Abstract
The peels extracted from various citrus species are major source of phenols, flavonoids and anti-microbial agents. The purpose of this study was a detailed investigation of the phytochemical and pharmacological character of the ethanolic (80%), methanolic and acetone extracts of the peel of local variants of orange (lemon, grape fruit, mousami, fruiter, and shikri malta). The extracts were studied to find out the total phenolic contents (TPC), and total flavonoids (TF) present. The antioxidant activities were assessed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging effect, and the reducing power was determined through free radical scavenging activity (FRAP) assays. The sensitivity of four bacterial strains to peels extracts was examined by applying the diffusion disc on agar medium method. It was found that ethanol was the best extracting agent for TPC and TF in fruit peels under study. The highest TPC (21.33 ± 0.06 mg GAE/g) was quantified in orange peels, whereas fruiter contained the lowest TPC (20.40 ± 0.03 mg GAE/g) in ethanolic extract. The highest amount of TF (2.02 ± 0.08 mg QE/g) was quantified in lemon peels, whereas shikri malta contained lowest quantity of TF (1.04 ± 0.02 mg QE/g). The highest free radical scavenging activity (93.1%) of DPPH was exhibited by lemon peels, whereas the least activity (78.6%) was shown by mousami peels. Ethanolic extract of orange peels demonstrated more reducing power while showing an absorption of 1.98, followed by methanolic (1.11) and acetone (0.81) extracts. The inhibition effect of methanolic extract of lemon peels (inhibition zone = 18 mm) against B. subtilis was considerable and comparable to that of ciprofloxacin. Gas chromatography/mass spectrometry (GC/MS) was used to detect the compounds in ethanolic extract and up to 14 compounds were detected. These compounds were also assessed for their docking scores. Plausible binding modes with polyphenol oxidase and four best compounds were selected for molecular dynamics (MD) simulation to analyze their structural stability with receptor.
Keywords: Citrus peels, Phenolic contents, Flavonoids, Biological activities, Molecular docking
1. Introduction
Citrus fruits belong to the “Rutaceae” family, and are cultivated globally in tropical and subtropical regions with an approximate production of 102 million tons per year [1]. They contain large quantities of vitamin C as well as appreciable amounts of several other nutrients such as minerals, vitamins, micronutrients, and dietary fibers. These components are considered an extremely important part of human diet. Citrus fruits are an excellent source of antioxidants including phenolic compounds, ascorbic acid, essential oils, and carotenoids and thus can potentially prove helpful to prevent cellular oxidative damage [2]. The peels of citrus fruits make an almost 50–60% of the total weight of citrus fruits. The percentage of refined citrus fruits out of the total production is just 33%, whereas thousands of tons of citrus fruit peels ultimately become as agro-industrial waste [3].
The peels obtained from various citrus species are a major source of phenols, and flavonoids [4,5]. Phenolic compounds also show huge potential as therapeutic agents due to their antimicrobial, anti-inflammatory, anticancer, and cardio protective activities. Antioxidant compounds exhibit several health-related benefits, which make them potential candidates in the field of medicines [6]. Several previously reported studies also show that in most of the citrus fruits, the compounds responsible for antioxidant properties are mainly present in their peels rather than in their pulps [7,8]. Fruit peels can be used in the food sector, the cosmetics industry, and as a source for making useful medications. These compounds, which were obtained from peels, shown notable antibacterial activity against the pathogenic bacteria that causes food poisoning [9]. Citrus fruit peels are used in traditional Chinese medicine to treat high blood pressure, cough, stomachaches, and muscle soreness [10]. The studies, therefore, suggest that the peels of these fruits should be included in the preparation of various breakfast cereals, dietary supplements, and in the formulation of nutraceuticals [11]. The inclusion of antioxidants to food products is required to extend their shelf-life considerably for consumption over an extended period of time, and to avoid any alterations in their flavor, odor or texture [12,13]. To avoid the food spoilage, synthetic preservatives are most commonly employed but the use of these synthetic preservatives is harmful for human beings as well as for the environment [14,15]. Citrus peel extract, according to recent studies, has a stronger inhibitory effect on lipid oxidation and a better antioxidant activity than synthetic antioxidants. Synthetic antioxidants like butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and tertiary butylhydroquinone (TBHQ) are also unstable at high temperatures [3]. Lipid peroxidation is a main reason of the mustiness of foods, which are immensely sensitive to free oxygen radicals. Antioxidant food additives do not alter the organoleptic characteristics of the products and are usually exceptionally stable. Citrus peels include a variety of bioactive compounds including phenolic compounds, polysaccharides, flavonoids, and limonoids that have antioxidant properties. These compounds scavenge hydroxyl radicals, single oxygen atoms, and lipid peroxyl radicals [16]. The peels of lemon and grapefruit are a primary source of polyphenols, flavonoids and flavones. Grapefruit peels are primarily employed for the oil extraction purpose. An amount of 200 tons of grapefruit oil is produced in the world every year [17]. In addition to oil, lemon peels contain a large number of bioactive compounds including ascorbic acid, phenolic acids (ferulic acid, p-coumaric acid, and sinapic acid), and flavonoids (flavonols, flavanones, and flavones) [3], which have been linked to the anti-microbial [18], and anti-oxidant characteristics [19]. Orange peel is being utilized as a folklore and traditional drug to treat a large number of diseases such as stomach ache, cancer, diuretic, cold, immune system diseases, viral and bacterial infections, digestive system diseases, and vitamin deficiencies [20,21].
This research was aimed to investigate the phytochemical components of the peel extracts of locally available citrus fruits such as orange (Citrus sinensis), lemon (Citrus limon), grape fruit (Citrus paradise), mousami (Citrus limetta), fruiter, and shikri malta (local names). The extraction was done with ethanol, methanol, and acetone. Subsequently, GC-MS (gas chromatography coupled with mass spectrometry) was employed to find the phytochemicals. Furthermore, the current research screened the citrus fruit peels' extracts potentials for their antioxidant and antimicrobial properties, whereas in silico studies were performed to check the plausible bonding mode of different phytochemicals as ligands versus polyphenol oxidases (PPOs) receptor proteins.
2. Method and materials
2.1. Collection of samples
Fresh citrus fruit samples including orange (Citrus sinensis), lemon (Citrus limon), grape fruit (Citrus paradise), mousami (Citrus limetta), fruiter, and shikri malta (local names), were collected from a local market of Lahore, Punjab, Pakistan. Three packs were selected, and each pack contained three fruits of each species. Taxonomists at the University of the Punjab's Institute of Agriculture Sciences in Lahore, Pakistan, verified the plant species' authenticity. A voucher specimen was submitted to the Institute's herbarium (DCUET-020220). Before peeling, each sample was thoroughly washed with deionized water.
2.2. Preparation of crude extract
In an air oven, the peels were dried for 72 h at 60 °C and a knife mill was used to grind the dried peels. Then, the ground peels were filtered through a 100 US mesh screen and were kept in airtight pouches at 4 °C till further use. 40 g of each pulverized powder was steeped in 0.2 L of ethanol (80%), methanol and acetone separately for 24 h while being occasionally stirred before performing, to ascertain the crude extracts' phytochemical analysis, and to find out the antibacterial and antioxidant properties of the respective extract. The plant components that had been soaked in different solvents were filtered using a filter paper (Whatman no. 42). To evaporate the solvent, a rotary evaporator was used. Under the chemical hood, the residues were air dried before their final collection in tiny microfuge tubes. Finally, a stock solution (5 mg/mL in DMSO) of above different residues was prepared, and various quantities of it were diluted subsequently.
2.3. Phytochemical analysis
2.3.1. Determination of total phenolic contents
Folin-Ciocalteu reagent technique was employed to determine the total phenolic contents (TPC). Folin-Ciocalteu (1.5 mL) was added to 300 μL of the extract. To this solution, 1.2 mL of 2% (w/v) aqueous Na2CO3 solution was added after 3 min. This mixture was kept in dark for 90 min and the absorbance was taken against blank at 765 nm. The results were manifested in mg GAE/g of extract in triplicate, while employing gallic acid as a standard [22].
