Abstract
Background
Palbociclib, the first available cyclin-dependent kinase 4/6 inhibitor, plus endocrine therapy is approved for hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) metastatic breast cancer (MBC). This study compared real-world effectiveness of palbociclib plus letrozole versus letrozole in older patients with MBC in US clinical practice.
Methods
This retrospective analysis included patients from the Flatiron Health longitudinal database. Overall, 796 women with HR+/HER2− MBC aged ≥65 years starting palbociclib plus letrozole or letrozole as first-line therapy between February 2015 and September 2018 were included. Patients were evaluated from treatment start until December 2018, death, or last visit, whichever came first. Real-world progression-free survival (rwPFS), overall survival (OS), and real-world best tumor responses (rwBTR) were endpoints. Stabilized inverse probability treatment weighting (sIPTW) balanced patient characteristics.
Results
After sIPTW, 450 patients treated with palbociclib plus letrozole and 335 treated with letrozole were included; median age was 74.0 years. Median rwPFS was 22.2 (95% CI, 20.0–30.4) months for palbociclib plus letrozole versus 15.8 (12.9–18.9) months for letrozole (hazard ratio, 0.59 [0.47–0.74]; P<0.001). Median OS was not reached for palbociclib plus letrozole versus 43.4 months (30.0–not estimable) with letrozole (hazard ratio, 0.55 [0.42–0.72]; P<0.001). No interactions between age groups (65–74 and ≥75 years) and treatment groups were observed for rwPFS or OS. Rate of rwBTR was significantly higher for palbociclib plus letrozole (52.4%) versus letrozole (22.1%; odds ratio, 2.0 [1.4–2.7]; P<0.001).
Conclusion
This analysis demonstrates the effectiveness of palbociclib combination therapy as standard-of-care for older patients with HR+/HER2− MBC in the first-line setting.
Keywords: Real-world, Palbociclib, Letrozole, Metastatic breast cancer
Highlights
-
•
796 elderly patients with HR+/HER2− MBC from the Flatiron database were included.
-
•
Median PFS was longer with palbociclib + letrozole vs letrozole (HR, 0.59; P<0.001).
-
•
Median OS was not reached with palbociclib + letrozole vs 43 months with letrozole.
-
•
Palbociclib combination therapy was effective for older patients with MBC.
Abbreviations
- AI
aromatase inhibitor
- CDK4/6
cyclin-dependent kinase 4/6
- EHR
electronic health record
- HER2–
human epidermal growth factor receptor 2–negative
- HR+
hormone receptor–positive
- MBC
metastatic breast cancer
- NE
not estimable
- OS
overall survival
- PFS
progression-free survival
- rwBTR
real-world best tumor response
- rwPFS
real-world progression-free survival
- sIPTW
stabilized inverse probability treatment weighting
1. Background
Estimates project that there will be 287,850 new cases of female breast cancer and 43,250 associated deaths in the United States in 2022; in recent years, approximately 45% of these cases and 62% of deaths due to breast cancer occurred in women aged ≥65 years [1]. The most common biologic breast cancer subtype is hormone receptor–positive (HR+)/human epidermal growth factor receptor 2–negative (HER2–) [1]. For patients with HR+/HER2– metastatic breast cancer (MBC), treatment recommendation includes sequential endocrine therapy in combination with targeted agents, such as cyclin-dependent kinase 4/6 (CDK4/6) inhibitors, followed by sequential single-agent chemotherapy [2].
Palbociclib, the first oral CDK4/6 inhibitor approved, is indicated for HR+/HER2– MBC combined with an aromatase inhibitor (AI) or fulvestrant [3]. Palbociclib demonstrated improved median progression-free survival (PFS) in 3 pivotal clinical trials: PALOMA-1 (palbociclib plus letrozole versus letrozole alone), the confirmatory randomized controlled trial PALOMA-2 (palbociclib plus letrozole versus placebo plus letrozole), both as initial endocrine-based therapy, and PALOMA-3 (palbociclib plus fulvestrant versus placebo plus fulvestrant) after progression following endocrine therapy [4]. In these trials efficacy trends were relatively consistent across older and younger women [5,6]. In addition, a pooled analysis of the PALOMA trials found palbociclib plus letrozole prolonged PFS versus letrozole with or without placebo in patients aged <65 years, 65–74 years, and ≥75 years (hazard ratios, 0.50, 0.66, and 0.31, respectively) [7].
