This case series examines data for patients from 8 US medical centers to determine and describe the characteristics associated with progressive multifocal leukoencephalopathy in the context of sarcoidosis, including neurological symptoms and changes seen on brain magnetic resonance imaging.
Key Points
Question
What are the characteristics of patients who develop progressive multifocal leukoencephalopathy in the context of sarcoidosis (S-PML) without immunosuppression?
Findings
In this case series, patients with systemic sarcoidosis developed PML in the absence of therapeutic immune suppression, and their peripheral blood proxies of immune function were often only mildly abnormal. Systemic sarcoidosis flares may rarely herald the onset of S-PML.
Meaning
Clinicians should consider PML for any patient with systemic sarcoidosis and new neurological symptoms associated with white matter changes seen on brain magnetic resonance imaging even if the presentation is not typical of the disease.
Abstract
Importance
Progressive multifocal leukoencephalopathy can occur in the context of systemic sarcoidosis (S-PML) in the absence of therapeutic immune suppression and can initially be mistaken for neurosarcoidosis or other complications of sarcoidosis. Earlier recognition of S-PML could lead to more effective treatment of the disease.
Objective
To describe characteristics of patients with S-PML.
Design, Setting, and Participants
For this case series, records from 8 academic medical centers in the United States were reviewed from 2004 to 2022. A systematic review of literature from 1955 to 2022 yielded data for additional patients. Included were patients with S-PML who were not receiving therapeutic immune suppression. The median follow-up time for patients who survived the acute range of illness was 19 months (range, 2-99). Data were analyzed in February 2023.
Exposures
Sarcoidosis without active therapeutic immune suppression.
Main Outcomes and Measures
Clinical, laboratory, and radiographic features of patients with S-PML.
Results
Twenty-one patients with S-PML not receiving therapeutic immune suppression were included in this study, and data for 37 patients were collected from literature review. The median age of the 21 study patients was 56 years (range, 33-72), 4 patients (19%) were female, and 17 (81%) were male. The median age of the literature review patients was 49 years (range, 21-74); 12 of 34 patients (33%) with reported sex were female, and 22 (67%) were male. Nine of 21 study patients (43%) and 18 of 31 literature review patients (58%) had simultaneous presentation of systemic sarcoidosis and PML. Six of 14 study patients (43%) and 11 of 19 literature review patients (58%) had a CD4+ T-cell count greater than 200/μL. In 2 study patients, a systemic flare of sarcoidosis closely preceded S-PML development. Ten of 17 study patients (59%) and 21 of 35 literature review patients (60%) died during the acute phase of illness. No meaningful predictive differences were found between patients who survived S-PML and those who did not.
Conclusions and Relevance
In this case series, patients with sarcoidosis developed PML in the absence of therapeutic immune suppression, and peripheral blood proxies of immune function were often only mildly abnormal. Systemic sarcoidosis flares may rarely herald the onset of S-PML. Clinicians should consider PML in any patient with sarcoidosis and new white matter lesions on brain magnetic resonance imaging.
Introduction
Sarcoidosis is a granulomatous inflammatory disorder that can affect multiple organ systems1 and is associated with both treatment-related immune suppression and intrinsic immune dysregulation.2 Progressive multifocal leukoencephalopathy (PML) is a devastating disease of the central nervous system caused by JC virus (JCV) infection of oligodendrocytes, often seen in the context of cell-mediated immune compromise.3 PML was first described in 1958 in a patient with lymphoma4 but is now most commonly associated with AIDS, cancer, and treatment-related immune modulation.5,6,7,8,9 Sarcoidosis associated with a PML-like disease was described in 1955,10 although in this report, 1 of the defining histopathologic features of the disease (enlarged oligodendrocyte nuclei) was not described. In the intervening 7 decades, the occurrence of PML associated with systemic sarcoidosis (S-PML) in the absence of therapeutic immune suppression has been described in 37 patients.11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45 Recent research for possible PML treatment includes infusion of interleukin (IL) 246,47 and IL-7,48 checkpoint inhibitors,49,50,51,52,53,54 polyoma virus-specific T-cell therapy (PyVST),55,56,57 and infliximab, the last of which was used for patients with sarcoidosis specifically.21 For now, the only effective treatment is to reverse immune suppression promptly. Thus, it is crucial to identify this cohort early and accurately.
In this study we retrospectively assessed the clinical, laboratory, and radiographic characteristics of patients with PML and sarcoidosis at multiple tertiary care centers over the past 2 decades. We also performed a systematic literature review of all published cases to provide an updated, comprehensive analysis and description of S-PML.
Methods
Data Collection and Consent
Patient data were collected from Mass General Brigham network hospitals, Boston, Massachusetts; National Institutes of Health (NIH); Beth Israel Deaconess Medical Center, Boston; Columbia University Irving Medical Center–New York Presbyterian Hospital, New York; University of California, San Francisco; University of Michigan, Ann Arbor; Mayo Clinic, Rochester, Minnesota; and Boston Medical Center, Boston. All NIH patients were enrolled in the institutional review board–approved natural history study of PML (NCT01730131) and provided written informed consent. Retrospective data collection was approved by institutional review boards or other relevant regulatory agencies as required by other institutions, in which cases consent was not obtained directly from patients in accordance with institutional policies. The study was conducted and reported according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)58 and Enhancing the Quality and Transparency of Health Research (EQUATOR)59 guidelines.
Patient Selection Criteria
Medical records were searched electronically using diagnosis codes for sarcoidosis and PML in the academic medical centers. Twenty-eight patients were found from all participating centers, and 7 were excluded for receiving immune suppressive medication prior to neurologic symptom onset (NSO). All patients met American Academy of Neurology criteria for definite PML, including typical imaging and exclusion of alternative diagnoses in conjunction with either characteristic histopathological features or positive polymerase chain reaction for JCV in the cerebrospinal fluid.60 The diagnosis of sarcoidosis was based on retrospective application of the 2020 American Thoracic Society Clinical Practice Guideline using a combination of compatible clinical and radiographic manifestations, exclusion of other relevant disease, and histopathologic detection of noncaseating granulomas.61
Patients were excluded from the primary analysis if they received therapeutic immune suppression before PML development. For patients who were transferred to a tertiary care center partway through their course of illness, we based inclusion on presence or absence of immune suppression medications in their admission history and medicine reconciliation. In some patients, immune suppression was initiated very shortly before NSO. A case-by-case decision for inclusion or exclusion was made for these patients. Two patients who began receiving immune suppression within 1 month or less of NSO were included in this study, while 5 patients who received immune suppression beginning 10 weeks or more before NSO were excluded. Race and ethnicity data, self-reported by patients, were gathered in order to provide epidemiologic information on S-PML.
