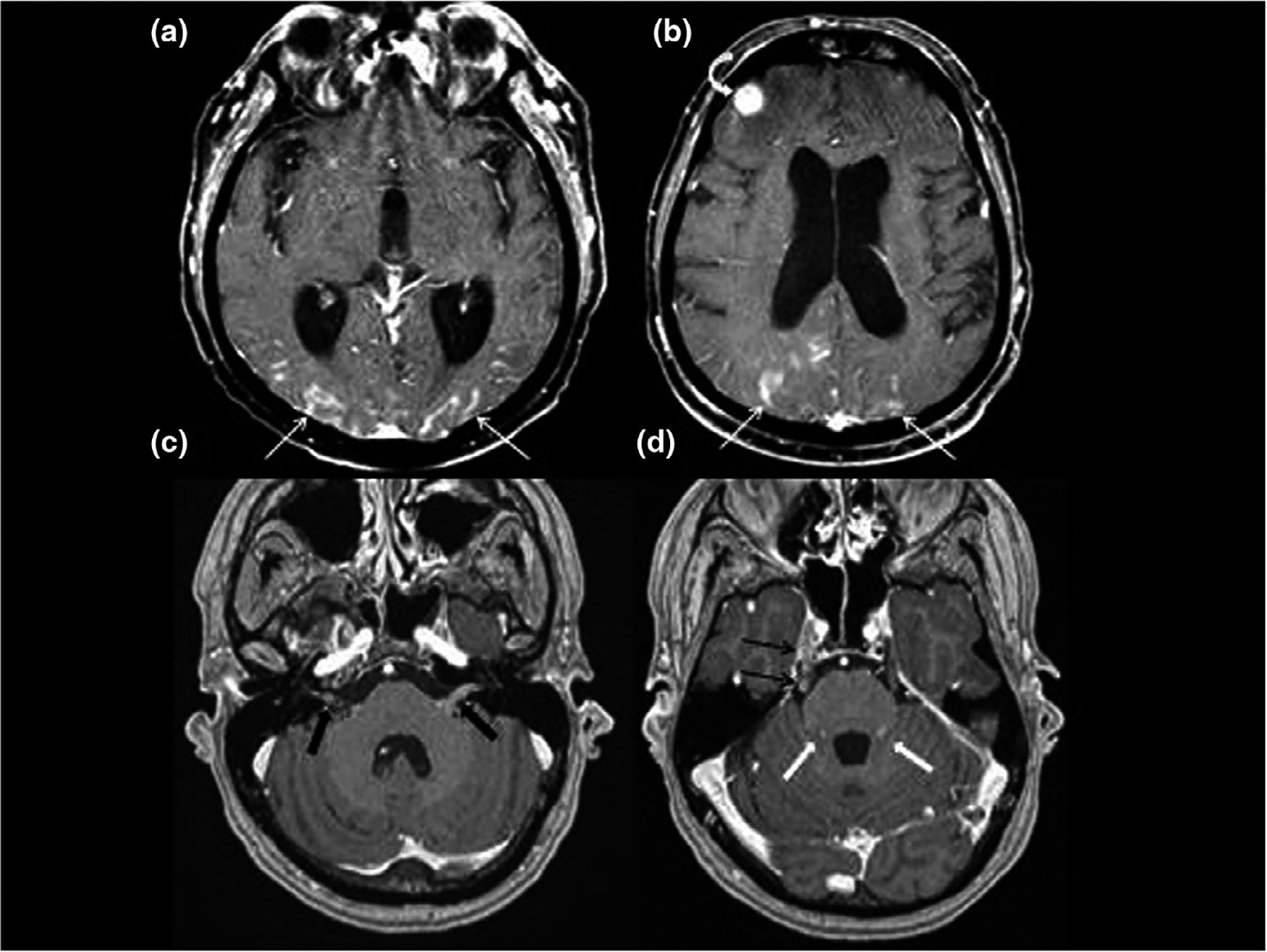
Contrast enhanced axial imaging of the supratentorial brain (a,b) demonstrates curvilinear enhancement of the pial surface of the brain from leptomeningeal disease (LMD). Extensive pial enhancement involves the occipital and parietal cortex bilaterally (white arrows) as well as a focal nodular deposit involving the right frontal cortex (white curved arrow). Contrast enhanced axial imaging of the posterior fossa (c,d) demonstrates nodular enhancing LMD involving cranial nerves VII and VIII within the IAC and CPA (black block arrows), right cranial nerve V in lateral pontine cistern and Meckel’s cave (black arrows), as well as the folia of the cerebellum (white block arrows)
FIGURE 3.
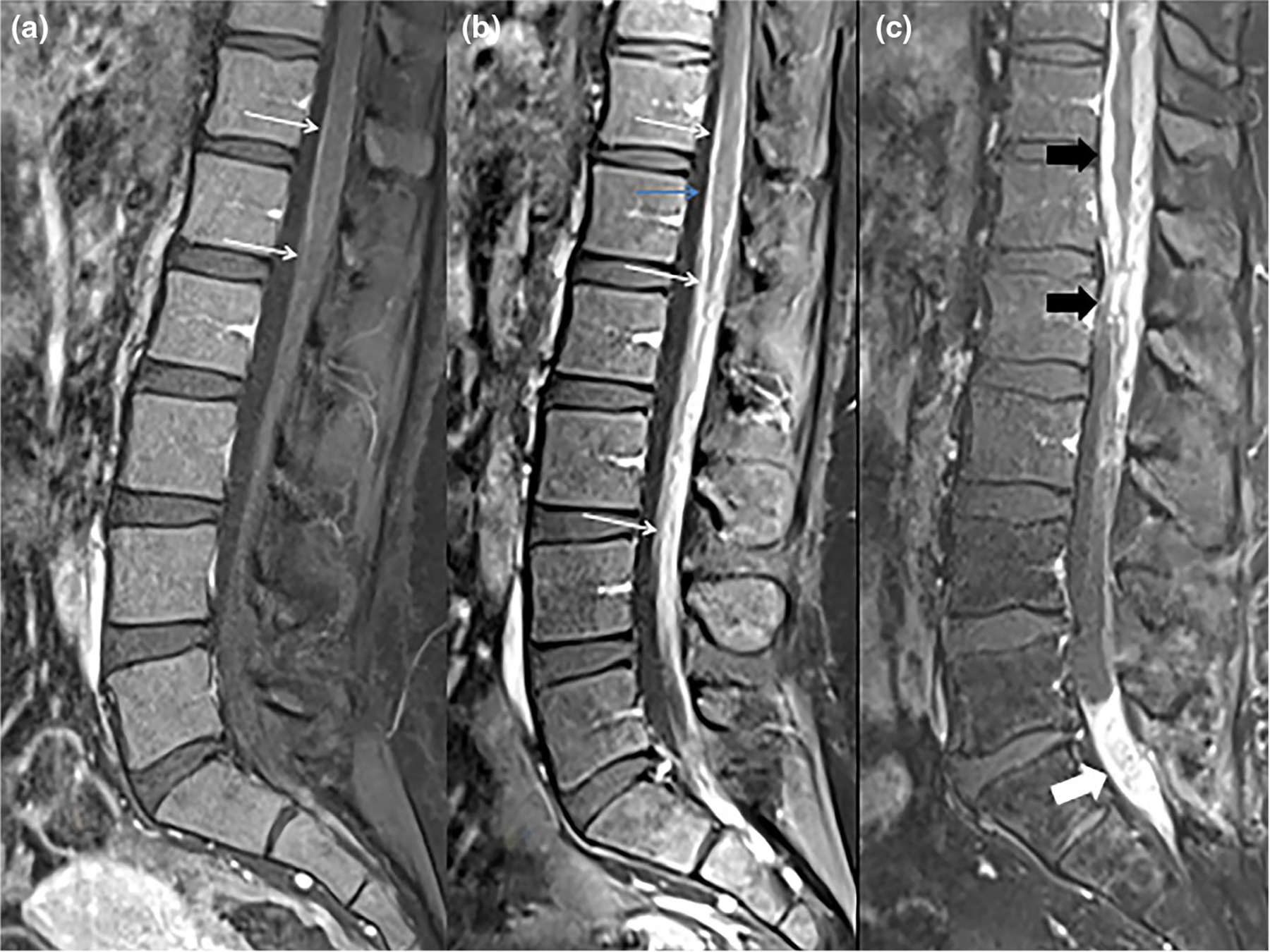
Contrast enhanced sagittal images of three different patients. First patient (a) does not have leptomeningeal disease (LMD) and demonstrates normal minimal enhancement involving the pial surface of the lower thoracic cord and conus (white arrows). Patient b has extensive smooth LMD involving the conus and cauda equine (white arrows). Patient c has more extensive and nodular LMD involving the lower thoracic cord and conus (black block arrows) and extensive focal leptomeningeal disease involving the sacral nerve roots (white block arrow)
Lumbar puncture should be performed after the MRI. Opening pressure (OP) should be obtained and can be very informative. Typical findings in LMD patient include elevated OP, high CSF protein concentration, low CSF glucose concentration, high CSF WBC count with lymphocytic pleocytosis, and positive cytology. The CSF is rarely completely normal in a patient with LMD (Le Rhun, Taillibert, et al., 2013; Taillibert & Chamberlain, 2018; Wang et al., 2018).