Since the approval of palbociclib in February 2015 [3], CDK4/6 inhibitors have become a recommended treatment option for women of all ages with HR+/HER2– MBC [2], and the rapid adoption of this class of agents has facilitated collection of real-world data documenting patient experiences. Recent real-world data analyses have shown that palbociclib is effective in patients with HR+/HER2– MBC in routine US clinical practice, but detailed information about the use of CDK4/6 inhibitors specifically in older patients is limited [[8], [9], [10], [11], [12]]. The available real-world studies including older European patients (≥70 or ≥75 years) support the effectiveness or tolerability of palbociclib in these age groups [13,14]. A better understanding of treatment benefits and risks in older women in the real-world setting in the United States is needed.
In the current study, data from the Flatiron Health Analytic Database were used to evaluate patient characteristics and effectiveness of palbociclib plus letrozole versus letrozole alone as first-line endocrine-based therapy in patients aged ≥65 years with HR+/HER2– MBC. The primary objectives of this study were to evaluate the real-world PFS (rwPFS), overall survival (OS), and real-world best tumor response (rwBTR) in this patient population in routine clinical practice in the United States.
2. Materials and methods
2.1. Study design and database
This retrospective cohort study was designed to use data derived from the Flatiron database to describe patient characteristics and effectiveness of palbociclib plus letrozole as initial endocrine-based therapy in HR+/HER2– MBC. The Flatiron database is a longitudinal database that is demographically and geographically diverse and subject to rigorous data curation and abstraction [15,16]. It includes de-identified structured and unstructured data from EHRs from >280 cancer clinics, representing >3 million records of US patients with cancer [16]. This database has been widely used for real-world studies in cancer, including breast cancer, and has validated endpoints including overall survival [11,[17], [18], [19], [20]]. The patient population in this study (women aged ≥65 years) is a subgroup of the previously reported Flatiron database analyses in women aged ≥18 years [11]; data presented here are a comparative analysis of palbociclib plus letrozole vs letrozole in patients aged 65 or above. In this comparative analysis, a stabilized inverse probability treatment weighting (sIPTW) method was used to adjust for the potential confounding effects of patient baseline demographics and clinical characteristics.
The study period ran from February 3, 2015, through the end of December 2018 (or data cut-off date). The initiation date of first-line palbociclib plus letrozole or letrozole alone between February 3, 2015, and September 30, 2018, was defined as the index date. Patients were followed up retrospectively from index date to December 30, 2018 (or study cut-off date), death, or last visit, whichever came first. This study is exempt from institutional review board approval because it is retrospective, non-interventional, and uses anonymized data provided by Flatiron Health.
2.2. Patients
Women aged ≥65 years diagnosed with HR+/HER2– MBC and initiating palbociclib plus letrozole or letrozole alone in the first-line setting between February 2015 and September 2018 were included. Patients were excluded if they had previously been treated with another CDK4/6 inhibitor, an AI, tamoxifen, raloxifene, toremifene, or fulvestrant for MBC. Patients were also excluded if they had been treated with a CDK4/6 inhibitor as part of a clinical trial. Those whose first structured activity was >90 days after their MBC diagnosis date were also excluded. Eligible MBC patients were assigned to 1 of 2 cohorts: patients who were treated with palbociclib plus letrozole as the initial endocrine-based therapy and patients who were treated with letrozole alone.
2.3. Outcomes
Real-world PFS was defined as the time from the start of treatment to death or disease progression [11,12]. Disease progression was determined by the treating clinician based on radiology, pathology, laboratory evidence, or clinical assessment, whichever came first. Patients who did not die and did not experience disease progression were censored either at the date of initiation of the next line of therapy for patients with ≥2 lines of therapy or at the date of their last visit during the study period for patients with only 1 line of therapy.
Overall survival was defined as the time from the start of treatment to the date of death due to any cause [11]. The date of death was found by using multiple mortality data sources, which were benchmarked against against the National Death Index [18]. Patients who did not die during that period were censored at data cutoff.
Patients with rwBTR occurring ≥30 days after the initiation of treatment were assessed based on the treating clinician's assessment of radiologic evidence for change in burden of disease over the course of treatment [12]. Real-world BTR included obtaining a complete response, defined as the complete resolution of all visible disease, or a partial response, defined as a partial reduction in the size of visible disease in some or all areas, without any areas of increase in visible disease. Stable disease was reported as no change in the overall size of visible disease; it also included cases in which some lesions increased in size and some lesions decreased in size. Finally, progressive disease was defined as an increase in visible disease and/or the presence of any new lesions; included cases in which the clinician indicated progressive disease. If the treating clinicians stated that they could not make an assessment of outcome, response was classified as indeterminate [12].