Literature Review
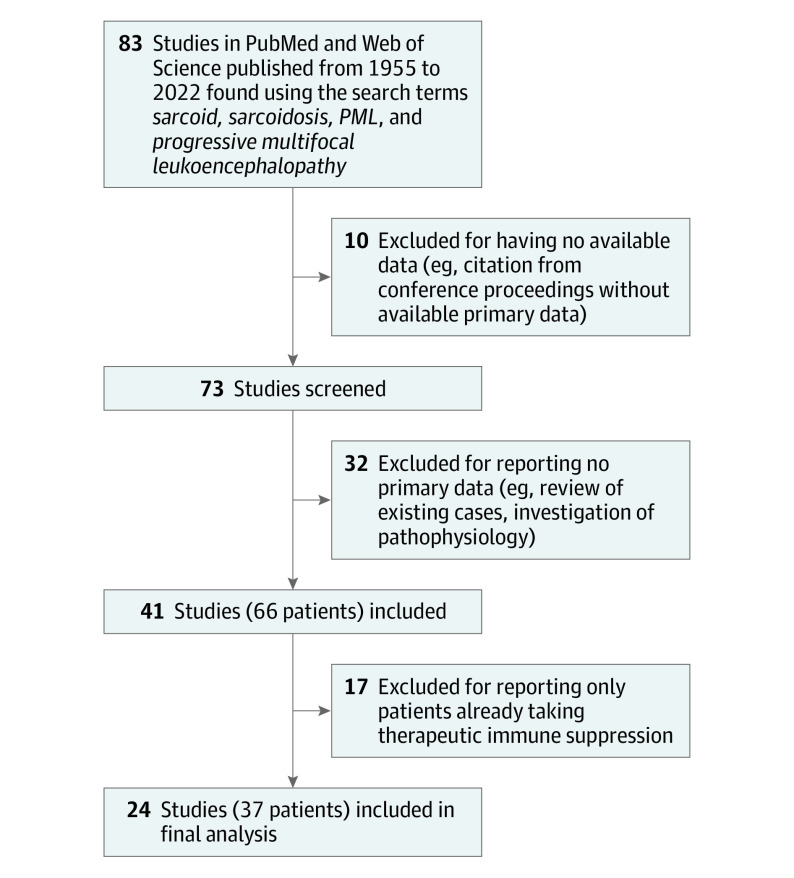
We reviewed publications from PubMed and Web of Science using permutations of the search terms sarcoid, sarcoidosis, PML, and progressive multifocal leukoencephalopathy from 1955 to August 2022. One author (C.R.S.M.) reviewed studies for inclusion individually without automated tools. English and Spanish language articles were read and interpreted directly, and articles in other languages were read with the assistance of a qualified medical interpreter. Studies included in the review required a unique report of a patient with probable or definite sarcoidosis, definite PML, and meaningful description of the relevant patient’s clinical course, laboratory values, or radiographic values. Missing or unclear values were not included in data collection. Of 83 unique articles returned through the literature search, 41 studies with 66 unique patients were reviewed. Ten studies were excluded for having no available data, 32 studies were excluded for reporting no primary data, and 17 were excluded for reporting only patients taking therapeutic immune suppression (Figure 1). The review was not registered in a national database.
Figure 1. Flow Diagram of Study Inclusion From the Literature Review.
Statistical Analysis
Data were analyzed using SPSS version 29.0.0.0 (IBM). Study cohort and literature review patients were aggregated, and a binary logistic regression model using the Hosmer and Lemeshow goodness-of-fit test was performed to evaluate for factors that may be predictive of patient mortality. A 2-sided t test was used to compare continuous variables between patient groupings.
Results
Patient Characteristics
Our study cohort included 21 patients with S-PML with median age of 56 years (range, 33-72) at sarcoidosis diagnosis and median age of 56 years (range, 33-75) at NSO. Four patients (19%) in our cohort were female, and 17 (81%) were male. Of 37 literature review patients, the median age at NSO was 49 years (range, 23-78), 12 of 34 patients (33%) with sex reported were female, and 22 (67%) were male (eTable in Supplement 1).
Latency From Sarcoidosis to PML Diagnosis
The median time from sarcoidosis diagnosis to NSO (S-PML latency) in the study cohort was 1 month. Nine patients (43%) had simultaneous diagnoses of sarcoidosis and PML; they presented with neurologic symptoms consistent with PML that led to a diagnosis of sarcoidosis. The maximum S-PML latency in our cohort was 87 months.
Of the 31 patients with S-PML latency listed in the literature review cohort, 18 (58%) had simultaneous diagnoses. Maximum S-PML latency was 300 months (25 years).
Characteristics of Sarcoidosis
All patients had pulmonary sarcoidosis. Fourteen patients (67%) had sarcoidosis in the lungs only. Of 7 patients with multisystemic disease, 5 (24%) had hepatic involvement, 2 (10%) had cutaneous involvement, 1 (5%) had kidney involvement, 1 (5%) had uveitis, and 1 (5%) had cardiac involvement.
Of 20 literature review patients with known sites of sarcoidosis, 8 (40%) had isolated pulmonary involvement, and 12 (60%) had multisystem involvement including the lungs (Table and eTable in Supplement 1).
Table. Characteristics of Patients From the Study Cohort and Literature Review.
| Characteristic | No./total No. (%)a | ||
|---|---|---|---|
| Study cohort | Literature review patients | All patients with S-PML | |
| Sex | |||
| Female | 4/21 (19) | 12/34 (33) | 26/55 (47) |
| Male | 17/21 (81) | 22/34 (67) | 29/55 (53) |
| Race and ethnicityb | |||
| Black | 8/21 (38) | NA | |
| White, non-Hispanic | 13/21 (62) | NA | |
| Age at NSO, y | |||
| <40 | 3/21 (14) | 8/35 (19) | 11/56 (20) |
| 40-60 | 11/21 (52) | 20/35 (62) | 31/56 (55) |
| >60 | 7/21 (33) | 7/35 (19) | 14/56 (25) |
| Method of PML diagnosis | |||
| Biopsy | 9/20 (45) | 14/26 (75) | 23/46 (50) |
| CSF | 11/20 (55) | 12/26 (25) | 23/46 (50) |
| S-PML latency, mo | |||
| Simultaneous | 9/21 (46) | 18/31 (58) | 27/52 (52) |
| 1-3 | 3/21 (14) | 1/31 (3) | 4/52 (7) |
| 4-24 | 2/21 (10) | 4/31 (13) | 6/52 (12) |
| 25-48 | 3/21 (14) | 1/31 (3) | 4/52 (7) |
| 49-84 | 3/21 (10) | 2/31 (6) | 4/52 (7) |
| 85-168 | 1/21 (5) | 2/31 (6) | 3/52 (6) |
| 169-336 | 0/21 | 3/31 (10) | 3/52 (6) |
| Sarcoidosis sites | |||
| Lung only | 14/21 (67) | 4/23 (15) | 18/44 (41) |
| Multisystem (≥2 sites) | 7/21 (33) | 19/23 (85) | 26/44 (59) |
| Serum CD4+ count at PML diagnosis | |||
| <200/μL | 8/14 (57) | 8/19 (42) | 16/33 (48) |
| 201/μL-500/μL | 6/14 (43) | 11/19 (58) | 17/33 (52) |
| Serum absolute lymphocyte count at PML diagnosis | |||
| ≤499/μL | 2/18 (11) | 2/14 (21) | 4/32 (13) |
| 500/μL-1000/μL | 11/18 (61) | 7/14 (50) | 18/32 (56) |
| ≥1001/μL | 5/18 (28) | 5/14 (29) | 10/32 (31) |
| MRI features | |||
| Contrast enhancement | 6/21 (29) | 2/31 (6) | 8/52 (15) |
| Infratentorial lesions only | 6/20 (30) | 2/27 (27) | 8/47 (17) |
| Supratentorial lesions only | 8/20 (40) | 25/27 (58) | 33/47 (70) |
| Mixed supratentorial and infratentorial lesions | 6/20 (30) | 0/27 | 6/47 (13) |
| Received IM after NSO | 4/20 (20) | 17/25 (68) | 21/45 (47) |
| PML treatment | |||
| Pembrolizumab | 5/21 (24) | 1/25 (4) | 6/46 (13) |
| Interleukins | 0/21 | 2/25 (8) | 2/46 (4) |
| PyVST | 2/21 (10) | 0/25 | 2/46 (4) |
| Cytarabine | 2/21 (10) | 1/25 (4) | 3/46 (7) |
| Infliximab | 3/21 (14) | 4/25 (16) | 7/46 (15) |
| Mirtazapine | 9/21 (43) | 15/25 (56) | 24/46 (52) |
| Mefloquine | 6/21 (29) | 9/25 (32) | 15/46 (33) |
| Cidofovir | 1/21 (5) | 5/25 (52) | 6/46 (13) |
| Patient outcome | |||
| mRS score 0-1 (no symptoms or no significant disability) | 1/17 (6) | 0/18 | 1/35 (3) |
| mRS score 2-3 (slight or moderate disability) | 3/17 (18) | 3/18 (12) | 6/35 (17) |
| mRS score 4-5 (moderately severe or severe disability) | 3/17 (18) | 4/18 (24) | 7/35 (20) |
| Mortality (mRS score 6) | 10/17 (59) | 11/18 (65) | 21/35 (60) |
Abbreviations: CSF, cerebrospinal fluid; IM, immune modulation; MRI, magnetic resonance imaging; mRS, modified Rankin scale; NA, not available; NSO, neurologic symptom onset; PML, progressive multifocal leukoencephalopathy; PyVST, polyoma virus–specific T-cell therapy; S-PML, progressive multifocal leukoencephalopathy in the context of sarcoidosis.