2.3.2. Determination of total flavonoids contents
A colorimetric method was employed to find out total flavonoid (TF) contents. 0.2 mL of the extract was transferred to 0.3 mL of a solution containing 5% (w/v) sodium nitrate solution. 0.6 mL of AlCl3 (10% methanolic solution) was added to the aforementioned mixture. 2 mL of 1 M NaOH was added to the mixture after 6 min, and the color variation was noticed. Next, the absorbance measurement was performed at 510 nm. Using quercetin as the standard, the outcomes were tested in triplicate and displayed as mg QE/g of extract [23].
2.4. Antioxidant activities essays
2.4.1. DPPH free radical scavenging assay
The free radical scavenging activity was calculated using methanol solution of DPPH [[24], [25], [26]]. Methanolic solution (1.0 mL) of DPPH was mixed with 10 μL of extract that was previously dissolved in DMSO at a concentration of 4 mg/mL. After shaking vigorously, the solution was allowed to stay in the dark at room temperature for 30 min. The absorbance measurement was performed at 515 nm, whereas the scavenging activity was calculated by using the following equation.
DMSO was used as a negative control, whereas Ascorbic acid was employed as a positive control. Ascorbic acid as well as extracts with FRSA ≥ 50% were investigated further at lower concentrations ranging from 7.81 to 1000 μg/mL (7.81, 15.62, 31.25, 62.5, 125, 250, 500, 1000 μg/mL) to determine their 50% inhibitory concentration (IC50) in μg.mL−1. IC50 was calculated by using GraphPad Prism 7.0 Software (Graph Pad Software Inc.) and all the analysis was performed in triplicate.
2.4.2. Reducing power assay (FRAP)
The extracts' reducing capacity was assessed using a common potassium ferricyanide colorimetric test [24,25]. 2.5 mL of the extract samples (4 mg/mL DMSO) were combined with 2.5 mL of each of the phosphate buffer (pH 6.6, 0.2 M) and 1% (w/v) K3 [Fe(CN)6] and then the incubation of mixture was done at 50 °C for 20 min, after this 2.5 mL of 10% (w/v) CCl3COOH was added. The centrifugation of mixture was performed for 10 min at 3000 rpm. Then, 0.5 mL of FeCl3 (0.1% w/v) was combined with 2.5 mL of each of supernatant and water, and the absorbance measurement was performed at 700 nm. Ascorbic acid as well as extracts were examined in the concentration range of 50–800 μg mL−1 (50, 100, 200, 400, 600, and 800 μg/mL) to evaluate the FRAP. The measurements were taken in triplicate.
2.5. Antibacterial activities
2.5.1. Bacterial strains and antibacterial assay
Disc diffusion method with minor modifications was employed to test the antibacterial activity of extracts against four bacterial strains [24,27,28]. For the said purpose, 2 g negative (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853) and 2 g positive (Staphylococcus aureus ATCC 25923, Bacillus subtilis ATCC 6051) bacteria were employed. The surface of nutrient agar media plates was swabbed slowly in three separate directions with 100 μL aliquot of inoculum that had been pre-adjusted (108 cells/mL) for seeding density. The plates' surface was covered with discs of sterile filter paper containing 5 μL (20 mg/mL DMSO) of extracts. The use of DMSO-infused discs as a negative control was contrasted with the use of cefixime-loaded discs as a positive control. The value of ZOI (zone of inhibition) was measured with a Vernier calliper (Starrett 799A-6/150, USA) following an incubation period of 24 h at 37 °C.
2.6. GC-MS analysis
GC-MS analysis was used to evaluate the considered extract. To determine the natural components of peel extracts, an Agilent gas chromatograph (model GC 7890A) paired with a mass spectrometer (model MS 5973A) was employed. The initial temperature of the apparatus was set at 70 °C and was maintained for 3 min. The temperature was then enhanced to 300 °C at the rate of 10 °C per min and was maintained for 9 min. The temperature at injection port was maintained at 250 °C. He gas (flow rate = 1.5 mL min−1) was employed as a carrier gas. A potential difference of 70 eV was used for ionization. Split mode was used for sample injection. The range of spectral scan was set at 40–700 m/z. The ion source temperature was kept at 230 °C and the interface temperature was maintained at 240 °C. The start and end time of mass spectrum were 3 min and 35 min respectively and solvent cut off time was of 3 min [29].
2.7. Molecular docking and simulation studies
The crystallographic coordinates of protein (PDB ID: 2Y9X, polyphenol oxidase) were downloaded from Protein Data Bank prepared by the protein preparation wizard of Maestro (Schrodinger’s suite) [30]. The receptor was preprocessed for structural integrity by adding hydrogen atoms, assigning bond orders for amino acids and ligands, creating zero-order bond to metals. Similarly, unnecessary chains, ligands and water molecules were removed. The structure was refined by adjusting tautomeric states and protonation at 7.4 pH. The geometry of the structure was optimized and then minimized by OPLS_2005 force field [31]. After minimization, a site-specific grid was generated by picking the co-crystal ligand atom. The internal grid volumes for X, Y, and Z were −10.02, −28.02, and −43.6 respectively. Similarly, all the ligands were prepared by the LigPrep tool. The ionization states of ligands were generated by Epik at pH 7.0. The stereoisomers of ligands were generated, and low energy conformers were generated by using OPLS_2005 forcefield. The low energy conformers were used for molecular docking. The docked ligands were ranked based on best binding modes and the compounds common in all extracts were selected.
The best binding poses among the compounds were selected for 50 ns MD simulation. The input files were prepared by CHARMM-GUI Web server. The protein structures were processed and ligand charm topology and parameter files were generated by Antechamber [32]. After generating the protein and ligand topology and parameter files, the complex systems were solvated in a rectangular box of 10 Å containing TIP3P water molecules. The systems were then neutralized by adding Na+ and Cl− counter ions. After neutralization, the systems were minimized by steepest decent technique for 5000 steps to exclude the steric clashes. After minimization, both systems were equilibrated at NVT and NPT ensemble for 50,000, and 100,000 steps, respectively, at 310 K temperature to set the systems for production run [33]. The simulations were conducted using Berendson thermostat and Parrinello-Rahman algorithms for maintaining constant temperature (310 K) and pressure (1 atm). The systems were relaxed by setting time at τ T = 0.1 ps and τ P = 2.0 ps. The bond lengths of hydrogen atoms were maintained at optimal bond lengths by using LINCS algorithm [34], while the non-bonded interactions were calculated by Verlet [35]. Particle Mesh Ewald method was used to calculate the electrostatic interactions beyond the short-range cutoff [36]. The Periodic boundary conditions were applied in x, y, and z dimensions, and systems were subjected to production run. The simulations were run by using Gromacs simulation package and applying CHARMM36 force field [37]. The trajectories of the production run were stored after every 10ps and analyzed by gromacs commands and BIO3D package of R [38].
3. Results and discussion
3.1. Phytochemical analysis
3.1.1. Determination of total phenolic and flavonoids contents
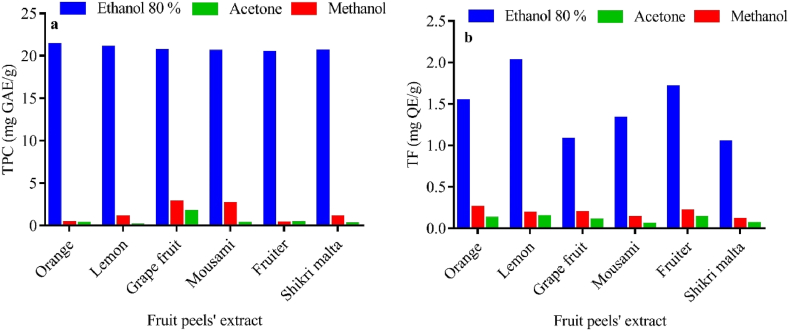
Nature has gifted plants to humans as an invaluable source of phytochemicals and these have different therapeutic actions against various phenotypes of disease models. Conventional herbal medicines are being utilized globally and their biological potential is assessed by measuring the phenolic compounds and total flavonoids present in them [39]. Fruit peels have ample amount of phenolic and flavonoid contents and received too much attention and interest of the researchers. Actually, the –OH groups present in the aromatic ring structure confer the antioxidant potential of these compounds. In these compounds, the non-coupled electrons around the ring can be used to eliminate free radicals [40]. TPC and TF quantified in fruit peels under investigation, using three different extraction methods, have been given in Fig. 1a and b.