4.3 |. CSF cytology
This is the gold standard for the diagnosis of LMD if it is not performed within two weeks of a craniotomy for tumor resection (which can lead to falsely positive cytology; Cagney et al., 2019). If possible, a high volume LP of 10–20 ml of CSF should be obtained and submitted for a cytospin (cell block). Cytology can be reported as positive, suspicious, atypical, or negative. We consider cytology to be diagnostic when it is positive or suspicious (Chamberlain et al., 2017; Glantz et al., 1998). There are no flow cytometric markers (as found in lymphoma) for melanoma. For patients where LMD is suspected, and where the MRI remains inconclusive, it is useful to repeat the CSF up to three times to increase the sensitivity to up to 98%. This also depends somewhat on the site from which the CSF is collected. For some patients, cytology from the CSF is negative when sampled from the ventricle but positive when obtained from the lumbar space. In others, the converse is true.
4.4 |. Leptomeningeal biopsy
In the absence of known systemic melanoma, evidence of an infection or inflammatory disorder of the CSF, and repeated negative cytology evaluations, it may be necessary to perform a meningeal biopsy. Biopsy should be performed from a nodular area of the leptomeninges and, if possible, subjected to DNA sequencing (e.g., BRAF mutation) and/or other diagnostic molecular techniques.
5 |. CIRCULATING TUMOR CELLS AND CELL-FREE DNA
5.1 |. Advanced diagnostics
Analysis of biological fluids, including blood, CSF, and urine, has undergone a revolution in the recent past. Limited by several technical constraints, previous somewhat coarse analyses provided limited diagnostic information or pathophysiologic insight. Modern, highly granular analytic approaches to CSF, or “liquid biopsies,” have the potential to greatly improve diagnostic sensitivity of LMD, guide treatment decisions, and drive discovery (Boire et al., 2019, 2017; Lin et al., 2017; Miller et al., 2019). There are two main liquid biopsy approaches currently in use in LMD: circulating tumor cell analysis (CTCs) and cell-free tumor DNA sequencing (ctDNA); these are reviewed in detail below. Other approaches include cell-free RNA (cfRNA), exosomes, proteomics, and metabolomics. Together, these approaches allow for simultaneous characterization of both the tumor and the microenvironment.
5.2 |. CSF-circulating tumor cells
New semi-automated cell assays have been applied with some success to melanoma as well as other solid tumors for the purpose of diagnosing LMD (Le Rhun, Tu, et al., 2013; Lin et al., 2017, #1447). In the largest cohort (n = 95, 36 breast, 31 lung, 28 others) to date, a cutoff of ≥1 CSF-CTC/ml provided the best threshold to diagnose LMD, achieving a sensitivity of 93%, specificity of 95%, positive predictive value 90%, and negative predictive value 97% (Lin et al., 2017). Other methods, such as flow cytometry, have proved disappointing due to inconsistent surface marker expression.
The Veridex Cellsearch® system detects melanoma tumor cells in the CSF through a combination of microscopy and cell surface markers (Le Rhun, Tu, et al., 2013). Briefly, CSF-CTCs are detected and defined as cells which are nucleated (and therefore immunofluorescent for the nuclear marker 4′6-diamidino-2-phenylindole dihydrochloride [DAPI]), do not express leukocyte markers [the leukocyte common antigen (LCA a.k.a. CD45)], and do express a melanoma marker such as high-molecular-weight-melanoma-associated antigen–associated chondroitin sulfate proteoglycan (HMW-MAA/MCSP; Le Rhun, Tu, et al., 2013). Unlike conventional cytology, this approach allows for a quantitative enumeration of the number of CSF-CTCs in the CSF, potentially allowing for quantitative measures of disease burden and/or therapeutic response.
Similar to the dilemma of repetitive CSF sampling for cytology by LP, the utility of serial CSF-CTC assessment as a marker of treatment response or clinical outcomes in patients with LMD has yet to be determined. For example, in a cohort of 21 patients with LMD and 11 patients without LMD, the sensitivity was a promising 100%, but the apparent specificity was only 73%, with three samples demonstrating CTCs but not positive cytologies (Law et al., 2018). One had melanoma brain metastases (MBM; possibly a source of CTCs “shed” into the CSF), another had extracranial metastatic melanoma but no known CNS involvement, and the third had benign intracranial hypertension and a history of systemic melanoma. Hence, until clear parameters for interpretation of this test are established through formal prospective study, CSF-CTCs in LMD must be interpreted with caution.
5.3 |. Cell-free tumor DNA
CSF ctDNA may also be useful and more reliably obtained than cell-associated DNA (Miller et al., 2019; Pentsova et al., 2016). Although less cell-free DNA (CFDNA) is captured from the CSF compared with the plasma, the CFDNA in the CSF is predominantly tumor-derived. CSF ctDNA may also be subjected to a number of sequencing approaches, including digital PCR, targeted exome sequencing, whole exome, and other evolving technologies (De Mattos-Arruda et al., 2015; Fan et al., 2018; Li et al., 2018; Marchio et al., 2017; Momtaz et al., 2016; Siravegna, Geuna, et al., 2017; Siravegna, Marsoni, Siena, & Bardelli, 2017).
As with CSF-CTCs, the presence of ctDNA in the CSF is not sufficient to make a diagnosis of LMD. LMD is defined as presence of viable, dividing cells within the CSF-filled leptomeningeal space. Parenchymal lesions abutting the leptomeningeal space or ventricular system may shed ctDNA into the CSF without viable cells invading this space; tumor-associated mutations (e.g., EGFR, BRAF, KRAS, PTEN, MET) were detected in the CSF in up to 63% of patients with parenchymal brain metastases (BM) without obvious LMD (De Mattos-Arruda et al., 2015; Pentsova et al., 2016; Siravegna, Marsoni, et al., 2017). Hence, the presence of tumor-associated mutations in CSF-cfDNA may reflect BM, LMD, or both. Momtaz et al. found that cfDNA was isolated in 3/3 (of patients with radiographic evidence of LMD and in 2/5 (40%) of patients with only parenchymal BM (Momtaz et al., 2016).
A recent report in melanoma patients with confirmed LMD highlighted the strong correlation of mutation detection by droplet-digital PCR (ddPCR), the presence of circulating tumor cells in CSF, and abnormalities in the MRI. However, approximately 30% of CSF samples that were negative or indeterminate for the presence of tumor cells by cytology were positive for CSF ctDNA by ddPCR (Ballester et al., 2018).
In a separate case report of a patient with BRAF-positive LMD, ddPCR detected a BRAF V600E mutation in the CSF and the mutant ctDNA fraction decreased with treatment and increased with treatment progression; interestingly, a PTEN mutation was detected at recurrence. Although this needs to be validated in larger studies, it suggests that ctDNA from CSF may be used to monitor treatment response (Li et al., 2016).
6 |. SURVIVAL AND PROGNOSTIC FACTORS
Survival has not changed over the last several decades and is still typically measured in weeks to a few months (Le Rhun, Taillibert, et al., 2013). Most studies of prognostic factors have included relatively small numbers of patients (i.e., <30–40). The analysis of the largest cohort (n = 178) did identify several factors independently associated with worse survival, including Eastern Cooperative Oncology Group [ECOG] performance status >0 (HR 2.1, p < .0001), the presence of neurological symptoms (HR 1.6, p < .0001), and lack of concurrent systemic disease (HR 0.4, p < .0001; Ferguson et al., 2019). Treatment with any therapy directed at LMD was also associated with improved outcomes (HR 0.4, p = .0024), as was treatment with targeted therapy (HR 0.6, p = .0060) and treatment with intrathecal (IT) therapy (HR 0.5, p = .0019). Only 12 patients in this group received systemic immunotherapy, which had no impact on survival (HR 1.2, p = .59). Similar findings were found in an independent cohort of 39 patients with LMD melanoma (Geukes Foppen et al., 2016). In addition, patients with elevated serum LDH levels at the diagnosis of LMD had worse outcomes than LMD patients with normal LDH levels (p < .001). Notably, treatment with radiation did not impact survival in either study (Ferguson et al., 2019; Geukes Foppen et al., 2016).
7 |. STANDARD APPROACH TO LMD
Currently, there is a lack of consensus guidelines for the treatment of melanoma patients with LMD, and NCCN guidelines are non-specific. Providing treatment on a clinical trial should be the highest priority but these are not widely available. In patients appropriate for IT therapy, we recommend an Ommaya reservoir/VP shunt be placed immediately after the diagnosis of LMD to relieve symptoms and to facilitate safe access to the CSF.
The major treatment modalities for LMD currently are radiotherapy (RT) and systemic therapy (e.g., BRAF and MEK inhibitors [BRAF/MEKi] and immunotherapy; see “Clinical Trials” below), and treatment may be selected for administration by either the systemic or IT route. All these treatment modalities will be discussed in greater detail below. Although LMD is diffuse, we recommend radiating symptomatic areas (e.g., brain or the spine) or those with bulky disease that make it unlikely these will be controlled by systemic or IT therapy approaches. The risk of significant side effects from entire neuraxis RT generally outweighs the benefits in this relatively radio-resistant tumor. It should be noted that a recent review did not find that RT had a positive impact on survival as monotherapy (Ferguson et al., 2019) while another showed longer survival when RT was used in combination with systemic therapies (Geukes Foppen et al., 2016).
Systemic therapy that penetrates the CSF, such as the BRAF and MEK inhibitors (which poorly penetrate an intact BBB), has activity in patients with parenchymal BM from BRAFV600-mutant melanoma. Systemically delivered immunotherapies (e.g., checkpoint inhibitors) have been said to be useful in some case reports Finally, while it was popular for a time to use temozolomide, an alkylating agent with high CNS penetration and modest activity in primary brain tumors, for patients with melanoma metastatic to the brain (and by analogy, the meninges), this drug confers no more than a 10% response rate for melanoma and is typically not recommended (Atkins et al., 2008; Margolin et al., 2002).