Cohort descriptions of patients from this study (study cohort), patients from the literature review, and summed totals of patients from the study cohort and literature review (all patients with S-PML).
Race and ethnicity were self-reported by the patients in the study cohort.
Peripheral Blood Immune Markers
Of 18 study patients with absolute lymphocyte count (ALC) available at time of NSO, median ALC was 795/μL (range, 260-1280). Five patients (28%) had ALC in the normal range of greater than 1000/μL.
Of 14 patients who had CD4+ T-cell count measured at NSO, 6 of 14 patients (43%) had a count higher than 200/μL. Six of 14 patients had CD8+ T-cell counts in the normal range of greater than 150/μL. The CD4+:CD8+ cell ratio was in the normal range of 1 or more in 12 of 15 patients (80%).
In the literature review cohort, ALC at NSO was reported for 14 patients with a median value of 890/μL (range, 240-4100). Two patients (14%) had ALC of 500/μL or less, 7 (50%) had ALC from 501/μL to 1000/μL, and 5 (36%) had ALC in the normal range of greater than 1000/μL. CD4+ count was reported for 20 patients with a median count of 218/μL (range, 88-820). Eleven patients (55%) had CD4+ T-cell counts greater than 200/μL. Nine patients from the literature review had CD8+ T-cell counts available, with a median count of 224/μL (range, 29-331).
Imaging Characteristics
Of 20 study patients with magnetic resonance imaging (MRI) available, 6 patients (30%) had T2 fluid-attenuated inversion recovery (FLAIR) hyperintense, T1 hypointense lesions limited to the posterior fossa, 8 (40%) had isolated supratentorial lesions, and the remaining 6 (30%) had mixed lesions.
Of 27 literature review patients with imaging reported, at least 2 (7%) had infratentorial lesions and at least 25 (93%) had supratentorial lesions. Of 10 patients in 1 study11 (not included in the above counts), 4 were noted to have cerebellar lesions, but it is not specified whether these were isolated infratentorial lesions or mixed infratentorial and supratentorial.
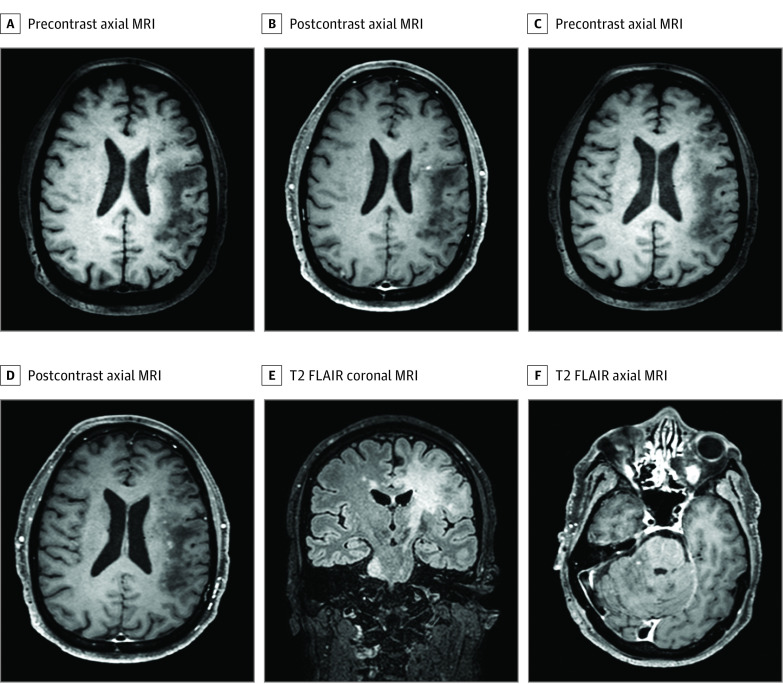
Brain MRI at the time of NSO showed contrast enhancement in 6 of 21 study patients (29%). Of 31 literature review patients with available data, 2 (6%) had contrast enhancement at NSO. Figure 2 and Figure 3 show representative images from patients in the study cohort.
Figure 2. Enhancing Lesions on Brain Magnetic Resonance Imaging (MRI) of a Male Patient in the Sixth Decade of Life With Progressive Multifocal Leukoencephalopathy in the Context of Sarcoidosis (S-PML).
Brain MRI with corresponding T1 precontrast and postcontrast axial images and T2 fluid-attenuated inversion recovery (FLAIR) coronal and axial images of a male patient in the sixth decade of life who developed PML 7 years after pulmonary sarcoidosis. Punctate enhancement on a milky-way background is seen in panels B and D.
Figure 3. Isolated Infratentorial Lesions on Brain Magnetic Resonance Imaging (MRI) of a Male Patient in the Seventh Decade of Life With Progressive Multifocal Leukoencephalopathy in the Context of Sarcoidosis (S-PML).