Fig. 1.
TPC (a) and TF (b) present in different extracts of different fruit peels.
For 80% ethanolic extract, the highest TPC was quantified in orange peels that was equal to 21.33 ± 0.06 mg GAE/g, while fruiter contained the lowest TPC (20.40 ± 0.03 mg GAE/g). For methanolic extract, grapefruit peels contained the highest TPC (2.77 ± 0.01 mg GAE/g), while the lowest TPC were found in fruiter peels (0.32 ± 0.07 mg GAE/g). For acetone extract, grapefruit peels showed the highest value of TPC (1.64 ± 0.01 mg GAE/g), whereas the lowest TPCs were quantified in lemon peels (0.06 ± 0.01 mg GAE/g). For 80% ethanolic extract, the highest TF (2.02 ± 0.08 mg QE/g) was quantified in lemon peels, while shikri malta contained the lowest TF (1.04 ± 0.02 mg QE/g). For methanolic extract, orange peels contained the highest TF (0.25 ± 0.06 mg QE/g), while the lowest TF (0.11 ± 0.01 mg QE/g) were found in shikri malta. For acetone extract, lemon peels showed the highest value of TF (0.14 ± 0.01 mg QE/g), whereas the lowest TF (0.05 ± 0.01 mg QE/g) were quantified in mousami peels.
It was observed that the 80% ethanolic extract was the best extracting agent for TPC and TF in fruit peels under study. Types of extracting agents were found to have a direct effect on the extraction yields of TPC and TF. The results indicated that polar extracts show much higher values of TPC and TF in their structures as compared to the least polar extracts such as acetone. TPC reported in orange peels' extract was 0.45 ± 0.03 mg GAE/g, while using ethanol as an extracting agent [41]. This amount is much lower than the one we found (21.33 ± 0.06 mg GAE/g). Previous studies revealed that the ethanolic extract of lemon peels contained the TPC in the range of 59.77–183.78 mg GAE/g, while employing 72% (v/v) ethanol for extraction [42]. This value is higher than the one that obtained in this studies. TFC content determined in the orange peel’s acetone extract was 21.87 mg QE/g (dry weight), whereas the same in the lemon peel of acetone extract was 380 mg QE/g (dry weight) [43,44]. Thus, the reported value is higher than the one we determined. This difference in the values is likely due to a number of different factors such as solubility degree in the solvents, environmental settings, seasonal differences, and degrees of polymerization of flavonoid and phenolic compounds [45].
3.2. Antioxidant activities essays
Natural oxidants play a much helpful and effective role against the oxidative stress. As compared to their synthetic counterparts, the medicines obtained (or derived) from the plant products are safer to use. Therefore, scientists and researchers are giving much attention to the field of exploration of natural antioxidant compounds. The antioxidant response of phenolic compounds depends on their chemical (or molecular) structure and thus can differ considerably. There are several different categories of phenolic compounds present in plants. One of the chief categories of these compounds is the flavonoids. Flavonoids are much famous because of having several beneficial and positive impacts on human health. In conclusion, antioxidant activity and the free radical scavenging of flavonoids depends on various features of the chemical structure and the position of –OH group [46,47]. In this study, two widely used techniques (FRSA and the reducing power of plant extracts) were employed to assess the antioxidant activity. The first approach is based on the trapping of the DPPH radicals by the ability of hydrogen donation. In the second method, the reducing power of a material in the extract is measured by reducing Fe3+ ions into Fe2+ ions. The electron donating potential is often measured by iron reducing and is a suitable technique to assess the antioxidant activity of phenolic compounds [25,48,49].
3.2.1. DPPH free radical scavenging assay
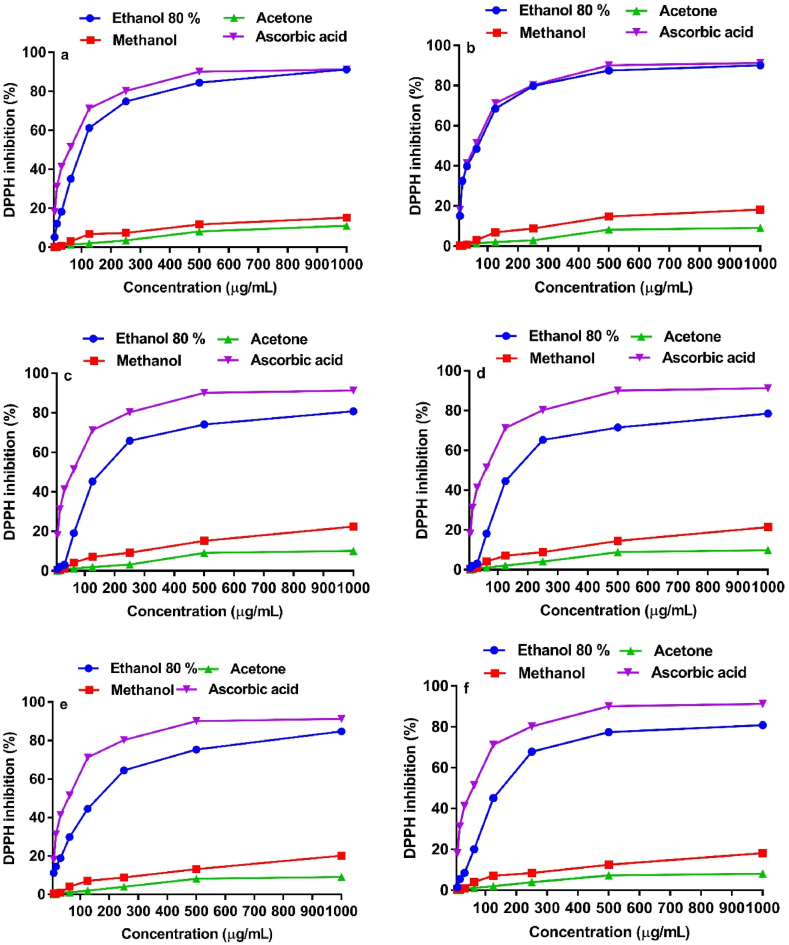
DPPH can donate a hydrogen in a radical-scavenging activity that is considered helpful against the detrimental effects of free radicals in various diseases. The antioxidant activity of a plant extract can thus be assessed by employing the accepted mechanism of DPPH-free radical scavenging [50]. Fig. 2 exhibits the profiles of the DPPH free radical scavenging activity measured for the different extracts (80% ethanol, methanol, and acetone) of fruit peels (orange, lemon, grape fruit, mousami, fruiter, and shikri malta) besides that of ascorbic acid standard. The IC50 values of standards and each of the extracts were also measured (Table 1). Free radical scavenging efficacies (Fig. 2a–f) of all the extracts depicted a dose-dependent gradual enhancement from 7.81 to 1000 μg/mL. The results showed that all the tested extracts had a DPPH free radical scavenging activity at 1000 μg/mL, the maximum concentration. 80% ethanolic extract of all the fruit peels exhibited the highest DPPH free radical scavenging activity (78.6–93.1%), followed by methanol (15.3–41.3%) and then acetone (11.1–18.1%). The highest scavenging activity of DPPH free radicals was exhibited by lemon peels (Fig. 2b), while the least activity was shown by mousami peels (Fig. 2d). In the previous studies, ethanolic, methanolic and acetone peel extract of orange demonstrated the DPPH free radical scavenging activity 79.32 ± 1.05%, 76.56 ± 0.70% and 55.70 ± 0.95% respectively. Whereas 71.90 ± 1.80%, 72.33 ± 1.17% and 47.63 ± 0.92% DPPH free radical scavenging activity was exhibited by lemon peel extract using ethanol, methanol and acetone as extracting solvent respectively [51]. The previous results of lemon extract for DPPH free radical scavenging activity are comparable with the present study.