2.4. Statistical analyses
For categorical variables (eg, region, race, and stage at initial diagnosis), data collection included the frequency (number) of cases and percentage of total patients observed in each category. Continuous variables (eg, age and time from initial breast cancer diagnosis to metastatic diagnosis) are presented as mean, standard deviation (SD), median, 25th and 75th percentiles, and range (minimum and maximum). Kaplan-Meier curves were performed to estimate rwPFS and OS. Comparative analyses were conducted with both an unadjusted method (without controlling for confounders) and sIPTW to balance patient baseline demographics and clinical characteristics. To compare the risk of rwPFS and OS between the study cohorts, Cox proportional hazards models with a robust sandwich estimator were used. Logistic regression was used to estimate the odds of real-world tumor responses in the group receiving palbociclib plus letrozole compared with the group receiving letrozole alone.
3. Results
3.1. Patients
A total of 796 women aged ≥65 years with HR+/HER2– MBC were included. In the unadjusted cohort, patient demographic and clinical characteristics differed between the groups receiving palbociclib plus letrozole and letrozole alone (Table 1). The majority of patients (82%) received 125 mg of palbociclib as the initial dose. After sIPTW, patient demographic and clinical characteristics were generally well balanced. The median age was 74.0 years in each treatment group, and approximately 71% of patients were White. Patients were typically (96%) from a community practice setting, approximately 40% had stage IV disease at initial diagnosis, and nearly half of patients had only 1 metastatic site (Table 1). The median duration of follow-up was 20.2 months in the group receiving palbociclib plus letrozole and 18.6 months in the group receiving letrozole alone.
Table 1.
Patient characteristics.
| Characteristic |
Unadjusted |
sIPTW |
||||
|---|---|---|---|---|---|---|
|
Letrozole (n = 406) |
Palbociclib + Letrozole (n = 390) |
Standardized Difference |
Letrozole (n = 335) |
Palbociclib + Letrozole (n = 450) |
Standardized Difference |
|
| Age, y | ||||||
| Mean (SD) | 75.9 (6.2) | 73.2 (5.9) | 0.444 | 74.9 (5.7) | 74.4 (6.5) | 0.077 |
| Median (IQR) | 77.0 (12.0) | 72.0 (10.0) | 74.0 (12.0) | 74.0 (10.0) | ||
| Age group, y | ||||||
| 65–74 | 175 (43.1) | 245 (62.8) | −0.403 | 175 (52.3) | 231 (51.4) | 0.018 |
| ≥75 | 231 (56.9) | 145 (37.2) | 160 (47.7) | 219 (48.6) | ||
| Race | ||||||
| White | 287 (70.7) | 269 (69.0) | 0.037 | 237 (70.8) | 318 (70.7) | 0.002 |
| Black | 32 (7.9) | 24 (6.2) | 0.068 | 23 (6.8) | 27 (6.0) | 0.034 |
| Asian | 6 (1.5) | 8 (2.1) | −0.044 | 5 (1.4) | 7 (1.5) | −0.013 |
| Hispanic or Latino | 11 (2.7) | 9 (2.3) | 0.026 | 8 (2.5) | 11 (2.4) | 0.005 |
| Other/unknown | 70 (17.2) | 80 (20.5) | −0.084 | 62 (18.6) | 87 (19.4) | −0.021 |
| Practice type | ||||||
| Community | 389 (95.8) | 375 (96.2) | −0.017 | 322 (96.4) | 435 (96.5) | −0.010 |
| Academic | 17 (4.2) | 15 (3.8) | 12 (3.6) | 16 (3.5) | ||
| Disease stage at initial diagnosis | ||||||
| I or II | 145 (35.7) | 145 (37.2) | −0.030 | 117 (35.1) | 163 (36.1) | −0.022 |