8 |. RADIATION THERAPY
Palliative RT, typically in the form of WBRT, may be an option in the symptom management of LMD. Similarly, focal radiation approaches may be utilized for symptomatic relief in patients with focal involvement at any site of the neuroaxis as well as for parenchymal lesions in the brain. Focused radiation may also be used to reduce tumor bulk and resolve obstructive hydrocephalus, which can both alleviate symptoms and improve IT therapy flow through the CSF. Radiation of the complete craniospinal column is typically not used in the management of melanoma LMD due to the toxicities associated with this approach in the absence of evidence of significant clinical benefit.
The late side effects of WBRT may include increased somnolence and memory loss; however, given the prognosis of LMD is so poor, the majority of patients do not survive long enough to experience these late effects. Techniques such as hippocampal-sparing radiation and concurrent treatment with cholinesterase inhibitors such as memantine have both demonstrated encouraging results in reducing neurocognitive decline in prospective trials in patients receiving WBRT (Brown et al., 2013; Gondi et al., 2014). However, patients with LMD have been excluded from such trials due to their poor prognosis and extensive disease burden, and studies assessing the use of memantine in appropriately selected LMD patients are warranted.
Radiation therapy may work synergistically with systemic and IT therapies in the management of LMD. Several studies have pointed to the increased permeability of the blood–brain barrier following the delivery of radiation therapy (Cao et al., 2005). In addition, there is significant interest within the field of radiation oncology on potential synergy for combinations of radiation therapy and immune checkpoint inhibitors (Ahmed et al., 2016; Twyman-Saint Victor et al., 2015; Williams et al., 2017). This hypothesis is being tested in numerous ongoing prospective studies in various primary and malignant tumors, including patients with BM. NCT03719768 tests the hypothesis that low-dose WBRT may increase T-cell trafficking into the CSF in LMD (see section “Clinical Trials”).
9 |. TARGETED THERAPY
Approximately 50% of advanced melanomas harbor an activating (most frequently position 600) mutation in the serine/threonine kinase BRAF (Fedorenko, Gibney, Sondak, & Smalley, 2015). There is evidence that melanomas driven by BRAF mutations have a slightly higher rate of CNS metastases (Arasaratnam et al., 2018).
While targeted therapies can reach the CSF, it is unclear if this is within therapeutic concentrations (Sakji-Dupre et al., 2015). The CNS is generally thought to have only low levels of drug being able to penetrate the blood–CSF barrier (B-CSF-B). There is also patient-to-patient variability with PK studies of vemurafenib showing highly variable levels in the CSF and not strongly correlated with plasma levels—a possible reflection of differing levels of B-CSF-B integrity between patients (Sakji-Dupre et al., 2015). Other interventions, such as radiotherapy and surgery, can influence B-CSF-B permeability as the highest CSF levels of vemurafenib were found in patients with prior stereotactic radiosurgery (SRS; Sakji-Dupre et al., 2015). Six case reports have found responses to BRAFi and BRAF/MEKi therapy, and the onset of response/clinical benefit can be rapid (Arasaratnam et al., 2018; Floudas, Chandra, & Xu, 2016; Glitza, Ferguson, & Guha-Thakurta, 2017; Kim et al., 2015; Lee et al., 2013; Schafer et al., 2013; Wilgenhof & Neyns, 2015).
A recent case series described a cohort of 14 metastatic melanoma patients with LMD (Arasaratnam et al., 2018). Of note, 11 of the 14 patients evaluated had BRAF-mutant melanoma, 7 of whom developed LMD during BRAFi therapy, and one of whom continued on BRAFi as their extracranial disease remained controlled. The other 4 BRAF-mutant patients received BRAFi after the diagnosis of LMD, and they had a median survival of 7.2 months. As this is longer than survival reported in several historical cohorts of patients with LMD, this suggests possible survival benefit with the BRAFi (Arasaratnam et al., 2018). Responses were also reported in patients treated with concurrent BRAFi and immunotherapy, although the number of patients was too small to meaningfully assess whether there was additional benefit.
The numbers of patients treated with BRAFi and the BRAF/MEKi combination are too small to determine whether response rates/duration are equivalent to those observed at extracranial sites. In the case of BRAF-mutant melanoma brain metastases (MBMs), patients respond to BRAF/MEKi therapy at a similar rate as at extracranial sites but for shorter duration (Davies et al., 2017). While there has been some suggestion from preclinical studies on rat CSF, and through melanoma cell-astrocyte co-culture experiments, that there may be factors in the CSF or brain environment that are protective for melanoma cells, the role of the PI3K-AKT pathway, which is important in BRAF-mutant melanomas, remains poorly defined (Niessner et al., 2013; Seifert et al., 2016).
10 |. IV THERAPY
Recent clinical trials have demonstrated that checkpoint inhibitors, such as ipilimumab, nivolumab, and pembrolizumab, can safely achieve durable clinical responses in patients with MBM (Eroglu et al., 2019). Response rates in MBM patients have been as high as ~55%, and MBM responses are almost always concordant with systemic responses. Importantly, no increased CNS toxicities were observed. Almost all of these prospective studies excluded patients with LMD. Thus, data regarding outcomes with checkpoint inhibitors in patients with LMD are essentially limited to case reports. There is one report of a patient with metastatic melanoma and LMD who received WBRT with no improvement and then had a complete response after treatment with ipilimumab (Smalley, Fedorenko, Kenchappa, Sahebjam, & Forsyth, 2016). A second case report describes two melanoma LMD patients in whom systemic therapy with anti-PD1 therapy led to neurologic improvement (Glitza & Bucheit, 2017). The recently published ABC Phase II study allowed patients with LMD, symptomatic neurologic symptoms or in whom local therapy (i.e., radiation) for MBMs had failed, to be treated in with single-agent nivolumab (Cohort C; n = 16; Long et al., 2018). The other cohorts included patients with asymptomatic brain metastases with no previous local brain that were randomized to either received treatment with nivolumab plus ipilimumab (cohort A) or nivolumab single agent (cohort B). Cohort C included 4 patients with LMD, none of whom responded. The median overall survival in this cohort was 5.1 months (1.8- not reached), which was in stark contrast to the outcomes of the other cohorts, where the median OS had not been reached at time of the analysis (Cohort A: 8.5 months—NR; Cohort B: 6.9 months—NR). Therapy was well-tolerated without unique CNS toxicities (trials are ongoing, e.g., NCT02939300; NCT03091478; NCT03719768 [clinicaltrials.gov]).
11 |. INTRATHECAL IMMUNOTHERAPY
An overview of IT immunotherapy that has been previously used in melanoma patients with LMD is summarized in Table 1. The use of intrathecal immunotherapy was first reported in the late 1980s, when it was found that the concentration of recombinant interleukin-2 (rIL-2) in the CSF was only half as high as in the plasma of patients who received high-dose interleukin-2 (IL-2) for the treatment of their metastatic disease (Shapiro, Chernik, & Posner, 1973). In addition, interferon-alpha had also been tested in this setting (Table 1; Dorval et al., 1992; Misset, Mathe, & Horoszewicz, 1981).
TABLE 1.
Overview of intrathecal immunotherapy used in melanoma patients with leptomeningeal disease
| Author | Year | Melanoma patients (n) | Treatment used | (Median Overall) Survival |
|---|---|---|---|---|
| Moser, Bruner, and Grimm (1991) | 1991 | 9 | IL-2 | 2/12 had neurological improvement |
| Meyers, Obbens, Scheibel, and Moser (1991) | 1991 | 4 | Interferon- alpha | No clinical improvement |
| Heilmans et al. (1991) | 1991 | 1 | IL-2 and LAK cells | NR |
| Dorval et al. (1992) | 1992 | 1 | Interferon- alpha | 3 months (died from systemic progression) |
| List et al. (1992) | 1992 | 11 | IL-2 | NR |
| Samlowski, Park, Galinsky, Ward, and Schumann (1993) | 1993 | 1 | IL-2 | 1 month |
| Rosener et al. (1993) | 1993 | 1 | IL-2 | NR |
| Meyers and Yung (1993) | 1993 | 1 | IL-2 | 4 years |
| Dippold, Bernhard, and Meyer zum Buschenfelde (1994) | 1994 | 2 | Ganglioside antibody R-24 | NR |
| Fathallah-Shaykh et al. (1996) | 1996 | 1 | IL-2 | 15 months alive at time of report |
| Chamberlain (2002) | 2002 | 2 | Interferon- alpha | NR, progressed after 4 weeks |
| Papadopoulos et al. (2002) | 19942002 | 46 | IL-2 | 3.8 months (0.5–90), response: 11.5 months (range 7–90), no response 3.5 (1.5–11), survival not evaluable, 1 month (0.5–7) |
| Clemons-Miller et al. (2001) | 2001 | 1 | CD8+ CTLs and IL-2 | At least 1 year |
| Heiss, Taha, Oldfield, and Ram (2011) | 2011 | 1 | NIH3T3 producer cells | 9 months |
| Shonka, Kessinger, and Aizenberg (2014) | 2014 | 1 | IL-2 | 170 days after start of IT IL-2 |
| Glitza, Haymaker, et al. (2015) and Glitza, Rohlfs, et al. (2015) | 2015 | 1 | IL-2 and TIL | 5 months after start of IT TIL |
| Ursu et al. (2015) | 2015 | 1 | Immunotherapy with CpG-ODN | 84 weeks, IT therapy given with fotemustine |
| Glitza et al. (2018) | 2018 | 43 | IL-2 | 7.8 months (range, 0.4–90.8 months) |
Abbreviations: IL-2, interleukin-2; LAK, lymphokine-activated killer cells; TIL, tumor-infiltrating lymphocytes.