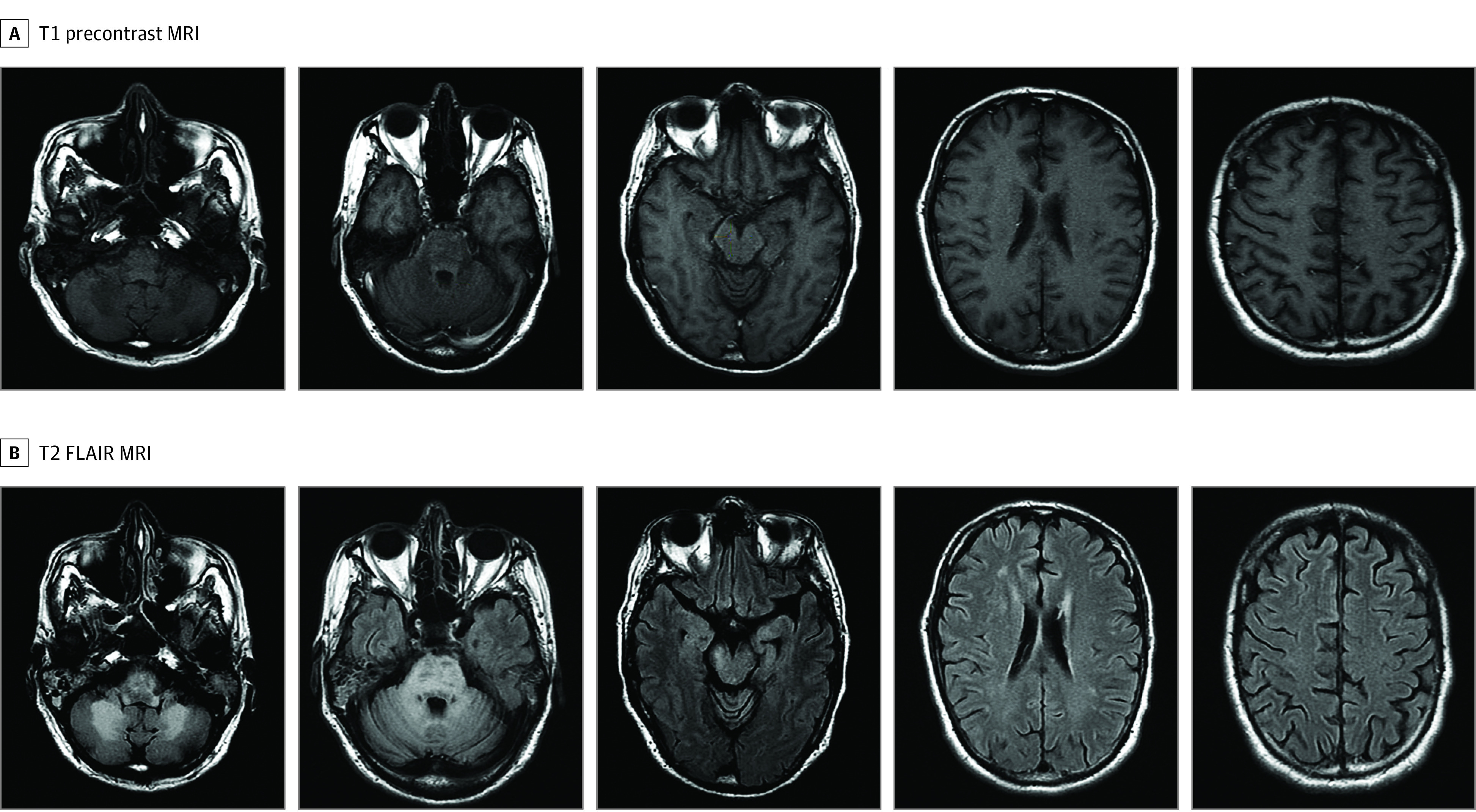
Brain MRI with corresponding T1 precontrast and T2 fluid-attenuated inversion recovery (FLAIR) axial images of a male patient in the seventh decade of life who developed PML 4 years after multisystemic (lung, skin, liver, heart) sarcoidosis and 6 months after starting prednisone for worsening dyspnea. T2-FLAIR hyperintensities with corresponding T1 hypointensities are seen in the cerebellum, medulla, midbrain, and pons. Nonspecific supratentorial white matter abnormalities were present, but no focal lesions were identified. No enhancement was noted (not shown).
Systemic Sarcoidosis Flares Before NSO
Two study patients as well as 2 patients who were excluded from analysis because of the length of immune suppression had received new or increased immune suppression for systemic symptoms shortly before NSO: 1 patient with 1-month S-PML latency was given prednisone, 60 mg daily, for a new systemic sarcoidosis diagnosis presenting with kidney insufficiency and hypercalcemia 1 month before NSO. One patient with 6-month S-PML latency was given prednisone (dose not listed) for dyspnea 1 month before NSO. One patient with 4-year S-PML latency was given prednisone, 40 mg daily, for clinicoradiographic progression of pulmonary disease 6 months before NSO. One patient with 6-year S-PML latency was given prednisone (dose not listed) for new dyspnea 10 weeks before NSO. The latter 2 patients were not included in the primary analysis.
Outcomes
Four of 21 total patients (19%) were lost to follow-up. Of the patients with available follow-up data, 10 (48%) progressed to death in the acute phase of illness, 3 (18%) had a Modified Rankin Scale (mRS) score nadir (measured at the most clinically severe time point) of 4 or 5, and 3 (18%) had an mRS score nadir of 2 or 3. Median time to follow-up was 19 months (range, 1-99) from acute presentation, at which the median change in mRS score was 0.
Subgroup Analyses
Based on the aggregated sample of study and literature review patients, a binary logistic regression model was performed to predict death as a function of age, sex, and CD4+ T-cell count at NSO. The full model containing all predictors was not statistically significant (χ23,58 = 4.32; P = .23), indicating that the model was not able to distinguish between patients who did or did not survive.
We examined characteristics of female vs male subgroups of the study cohort. An independent-samples t test with assumption of equal variance was used to compare means. When comparing female and male patients, we found no significant difference in age (female mean [SD] age, 57.9 years [15.4]; male, 49.5 years [11.0]; P < .22) or CD4+ T-cell count (female mean [SD] count, 362.9/μL [238]; male, 213.9/μL [128.0]; P < .06). We did find a significant difference in S-PML latency between female and male groups (female mean [SD] latency time, 75.3 months [100.8]; male, 7.9 months [35.6]; P < .001).
Discussion
Progressive multifocal leukoencephalopathy is a rarely diagnosed but potentially underrecognized complication of sarcoidosis that can emerge even without the use of therapeutic immune suppression. Distinct features of patients with S-PML include the following: (1) the epidemiology of S-PML compared with systemic sarcoidosis generally differs in terms of age and sex, (2) the degree of lymphopenia is often less severe than is traditionally associated with PML, and (3) a plurality of patients have no existing diagnosis of sarcoidosis before NSO. We identified a pattern seen in a handful of patients in which systemic sarcoidosis symptoms flared shortly before development of PML.
Our findings may inform laboratory surveillance in patients with sarcoidosis to help identify those patients at risk of developing S-PML. All patients in this study showed some degree of CD4+ lymphopenia, but nearly one-half had CD4+ T-cell counts above the 200/μL threshold often associated with infection diathesis, and not all patients had absolute lymphopenia. Lymphocyte subsets are not routinely checked in patients with sarcoidosis in the absence of absolute lymphopenia but may aid in diagnosis by identifying patients who are immunocompromised at the time of NSO. In contrast to our S-PML cohort, patients living with HIV/AIDS who develop PML (HIV-PML) have median CD4+ counts ranging from 54/μL to 106/μL.8,62,63 Only 11% to 13% of these patients have CD4+ counts above 200/μL at time of PML diagnosis.62,63 In published cases of PML associated with idiopathic CD4+ lymphopenia, the median CD4+ count was 119/μL, with CD4+ counts greater than 200/μL in 4 of 20 patients (20%).64 Our findings suggest that even patients with moderate rather than severe CD4+ lymphopenia may be at risk of S-PML.