| III | 46 (11.3) | 44 (11.3) | 0.002 | 39 (11.6) | 52 (11.6) | −0.000 |
| IV | 166 (40.9) | 159 (40.8) | 0.002 | 139 (41.5) | 180 (40.0) | 0.031 |
| Not documented | 49 (12.1) | 42 (10.8) | 0.041 | 40 (11.8) | 55 (12.3) | −0.014 |
| ECOG performance status | ||||||
| 0 | 93 (22.9) | 147 (37.7) | −0.326 | 100 (30.0) | 137 (30.4) | −0.008 |
| 1 | 88 (21.7) | 94 (24.1) | −0.058 | 78 (23.2) | 106 (23.6) | −0.009 |
| 2 | 41 (10.1) | 35 (9.0) | 0.038 | 32 (9.4) | 44 (9.7) | −0.010 |
| 3 or 4 | 18 (4.4) | 3 (0.8) | 0.232 | 9 (2.7) | 8 (1.9) | 0.053 |
| Not documented | 166 (40.9) | 111 (28.5) | 0.263 | 116 (34.7) | 155 (34.4) | 0.006 |
| Visceral diseasea | 137 (33.7) | 170 (43.6) | −0.203 | 129 (38.7) | 172 (38.3) | 0.008 |
| No visceral diseasea | 269 (66.3) | 220 (56.4) | 205 (61.3) | 278 (61.7) | ||
| Bone-only diseaseb | 162 (39.9) | 134 (34.4) | 0.115 | 124 (37.0) | 163 (36.2) | 0.017 |
| Brain metastases | 14 (3.4) | 8 (2.1) | 0.086 | 9 (2.8) | 10 (2.3) | 0.029 |
| Number of metastatic sitesc | ||||||
| 1 | 215 (53.0) | 182 (46.7) | 0.126 | 165 (49.4) | 214 (47.6) | 0.036 |
| 2 | 90 (22.2) | 118 (30.3) | −0.185 | 90 (26.8) | 119 (26.4) | 0.009 |
| 3 | 43 (10.6) | 58 (14.9) | −0.129 | 42 (12.7) | 57 (12.7) | 0.000 |
| 4+ | 22 (5.4) | 26 (6.7) | −0.052 | 19 (5.8) | 26 (5.9) | −0.003 |
ECOG, Eastern Cooperative Oncology Group; IQR = interquartile range; sIPTW = stabilized inverse probability treatment weighting; SD, standard deviation; y, years.
All data are n (%) unless otherwise noted.
Visceral disease was defined as metastatic disease in the lung and/or liver; patients could have had other sites of metastases. No visceral disease was defined as no lung or liver metastases.
Bone-only disease was defined as metastatic disease in the bone only.
Multiple metastases at the same site were counted as 1 site (eg, if a patient had 3 bone metastases in the spine, it was considered only 1 site).
3.2. Outcomes
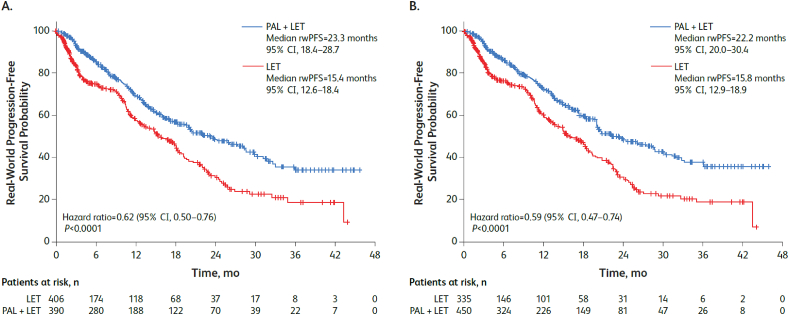
In the unadjusted analysis, median rwPFS was significantly prolonged among patients aged ≥65 years who received palbociclib plus letrozole compared with letrozole alone (23.3 [95% CI, 18.4–28.7] vs 15.4 [95% CI, 12.6–18.4] months; hazard ratio, 0.62 [95% CI, 0.50–0.76]; P<0.001). After sIPTW adjustment, median rwPFS was significantly prolonged in the palbociclib plus letrozole group versus letrozole alone group (22.2 [20.0–30.4] vs 15.8 [12.9–18.9] months; hazard ratio, 0.59 [0.47–0.74]; P<0.001; Fig. 1).
Fig. 1.
Kaplan-Meier curves of real-world progression-free survival in the (A) unadjusted and (B) sIPTW-adjusted analyses. LET = letrozole; PAL = palbociclib; PFS = progression-free survival; sIPTW = stabilized inverse probability treatment weighting.
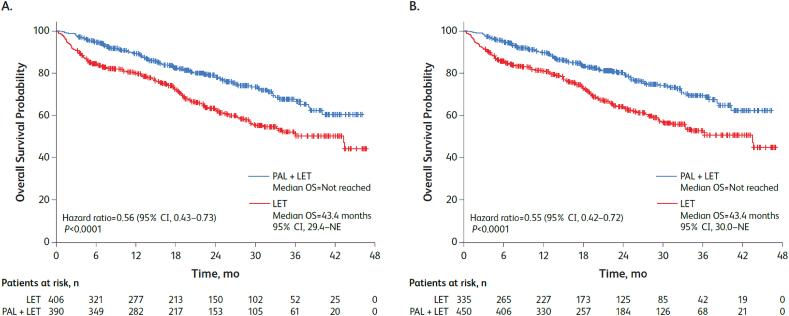
Median OS was significantly longer among patients aged ≥65 years in the palbociclib plus letrozole group versus letrozole alone group in the unadjusted analysis (not reached vs 43.4 [95% CI, 29.4–not estimable (NE)] months; hazard ratio, 0.56 [95% CI, 0.43–0.73], P<0.001) and in the sIPTW-adjusted analysis, median OS was not reached versus 43.4 (30.0–NE) months (hazard ratio, 0.55 [0.42–0.72]; P<0.001; Fig. 2).