Pharmacologic studies were first performed in patients (n = 37) who did not have any CNS involvement with cancer. They were treated with intravenous IL-2 at a dose of 105 U/kg every 8 hr and then hourly for an additional 6 hr. Samples for both CSF and plasma were taken at frequent time intervals, with IL-2 being first detectable 4–5 hr after the initial infusion, and typically cleared over 8–12 hr (Saris et al., 1988).
While peripheral blood lymphocytes appear to need a minimum dose of 6 IU/ml of IL-2 to be transformed into lymphokine-activated killer cells (LAKs) ex vivo, it appears that intravenous administration of IL-2 is not sufficient to generate or maintain LAK cells in vivo. In addition, the significant systemic toxicity of intravenous high-dose IL-2 is an important safety consideration, although meningismus, headaches, nausea and vomiting, transient change in mental status, and marked rise in intracranial pressure were all observed with the IT administration of IT IL-2 (Rosenberg et al., 2011, 1994).
Subsequently, intrathecal injection of LAK cells in murine mammary cancer models showed that these cells can prevent the development of LMD when given together with the cancer cells at a dose of the latter that is sufficient to establish tumor (Herrlinger, Weller, & Schabet, 1998). Multiple different groups then started using intrathecal patient-derived, ex vivo-generated LAK cells either in combination with either intravenous IL-2 or IT IL-2, across different tumor types (Heimans et al., 1991; Jacobs, O’Malley, Freeman, & Ekes, 1981; Mayumi et al., 2017; Okamoto et al., 1986).
For melanoma, the first report of using both IT IL-2 and LAK dates back to 1991 (Table 1), when a patient with advanced melanoma and cytologically proven LMD was treated with 5 × 109 LAK cells derived via leukapheresis and ex vivo exposure to IL-2. This patient had a transient fall in CSF malignant cell count and a rise in CSF lymphocytosis but did not otherwise benefit from therapy and was also unresponsive to the systemically administered IL-2.
In the largest reported treatment cohort for melanoma LMD patients (n = 46), patients treated with IT IL-2 had reported OS rates at 1, 2, and 5 years of 36%, 26%, and 13%, respectively. These patients were treated with an induction scheme of IT IL-2 (1.2 mIU) up to five times per week for 4 weeks; patients with good tolerance and clinical benefit received maintenance IT IL-2 every 1–3 months thereafter (Glitza, Rohlfs, et al., 2015). The median overall survival from initiation of IT IL-2 was 7.8 months (range, 0.4–90.8 months). The presence of neurological symptoms (HR 2.1, p = .03) at diagnosis, positive baseline CSF cytology (HR 4.1, p = .001), and concomitant use of targeted therapy (HR 3.0, p = .02) was associated with shorter OS on univariate analysis. It is important to note that all patients were treated on the inpatient unit during the induction period and that all patients had significant clinical decline from treatment-related toxicities. However, there were no treatment-related deaths.
Finally, IT chemotherapy is still used despite its limited efficacy. There has never been a clinical trial in LMD from melanoma that showed a significant benefit from IT chemotherapy (Pape et al., 2012).
12 |. CURRENT CLINICAL TRIALS IN LMD
Current clinical trials are summarized in Table 2. Virtually, all trials for LMD from melanoma involve immunotherapy with checkpoint inhibitors (CPIs), which is reasonable given the dramatic results seen in parenchymal MBM (Eroglu et al., 2019). Few of these trials (only 4) are specifically designed for melanoma patients, and most involve systemic administration of a CPI with the hope that peripheral activation of T cells will lead to a response in the CSF space. However, little is known about the migration of T cells in and out of the CSF, though evidence of T-cell passage has been reported (Mohammad et al., 2014; Strazielle, Creidy, Malcus, Boucraut, & Ghersi-Egea, 2016). This aspect is significant, as only a relatively small number of T cells (~35/mm3 = 35,000/ml) are routinely found in the CSF in LMD and may not be sufficiently activated/or activatable to have beneficial anti-tumor activity. It is therefore critically important for all LMD trials to longitudinally monitor CSF understand tumor microenvironment and how both systemic and IT therapies can alter this.
TABLE 2.
Active clinical trials for patients with leptomeningeal disease from melanoma
| NCT# PI | Phase | Tumor types | Drug or intervention | Primary outcomes | Secondary outcomes |
|---|---|---|---|---|---|
| NCT03719768 Forsyth | 1 | Melanoma Breast NSCLC | Avelumab + WBRT | Safety + OS | Bio. endpoints (e.g., single cell) CSF/Blood |
| NCT03025256 Glitza | 1 | Melanoma | Nivo IT + IV IT alone for C1 then IT + IV for ≥C2 | Safety + IT dose of Nivo | OS; CSF/Blood for Transl. studies |
| NCT02939300 Brastianos | 2 | Melanoma | Ipi + Nivo IV | OS | Response + toxicities |
| NCT02886585 Brastianos | 2 | Multi. Histol. | Pembro IV | OS, ORR | Toxicities, Bio. endpoints (e.g., single cell) |
| NCT03091478 Naidoo | 2 | Multi. Histol. Solid Tumors | Pembro IV | ORR | Toxicities |
| NCT0338377 Amaria | 2 | Systemic Melanoma and LMD in Cohort D | IT TILs + IT IL-2 with lymphodepletion in the LMD Cohort D | Safety | IC ORR, Bio. endpoints (e.g., TIL persistence in CSF, CSF- CTCs, cytokines etc.) |
| NCT02308020 Eli Lilly | 2 | Multi. Histol. + Melanoma | Abemaciclib P.O. | IC ORR | Response + toxicities + PK |
| NCT02910700 Tawbi | 2 | Melanoma | Nivo + Dabra + Tram. LMD pts are allowed | Safety, IC ORR | OS, PFS, PK |
| NCT03520504 Yang J | 1b | Multi. Histol. + Melanoma | Proton Beam CSI | DLT |
Abbreviations: Active, Study is Active but no longer recruiting; Bio, Biological; BM, Brain Metastasis (es); C, Cycle; CSI, CranioSpinal Irradiation; Dabra, Dabrafenib; IC, Intracranial; IL-2, interleukin-2; IT, Intrathecal; IV, Intravenous; LAK, lymphokine-activated killer cells; LMD, Leptomeningeal Metastasis Disease; Multi. Histol., Multiple Histologies or tumor types; Nivo, Nivolumab; NSCLC, Non-Small-Cell Lung Cancer; ORR, Objective Response Rate; OS, Overall Survival; PFS, Progression-Free Survival; PK, Pharmacokinetics; RR, Response Rate; RT, Radiotherapy; TIL, tumor-infiltrating lymphocytes; TILs, Tumor-Infiltrating Lymphocytes; Tram, Trametinib; Transl., Translational; WBRT, Whole-Brain Radiation Therapy.
Innovative approaches include the IT administration of a CPI with systemic administration, (NCT03025256), the IT administration of TILs and IL-2 (NCT0338377) in melanoma patients with LMD, and the combination of BRAF/MEKi in combination with nivolumab (NCT02910700).
Time will tell if these approaches are effective, but each prospective trial will further inform us about the biology of LMD in melanoma patients and will allow for the development of further treatment strategies that will also incorporate the efforts of translational researchers to better understand the biology and vulnerabilities of this disease.
13 |. NEUROSURGICAL AND OTHER CLINICAL ISSUES
13.1 |. Ommaya reservoir placement
Dr. Ayub Ommaya first described the placement of a subcutaneous reservoir attached to an intraventricular catheter in 1963 (Ommaya, 1963). Since then, this approach has become the mainstay for the delivery of IT therapy, as it can be used as soon as one day after placement.
Despite being a relatively simple neurosurgical procedure, there are several key issues to consider in the placement of an Ommaya reservoir for patients with LMD. The catheter tip can be malpositioned and not reside in the intraventricular CSF, a complication that may occur in 3%–13% of patients (Lau et al., 2019). Strategies to improve this include the use of intraoperative CT scan or MRI, intraventricular pneumocephalograms (widely available), and stereotactic navigation (Lau et al., 2019). There is a 1%–3.4% risk of intracerebral hemorrhage during catheter placement (Lau et al., 2019). Intracranial hemorrhage can be delayed for up to 28 days following surgery (Sandberg, Bilsky, Souweidane, Bzdil, & Gutin, 2000).
13.2 |. Infections associated with an Ommaya
The most common complication is infection, which occurs in 1.9%–12% of patients, primarily due to gram-positive bacteria introduced at the time of placement and/or later instrumentation of the device (Lau et al., 2019; Mead, Safdieh, Nizza, Tuma, & Sepkowitz, 2014; Sandberg et al., 2000). Treatment of infection remains controversial regarding the need to remove the device in addition to the requirement for antibiotics for all patients (Chamberlain, Kormanik, & Barba, 1997; Dinndorf & Bleyer, 1987; Mead et al., 2014; Szvalb et al., 2014). The largest review of Ommaya reservoir placement (n = 616) reported an infection rate of 5.5%. One third of the infections were related to placement, and the remainder were associated with subsequent reservoir access (Mead et al., 2014). The updated guidelines of the Infectious Disease Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis recommend replacement of hardware at three or seven days following initiation of antibiotics, depending on the degree of CSF pleocytosis with negative CSF cultures for coagulase-negative staphylococcus or Propionibacterium acnes. For staphylococcus aureus or gram-negative bacillary infections, hardware can be replaced in 10–14 days from the initiation of antibiotics with negative CSF cultures (Tunkel et al., 2017).