Fig. 2.
DPPH radical scavenging activities of different extract of (a) orange peel, (b) lemon peel, (c) grape fruit peel, (d) mousami peel, (e) fruiter peel, (f) shikri malta peel, and ascorbic acid (standard) at various concentrations. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Results of IC50 of fruit peel extracts versus antioxidant standards.
| IC50 (μg/mL, Fruit peel type) | ||||||
|---|---|---|---|---|---|---|
| Extract type | Orange | Lemon | Grape fruit | Mousami | Fruiter | Shikri malta |
| 80% ethanol | 90.42 | 59.82 | 115.9 | 114.6 | 133.9 | 111.5 |
| Methanol | 197.1 | 97.33 | 266.2 | 260.9 | 254.1 | 230.4 |
| Acetone | 309.9 | 277.5 | 289.7 | 253.5 | 241.9 | 222.8 |
| Ascorbic acida | 62.28 | |||||
Standard DPPH inhibitor.
Moreover, 80% ethanolic extract of lemon peels produced an IC50 value of 59.82 μg/mL and was found to be the most active extract followed by methanolic extract with an IC50 value of 97.33 μg/mL. Previous studies revealed that the 70% ethanolic extract of lemon peels demonstrated EC50 value of 1.30 mg/mL [52]. The acetone extract produced an IC50 value of 277.5 μg/mL and thus shows the lowest anti-free radical potential (Table 1). Acetone extract of shikri malta was found to be the most active (IC50 = 222.8 μg/mL) among all other fruit peels, whereas methanolic extract of lemon peels was most active with an IC50 value of 97.33 μg/mL (Table 1). For comparative studies, ascorbic acid was employed as antioxidant standards, which demonstrated an interesting anti-free radical activity with an IC50 value of 62.28 μg/mL. Since ascorbic acid is a pure standard and therefore it has more antioxidant activity whereas, the peel extracts are in crude form.
As an action, antioxidants scavenge the reactive oxygen species and protect the antioxidant defense mechanisms. DPPH assay can help to measure the electron donating ability of the natural products. The decolonization of DPPH solution indicates the scavenging of DPPH by adding a radical species or an antioxidant [53]. The degree of the change in color is directly related to the potency and amount of the antioxidants. The free radical scavenging potential of a compound can be found out by measuring a decrease in the absorbance value of a reaction mixture. Our findings indicate that the peels extracts have active components that can donate hydrogen to a free radical to prevent the potential damage [54].
3.2.2. Reducing power assay (FRAP)
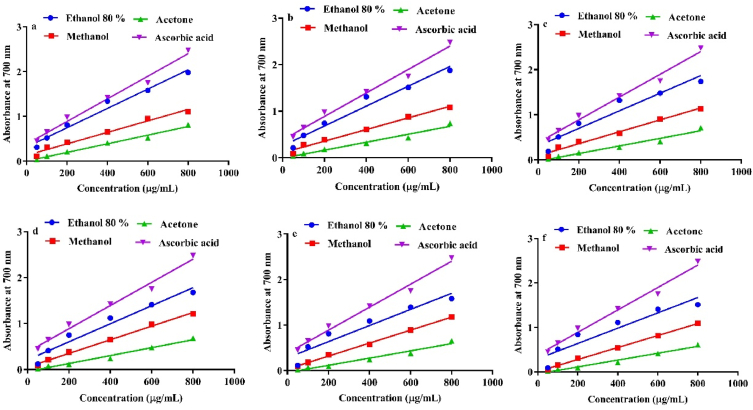
As Fig. 3 shows, the reducing powers (absorbance at 700 nm) of all the fruit peels extracts are maximum at 800 μg/mL concentrations. Moreover, 80% ethanolic extract of orange peels exhibited more reducing power with an absorption of 1.98 followed by the methanolic extract with an absorption of 1.11. The acetone extract gave an absorption of 0.81 and hence demonstrated the lowest reducing power (Fig. 3a–f). Methanolic extract of mousami was found to have more reducing power with an absorption of 1.21 among all other fruit peels, whereas acetone extract of orange peels had more reducing power with an absorption of 0.81 (Fig. 3a). For comparative studies, ascorbic acid was employed as FRAP standards and demonstrated an interesting reducing power with an absorption of 2.48. All the extracts tested were found to have less FRAP than that of ascorbic acid. The antioxidant potential of a compound can reliably be determined by measuring its reducing power. A higher reducing power indicates a better ability to donate the electron. Thus, free radicals accept the donated electron and form stable substances, which results in the termination of radical chain reactions [55].
Fig. 3.
FRAP of different extract of (a) orange peel, (b) lemon peel, (c) grape fruit peel, (d) mousami peel, (e) fruiter peel, (f) shikri malta peel, and ascorbic acid (standard) at various concentrations. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Several studies have been conducted on the flavonoids and polyphenolic contents of various citrus plant peel extracts and these studies have demonstrated that these compounds are extremely important in the development of food supplements and drugs. The antioxidant activity of these extracts is being exploited in the conventional medicines since ancient times. A considerable number of studies indicate that oxidative stress plays a main role in the health complications and manifestations of different diseases by reactive oxygen species (ROS) mechanism [56]. Flavonoids and polyphenols can be utilized as antioxidant agents against various stress-induced diseases. Oxidative stress causes many intestinal diseases. Basically, oxidative stress gives rise to a defective barrier function which ultimately leads to several intestinal ailments. Flavonoids and polyphenols demonstrate protective effects versus H2O2-induced damage in Caco-2 intestinal epithelial cells [57]. These compounds can decrease ROS through increased antioxidant enzymes' expression. Thus in current studies, the compounds found in the fruit peels extract were docked against the 2Y9X protein to find their virtual binding affinities and plausible binding modes and details are provided in docking and simulation studies.