Our cohort differs from the broader population of patients with sarcoidosis described in the literature in key ways. Age at sarcoidosis diagnosis is heterogeneous depending on genetic background and geographic region but is typically anywhere from the third to fifth decades of life, often with a slightly later onset in women.65,66 Patients with S-PML had peak incidence of NSO in the fifth and sixth decades of life (Table), suggesting an older age at onset than is generally described in sarcoidosis. Sex ratio was also distinct in patients with S-PML; in the study cohort, the male to female ratio was 3:1 and in the literature review, it was 2:1, whereas patients with systemic sarcoidosis are more likely to be female than male.66
Nine patients (43%) in this cohort were diagnosed with systemic sarcoidosis at the time of NSO. There was no overt second peak in timing of NSO. It is possible that sarcoidosis may have gone undiagnosed with minimal or absent symptoms in these patients for a significant time before NSO.
While this study focuses on patients who developed PML in the context of no prior immune suppression, we find it important to note that 4 patients had documented systemic flares of sarcoidosis shortly before developing PML. Two of these patients received steroids no more than a month prior to NSO, while 1 patient received steroids at 10 weeks and 1 patient at 6 months prior to NSO. The latency from steroid administration to NSO in these former 2 patients is too short for a causal role of steroids in PML development. It is more likely that the systemic flares of sarcoidosis reflected acute immune dysregulation specific to this disease and associated with risk of developing PML, while steroids were incidental to neurologic disease development. Even in the latter 2 patients, who were excluded from analysis, it is unlikely that 2 to 6 months of receiving steroids would predispose to PML without extant underlying immune dysregulation. Regardless of etiology, clinicians who treat patients with systemic sarcoidosis should be aware that, although rarely, systemic disease flares may act as a heralding sign for the development of PML. They should be aware of this potential and seek neurologic consultation if patients develop new neurologic symptoms in this context.
Fourteen of 20 patients in our study cohort (70%) had PML lesions involving the posterior fossa, including 6 (30%) with lesions limited to posterior fossa. Posterior fossa lesions occurred in only 2 of 27 literature review patients (7%), although 1 study did note 4 additional patients with cerebellar lesions that were not included in this analysis because it was not specified whether the patients had or had not received immune suppression.11 We do not have a clear explanation for this finding, although it is interesting to consider whether this could correlate with a higher frequency of JCV granule cell neuronopathy (JCV GCN) in addition to white matter findings in patients with S-PML. While infratentorial lesions occur in up to 32% to 60% of cases of HIV-PML, isolated infratentorial lesions (with no supratentorial lesions also present) are rare, occurring in 4% to 6% of patients.62,67,68 Isolated posterior fossa involvement has been reported in 4 of 20 patients (20%) with idiopathic CD4+ lymphopenia and PML to date.64 No data were available regarding the presence of cerebellar atrophy, as is seen in patients with JCV GCN, but this may be an area of future investigation, especially in patients with S-PML who have been immunosuppressed.69
Contrast enhancement was seen in 6 of 21 study patients (29%), although only in 2 of 31 literature review patients (6%). The contrast enhancement seen on MRI in our study patients featured a punctate pattern within a larger T2-FLAIR hyperintense lesion similar to the milky-way pattern that has been reported in natalizumab-associated PML,70,71,72 up to 25% to 43% of whom have contrast-enhancing lesions.70,73,74 Enhancement has been documented in approximately 10% of patients with HIV-PML at time of PML presentation,75 although it is often seen at the periphery of the PML lesions rather than distributed within the lesions as in this cohort. This has been attributed variously to immune reconstitution inflammatory syndrome–like response in the context of active antiretroviral therapy vs possibly direct viral effects from the HIV rather than JCV.76,77
Mortality in patients with S-PML appears to be worse than in patients with HIV-PML and comparable CD4+ T-cell counts at NSO and about the same as in those with idiopathic CD4+ lymphopenia to date. Ten of 17 patients in our study cohort with known outcomes (59%) died during the acute phase of the illness, and in the literature review cohort, 11 of 18 patients (61%) died. By comparison, 45% to 50% of patients with HIV-PML in the era of highly active antiretroviral therapy (HAART) survive 1 year after diagnosis. Pre-HAART PML mortality, by contrast, was 80% to 90% by 1 year after diagnosis.78 Of the limited patients reported with PML due to idiopathic CD4+ lymphopenia, 7 of 19 (37%) died during the acute phase of illness.64
Study and literature review patients received a variety of treatments that had no significant correlation with survival. However, research over the past decade offers increasingly promising possibilities for treatment of PML when restoration of immune response is not possible. Infliximab administration to treat S-PML resulted in continuous clinical and cerebrospinal fluid proxies of improvement in 3 of 6 patients in a recent study.21 Checkpoint inhibitors have shown some potential to decrease morbidity and mortality in PML,54,79,80 although outcomes are heterogeneous.53,81,82,83 Polyoma virus–directed T-cell therapies are also a promising treatment that could offer benefit to patients with S-PML.55,56,57,84 While these areas remain under investigation, early recognition of S-PML is nevertheless necessary to allow the greatest chance for treatment to be initiated before severe morbidity or mortality.
Limitations
This study has some limitations. As in most retrospective studies, data were obtained from multiple institutions across which clinical, laboratory, and imaging testing practices are not standardized. Second, common variable immune deficiency and sarcoidosis can be indistinguishable if relevant screening tests, most often serum immunoglobulins, are not assessed to ensure diagnostic accuracy.85,86 Not all patients in this cohort had these screening tests drawn, so it is possible some patients were misdiagnosed as having sarcoidosis when in fact they had common variable immunodeficiency, especially in younger patients.9
Conclusions
In this case series, patients with sarcoidosis developed PML in the absence of therapeutic immune suppression, and peripheral blood proxies of immune function were often only mildly abnormal. The association of PML with sarcoidosis is not widely recognized, particularly in patients who have not been exposed to immunosuppressive medications. S-PML may have frequent diagnostic delays and could be underdiagnosed, which can lead to administration of potentially harmful immune suppression or modulation. Systemic flares of sarcoidosis may rarely act as a heralding sign of further immune dysregulation and should prompt suspicion for PML as a cause for new neurological signs and symptoms. Patients with S-PML may have atypical laboratory features, including mild or absent absolute lymphopenia and mild CD4+ lymphopenia, and PML onset can occur any time after sarcoidosis diagnosis, even after many years of stable disease. We thus emphasize that PML should be considered in any patient with sarcoidosis and new neurological symptoms associated with white matter changes seen on brain MRI, even if the presentation is not classically consistent with PML.