Fig. 2.
Kaplan-Meier curves of overall survival in the (A) unadjusted and (B) sIPTW-adjusted analyses. LET = letrozole; NE = not estimable; OS = overall survival; PAL = palbociclib; sIPTW = stabilized inverse probability treatment weighting.
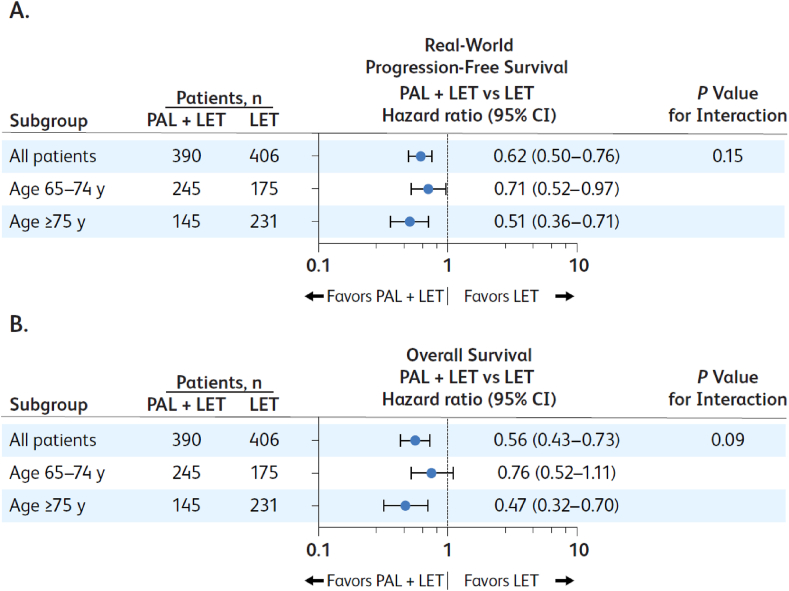
Similar results were observed when patients were stratified by age group. Median rwPFS and median OS were longer in patients included in the 65- to 74-year-old and the ≥75-year-old age groups who received palbociclib plus letrozole versus letrozole alone (Fig. 3). No significant interactions between age groups and treatment groups were observed for rwPFS or OS (Fig. 3).
Fig. 3.
Forest plots of unadjusted (A) real-world progression-free survival and (B) overall survival by age group; LET = letrozole; PAL = palbociclib; y = years.
The rwBTR rate (complete response plus partial response) in the unadjusted analysis was significantly higher among patients aged ≥65 years who received palbociclib plus letrozole versus letrozole alone (52.1% vs 21.4%; odds ratio, 2.2 [95% CI, 1.6–3.1]; P<0.001; Table 2). After sIPTW adjustment, the rwBTR rate was significantly higher: 52.4% versus 22.1% in the palbociclib plus letrozole group versus letrozole alone group (odds ratio, 2.0 [95% CI, 1.4–2.7]; P<0.001).
Table 2.
Real-world best tumor responses.
| Response |
Unadjusted |
sIPTW |
||||
|---|---|---|---|---|---|---|
|
Letrozole (n = 406) |
Palbociclib + Letrozole (n = 390) |
P Value |
Letrozole (n = 335) |
Palbociclib + Letrozole (n = 450) |
P Value | |
| CR | 17 (4.2) | 32 (8.2) | <0.001 | 13 (4.0) | 45 (10.1) | <0.001 |
| PR | 70 (17.2) | 171 (43.8) | 60 (18.0) | 190 (42.3) | ||
| Stable disease | 53 (13.1) | 86 (22.1) | 44 (13.0) | 98 (21.8) | ||
| Progressive disease | 68 (16.8) | 47 (12.1) | 52 (15.6) | 57 (12.7) | ||
| Indeterminate | 13 (3.2) | 8 (2.1) | 10 (3.0) | 7 (1.6) | ||
| Missing | 185 (45.6) | 46 (11.8) | 155 (46.4) | 52 (11.5) | ||
| Best overall response (CR + PR) | 87 (21.4) | 203 (52.1) | <0.001 | 74 (22.1) | 236 (52.4) | <0.001 |
CR = complete response; PR = partial response; sIPTW = stabilized inverse probability treatment weighting.
All data are n (%) unless otherwise noted.