3.3. Antibacterial activities
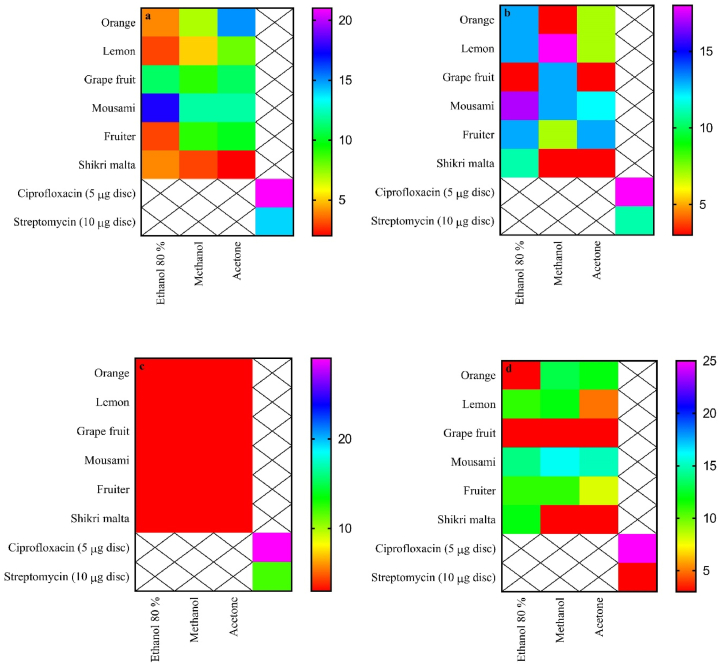
Plants are extensively studied as a prime source for the preparation of novel therapeutic agents. In addition, the possibility of using crude extracts of various plants in the treatment of different pathologies is also being vastly investigated. The exact working of the antimicrobial action of polyphenols is still not known. However, several studies suggest that polyphenols, via a H-bond, connect to bacterial enzymes at the cellular level, which induces a number of changes and modifications in the cell membrane’s permeability and the integrity of cell wall [58]. Fig. 4a–d depicts most of the effects of the ethanolic (80%) and methanolic extracts on the studied microbial strains. The results demonstrated that under the given experimental setup and conditions, all the extracts showed an inhibiting effect on all the tested microbial strains except E. coli ATCC 25922 (Fig. 4c). The inhibition effect of methanolic extract of lemon peels against B. subtilis ATCC 6051 (Fig. 4b) was considerable and comparable (zone of inhibition = 18 mm) to that of some synthetic antibiotics (ciprofloxacin, zone of inhibition = 18 mm), while more active than streptomycin (zone of inhibition = 11 mm) used as standards (Fig. 4b). The antimicrobial activities observed in the lemon peel extract are mainly because of the flavonoid content [59]. However, any of the peels extract did not show any activity against E. coli ATCC 25922. Jayaprakasha et al. reported that the ethanol-soluble fractions in citrus peels were found to be more effective against all the studied microorganisms including S. aureus, B. subtilis, E. coli, and P. aeruginosa. The acetone extract was least effective than all other fractions [60]. Previous studies revealed that the strain Bacillus cereus ATCC 49064 was more sensitive to the extracts of citrus peels with zone of inhibition 22.33 ± 1.04 mm, 17.00 ± 0.86 mm and 18.50 ± 1.02 mm for orange, lemon and grapefruit respectively [51].
Fig. 4.
Zone of inhibition (mm) of fruit peel extract against (a) Staphylococcus aureus ATCC 25923, (b) Bacillus subtilis ATCC 6051, (c) Escherichia coli ATCC 25922, (d) Pseudomonas aeruginosa ATCC 27853, and ciprofloxacin and streptomycin (standard drugs).
Antibacterial results may vary because of plant parts, different solvents used for herbal extraction, microorganisms in the environment, method, the area where the plants are grown, and the harvesting seasons of the plants. The ratios of phytochemical components such as polyphenols and flavonoids in the extract of a plant directly influence the antimicrobial characteristics of a plant. The extract of fruit peels showed that the antimicrobial potential of these extracts are because of different phytochemicals that may bind to proteins of membrane by hydrophobic and hydrogen bonding, and thus altering the permeability of membranes [61]. Flavonoids are also hydroxylated phenolic substances but is present as a C6–C3 unit connected to an aromatic ring. Since plants produce flavonoids in response to microbial infections, it is not surprising that they prove quite effective versus a large range of microorganisms. The main reason behind their activity is probably their ability to complex with soluble and extracellular proteins and with bacterial cell walls [62]. Flavonoids may not target specifically but most probably act nonspecifically on the respiratory chain or on the bilayer of cell membrane to kill bacteria [63].
Scientists and researchers are looking for new antimicrobial drugs because of an increased microbial resistance to existing drugs. Screening of medicinal plants for novel and advanced antimicrobial principles is a current scientific approach. In the drug discovery and design process, the bioactive natural products of plants are proving much helpful [64].
3.4. GC-MS analysis
Among three different types of extracts of fruit peels under investigation, ethanolic extracts revealed the post potent antioxidant activities. Therefore, the ethanolic extract of each fruit peel was screened for phyto-compounds through GC-MS method. The spectral data acquired from GC-MS was analyzed and compared with the information of the National Institute of Standard and Technology (NIST) to recognize different compounds. The name of the compound, retention time (RT), formula, Mw (g/mol), and the structure of the peel extract are given in Table 2. According to GC/MS study, 9–14 compounds were identified (Individual data of fruit peel is presented in supplementary data, Table S1–S6).
Table 2.
Phytocomponents recognized by GC/MS technique in peel extracts.
| Sr. No | RT (min.) | Compound | Formula | MW (g/mol) | Orange | Lemon | Grape fruit | Mousami | Fruiter | Shikri malta |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.15 | 2(3H)-Furanone, 5-methyl- | C5H6O2 | 98.10 | + | + | + | |||
| 2 | 4.02 | Cyclohexanol, 1-methyl-4-(1-methylethenyl)-, acetate | C12H20O2 | 196.29 | + | + | ||||
| 3 | 5.22 | Butanoic acid, 3-oxo-, 1-methylethyl ester | C7H12O3 | 144.17 | + | + | ||||
| 4 | 5.30 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144.13 | + | + | + | + | ||
| 5 | 5.75 | Ethane, 1,1,1-triethoxy- | C8H18O3 | 162.22 | + | + | + | |||
| 6 | 6.27 | 1,2,3-Propanetriol, 1-acetate | C5H10O4 | 134.13 | + | + | + | + | ||
| 7 | 6.59 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.18 | + | + | + | |||
| 8 | 7.17 | 2-Butenethioic acid, 3-(ethylthio)-, S-(1-methylethyl) ester | C9H16OS2 | 204.35 | + | + | + | |||
| 9 | 8.28 | 1,2,4-Cyclopentanetrione, 3-(2-pentenyl)- | C10H12O3 | 180.20 | + | |||||
| 10 | 9.79 | Sulfurous acid, 2-methyl-4-methoxybutyl pentyl ester | C11H24O4S | 252.37 | + | |||||
| 11 | 10.39 | 5,7-Diethyl-1,3-diazaadamantan-6-one | C12H20N2O | 208.31 | + | |||||
| 12 | 10.82 | n-Decanoic acid | C10H20O2 | 172.27 | + | + | + | |||
| 13 | 11.48 | 2H-1-Benzopyran-2-one, 5,7-dimethoxy- | C11H10O4 | 206.20 | + | |||||
| 14 | 12.29 | 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 280.44 | + | + | + | + | + | + |
| 15 | 12.46 | Tetracyclo [7.3.1.0(2,7).1(7,11)]tetradecane | C14H22 | 190.33 | + | |||||
| 16 | 17.28 | 2H-1-Benzopyran-2-one, 7-[(3,7-dimethyl-2,6-octadienyl)oxy]-, (E)- | C19H22O3 | 298.38 | + | |||||
| 17 | 21.81 | dl-.alpha.-Tocopherol | C29H50O2 | 430.72 | + | + | ||||
| 18 | 22.41 | Methyl 2-[4-[2-[4-(methoxycarbonylmethyl)phenyl]ethyl]phenyl]acetate | C20H22O4 | 326.39 | + | + | + | + | ||
| 19 | 22.89 | 4′,5,7,8-Tetramethoxyflavone | C19H18O6 | 342.34 | + | |||||
| 20 | 23.85 | 4H-1-Benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-5-hydroxy-3,6,7-trimethoxy- | C20H20O8 | 388.37 | + | |||||
| 21 | 24.04 | Butylphosphonic acid, pentyl 4-(2-phenylprop-2-yl)phenyl ester | C24H35O3P | 402.51 | + | + | + | + | ||
| 22 | 24.29 | 4H-1-Benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-5,6,7-trimethoxy- | C20H20O7 | 372.37 | + |
*The details of phytochemicals of individual fruit peels is given in supplementary data.