14 |. CAUTION IN PLACING AN OMMAYA WITH PRE-EXISTING LEUKOENCEPHALOPATHY
A consideration prior to placement of an Ommaya reservoir is chemotherapy-related leukoencephalopathy. This may be due to stagnation of IT chemotherapy due to a lack of proper CSF flow caused by obstruction secondary to tumor deposits in arachnoid granulations that may or may not be apparent on neuroimaging (Sandberg et al., 2000). Some advocate the use of 111In-diethylenetriamine pentaacetic acid (DPTA) flow studies to verify normal passage of CSF prior to implantation of an Ommaya reservoir and initiation of IT chemotherapy (Chamberlain et al., 1999).
15 |. CNS COMPLICATIONS ASSOCIATED WITH LMD
15.1 |. Hydrocephalus
Leptomeningeal disease often provokes hydrocephalus. This may occur either in a “communicating” fashion (in which tumor cells block CSF flow at narrow points in the ventricular system, e.g., aqueduct of Sylvius) or more often in a “non-communicating” fashion when cells collect on arachnoid granulations. These granulations serve as sites of bulk CSF resorption into the venous system and hence, when impeded, leads to CSF accumulation. Up to half of patients with LMD develop hydrocephalus at some point in their course. This can mild and well-tolerated or may be more symptomatic way with the classic symptoms of increased intracranial pressure (nausea, vomiting, headache, and papilledema). Note that changes in vision may also occur due to leptomeningeal deposits of tumor on the optic, oculomotor, abducens, or trochlear nerves; thus, changes in vision should not automatically trigger a diagnosis of hydrocephalus. A neurologic examination including fundoscopy and cranial nerve assessments is therefore essential in the management of LMD.
Endoscopic third ventriculostomy (ETV) is frequently used to treat hydrocephalus from other causes, but it is not recommended for the hydrocephalus caused by LMD. The success of an ETV depends on good reabsorption of CSF at the arachnoid granulations, a feature typically impaired by micro- or macro-tumor deposits from melanomatous LMD.
The hydrocephalus caused by LMD can in some cases be quite subtle and show only a slight increase in ventricular size or no increase at all; physicians treating patients with LMD should have a high index of suspicion for the presence of hydrocephalus when clinical symptoms or signs of increased intracranial pressure are present, whether or not the radiologist has detected hydrocephalus from the appearance of the scan. Fundoscopic changes frequently precede changes on MRI. This incongruence between ventricular size and intraventricular pressure probably arises from changes in cerebral compliance induced by tumor in the subarachnoid space or in brain perfusion caused by the effect of LMD on cortical blood vessels. Symptomatic hydrocephalus in a patient with LMD is treated by placement of a ventriculoperitoneal shunt. We find ventriculoperitoneal shunts much easier to assess and maintain than lumboperitoneal shunts, which we deem a less desirable alternative, and yield rapid improvement in signs and symptoms in 90% of patients (Mitsuya et al., 2019).
There is some controversy about whether treating the hydrocephalus increases survival, but the symptomatic improvement offered by shunting makes such a procedure excellent palliation and may allow time for novel therapies to work (Lin et al., 2011).
Shunting a patient with LMD does raise the theoretical concern that CSF diversion may enable the transfer of tumor cells from the CSF spaces into the peritoneal cavity (Lee & Lee, 2011). However, in practice such transfer is rarely clinically significant.
15.2 |. Intracranial hemorrhages
Bleeding into the cerebral parenchyma or into the intraventricular, subarachnoid, or even subdural space can occur through rupture of delicate vessels associated with focal deposits of LMD. Intracranial hemorrhage should be evaluated by CT and/or MRI scan, neurology/neurosurgical specialists and if necessary be considered for surgical evacuation.
16 |. CONCLUSIONS—ACCELERATING DISCOVERIES AND IMPROVING CARE
Rarely are we confronted with a disease about which we know so little and whose outcomes are so dismal. Below are key challenges and suggested actions to improve LMD patient outcomes (modified from Cohen et al., 2016):
16.1 |. The biology of LMD is unknown—develop animal and tissue-based models of LMD
Unlike other tumors, including MBMs, there are no spontaneously arising immunocompetent models of LMD. This would help uncover mechanisms of LMD development as well as therapeutic targets. The development of PDXs from CSF-CTCs either from patients or from LMD tumor deposits (either at surgery or from a rapid autopsy) has been difficult and not yet fully successful. This may be appropriate for preclinical testing albeit in immunocompromised mice. Xenografts of established melanoma cell lines into the IT space in immunocompromised animals may be useful for functional testing, though it must be assumed these do not completely recapitulate the cells in LMD.
16.2 |. We need better insights about pathogenesis and treatment of LMD—close collaboration between clinicians, clinician scientists, and scientists in other disciplines may help
Novel ideas may arise from interactions between scientists and clinicians and suggest new insights into the diagnosis and treatment of LMD.
16.3 |. Matched “tissues” are scarce in LMD—develop multi-institutional collaborative specimen banks
CSF offers the ideal opportunity to sample both tumors (the “seed”) and tumor microenvironment (TME or the “soil”), and yet, it is rare to have access to these or the corresponding matched samples from MBM (if they exist), blood (to capture cells that spread hematogenously to the LMD), the systemic metastases, and any primary tumors. Profiling with various platforms may uncover novel targets for therapy (e.g., if certain oncogene drivers or secreted oncogenic proteins for which therapeutic antibodies exist, etc.). Pooling of resources could occur between major melanoma research centers to facilitate research, validation, and discovery. Rapid autopsies are also useful in providing matched tissues.
16.4 |. We need more clinical trials & with tissue interrogation—understand the biology, include LMD cohorts in melanoma clinical trials, routinely collect CSF and tissue of patients on clinical trials
We need more trials designed specifically for LMD as well as including LMD patients in clinical trials for systemic melanoma. Integration of correlative CSF, plasma, and tissue collections throughout the course of the trial is essential. This will enable interrogation of CSF and blood for PK, target modulation, and other markers of response and resistance (e.g., single cells, proteomics, metabolomics etc). New technologies offer the hope of identifying the best potential treatment targets and then, by serial sampling, predict resistance early, potentially in a single cell, well before it is clinically apparent.
16.5 |. What are the best trial endpoints in LMD?—incorporate suggestions from RANO and other groups and prospectively evaluate them
Frustratingly, the criteria for diagnosis, response, and progressive disease in LMD remained difficult to assess and somewhat controversial. Prospectively including putative response schemes from RANO and other groups would help to prospectively evaluate their possible utility and speed to clinical evaluation of novel therapies.
16.6 |. LMD patients are very complex and require multidisciplinary care
Multidisciplinary care and well weighing collaborative teams with medical oncologist, neurosurgeons, neuro-oncologists, neuroradiologists, neurologists, a neuropathologist, radiation oncologists, and early phase drug developers are necessary to make a diagnosis, make treatment decisions, and manage complications and neurologic symptoms. The already complex neurologic environment of LMD becomes much more so when treatments such as RT or immunotherapies or other novel therapies are added with the potential consequences on the normal nervous system.
We remain optimistic that armed with knowledge about the biology of LMD, new technologies, novel treatments, enhanced cooperation among melanoma centers, and hope that we will make a difference to patients with LMD.
CONFLICTS OF INTEREST
Isabella C. Glitza received research funding from Bristol-Myers Squibb and Merck and served as consultant for Bristol-Myers Squibb, Novartis, and Array. Priscilla K. Brastianos served as consultant for Genentech-Roche, Tesaro, Angiochem, and Lilly; received speaker’s honoraria from Merck and Genentech-Roche; and received research funds from Lilly, Merck, Pfizer, and BMS. Michael A. Davies served as PI of research grants to my institution from AstraZeneca, Roche/Genentech, GlaxoSmithKline, Myriad, Oncothyreon, and Sanofi-Aventis and served as consultant for GlaxoSmithKline, Roche/Genentech, Novartis, Array, BMS, Sanofi-Aventis, and Vaccinex. James K.C. Liu received grant support from Celgene and served as advisory board member for Novocure. Kamran A. Ahmed received research funding from Bristol-Myers Squibb and Genentech. Zeynep Eroglu received research funding from Novartis and served as consultant for Array and Regeneron. Nikhil Khushalani served as advisory board member for BMS, HUYA, Immunocore, Regeneron, Array, EMD Serono, and Merck; received honoraria from Sanofi. served as Data Safety Monitoring Board member for AstraZeneca and Incyte; received research funding (to Institute) from BMS, Celgene, HUYA, Regeneron, GSK, Novartis, and Merck; and hold common stock in Bellicum, TransEnterix, Mazor Robotics, Amarin. Kim Margolin served as consultant for Nektar, Iovance, and ImaginAb. Harriet Kluger received grant support from Merck, Bristol-Myers Squibb, and Apexigen and personal fees from Alexion, Corvus, Nektar, Biodesix, Roche-Genentech, and Pfizer. Michael B. Atkins served as advisory board member for BMS, Merck, Novartis, Arrowhead, Pfizer, Galactone, Werewolf, Fathom, Pneuma, and Leads; served as consultant for BMS, Merck, Novartis, Pfizer, Genentech-Roche, Exelixis, Eisai, Aveo, Array, AstraZeneca, Ideera, Aduro, ImmunoCore, Boehringer-Ingelheim, Iovance, Newlink, Pharma, Surface, Alexion, Acceleron, Cota, and Amgen; received research support (for institution) from BMS, Merck, Pfizer, and Genentech; and hold stock options in Werewolf and Pyxis Oncology. Hussein Tawbi received grant/research support from Genentech, BMS, Merck, and GSK and served as consultant for Genentech, BMS, Novartis, Merck, and Array. Adrienne Boire served as consultant for Arix Biosciences; served as advisory board member for Evren Scientific; and received patents: Boire A and J Massagué, inventors. Sloan Kettering Institute, assignee. Modulating Permeability of the Blood Cerebrospinal Fluid Barrier. United States Provisional Application No.: 62/258,044. November 20, 2015, and Boire A, Chen Q and J Massagué, inventors. Sloan Kettering Institute, assignee. Methods for Treating Brain Metastasis. United States Provisional 62/052,966. September 19, 2014. Peter Forsyth received grant support from NIH/NCI, CDMRP, Department of Defense, Pfizer, State of Florida Bankhead Coley, and Moffitt Cancer Center Celgene Project; served as advisory board member for Novocure, BTG, Inovio, and Bayer; and served as consultant for AbbVie, Inc., Ziopharm, Novellus, NCI Neuro-Oncology Branch (NOB), Physical Sciences Oncology Network (PSON), and Tocagen. Keiran S. M. Smalley, Ian McCutcheon, John A. Arrington, Brittany R. Evernden, and Inna Smalley declare no conflict of interest.