eTable. Characteristics of S-PML Patients in Scientific Literature 1955-2022
Data sharing statement
References
- 1.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155-1167. doi: 10.1016/S0140-6736(13)60680-7 [DOI] [PubMed] [Google Scholar]
- 2.Daniele RP, Dauber JH, Rossman MD. Immunologic abnormalities in sarcoidosis. Ann Intern Med. 1980;92(3):406-416. doi: 10.7326/0003-4819-92-3-406 [DOI] [PubMed] [Google Scholar]
- 3.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425-437. doi: 10.1016/S1474-4422(10)70040-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astrom KE, Mancall EL, Richardson EP Jr. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain. 1958;81(1):93-111. [DOI] [PubMed] [Google Scholar]
- 5.Cinque P, Koralnik IJ, Gerevini S, Miro JM, Price RW. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect Dis. 2009;9(10):625-636. doi: 10.1016/S1473-3099(09)70226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aksamit AJ Jr. Progressive multifocal leukoencephalopathy. Continuum (Minneap Minn). 2012;18(6 Infectious Disease):1374-1391. doi: 10.1212/01.CON.0000423852.70641.de [DOI] [PubMed] [Google Scholar]
- 7.Iacobaeus E, Burkill S, Bahmanyar S, et al. The national incidence of PML in Sweden, 1988-2013. Neurology. 2018;90(6):e498-e506. doi: 10.1212/WNL.0000000000004926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand P, Hotan GC, Vogel A, Venna N, Mateen FJ. Progressive multifocal leukoencephalopathy: a 25-year retrospective cohort study. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e618. doi: 10.1212/NXI.0000000000000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills EA, Mao-Draayer Y. Understanding progressive multifocal leukoencephalopathy risk in multiple sclerosis patients treated with immunomodulatory therapies: a bird’s eye view. Front Immunol. 2018;9:138. doi: 10.3389/fimmu.2018.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen E, Fog M. A case of Schilder’s disease in an adult with remarks to the etiology and pathogenesis. Acta Psychiatr Neurol Scand. 1955;30(1-2):141-154. doi: 10.1111/j.1600-0447.1955.tb06054.x [DOI] [PubMed] [Google Scholar]
- 11.Jamilloux Y, Néel A, Lecouffe-Desprets M, et al. Progressive multifocal leukoencephalopathy in patients with sarcoidosis. Neurology. 2014;82(15):1307-1313. doi: 10.1212/WNL.0000000000000318 [DOI] [PubMed] [Google Scholar]
- 12.Graf LM, Rosenkranz SC, Hölzemer A, et al. Clinical presentation and disease course of 37 consecutive cases of progressive multifocal leukoencephalopathy (PML) at a German tertiary-care hospital: a retrospective observational study. Front Neurol. 2021;12:632535. doi: 10.3389/fneur.2021.632535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbloom MA, Uphoff DF. The association of progressive multifocal leukoencephalopathy and sarcoidosis. Chest. 1983;83(3):572-575. doi: 10.1378/chest.83.3.572 [DOI] [PubMed] [Google Scholar]
- 14.Davis MJ, Khan A, Royal W III. Progressive multifocal leukoencephalopathy as the first manifestation of occult sarcoidosis: case report and review of the literature. Neurologist. 2013;19(1):26-29. doi: 10.1097/NRL.0b013e31827c6c3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Raedt S, Lacor P, Michotte A, Flamez A, Ebinger G. Progressive multifocal leukoencephalopathy as first manifestation of sarcoidosis. Clin Neurol Neurosurg. 2008;110(2):186-189. doi: 10.1016/j.clineuro.2007.09.012 [DOI] [PubMed] [Google Scholar]
- 16.Elovaara I, Haltia M, Suoranta H. Multifocal neurological signs and pulmonary sarcoidosis [in Finnish]. Duodecim. 1989;105(3):293-302. [PubMed] [Google Scholar]
- 17.Yagi T, Hattori H, Ohira M, et al. Progressive multifocal leukoencephalopathy developed in incomplete Heerfordt syndrome, a rare manifestation of sarcoidosis, without steroid therapy responding to cidofovir. Clin Neurol Neurosurg. 2010;112(2):153-156. doi: 10.1016/j.clineuro.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 18.Völker H-U, Kraft K, Arnold E, Steinhoff S, Kolios G, Sommer S. Progressive multifocal leukoencephalopathy developing in advanced pulmonal sarcoidosis. Clin Neurol Neurosurg. 2007;109(7):624-630. doi: 10.1016/j.clineuro.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 19.Trentalange A, Calcagno A, Ghisetti V, et al. Clearance of cerebrospinal fluid JCV DNA with mirtazapine in a patient with progressive multifocal leukoencephalopathy and sarcoidosis. Antivir Ther. 2016;21(7):633-635. doi: 10.3851/IMP3032 [DOI] [PubMed] [Google Scholar]
- 20.Scholten P, Kralt P, Jacobs B. Posterior fossa progressive multifocal leukoencephalopathy: first presentation of an unknown autoimmune disease. BMJ Case Rep. 2017;2017:bcr2017220990. doi: 10.1136/bcr-2017-220990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenkranz SC, Häußler V, Kolster M, et al. Treating sarcoidosis-associated progressive multifocal leukoencephalopathy with infliximab. Brain Commun. 2021;4(1):fcab292. doi: 10.1093/braincomms/fcab292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rath T, Hackenbroch M, Saech J, Hallek M, Rubbert A. Longterm remission of more than 5 years in a patient with progressive multifocal leucoencephalopathy and sarcoidosis. Clin Neurol Neurosurg. 2012;114(7):1057-1058. doi: 10.1016/j.clineuro.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 23.Primicerio GC, Muroni A, Cimino P, Defazio G. Progressive multifocal leukoencephalopathy in an ever-treated pulmonary sarcoidosis. J Neurol Sci. 2021;429:118912. doi: 10.1016/j.jns.2021.118912 [DOI] [Google Scholar]
- 24.Patel L, Elavarasi A, Garg A, Nambirajan A. Cerebellar progressive multifocal leukoencephalopathy associated with pulmonary sarcoidosis. BMJ Case Rep. 2021;14(9):e245271. doi: 10.1136/bcr-2021-245271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha RK, Li H-P. Infections in sarcoidosis. Int J Sci Inventions Today. 2017;6(4):320-328. http://www.ijsit.com/admin/ijsit_files/INFECTIONS%20IN%20SARCOIDOSIS_IJSIT_6.4.6.pdf [Google Scholar]
- 26.Park JH, Ryoo S, Noh HJ, et al. Dual therapy with cidofovir and mirtazapine for progressive multifocal leukoencephalopathy in a sarcoidosis patient. Case Rep Neurol. 2011;3(3):258-262. doi: 10.1159/000333780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallin M, O’Sullivan C, Dodd JD, et al. A case of progressive multifocal leukoencephalopathy in a patient with sarcoidosis. QJM. 2012;105(10):1011-1016. doi: 10.1093/qjmed/hcr154 [DOI] [PubMed] [Google Scholar]
- 28.Owczarczyk K, Hilker R, Brunn A, Hallek M, Rubbert A. Progressive multifocal leucoencephalopathy in a patient with sarcoidosis: successful treatment with cidofovir and mirtazapine. Rheumatology (Oxford). 2007;46(5):888-890. doi: 10.1093/rheumatology/kem049 [DOI] [PubMed] [Google Scholar]
- 29.Olindo S, Guillon B, Faighel M, Fève JR. Progressive multifocal leukoencephalopathy and pulmonary sarcoidosis [in French]. Rev Neurol (Paris). 2000;156(11):1013-1016. [PubMed] [Google Scholar]