3.5. Molecular docking and simulation studies
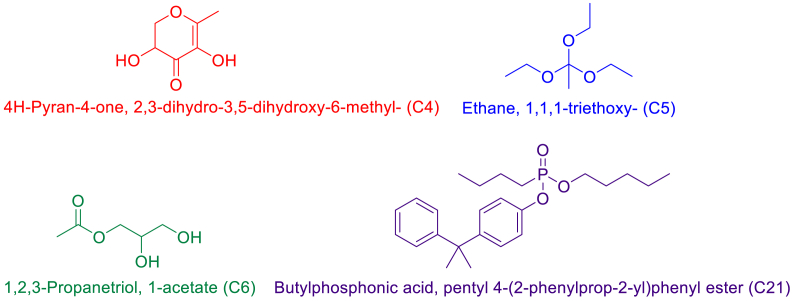
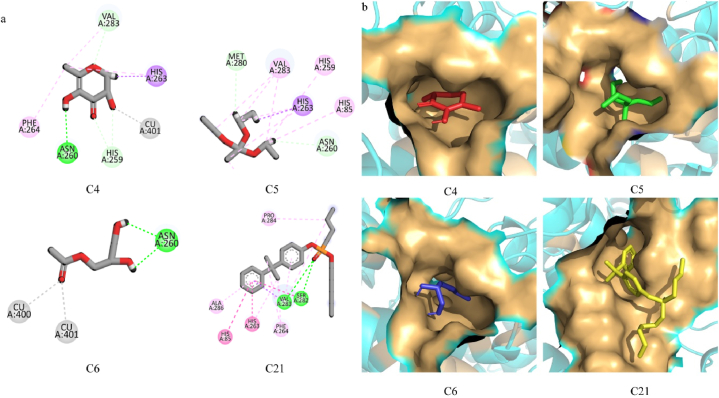
The components from the peel extracts were docked against the 2Y9X protein to find their virtual binding affinities and plausible binding modes. The binding affinities were in the range of −7.232 to −4.221 kcal/mol. The binding modes of the docked ligands were analyzed and based on the interactions with the protein binding pocket, four compounds i.e., C4, C5, C6, and C21 were selected (Fig. 5). C4 made one hydrogen bond with Asn260, one Pi-Sigma bond with His263 and a hydrophobic interaction with Phe264. C5 was involved in hydrophobic interactions with His85, His259, Phe264, and Val283 and a Pi-Sigma bond with His263. The C6 compound only formed one hydrogen bond with Asn260 while C21 formed two hydrogen bonds with Ser282 and Val283 and five hydrophobic interactions with His85, His263, Phe264, Pro284, and Ala286. The interactions and plausible binding modes of these compounds are shown in Fig. 6a–b.
Fig. 5.
The selected compounds based on the plausible binding modes.
Fig. 6.
(a) The interactions of selected compounds with protein binding pocket. Green lines show hydrogen bonding, magenta shows hydrophobic interactions, purple shows Pi-Sigma bond and grey shows metal interactions. (b) The binding modes of the selected compounds. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
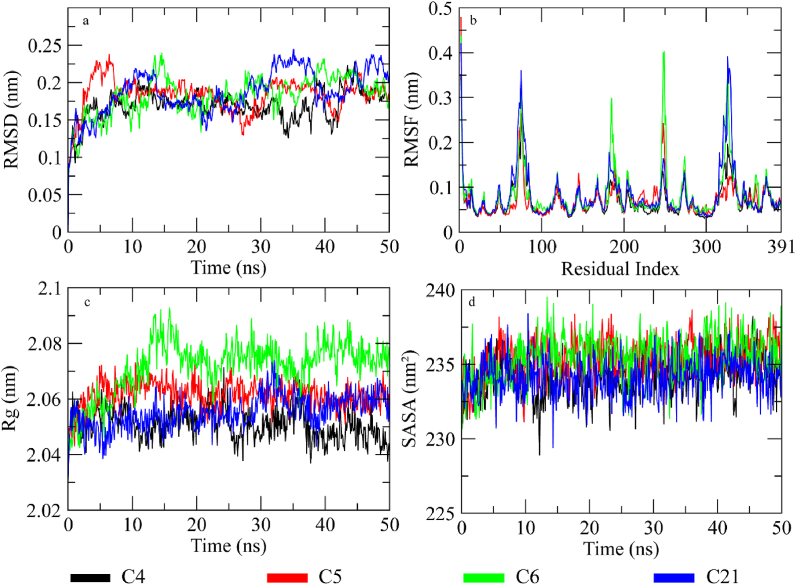
In order to find the stability of protein-ligand complex, the root mean square deviation (RMSD) of C-alpha atoms and ligand atoms was calculated over the simulation time (Fig. 7a) [65]. It can be observed that all complexes equilibrated at 5 ns and then attained stability in RMSD values in the range of 0.15–0.2 nm till 30 ns with minor deviations in C6 complex. After 30 ns, the RMSD of C21 complex increased to ∼0.25 nm and then gradually decreased to ∼0.17 nm at 40 ns. It remained in this range till 45 ns and then increased to 0.22 nm towards the end of simulation. The RMSD of other three complexes dropped to ∼0.15 nm in time period of 30–40 ns and then attained stability at ∼0.17 nm at the end of simulation. The small deviations in RMSD values indicated that the complexes were stable during simulation. The Root Mean Square fluctuations describe the flexible residues of the protein [66]. The higher RMSF value shows the flexible regions such as loops while lower value indicates the rigid residues such as helices and sheets. The RMSF plots of studied systems are shown in Fig. 7b. The starting residues showed higher RMSF values due to presence of N-terminal. The residues from 60 to 80, 180 to 200, 240 to 250, and 320 to 340 showed major fluctuations as these formed the loops in protein structure. The other residues did not show such fluctuations indicating the rigid portion of the protein. The RMSF plots for all complexes were same except for C6 complex which showed higher flexibility in residues from 240 to 250 than other complexes. Similarly, the Radius of gyration (Rg) was calculated to check the compactness of the protein when the ligands were bound to it [67]. The higher Rg values indicate the unfolding events in protein structure during simulation. The Rg plots of the systems are shown in Fig. 7c. It can be observed that the Rg value of C4, C5, and C21 complexes attainted stability in the range of 2.04–2.07 after being equilibrated at 5 ns. The Rg value of C6 complex showed a deviation of 0.03 nm at 10 ns and then remained in the range of ∼2.07–2.09 nm till the end of simulation. The stable Rg values showed that the protein did not undergo any unfolding event during simulation when bound to the compounds. Lastly, solvent accessible surface area of protein was estimated during simulation to check the exposed surface of protein to solvent [68]. The higher SASA values indicate that the protein undergo some confirmational changes due to which the exposed surface to solvent increase. The SASA plots of the complexes showed that the initial value was ∼232 nm2 which gradually increased to 235 nm2 after equilibration (Fig. 7d). The SASA values of all complexes remained in range of 233–237 nm2 throughout the simulation which indicate that the protein did not undergo confirmational changes when bound to the ligands.
Fig. 7.
The MD trajectory analysis of the complexes. (a) The RMSD of C-alpha atoms of protein when bound to selected compounds. (b) The residual flexibility of the protein. (c) The Rg calculation of C-alpha atoms to analyze the compactness of protein. (d) Solvent Accessible Surface Area calculation of protein bound to ligands during simulation.
This study reveals that a large number of biologically active natural compounds are present in fruit peels which can have potentially beneficial effects on human health. Furthermore, docking and simulation studies proved well binding with PPOs. These findings support the strength of present study. The research conducted here makes it abundantly evident that the pharmaceutical sector has the ability to make use of these bioactive components as new therapeutic agents. Although the in vitro biological activities were performed to explore the therapeutic potential of fruit peels. But in vivo studies need to perform to authenticate their therapeutic targets, which is limitation of this study.
4. Conclusion
The current study provide an insight into the pharmacological uses of the extract of fruit peels as potentially antibacterial and antioxidant agent. The ethanolic extracts demonstrate some really promising results. The investigated fruit peels can serve as a rich source of natural oxidants because of the presence of quite appreciable quantities of supportive phytochemicals including flavonoids and phenolic contents. Furthermore, considerable scavenging activities of the studied extracts were observed. The lemon peel extract showed an interesting anti-free radical activity and even this activity was greater than that of ascorbic acid. Besides, antibacterial potency of all three extracts against four bacterial species was investigated. The studies also reveal that the ethanolic and methanolic extract of orange and mousami against gram (+) bacteria exhibit convincing antibacterial potential. Molecular docking of these compounds displayed that C4, C5, C6, and C21 compounds were bound with reasonably well binding modes with oxidase and their binding stability was explored by the MD Simulation. The findings of the present study may prove helpful to define novel research directions in terms of utilization and applications of the studied extracts as a pharmaceutical and nutraceutical agents. In a nut shell, the antioxidant potential of the studied fruit peel extracts can prove helpful to facilitate their applications in pharmaceutical industry. They can also find an appreciable acceptance because of their natural character and characteristics. In the last but not least, these fruit peel extracts can greatly reduce the impact of ailments caused by oxidation processes.