REFERENCES
- Ahmed KA, Stallworth DG, Kim Y, Johnstone P, Harrison LB, Caudell JJ, … Gibney GT (2016). Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Annals of Oncology, 27(3), 434–441. 10.1093/annonc/mdv622 [DOI] [PubMed] [Google Scholar]
- Arasaratnam M, Hong A, Shivalingam B, Wheeler H, Guminksi AD, Long GV, & Menzies AM (2018). Leptomeningeal melanoma-A case series in the era of modern systemic therapy. Pigment Cell & Melanoma Research, 31(1), 120–124. 10.1111/pcmr.12652 [DOI] [PubMed] [Google Scholar]
- Atkins MB, Sosman JA, Agarwala S, Logan T, Clark JI, Ernstoff MS, … Margolin KA (2008). Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: A phase II Cytokine Working Group study. Cancer, 113(8), 2139–2145. 10.1002/cncr.23805 [DOI] [PubMed] [Google Scholar]
- Ballester LY, Glitza Oliva IC, Douse DY, Chen MM, Lan C, Haydu LE, … Davies MA (2018). Evaluating circulating tumor DNA from the cerebrospinal fluid of patients with melanoma and leptomeningeal disease. Journal of Neuropathology and Experimental Neurology, 77(7), 628–635. 10.1093/jnen/nly046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire A, Brandsma D, Brastianos PK, Le Rhun E, Ahluwalia M, Junck L, … Soffietti R (2019). Liquid biopsy in central nervous system metastases: A RANO review and proposals for clinical applications. Neuro-Oncology, 21(5), 571–584. 10.1093/neuonc/noz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire A, Zou Y, Shieh J, Macalinao DG, Pentsova E, & Massague J (2017). Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell, 168(6), 1101–1113.e13. 10.1016/j.cell.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, … Watkins-Bruner D (2013). Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-Oncology, 15(10), 1429–1437. 10.1093/neuonc/not114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagney DN, Lamba N, Sinha S, Catalano PJ, Bi WL, Alexander BM, & Aizer AA (2019). Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncology, 5(5), 703. 10.1001/jamaoncol.2018.7204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Tsien CI, Shen Z, Tatro DS, Ten Haken R, Kessler ML, … Lawrence TS (2005). Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. Journal of Clinical Oncology, 23(18), 4127–4136. 10.1200/JCO.2005.07.144 [DOI] [PubMed] [Google Scholar]
- Chamberlain MC (2002). A phase II trial of intra-cerebrospinal fluid alpha interferon in the treatment of neoplastic meningitis. Cancer, 94(10), 2675–2680. 10.1002/cncr.10547 [DOI] [PubMed] [Google Scholar]
- Chamberlain M, Junck L, Brandsma D, Soffietti R, Rudà R, Raizer J, … Jaeckle KA (2017). Leptomeningeal metastases: A RANO proposal for response criteria. Neuro-Oncology, 19(4), 484–492. 10.1093/neuonc/now183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain MC, Kormanik PA, & Barba D (1997). Complications associated with intraventricular chemotherapy in patients with leptomeningeal metastases. Journal of Neurosurgery, 87(5), 694–699. 10.3171/jns.1997.87.5.0694 [DOI] [PubMed] [Google Scholar]
- Chamberlain MC, Kormanik P, Jaeckle KA, Glantz M, Mason WP, Yeh SD, & DeAngelis LM (1999). 111Indium-diethylenetriamine pentaacetic acid CSF flow studies predict distribution of intrathecally administered chemotherapy and outcome in patients with leptomeningeal metastases. Neurology, 52(1), 216–217. 10.1212/WNL.52.1.214-b [DOI] [PubMed] [Google Scholar]
- Chang PC, Fischbein NJ, McCalmont TH, Kashani-Sabet M, Zettersten EM, Liu AY, & Weissman JL (2004). Perineural spread of malignant melanoma of the head and neck: Clinical and imaging features. AJNR. American Journal of Neuroradiology, 25(1), 5–11. 10.1016/j.ajo.2004.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons-Miller AR, Chatta GS, Hutchins L, Angtuaco EJ, Ravaggi A, Santin AD, & Cannon MJ (2001). Intrathecal cytotoxic T-cell immunotherapy for metastatic leptomeningeal melanoma. Clinical Cancer Research, 7(3 Suppl), 917s–924s. [PubMed] [Google Scholar]
- Cohen JV, Tawbi H, Margolin KA, Amravadi R, Bosenberg M, Brastianos PK, … Kluger HM (2016). Melanoma central nervous system metastases: Current approaches, challenges, and opportunities. Pigment Cell & Melanoma Research, 29(6), 627–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, … Bedikian A (2011). Prognostic factors for survival in melanoma patients with brain metastases. Cancer, 117(8), 1687–1696. 10.1002/cncr.25634 [DOI] [PubMed] [Google Scholar]
- Davies MA, Saiag P, Robert C, Grob J-J, Flaherty KT, Arance A, … Long GV (2017). Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. The Lancet Oncology, 18(7), 863–873. 10.1016/S1470-2045(17)30429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mattos-Arruda L, Mayor R, Ng CKY, Weigelt B, Martínez-Ricarte F, Torrejon D, … Seoane J (2015). Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nature Communications, 6, 8839. 10.1038/ncomms9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinndorf PA, & Bleyer WA (1987). Management of infectious complications of intraventricular reservoirs in cancer patients: Low incidence and successful treatment without reservoir removal. Cancer Drug Delivery, 4(2), 105–117. 10.1089/cdd.1987.4.105 [DOI] [PubMed] [Google Scholar]
- Dippold W, Bernhard H, & Meyer zum Buschenfelde KH (1994). Immunological response to intrathecal and systemic treatment with ganglioside antibody R-24 in patients with malignant melanoma. European Journal of Cancer, 30(2), 137–144. 10.1016/0959-8049(94)90073-6 [DOI] [PubMed] [Google Scholar]
- Dorval T, Beuzeboc P, Garcia-Giralt E, Jouve M, Palangie T, & Pouillart P (1992). Malignant melanoma: Treatment of metastatic meningitis with intrathecal interferon alpha-2b. European Journal of Cancer, 28(1), 244–245. 10.1016/0959-8049(92)90420-7 [DOI] [PubMed] [Google Scholar]
- Eroglu Z, Holmen SL, Chen Q, Khushalani NI, Amaravadi R, Thomas R, … Smalley KSM (2019). Melanoma central nervous system metastases: An update to approaches, challenges, and opportunities. Pigment Cell & Melanoma Research, 32(3), 458–469. 10.1111/pcmr.12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Zhu X, Xu Y, Lu X, Xu Y, Wang M, … Hu M (2018). Cell-Cycle and DNA-damage response pathway is involved in leptomeningeal metastasis of non-small cell lung cancer. Clinical Cancer Research, 24(1), 209–216. 10.1158/1078-0432.Ccr-17-1582 [DOI] [PubMed] [Google Scholar]
- Fathallah-Shaykh HM, Zimmerman C, Morgan H, Rushing E, Schold SC Jr, & Unwin DH (1996). Response of primary leptomeningeal melanoma to intrathecal recombinant interleukin-2. A case report.Cancer, 77(8), 1544–1550. [DOI] [PubMed] [Google Scholar]
- Fedorenko IV, Gibney GT, Sondak VK, & Smalley KSM (2015). Beyond BRAF: Where next for melanoma therapy? British Journal of Cancer, 112(2), 217–226. 10.1038/bjc.2014.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SD, Bindal S, Bassett RL, Haydu LE, McCutcheon IE, Heimberger AB, … Glitza IC (2019). Predictors of survival in metastatic melanoma patients with leptomeningeal disease (LMD). Journal of Neuro-Oncology, 142(3), 499–509. 10.1007/s11060-019-03121-2 [DOI] [PubMed] [Google Scholar]
- Floudas CS, Chandra AB, & Xu Y (2016). Vemurafenib in leptomeningeal carcinomatosis from melanoma: A case report of near-complete response and prolonged survival. Melanoma Research, 26(3), 312–315. 10.1097/CMR.0000000000000257 [DOI] [PubMed] [Google Scholar]
- Franklin C, Livingstone E, Roesch A, Schilling B, & Schadendorf D (2017). Immunotherapy in melanoma: Recent advances and future directions. European Journal of Surgical Oncology, 43(3), 604–611. 10.1016/j.ejso.2016.07.145 [DOI] [PubMed] [Google Scholar]
- Geukes Foppen MH, Brandsma D, Blank CU, van Thienen JV, Haanen JB, & Boogerd W (2016). Targeted treatment and immunotherapy in leptomeningeal metastases from melanoma. Annals of Oncology, 27(6), 1138–1142. 10.1093/annonc/mdw134 [DOI] [PubMed] [Google Scholar]
- Glantz MJ, Cole BF, Glantz LK, Cobb J, Mills P, Lekos A, … Recht LD (1998). Cerebrospinal fluid cytology in patients with cancer: Minimizing false-negative results. Cancer, 82(4), 733–739. [DOI] [PubMed] [Google Scholar]
- Gleissner B, & Chamberlain MC (2006). Neoplastic meningitis. The Lancet Neurology, 5(5), 443–452. 10.1016/s1474-4422(06)70443-4 [DOI] [PubMed] [Google Scholar]
- Glitza IC, & Bucheit AD (2017). Clincial response of central nervous system melanoma to anti-PD1 therapy in 2 melanoma patients. Archives of Immunology, 1(1), 1–3. [Google Scholar]