4. Discussion
In our study in older patients with a median age of 74 years, first-line palbociclib plus letrozole significantly prolonged median rwPFS (22.2 [95% CI, 20.0–30.4] vs 15.8 [95% CI, 12.9–18.9] months; hazard ratio, 0.59 [95% CI, 0.47–0.74]; P<0.001) and OS (not reached vs 43.4 [95% CI, 29.4–NE] months; hazard ratio, 0.56 [95% CI, 0.43–0.73], P<0.001) compared with letrozole alone. In addition, rwBTR was significantly higher in the palbociclib plus letrozole group versus the letrozole alone group (52.4% vs 22.1%, odds ratio, 2.0 [95% CI, 1.4–2.7], P<0.001).
Cyclin-dependent kinase 4/6 inhibitors have significantly changed clinical practice for the treatment of HR+/HER2– MBC, as supported by results of the PALOMA studies [4,5,[21], [22], [23]]. Evaluation of real-world treatment patterns and effectiveness analysis of first-line treatment for HR+/HER2– MBC have consistently demonstrated that elderly patients are less likely to be treated with CDK4/6 inhibitor combination treatment than younger patients with the same clinical characteristics [11,12]. To gain a better understanding of the effectiveness of palbociclib in the older population, the Flatiron database was used to help evaluate clinical outcomes of treatment in US patients aged ≥65 years with HR+/HER2– MBC. In the analysis of 796 patients aged ≥65 years in the Flatiron database, rwPFS and OS were significantly longer in those receiving palbociclib plus letrozole versus those receiving letrozole alone in both unadjusted and sIPTW-adjusted analyses, including in the subgroup of patients ≥75 years of age. Similarly, the rwBTR was significantly higher in patients receiving palbociclib plus letrozole.
These findings confirm the significant improvement in PFS associated with palbociclib plus letrozole treatment versus letrozole alone as observed in the PALOMA-2 trial [6]. In the PALOMA-2 trial, palbociclib plus letrozole vs letrozole alone numerically prolonged OS (53.9 vs 51.2 months) but it was not statistically significant (P > 0.05) [24]. In the subanalysis of PALOMA-2 on older patients aged 65 or above, the median OS was 58.6 vs 47.4 months in palbociclib plus letrozole and letrozole alone patients (HR = 0.87, 95% CI = 0.62–1.22) [24]. In terms of safety, several clinical studies have reported the manageable toxicity of CDK4/6 inhibitors in the geriatric population [[13], [14], [25]]. Palbociclib specifically was well-tolerated with low levels of clinically significant toxicities in elderly patients [13,14]. Thus, data from both prospective trials and the real-world findings presented here collectively support use of first-line CDK4/6 inhibitors in combination with endocrine therapy as treatment for HR+/HER2– MBC in the older population.
In this study after sIPTW, median rwPFS was 22.2 months versus 15.8 months in the palbociclib plus letrozole versus letrozole alone group. This median rwPFS was slightly shorter than that reported for patients 65–74 years of age in the PALOMA clinical trials (27.5 [95% CI, 24.2–NE] vs 21.8 [95% CI, 16.3–31.3]) [7] but similar to that reported in the real-world analysis in the US adult population aged ≥18 years and treated primarily in community settings (sIPTW-adjusted analysis: 20.0 [95% CI, 17.5–21.9] vs 11.9 [95% CI, 10.5–13.7] months) [11]. Real-world evaluation of older patients treated in an academic setting revealed a substantially shorter PFS (∼12 months) [26], possibly reflecting that patients treated in an academic versus community may have a heavier burden of disease or other complicating comorbidity.
We also found that median OS after sIPTW analysis was not reached versus 43.4 months among patients who received palbociclib plus letrozole versus letrozole alone, respectively, and that associated median OS was significantly longer in the palbociclib plus letrozole group in both the 65- to 74-year-old and the ≥75-year-old age groups. These results are consistent with those reported in the real-world analysis from the Flatiron database in the US adult population aged ≥18 years (sIPTW-adjusted analysis: not reached vs 43.1 months [95% CI, 34.3–NE]) [11].
Finally, in this study rwBTR was significantly higher in the group that received palbociclib plus letrozole versus the group that received letrozole alone in both the unadjusted and sIPTW adjusted analyses (sIPTW, 52.4% vs 22.1%). The rwBTR is slightly lower than that reported in a real-world analysis in the US adult population aged ≥18 years (sIPTW, 59.3% vs 41.5%) [12] but higher than that reported in a UK analysis in patients aged ≥75 years (complete response of 2.0% and partial response of 32.9%, providing a rwBTR of 34.9%), possibly because in the UK study a response duration of ≥24 weeks was required [14].