Credit authors statement
Muhammad Saleem: Conceived and designed the experiments, Performed the experiments Arjumand Iqbal Durani: Contributed reagents, materials, analysis tools or data.
Asnuzilawati Asari: Analyzed and interpreted the data.
Mahmood Ahmed: Wrote the paper, Analyzed and interpreted the data.
Muhammad Ahmad: Contributed reagents, materials, analysis tools or data.
Nouman Yousaf: Wrote the paper, Analyzed and interpreted the data.
Muhammad Muddassar: Analyzed and interpreted the data.
Data availability
All data generated or analyzed during this study are included in this published article.
Funding
The authors received no specific funding for this work.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15433.
Contributor Information
Asnuzilawati Asari, Email: asnu@umt.edu.my.
Mahmood Ahmed, Email: mahmoodresearchscholar@gmail.com.
Muhammad Muddassar, Email: mmuddassar@comsats.edu.pk.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Zou Z., et al. Antioxidant activity of Citrus fruits. Food Chem. 2016;196:885–896. doi: 10.1016/j.foodchem.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 2.Vlaicu P.A., et al. Nutritional characterization and the antioxidant properties of sweet orange and red grapefruit peels. Lucrări Științifice-Universitatea de Științe Agricole şi Medicină Veterinară. Seria Zootehnie. 2020;73:112–118. [Google Scholar]
- 3.Singh B., et al. Food Research International; 2020. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. [DOI] [PubMed] [Google Scholar]
- 4.Hasija S., Ibrahim G., Wadia A. Antimicrobial activity of Citrus sinensis (Orange), Citrus limetta (Sweet lime) and Citrus limon (lemon) peel oil on selected food borne pathogens. Int. J. Libr. Sci. Res. 2015;3(3):35–39. [Google Scholar]
- 5.Chung D., Cho T.J., Rhee M.S. Citrus fruit extracts with carvacrol and thymol eliminated 7-log acid-adapted Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes: a potential of effective natural antibacterial agents. Food Res. Int. 2018;107:578–588. doi: 10.1016/j.foodres.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Cory H., et al. The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czech A., et al. Bioactive substances, heavy metals, and antioxidant activity in whole fruit, peel, and pulp of citrus fruits. Int. J. Food Sci. 2021:2021. doi: 10.1155/2021/6662259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morais D.R., et al. Proximate composition, mineral contents and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in Brazil. J. Braz. Chem. Soc. 2017;28(2):308–318. [Google Scholar]
- 9.Hussain H., et al. Fruit peels: food waste as a valuable source of bioactive natural products for drug discovery. Curr. Issues Mol. Biol. 2022;44(5):1960–1994. doi: 10.3390/cimb44050134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyun J.M., et al. Tetramethyl-O-scutellarin isolated from peels of immature Shiranuhi fruit exhibits anti-inflammatory effects on LPSinduced RAW264. 7 cells. Trop. J. Pharmaceut. Res. 2017;16(9):2197–2205. [Google Scholar]
- 11.Czech A., et al. Mineral content of the pulp and peel of various Citrus fruit cultivars. Biol. Trace Elem. Res. 2020;193(2):555–563. doi: 10.1007/s12011-019-01727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phong W.N., et al. Methods used for extraction of plant volatiles have potential to preserve truffle aroma: a review. Compr. Rev. Food Sci. Food Saf. 2022;21(2):1677–1701. doi: 10.1111/1541-4337.12927. [DOI] [PubMed] [Google Scholar]
- 13.Borella T.G., et al. Effect of rosemary (Rosmarinus officinalis) antioxidant in industrial processing of frozen‐mixed hamburger during shelf life. J. Food Process. Preserv. 2019;43(9) [Google Scholar]
- 14.Singh B.K., Tiwari S., Dubey N.K. Essential oils and their nanoformulations as green preservatives to boost food safety against mycotoxin contamination of food commodities: a review. J. Sci. Food Agric. 2021;101(12):4879–4890. doi: 10.1002/jsfa.11255. [DOI] [PubMed] [Google Scholar]
- 15.Nasrollahzadeh A., et al. Antifungal preservation of food by lactic acid bacteria. Foods. 2022;11(3):395. doi: 10.3390/foods11030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imeneo V., et al. Functionalized biscuits with bioactive ingredients obtained by Citrus lemon pomace. Foods. 2021;10(10):2460. doi: 10.3390/foods10102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan U.M., et al. Citrus genus and its waste utilization: a review on health-promoting activities and industrial application. Evid. base Compl. Alternative Med. 2021;2021:1–17. doi: 10.1155/2021/2488804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Dhanavade M.J., et al. Study antimicrobial activity of lemon (Citrus lemon L.) peel extract. Br. J. Pharmacol. Toxicol. 2011;2(3):119–122. [Google Scholar]
- 19.Chen Y., et al. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021;364 doi: 10.1016/j.foodchem.2021.130413. [DOI] [PubMed] [Google Scholar]
- 20.Liu N., et al. A review of chemical constituents and health-promoting effects of citrus peels. Food Chem. 2021;365 doi: 10.1016/j.foodchem.2021.130585. [DOI] [PubMed] [Google Scholar]
- 21.Yerou K.O., et al. The use of orange (citrus sinensis) peel as antimicrobial and anti-oxidant agents. J. Fund. Appl. Sci. 2017;9(3):1351–1357. [Google Scholar]
- 22.Ahmed M., Khaleeq A., Ahmad S. Antioxidant and antifungal activity of aqueous and organic extracts of liquorice. World Appl. Sci. J. 2014;30(11):1664–1667. [Google Scholar]
- 23.Siddique M.H., et al. Antidiabetic and antioxidant potentials of Abelmoschus esculentus: in vitro combined with molecular docking approach. J. Saudi Chem. Soc. 2022;26(2) [Google Scholar]
- 24.Kazmi S.T.B., et al. Phytochemical analysis and comprehensive evaluation of pharmacological potential of Artemisia brevifolia Wall. ex DC. Saudi Pharmaceut. J. 2022;30(6):793–814. doi: 10.1016/j.jsps.2022.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadeghi M., et al. α-glucosidase inhibitory, antioxidant activity, and GC/MS analysis of Descurainia sophia methanolic extract: in vitro, in vivo, and in silico studies. Arab. J. Chem. 2022;15(9) [Google Scholar]
- 26.Ahmed M., et al. Exploring the antioxidant potential of some common marketed nutraceuticals/drugs in Pakistan by different in vitro models. Lat. Am. J. Pharm. 2020;39:372–375. [Google Scholar]
- 27.Mohammed I.A., et al. Synthesis of 1, 3-benzoxazines based on 2, 4, 4-trimethyl-7, 2', 4'-trihydroxy flavan: antibacterial, anti-inflammatory, cyclooxygenase-2 inhibition and molecular modelling studies. Lett. Drug Des. Discov. 2019;16(1):58–65. [Google Scholar]
- 28.Ahmed M., et al. Synthesis, characterization, biological activities and molecular modeling of Schiff bases of benzene sulfonamides bearing curcumin scaffold. Arab. J. Chem. 2019;12(1):41–53. [Google Scholar]
- 29.Ghosh G., et al. GC-MS analysis of bioactive compounds in the methanol extract of Clerodendrum viscosum leaves. Pharmacogn. Res. 2015;7(1):110. doi: 10.4103/0974-8490.147223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schrödinger, L.J.S.S., Schrödinger, LLC; New York, NY: 2017. 2017. vol. 2: pp. 2017–1.