- 30.Nicoletti T, Gaudino S, Colacicco G, et al. A man with sarcoidosis and slurred speech. Eur J Neurol. 2020;27(1):e7-e8. doi: 10.1111/ene.14067 [DOI] [PubMed] [Google Scholar]
- 31.Neeb L, Diekmann S, Blechschmidt C, et al. Infratentorial progressive multifocal leukencephalopathy in a patient with pulmonary sarcoidosis. J Neurol. 2009;256(11):1936-1938. doi: 10.1007/s00415-009-5265-5 [DOI] [PubMed] [Google Scholar]
- 32.Matsudaira T, Araki K, Oishi W, et al. Mirtazapine treatment ceased the progression of progressive multifocal leukoencephalopathy associated with systemic sarcoidosis. Neurol Clin Neurosci. 2014;2(5):158-160. doi: 10.1111/ncn3.108 [DOI] [Google Scholar]
- 33.Marriott PJ, O’Brien MD, Mackenzie IC, Janota I. Progressive multifocal leucoencephalopathy: remission with cytarabine. J Neurol Neurosurg Psychiatry. 1975;38(3):205-209. doi: 10.1136/jnnp.38.3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Guilloux J, Roubiou C, Mallet J, et al. Atypical progressive multifocal leukoencephalopathy (PML) in a patient with pulmonary sarcoidosis [in French]. Rev Neurol (Paris). 2010;166(3):341-345. doi: 10.1016/j.neurol.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 35.Hohlfeld SK, Günthard HF, Zeitz J, Locher P, Bachli E. Progressive multi-focal leukoencephalopathy as a rare lethal complication in untreated sarcoidosis. BMJ Case Rep. 2012;2012:bcr0320114036. doi: 10.1136/bcr.03.2011.4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haegele-Link S, Stoeter P, Klimpe S, Dieterich M. Progressive multifocal leukoencephalopathy successfully treated with cidofovir in a patient with pulmonary sarcoidosis. Aktuelle Neurologie. 2006;33(S1):P599. doi: 10.1055/s-2006-953423 [DOI] [Google Scholar]
- 37.Guffroy A, Solis M, Gies V, et al. Progressive multifocal leukoencephalopathy and sarcoidosis under interleukin 7: the price of healing. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e862. doi: 10.1212/NXI.0000000000000862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granot R, Lawrence R, Barnett M, et al. What lies beneath the tent? JC-virus cerebellar granule cell neuronopathy complicating sarcoidosis. J Clin Neurosci. 2009;16(8):1091-1092. doi: 10.1016/j.jocn.2008.07.091 [DOI] [PubMed] [Google Scholar]
- 39.Goldbecker A, Tountopoulou A, Wurster U, et al. Spontaneous recovery from progressive multifocal leukoencephalopathy in a patient with non-active sarcoidosis. Int J Infect Dis. 2010;14(suppl 3):e313-e316. doi: 10.1016/j.ijid.2010.02.2257 [DOI] [PubMed] [Google Scholar]
- 40.Gamperl I, Enzinger C, Pichler A, Feichtinger M, Schlager T, Fertl E. Can pulmonary sarcoidosis trigger a progressive multifocal leukoencephalopathy? considerations from a case series and a review of literature. Clin Case Rep. 2018;6(11):2121-2125. doi: 10.1002/ccr3.1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franzen D, Mathys C, Vesper J, Malzkorn B, Bohlen H, Seitz RJ. Progressive multifokale leukenzephalopathie. Dtsch Med Wochenschr. 2015;140(17):1291-1293. doi: 10.1055/s-0041-103278 [DOI] [PubMed] [Google Scholar]
- 42.Elbadri M, Plant G. Progressive multifocal leukoencephalopathy as the first presentation of sarcoidosis. BMJ Case Rep. 2020;13(8):e232636. doi: 10.1136/bcr-2019-232636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dohrn MF, Ellrichmann G, Pjontek R, et al. Progressive multifocal leukoencephalopathy and immune reconstitution inflammatory syndrome in seven patients with sarcoidosis: a critical discussion of treatment and prognosis. Ther Adv Neurol Disord. 2021;14:17562864211035543. doi: 10.1177/17562864211035543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galdys AL, Kale HA, Lacomis D, Vergidis P, Murdoch GH. Progressive right-sided hemiparesis in a man with sarcoidosis. Clin Infect Dis. 2016;62(9):1141-1142, 1186-1187. doi: 10.1093/cid/ciw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gottschalk A, Danz B, Völk M. Infratentorial progressive multifocal leukoencephalopathy in a patient with pulmonary sarcoidosis [in German]. Rofo. 2005;177(11):1583-1585. doi: 10.1055/s-2005-858653 [DOI] [PubMed] [Google Scholar]
- 46.Kunschner L, Scott TF. Sustained recovery of progressive multifocal leukoencephalopathy after treatment with IL-2. Neurology. 2005;65(9):1510-1510. doi: 10.1212/01.wnl.0000183064.10227.b5 [DOI] [PubMed] [Google Scholar]
- 47.Przepiorka D, Jaeckle KA, Birdwell RR, et al. Successful treatment of progressive multifocal leukoencephalopathy with low-dose interleukin-2. Bone Marrow Transplant. 1997;20(11):983-987. doi: 10.1038/sj.bmt.1701010 [DOI] [PubMed] [Google Scholar]
- 48.Alstadhaug KB, Rinaldo CH, Osnes L, Sereti I, Ofte HK. Progressive multifocal leukoencephalopathy treated with interleukin-7. Clin Infect Pract. 2020;7-8:100049. doi: 10.1016/j.clinpr.2020.100049 [DOI] [Google Scholar]
- 49.Volk T, Warnatz K, Marks R, et al. Pembrolizumab for treatment of progressive multifocal leukoencephalopathy in primary immunodeficiency and/or hematologic malignancy: a case series of five patients. J Neurol. 2022;269(2):973-981. doi: 10.1007/s00415-021-10682-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Möhn N, Wattjes MP, Adams O, et al. PD-1-inhibitor pembrolizumab for treatment of progressive multifocal leukoencephalopathy. Ther Adv Neurol Disord. 2021;14:1756286421993684. doi: 10.1177/1756286421993684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beudel M, Rövekamp F, van de Beek D, Brouwer M. Single-dose pembrolizumab treatment for progressive multifocal leukoencephalopathy. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1021. doi: 10.1212/NXI.0000000000001021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes A, Wellings T, Walsh O, Rowlings P. Progressive multifocal leukoencephalopathy associated with a lymphoproliferative disorder treated with pembrolizumab. J Neurovirol. 2020;26(6):961-963. doi: 10.1007/s13365-020-00899-0 [DOI] [PubMed] [Google Scholar]
- 53.Küpper C, Heinrich J, Kamm K, Bücklein V, Rothenfusser S, Straube A. Pembrolizumab for progressive multifocal leukoencephalopathy due to primary immunodeficiency. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e628. doi: 10.1212/NXI.0000000000000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cortese I, Muranski P, Enose-Akahata Y, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med. 2019;380(17):1597-1605. doi: 10.1056/NEJMoa1815039 [DOI] [PubMed] [Google Scholar]
- 55.Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK virus–specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med. 2018;379(15):1443-1451. doi: 10.1056/NEJMoa1801540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson AS, Yalamarthi N, Yong MK, Blyth E. Beyond antivirals: virus-specific T-cell immunotherapy for BK virus haemorrhagic cystitis and JC virus progressive multifocal leukoencephalopathy. Curr Opin Infect Dis. 2021;34(6):627-634. doi: 10.1097/QCO.0000000000000794 [DOI] [PubMed] [Google Scholar]