- Glitza IC, Ferguson SD, & Guha-Thakurta N (2017). Rapid resolution of leptomeningeal disease with targeted therapy in a metastatic melanoma patient. Journal of Neuro-oncology, 133(3), 663–665. [DOI] [PubMed] [Google Scholar]
- Glitza IC, Haymaker C, Bernatchez C, Vence L, Rohlfs M, Richard J, … Hwu P (2015). Intrathecal administration of tumor-infiltrating lymphocytes is well tolerated in a patient with leptomeningeal disease from metastatic melanoma: a case report. Cancer Immunology Research, 3(11), 1201–1206. 10.1158/2326-6066.cir-15-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitza IC, Rohlfs M, Bassett R, John I, Richard J, Iqbal M, … Davies MA (2015). Therapeutic outcomes of intrathecal interleukin-2 in metastatic melanoma patients with leptomeningeal disease (LMD). Neuro-oncology, 17, v107–v112. [Google Scholar]
- Glitza IC, Rohlfs M, Guha-Thakurta N, Bassett RL, Bernatchez C, Diab A, … Davies MA (2018). Retrospective review of metastatic melanoma patients with leptomeningeal disease treated with intrathecal interleukin-2. ESMO Open, 3(1), e000283. 10.1136/esmoopen-2017-000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, … Mehta MP (2014). Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. Journal of Clinical Oncology, 32(34), 3810–3816. 10.1200/JCO.2014.57.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimans JJ, Wagstaff J, Schreuder WO, Wolbers JG, Baars JW, Polman CH, … Franks CR (1991). Treatment of leptomeningeal carcinomatosis with continuous intraventricular infusion of recombinant interleukin-2. Surgical Neurology, 35(3), 244–247. 10.1016/0090-3019(91)90079-O [DOI] [PubMed] [Google Scholar]
- Heiss JD, Taha S, Oldfield EH, & Ram Z (2011). Intrathecal gene therapy for treatment of leptomeningeal carcinomatosis. Journal of Neuro-oncology, 104(1), 365–369. 10.1007/s11060-010-0458-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlinger U, Weller M, & Schabet M (1998). New aspects of immunotherapy of leptomeningeal metastasis. Journal of Neuro-oncology, 38(2–3), 233–239. [DOI] [PubMed] [Google Scholar]
- Jacobs L, O’Malley J, Freeman A, & Ekes R (1981). Intrathecal interferon reduces exacerbations of multiple sclerosis. Science, 214(4524), 1026–1028. [DOI] [PubMed] [Google Scholar]
- Kim DW, Barcena E, Mehta UN, Rohlfs ML, Kumar AJ, Penas-Prado M, & Kim KB (2015). Prolonged survival of a patient with metastatic leptomeningeal melanoma treated with BRAF inhibition-based therapy: A case report. BMC Cancer, 15, 400. 10.1186/s12885-015-1391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JC, Kosteniuk SE, Walker T, Iansavichene A, Macdonald DR, & Megyesi JF (2019). Operative complications with and without image guidance: A systematic review and meta-analysis of the Ommaya reservoir literature. World Neurosurgery, 122, 404–414. 10.1016/j.wneu.2018.11.036 [DOI] [PubMed] [Google Scholar]
- Law V, Evernden B, Kenchappa R, Puskas J, Ryzhova E, Smalley I, … SNO abstract 2018 (2018). Detection, molecular profiling and culture of CSF-CTCs in Leptomeningeal disease (LMD) in melanoma to improve diagnosis and treatment strategies. Neuro-oncology, 20(suppl_6), vi63. [Google Scholar]
- Le Rhun E, Taillibert S, & Chamberlain MC (2013). Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surgical Neurology International, 4(Suppl 4), S265–S288. 10.4103/2152-7806.111304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rhun E, Tu Q, De Carvalho Bittencourt M, Farre I, Mortier L, Cai H, … Faure GC (2013). Detection and quantification of CSF malignant cells by the Cell Search technology in patients with melanoma leptomeningeal metastasis. Medical Oncology, 30(2), 538. 10.1007/s12032-013-0538-3 [DOI] [PubMed] [Google Scholar]
- Lee JM, Mehta UN, Dsouza LH, Guadagnolo BA, Sanders DL, & Kim KB (2013). Long-term stabilization of leptomeningeal disease with whole-brain radiation therapy in a patient with metastatic melanoma treated with vemurafenib: A case report. Melanoma Research, 23(2), 175–178. 10.1097/CMR.0b013e32835e589c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, & Lee JI (2011). Malignant ascites after subduroperitoneal shunt in a patient with leptomeningeal metastasis. Journal of Korean Neurosurgical Society, 50(4), 385–387. 10.3340/jkns.2011.50.4.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Jiang BY, Yang JJ, Zhang XC, Zhang Z, Ye JY, … Wu YL (2018). Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: A new medium of liquid biopsy. Annals of Oncology, 29(4), 945–952. 10.1093/annonc/mdy009 [DOI] [PubMed] [Google Scholar]
- Li Y, Pan W, Connolly ID, Reddy S, Nagpal S, Quake S, & Gephart MH (2016). Tumor DNA in cerebral spinal fluid reflects clinical course in a patient with melanoma leptomeningeal brain metastases. Journal of Neuro-oncology, 128(1), 93–100. 10.1007/s11060-016-2081-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N, Dunn IF, Glantz M, Allison DL, Jensen R, Johnson MD, … Kesari S (2011). Benefit of ventriculoperitoneal cerebrospinal fluid shunting and intrathecal chemotherapy in neoplastic meningitis: A retrospective, case-controlled study. Journal of Neurosurgery, 115(4), 730–736. 10.3171/2011.5.Jns101768 [DOI] [PubMed] [Google Scholar]
- Lin X, Fleisher M, Rosenblum M, Lin O, Boire A, Briggs S, … Pentsova EI (2017). Cerebrospinal fluid circulating tumor cells: A novel tool to diagnose leptomeningeal metastases from epithelial tumors. Neuro-Oncology, 19(9), 1248–1254. 10.1093/neuonc/nox066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- List J, Moser RP, Steuer M, Loudon WG, Blacklock JB, & Grimm EA (1992). Cytokine responses to intraventricular injection of interleukin 2 into patients with leptomeningeal carcinomatosis: Rapid induction of tumor necrosis factor alpha, interleukin 1 beta, interleukin 6, gamma-interferon, and soluble interleukin 2 receptor (Mr 55,000 protein). Cancer Research, 52(5), 1123–1128. [PubMed] [Google Scholar]
- Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, … McArthur GA (2018). Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. The Lancet Oncology, 19(5), 672–681. 10.1016/s1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- Marchiò C, Mariani S, Bertero L, Di Bello C, Francia Di Celle P, Papotti M, … Cassoni P (2017). Liquoral liquid biopsy in neoplastic meningitis enables molecular diagnosis and mutation tracking: A proof of concept. Neuro-Oncology, 19(3), 451–453. 10.1093/neuonc/now244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin K, Atkins M, Thompson J, Ernstoff M, Weber J, Flaherty L, … Johnson D (2002). Temozolomide and whole brain irradiation in melanoma metastatic to the brain: A phase II trial of the Cytokine Working Group. Journal of Cancer Research and Clinical Oncology, 128(4), 214–218. 10.1007/s00432-002-0323-8 [DOI] [PubMed] [Google Scholar]
- Mayumi A, Sawada A, Ioi A, Higuchi K, Shimizu M, Sato M, … Inoue M (2017). Intrathecal infusion of haploidentical nondonor lymphocytes for central nervous system leukemic relapse after haploidentical hematopoietic stem cell transplantation. Journal of Pediatric Hematology/Oncology, 40(2), e129–e132. 10.1097/mph.0000000000000937 [DOI] [PubMed] [Google Scholar]
- Mead PA, Safdieh JE, Nizza P, Tuma S, & Sepkowitz KA (2014). Ommaya reservoir infections: A 16-year retrospective analysis. Journal of Infection, 68(3), 225–230. 10.1016/j.jinf.2013.11.014 [DOI] [PubMed] [Google Scholar]
- Meyers CA, Obbens EA, Scheibel RS, & Moser RP (1991). Neurotoxicity of intraventricularly administered alpha-interferon for leptomeningeal disease. Cancer, 68(1), 88–92. [DOI] [PubMed] [Google Scholar]
- Meyers CA, & Yung WK (1993). Delayed neurotoxicity of intraventricular interleukin-2: A case report. Journal of Neuro-oncology, 15(3), 265–267. 10.1007/BF01050073 [DOI] [PubMed] [Google Scholar]
- Miller AM, Shah RH, Pentsova EI, Pourmaleki M, Briggs S, Distefano N, … Mellinghoff IK (2019). Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature, 565(7741), 654–658. 10.1038/s41586-019-0882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misset JL, Mathe G, & Horoszewicz JS (1981). Intrathecal interferon in meningeal leukemia. New England Journal of Medicine, 304(25), 1544. 10.1056/nejm198106183042513 [DOI] [PubMed] [Google Scholar]