- 31.Shivakumar D., et al. Improving the prediction of absolute solvation free energies using the next generation OPLS force field. J. Chem. Theor. Comput. 2012;8(8):2553–2558. doi: 10.1021/ct300203w. [DOI] [PubMed] [Google Scholar]
- 32.Sousa da Silva A.W., Vranken W.F. ACPYPE-Antechamber python parser interface. MBC Research Notes. 2012;5(1):1–8. doi: 10.1186/1756-0500-5-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qureshi K.A., et al. In vitro and in silico approaches for the antileishmanial activity evaluations of actinomycins isolated from novel streptomyces smyrnaeus strain UKAQ_23. Antibiotics. 2021;10(8):887. doi: 10.3390/antibiotics10080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hess B., et al. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 1997;18(12):1463–1472. doi: 10.1021/ct700200b. [DOI] [PubMed] [Google Scholar]
- 35.Grubmüller H., et al. Generalized Verlet algorithm for efficient molecular dynamics simulations with long-range interactions. Mol. Simulat. 1991;6(1–3):121–142. [Google Scholar]
- 36.Essmann U., et al. A smooth particle mesh Ewald method. J. Chem. Phys. 1995;103(19):8577–8593. [Google Scholar]
- 37.Huang J., MacKerell A.D., Jr. CHARMM36 all‐atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 2013;34(25):2135–2145. doi: 10.1002/jcc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grant B.J., Skjærven L., Yao X.Q.J.P.S. The Bio3D packages for structural bioinformatics. Protein Sci. 2021;30(1):20–30. doi: 10.1002/pro.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umaru I.J., Badruddin F.A., Umaru H.A. Phytochemical screening of essential oils and antibacterial activity and antioxidant properties of Barringtonia asiatica (L) leaf extract. Biochem. Res. Int. 2019;2019 doi: 10.1155/2019/7143989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khatiwora E., et al. Spectroscopic determination of total phenol and flavonoid contents of citrus limon peel from north eastern region of India. J. Drug Deliv. Therapeut. 2017;7(1):21–24. [Google Scholar]
- 41.Fejzić A., Ćavar S. Phenolic compounds and antioxidant activity of some citruses. Bull. Chem. Technol. Bosnia Herzegovina. 2014;42:1–4. [Google Scholar]
- 42.Li B., Smith B., Hossain M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006;48(2):182–188. [Google Scholar]
- 43.Hegazy A., Ibrahium M. Antioxidant activities of orange peel extracts. World Appl. Sci. J. 2012;18(5):684–688. [Google Scholar]
- 44.Ghasemi K., Ghasemi Y., Ebrahimzadeh M.A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009;22(3):277–281. [PubMed] [Google Scholar]
- 45.Ștefănescu B.E., et al. Phenolic compounds from five Ericaceae species leaves and their related bioavailability and health benefits. Molecules. 2019;24(11):2046. doi: 10.3390/molecules24112046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diab T.A., Donia T., Saad-Allah K.M. Characterization, antioxidant, and cytotoxic effects of some Egyptian wild plant extracts. Beni-Suef Univ. J. Basic Appl. Sci. 2021;10(1):1–13. [Google Scholar]
- 47.Croft K.D. The chemistry and biological effects of flavonoids and phenolic acids a. Ann. N. Y. Acad. Sci. 1998;854(1):435–442. doi: 10.1111/j.1749-6632.1998.tb09922.x. [DOI] [PubMed] [Google Scholar]
- 48.Franco R.R., et al. Antioxidant and anti-glycation capacities of some medicinal plants and their potential inhibitory against digestive enzymes related to type 2 diabetes mellitus. J. Ethnopharmacol. 2018;215:140–146. doi: 10.1016/j.jep.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 49.Đorđević N.O., et al. Antioxidant activity of selected polyphenolics in yeast cells: the case study of Montenegrin Merlot wine. Molecules. 2018;23(8):1971. doi: 10.3390/molecules23081971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prakash P., et al. In vitro and in silico toxicological properties of natural antioxidant therapeutic agent azima tetracantha. LAM. Antioxidants. 2021;10(8):1307. doi: 10.3390/antiox10081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shehata M.G., et al. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021;4:326–335. doi: 10.1016/j.crfs.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azman N.F.I.N., et al. Antioxidant properties of fresh and frozen peels of citrus species. Curr. Res. Nutr. Food Sci. J. 2019;7(2):331–339. [Google Scholar]
- 53.Ren Q., et al. In vitro antioxidant and immunostimulating activities of polysaccharides from Ginkgo biloba leaves. Int. J. Biol. Macromol. 2019;124:972–980. doi: 10.1016/j.ijbiomac.2018.11.276. [DOI] [PubMed] [Google Scholar]
- 54.Khan R.A., et al. Assessment of flavonoids contents and in vitro antioxidant activity of Launaea procumbens. Chem. Cent. J. 2012;6(1):1–11. doi: 10.1186/1752-153X-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumari S., et al. In vitro and in vivo antioxidant, anti-hyperlipidemic properties and chemical characterization of Centella asiatica (L.) extract. Front. Pharmacol. 2016;7:400. doi: 10.3389/fphar.2016.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burgos-Morón E., et al. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. J. Clin. Med. 2019;8(9):1385. doi: 10.3390/jcm8091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stagos D. Antioxidant activity of polyphenolic plant extracts. Antioxidants. 2019:19. doi: 10.3390/antiox9010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jubair N., et al. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR) Evid. base Compl. Alternative Med. 2021:2021. doi: 10.1155/2021/3663315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oikeh E.I., Oviasogie F.E., Omoregie E.S. Quantitative phytochemical analysis and antimicrobial activities of fresh and dry ethanol extracts of Citrus sinensis (L.) Osbeck (sweet Orange) peels. Clin. Phytosci. 2020;6(1):1–6. [Google Scholar]
- 60.Jayaprakasha G.K., et al. Antibacterial activity of Citrus reticulata peel extracts. Z. Naturforsch. C Biosci. 2000;55(11–12):1030–1034. doi: 10.1515/znc-2000-11-1230. [DOI] [PubMed] [Google Scholar]
- 61.Gonelimali F.D., et al. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018;9:1639. doi: 10.3389/fmicb.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hobson C., Chan A.N., Wright G.D. The antibiotic resistome: a guide for the discovery of natural products as antimicrobial agents. Chem. Rev. 2021;121(6):3464–3494. doi: 10.1021/acs.chemrev.0c01214. [DOI] [PubMed] [Google Scholar]
- 63.Yuan G., et al. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021;11(1):1–15. doi: 10.1038/s41598-021-90035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hafidh R.R., et al. Inhibition of growth of highly resistant bacterial and fungal pathogens by a natural product. Open Microbiol. J. 2011;5:96–106. doi: 10.2174/1874285801105010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sargsyan K., et al. How molecular size impacts RMSD applications in molecular dynamics simulations. J. Chem. Theor. Comput. 2017;13(4):1518–1524. doi: 10.1021/acs.jctc.7b00028. [DOI] [PubMed] [Google Scholar]
- 66.Martinez R., et al. Mitotic checkpoint kinase Mps1 has a role in normal physiology which impacts clinical utility. PLoS One. 2015;10(9):e0138616. doi: 10.1371/journal.pone.0138616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lobanov M.Y., Bogatyreva N., Galzitskaya O.J.M.B. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 2008;42(4):623–628. [PubMed] [Google Scholar]
- 68.Joshi T., et al. Molecular docking and molecular dynamics simulation approach to screen natural compounds for inhibition of Xanthomonas oryzae pv. Oryzae by Targeting Peptide Deformylase. J. Biomol. Struct. Dyn. 2021;39(3):823–840. doi: 10.1080/07391102.2020.1719200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.