4.1. Limitations
Electronic health records have the potential for missing or incomplete data, and the quality of information extracted from the EHR depends on the quality of data entered by the clinician. In the current study, tumor response assessments in routine practice were not scheduled, and tumor responses were limited by the clinician's interpretation and documentation of tumor response based on radiologic evidence for change in disease burden. Tumor response assessment was not based on Response Evaluation Criteria in Solid Tumors (RECIST). In addition, other variables unavailable in the database could not be statistically controlled. As with other database analyses, findings from patients in the Flatiron database may not be reflective of the general population nationally in terms of ethnic minorities, frailty, etc. Moreover, there is limited information on geriatric characteristics of physical performance of older adults, which can strongly influence treatment decisions in this population. Eastern Cooperative Oncology Group performance status information was available for the cohorts, but it is not optimal for determining performance in older adults [27]. Finally, this is a retrospective analysis of data from an EHR database, and causal relationships to treatment cannot be determined.
5. Conclusions
In older patients with a median age of 74 years, first-line palbociclib plus letrozole significantly prolonged median rwPFS (hazard ratio, 0.59 [95% CI, 0.47–0.74]; P<0.001) and OS (hazard ratio, 0.55 [95% CI, 0.42–0.72]; P<0.001) compared with letrozole alone. The rwBTR rate was also significantly higher among patients who received palbociclib plus letrozole versus letrozole alone (odds ratio, 2.0 [95% CI, 1.4–2.7]; P<0.001). In summary, this real-world comparative analysis of palbociclib plus letrozole versus letrozole alone provides evidence of effectiveness for palbociclib combination therapy in routine US clinical practice, supporting this treatment as a standard of care for older patients with HR+/HER2– MBC in the first-line setting.
Authors' contributions
All authors contributed to the conception and design, data analysis and interpretation, manuscript writing, and approved the final version of the article.
Declarations
Ethics approval and consent to participate: This study is exempt from institutional review board and ethics approval because it is retrospective, non-interventional, and uses anonymized data provided by the Flatiron Health longitudinal database.
Consent for publication
Not applicable.
Availability of data and materials
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Funding
This study was sponsored by Pfizer Inc.
Declaration of competing interest
Hope S. Rugo reports sponsored research to her institution from Pfizer Inc, Merck, Novartis, Eli Lilly, Roche, Daiichi-Sankyo, Seattle Genetics, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, AstraZeneca, Ayala, and Gilead and honoraria from PUMA, Samsung, and Mylan. Rachel M. Layman has received research funding to her institution from Pfizer Inc, Eli Lilly, Novartis, GlaxoSmithKline, Puma, Zentalis, and Celcuity; and advisory board/consulting fees from Eli Lilly, Novartis, and Celcuity. Adam Brufsky has received consulting fees from Pfizer Inc, Lilly, Novartis, Eisai, AstraZeneca, Roche, Daiichi, and Merck. Xianchen Liu, Benjamin Li, and Lynn McRoy are employees of and own stock in Pfizer Inc.
Acknowledgements
Editorial/medical writing support was provided by Jill Shults, PhD, ICON plc (Blue Bell, PA) and Oxford PharmaGenesis Inc. (Newtown, PA), and was funded by Pfizer Inc.
References
- 1.National Cancer Institute. Cancer stat facts: female breast cancer subtypes. Surveillance, Epidemiology, and End Results Program.