- Mitsuya K, Nakasu Y, Hayashi N, Deguchi S, Takahashi T, Murakami H, … Harada H (2019). Palliative cerebrospinal fluid shunting for leptomeningeal metastasis-related hydrocephalus in patients with lung adenocarcinoma: A single-center retrospective study. PLoS ONE, 14(1), e0210074. 10.1371/journal.pone.0210074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MG, Tsai VWW, Ruitenberg MJ, Hassanpour M, Li H, Hart PH, … Brown DA (2014). Immune cell trafficking from the brain maintains CNS immune tolerance. Journal of Clinical Investigation, 124(3), 1228–1241. 10.1172/jci71544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momtaz P, Pentsova E, Abdel-Wahab O, Diamond E, Hyman D, Merghoub T, … Chapman PB (2016). Quantification of tumor-derived cell free DNA(cfDNA) by digital PCR (DigPCR) in cerebrospinal fluid of patients with BRAFV600 mutated malignancies. Oncotarget, 7(51), 85430–85436. 10.18632/oncotarget.13397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser RP, Bruner JM, & Grimm EA (1991). Biological therapy of brain tumors. Cancer Bull, 43, 117–126. [Google Scholar]
- Nahed BV, Alvarez-Breckenridge C, Brastianos PK, Shih H, Sloan A, Ammirati M, … Olson JJ (2019). Congress of neurological surgeons systematic review and evidence-based guidelines on the role of surgery in the management of adults with metastatic brain tumors. Neurosurgery, 84(3), E152–E155. 10.1093/neuros/nyy542 [DOI] [PubMed] [Google Scholar]
- Niessner H, Forschner A, Klumpp B, Honegger JB, Witte M, Bornemann A, … Meier F (2013). Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Medicine, 2(1), 76–85. 10.1002/cam4.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojerholm E, Lee JY, Kolker J, Lustig R, Dorsey JF, & Alonso-Basanta M (2014). Gamma Knife radiosurgery to four or more brain metastases in patients without prior intracranial radiation or surgery. Cancer Medicine, 3(3), 565–571. 10.1002/cam4.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Shimizu K, Miyao Y, Yamada M, Ushio Y, Matsui Y, … Ikeda H (1986). Clinical studies of adoptive immunotherapy of human disseminated brain tumors with LAK cells and recombinant interleukin-2. No to Shinkei, 38(6), 593–598. [PubMed] [Google Scholar]
- Ommaya AK (1963). Subcutaneous reservoir and pump for sterile access to ventricular cerebrospinal fluid. Lancet, 2(7315), 983–984. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N, Gerber DL, Eton O, & Bedikian AY (2002). The role of intrathecal (IT) use of interleukin-2 (IL-2) in the treatment of leptomeningeal disease (LMD) in patients (pts) with melanoma. Proceedings of the American Society of Clinical Oncology, 21, 353a. [Google Scholar]
- Pape E, Desmedt E, Zairi F, Baranzelli MC, Dziwniel V, Dubois F, … Le Rhun E (2012). Leptomeningeal metastasis in melanoma: A prospective clinical study of nine patients. Vivo, 26(6), 1079–1086. [PubMed] [Google Scholar]
- Pentsova EI, Shah RH, Tang J, Boire A, You D, Briggs S, … Berger MF (2016). Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. Journal of Clinical Oncology, 34(20), 2404–2415. 10.1200/jco.2016.66.6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, … Dudley ME (2011). Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clinical Cancer Research, 17(13), 4550–4557. 10.1158/1078-0432.ccr-11-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, … White DE (1994). Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. Journal of the National Cancer Institute, 86(15), 1159–1166. 10.1093/jnci/86.15.1159 [DOI] [PubMed] [Google Scholar]
- Rosener M, Schwulera U, Menke G, Thrun A, Lissner R, Krauseneck P, … Martin R (1993). Tolerance and cerebrospinal fluid pharmacokinetics of intrathecally administered human natural interleukin-2: A phase I trial. European Cytokine Network, 4(3), 189–195. [PubMed] [Google Scholar]
- Sakji-Dupre L, Le Rhun E, Templier C, Desmedt E, Blanchet B, & Mortier L (2015). Cerebrospinal fluid concentrations of vemurafenib in patients treated for brain metastatic BRAF-V600 mutated melanoma. Melanoma Research, 25(4), 302–305. 10.1097/cmr.0000000000000162 [DOI] [PubMed] [Google Scholar]
- Samlowski WE, Park KJ, Galinsky RE, Ward JH, & Schumann GB (1993). Intrathecal administration of interleukin-2 for meningeal carcinomatosis due to malignant melanoma: Sequential evaluation of intracranial pressure, cerebrospinal fluid cytology, and cytokine induction. Journal of Immunotherapy with Emphasis on Tumor Immunology, 13(1), 49–54. 10.1097/00002371-199301000-00007 [DOI] [PubMed] [Google Scholar]
- Sandberg DI, Bilsky MH, Souweidane MM, Bzdil J, & Gutin PH (2000). Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery, 47(1), 49–54; discussion 54–45. [DOI] [PubMed] [Google Scholar]
- Saris SC, Rosenberg SA, Friedman RB, Rubin JT, Barba D, & Oldfield EH (1988). Penetration of recombinant interleukin-2 across the blood-cerebrospinal fluid barrier. Journal of Neurosurgery, 69(1), 29–34. 10.3171/jns.1988.69.1.0029 [DOI] [PubMed] [Google Scholar]
- Schäfer N, Scheffler B, Stuplich M, Schaub C, Kebir S, Rehkämper C, … Glas M (2013). Vemurafenib for leptomeningeal melanomatosis. Journal of Clinical Oncology, 31(11), e173–174. 10.1200/jco.2012.46.5773 [DOI] [PubMed] [Google Scholar]
- Seifert H, Hirata E, Gore M, Khabra K, Messiou C, Larkin J, & Sahai E (2016). Extrinsic factors can mediate resistance to BRAF inhibition in central nervous system melanoma metastases. Pigment Cell & Melanoma Research, 29(1), 92–100. 10.1111/pcmr.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro WR, Chernik NL, & Posner JB (1973). Necrotizing encephalopathy following intraventricular instillation of methotrexate. Archives of Neurology, 28(2), 96–102. [DOI] [PubMed] [Google Scholar]
- Shonka NA, Kessinger AM, & Aizenberg MR (2014). Intrathecal interleukin-2 for melanomatous meningitis. Journal of Clinical Oncology, 32(33), e111–e113. 10.1200/jco.2013.49.1100 [DOI] [PubMed] [Google Scholar]
- Siravegna G, Geuna E, Mussolin B, Crisafulli G, Bartolini A, Galizia D, … Montemurro F (2017). Genotyping tumour DNA in cerebrospinal fluid and plasma of a HER2-positive breast cancer patient with brain metastases. ESMO Open, 2(4), e000253. 10.1136/esmoopen-2017-000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siravegna G, Marsoni S, Siena S, & Bardelli A (2017). Integrating liquid biopsies into the management of cancer. Nature Reviews Clinical Oncology, 14(9), 531–548. 10.1038/nrclinonc.2017.14 [DOI] [PubMed] [Google Scholar]
- Smalley KS, Fedorenko IV, Kenchappa RS, Sahebjam S, & Forsyth PA (2016). Managing leptomeningeal melanoma metastases in the era of immune and targeted therapy. International Journal of Cancer, 139(6), 1195–1201. 10.1002/ijc.30147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazielle N, Creidy R, Malcus C, Boucraut J, & Ghersi-Egea JF (2016). T-Lymphocytes Traffic into the Brain across the Blood-CSF Barrier: Evidence Using a Reconstituted Choroid Plexus Epithelium. PLoS ONE, 11(3), e0150945. 10.1371/journal.pone.0150945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szvalb AD, Raad II, Weinberg JS, Suki D, Mayer R, & Viola GM (2014). Ommaya reservoir-related infections: Clinical manifestations and treatment outcomes. Journal of Infection, 68(3), 216–224. 10.1016/j.jinf.2013.12.002 [DOI] [PubMed] [Google Scholar]
- Taillibert S, & Chamberlain MC (2018). Leptomeningeal metastasis. Handbook of Clinical Neurology, 149, 169–204. 10.1016/b978-0-12-811161-1.00013-x [DOI] [PubMed] [Google Scholar]
- Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, … Zunt JR (2017). 2017 Infectious Diseases Society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clinical Infectious Diseases, 64(6), e34–e65. 10.1093/cid/ciw861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, … Minn AJ (2015). Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature, 520(7547), 373–377. 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu R, Taillibert S, Banissi C, Vicaut E, Bailon O, Le Rhun E, … Carpentier AF (2015). Immunotherapy with CpG-ODN in neoplastic meningitis: A phase I trial. Cancer Science, 106(9), 1212–1218. 10.1111/cas.12724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Bertalan MS, & Brastianos PK (2018). Leptomeningeal metastasis from systemic cancer: Review and update on management. Cancer, 124(1), 21–35. 10.1002/cncr.30911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgenhof S, & Neyns B (2015). Complete cytologic remission of V600E BRAF-mutant melanoma-associated leptomeningeal carcinomatosis upon treatment with dabrafenib. Journal of Clinical Oncology, 33(28), e109–e111. 10.1200/jco.2013.48.7298 [DOI] [PubMed] [Google Scholar]
- Williams NL, Wuthrick EJ, Kim H, Palmer JD, Garg S, Eldredge-Hindy H, … Shi W (2017). Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. International Journal of Radiation Oncology Biology Physics, 99(1), 22–30. 10.1016/j.ijrobp.2017.05.028 [DOI] [PubMed] [Google Scholar]