- 57.Hopfner F, Möhn N, Eiz-Vesper B, et al. Allogeneic BK virus-specific T-cell treatment in 2 patients with progressive multifocal leukoencephalopathy. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1020. doi: 10.1212/NXI.0000000000001020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1-9. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kempen JH. Appropriate use and reporting of uncontrolled case series in the medical literature. Am J Ophthalmol. 2011;151(1):7-10.e1. doi: 10.1016/j.ajo.2010.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430-1438. doi: 10.1212/WNL.0b013e31828c2fa1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(8):e26-e51. doi: 10.1164/rccm.202002-0251ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4(1):59-68. doi: 10.3109/13550289809113482 [DOI] [PubMed] [Google Scholar]
- 63.Casado JL, Corral I, García J, et al. Continued declining incidence and improved survival of progressive multifocal leukoencephalopathy in HIV/AIDS patients in the current era. Eur J Clin Microbiol Infect Dis. 2014;33(2):179-187. doi: 10.1007/s10096-013-1941-6 [DOI] [PubMed] [Google Scholar]
- 64.Aggarwal D, Tom JP, Chatterjee D, Goyal M. Progressive multifocal leukoencephalopathy in idiopathic CD4+ lymphocytopenia: a case report and review of literature. Neuropathology. 2019;39(6):467-473. doi: 10.1111/neup.12599 [DOI] [PubMed] [Google Scholar]
- 65.Arkema EV, Grunewald J, Kullberg S, Eklund A, Askling J. Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden. Eur Respir J. 2016;48(6):1690-1699. doi: 10.1183/13993003.00477-2016 [DOI] [PubMed] [Google Scholar]
- 66.Hillerdal G, Nöu E, Osterman K, Schmekel B. Sarcoidosis, epidemiology and prognosis: a 15-year European study. Am Rev Respir Dis. 1984;130(1):29-32. [DOI] [PubMed] [Google Scholar]
- 67.Kuchelmeister K, Gullotta F, Bergmann M, Angeli G, Masini T. Progressive multifocal leukoencephalopathy (PML) in AIDS: morphological and topographical characteristics [in German]. Verh Dtsch Ges Pathol. 1991;75:189-190. [PubMed] [Google Scholar]
- 68.Whiteman ML, Post MJ, Berger JR, Tate LG, Bell MD, Limonte LP. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology. 1993;187(1):233-240. doi: 10.1148/radiology.187.1.8451420 [DOI] [PubMed] [Google Scholar]
- 69.Rempe T, Wang Q, Wu Q, et al. Progressive multifocal leukoencephalopathy and granule cell neuronopathy with novel mutation flanking VP1 C-terminus in natalizumab-extended interval dosing. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e709. doi: 10.1212/NXI.0000000000000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hodel J, Darchis C, Outteryck O, et al. Punctate pattern: a promising imaging marker for the diagnosis of natalizumab-associated PML. Neurology. 2016;86(16):1516-1523. doi: 10.1212/WNL.0000000000002586 [DOI] [PubMed] [Google Scholar]
- 71.Wattjes MP, Verhoeff L, Zentjens W, et al. Punctate lesion pattern suggestive of perivascular inflammation in acute natalizumab-associated progressive multifocal leukoencephalopathy: productive JC virus infection or preclinical PML-IRIS manifestation? J Neurol Neurosurg Psychiatry. 2013;84(10):1176-1177. doi: 10.1136/jnnp-2013-304986 [DOI] [PubMed] [Google Scholar]
- 72.Yousry TA, Pelletier D, Cadavid D, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2012;72(5):779-787. doi: 10.1002/ana.23676 [DOI] [PubMed] [Google Scholar]
- 73.Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9(4):438-446. doi: 10.1016/S1474-4422(10)70028-4 [DOI] [PubMed] [Google Scholar]
- 74.Himedan M, Camelo-Piragua S, Mills EA, Gupta A, Aburashed R, Mao-Draayer Y. Pathologic findings of chronic PML-IRIS in a patient with prolonged PML survival following natalizumab treatment. J Investig Med High Impact Case Rep. 2017;5(3):2324709617734248. doi: 10.1177/2324709617734248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009;72(17):1458-1464. doi: 10.1212/01.wnl.0000343510.08643.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arbusow V, Strupp M, Pfister H-W, Seelos KC, Brückmann H, Brandt T. Contrast enhancement in progressive multifocal leukoencephalopathy: a predictive factor for long-term survival? J Neurol. 2000;247(4):306-308. doi: 10.1007/s004150050590 [DOI] [PubMed] [Google Scholar]
- 77.Sahraian MA, Radue EW, Eshaghi A, Besliu S, Minagar A. Progressive multifocal leukoencephalopathy: a review of the neuroimaging features and differential diagnosis. Eur J Neurol. 2012;19(8):1060-1069. doi: 10.1111/j.1468-1331.2011.03597.x [DOI] [PubMed] [Google Scholar]
- 78.Clifford DB, Yiannoutsos C, Glicksman M, et al. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology. 1999;52(3):623-625. doi: 10.1212/WNL.52.3.623 [DOI] [PubMed] [Google Scholar]
- 79.Rauer S, Marks R, Urbach H, et al. Treatment of progressive multifocal leukoencephalopathy with pembrolizumab. N Engl J Med. 2019;380(17):1676-1677. doi: 10.1056/NEJMc1817193 [DOI] [PubMed] [Google Scholar]
- 80.Hoang E, Bartlett NL, Goyal MS, Schmidt RE, Clifford DB. Progressive multifocal leukoencephalopathy treated with nivolumab. J Neurovirol. 2019;25(2):284-287. doi: 10.1007/s13365-019-00738-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinot M, Ahle G, Petrosyan I, et al. Progressive multifocal leukoencephalopathy after treatment with nivolumab. Emerg Infect Dis. 2018;24(8):1594-1596. doi: 10.3201/eid2408.180460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medrano C, Vergez F, Mengelle C, Faguer S, Kamar N, Del Bello A. Effectiveness of immune checkpoint inhibitors in transplant recipients with progressive multifocal leukoencephalopathy. Emerg Infect Dis. 2019;25(11):2145-2147. doi: 10.3201/eid2511.190705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pawlitzki M, Schneider-Hohendorf T, Rolfes L, et al. Ineffective treatment of PML with pembrolizumab: exhausted memory T-cell subsets as a clue? Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e627. doi: 10.1212/NXI.0000000000000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cortese I, Beck ES, Al-Louzi O, et al. BK virus-specific T cells for immunotherapy of progressive multifocal leukoencephalopathy: an open-label, single-cohort pilot study. Lancet Neurol. 2021;20(8):639-652. doi: 10.1016/S1474-4422(21)00174-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fasano MB, Sullivan KE, Sarpong SB, et al. Sarcoidosis and common variable immunodeficiency: report of 8 cases and review of the literature. Medicine (Baltimore). 1996;75(5):251-261. doi: 10.1097/00005792-199609000-00002 [DOI] [PubMed] [Google Scholar]
- 86.Verbsky JW, Routes JM. Sarcoidosis and common variable immunodeficiency: similarities and differences. Semin Respir Crit Care Med. 2014;35(3):330-335. doi: 10.1055/s-0034-1376862 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Characteristics of S-PML Patients in Scientific Literature 1955-2022
Data sharing statement