- 2.National Comprehensive Cancer Network . 2020. NCCN clinical practice guidelines in Oncology (NCCN Guidelines®) breast cancer version 5.https://seer.cancer.gov/statfacts/html/breast-subtypes.html 2020. Accessed 2023. [Google Scholar]
- 3.IBRANCE® capsules (palbociclib) Pfizer Inc; New York, NY: 2019. Full prescribing information. [Google Scholar]
- 4.Serra F., Lapidari P., Quaquarini E., Tagliaferri B., Sottotetti F., Palumbo R. Palbociclib in metastatic breast cancer: current evidence and real-life data. Drugs Context. 2019;8 doi: 10.7573/dic.212579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner N.C., Ro J., Andre F., Loi S., Verma S., Iwata H., et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 6.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.A., Gelmon K., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 7.Rugo H.S., Turner N.C., Finn R.S., Joy A.A., Verma S., Harbeck N., et al. Palbociclib plus endocrine therapy in older women with HR+/HER2− advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur J Cancer. 2018;101:123–133. doi: 10.1016/j.ejca.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Harbeck N., Bartlett M., Spurden D., Hooper B., Zhan L., Rosta E., et al. CDK4/6 inhibitors in HR+/HER2− advanced/metastatic breast cancer: a systematic literature review of real-world evidence studies. Future Oncol. 2021;17:2107–2122. doi: 10.2217/fon-2020-1264. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal C., Goyal P., Agarwal A., Tripathi R., Dodagoudar C., Baghmar S., et al. Multicentric real world evidence with palbociclib in hormone positive HER2 negative metastatic breast cancer in Indian population. Sci Rep. 2021;11 doi: 10.1038/s41598-021-95758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Cuerva C., del Rio Valencia J.C., Bermejo R.T. Effectiveness and safety of palbociclib plus endocrine therapy in hormone receptor-positive, HER2-negative metastatic breast cancer: real-world results. Can J Hosp Pharm. 2022;75:26–33. doi: 10.4212/cjhp.v75i1.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMichele A., Cristofanilli M., Brufsky A., Liu X., Mardekian J., McRoy L., et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2− metastatic breast cancer in US real-world clinical practice. Breast Cancer Res. 2021;23:37. doi: 10.1186/s13058-021-01409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brufsky A., Liu X., Li B., McRoy L., Layman R.M. Real-world tumor response of palbociclib plus letrozole versus letrozole for metastatic breast cancer in US clinical practice. Targeted Oncol. 2021;16:601–611. doi: 10.1007/s11523-021-00826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caillet P., Pulido M., Brain E., Falandry C., Desmoulins I., Ghebriou D., et al. PALOMAGE, a French real-world cohort of elderly women beyond age 70 with advanced breast cancer receiving palbociclib: baseline characteristics and safety evaluation. J Clin Oncol. 2021;39:1012. [Google Scholar]
- 14.El Badri S., Tahir B., Balachandran K., Bezecny P., Britton F., Davies M., et al. Palbociclib in combination with aromatase inhibitors in patients ≥75 years with oestrogen receptor-positive, human epidermal growth factor receptor 2 negative advanced breast cancer: a real-world multicentre UK study. Breast. 2021;60:199–205. doi: 10.1016/j.breast.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flatiron Health. Real-world Evidence. https://flatiron.com/real-world-evidence/. Accessed 2023.
- 16.Ma X., Long L., Moon S., Adamson B.J.S., Baxi S.S. Comparison of population characteristics in real-world cinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. 2020:2020. 03.16.20037143. [Google Scholar]
- 17.Liu R., Rizzo S., Whipple S., Pal N., Pineda A.L., Lu M., et al. Evaluating eligibility criteria of oncology trials using real-world data and AI. Nature. 2021;592:629–633. doi: 10.1038/s41586-021-03430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis M.D., Griffith S.D., Tucker M., Taylor M.D., Capra W.B., Carrigan G., et al. Development and validation of a high-quality composite real-world mortality endpoint. Health Serv Res. 2018;53:4460–4476. doi: 10.1111/1475-6773.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singal G., Miller P.G., Agarwala V., Li G., Kaushik G., Backenroth D., et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database. JAMA. 2019;321:1391–1399. doi: 10.1001/jama.2019.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q., Gossai A., Monroe S., Nussbaum N.C., Parrinello C.M. Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health Serv Res. 2021;56:1281–1287. doi: 10.1111/1475-6773.13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finn R.S., Crown J.P., Ettl J., Schmidt M., Bondarenko I.M., Lang I., et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res. 2016;18:67. doi: 10.1186/s13058-016-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finn R.S., Crown J.P., Lang I., Boer K., Bondarenko I.M., Kulyk S.O., et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 23.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.A., Masuda N., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 24.Finn R.S., Rugo H.S., Dieras V.C., Harbeck N., Im S.-A., Gelmon K.A., et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): analyses from PALOMA-2. J Clin Oncol. 2022;40 LBA1003-LBA. [Google Scholar]
- 25.Howie L.J., Singh H., Bloomquist E., Wedam S., Amiri-Kordestani L., Tang S., et al. Outcomes of older women with hormone receptor-positive, human epidermal growth factor receptor-negative metastatic breast cancer treated with a CDK4/6 inhibitor and an aromatase inhibitor: an FDA pooled analysis. J Clin Oncol. 2019;37:3475–3483. doi: 10.1200/JCO.18.02217. [DOI] [PubMed] [Google Scholar]
- 26.Clifton K., Min Y., Kimmel J., Litton J., Tripathy D., Karuturi M. Progression-free survival (PFS) and toxicities of palbociclib in a geriatric population. Breast Cancer Res Treat. 2019;175:667–674. doi: 10.1007/s10549-019-05181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simcock R., Wright J. Beyond performance status. Clin Oncol. 2020;32:553–561. doi: 10.1016/j.clon.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.