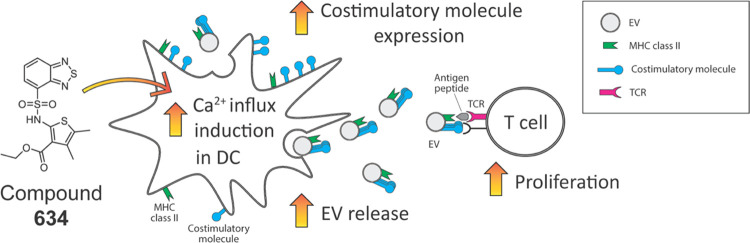
Abstract
Extracellular vesicles (EVs) transfer antigens and immunomodulatory molecules in immunologic synapses as a part of intracellular communication, and EVs equipped with immunostimulatory functions have been utilized for vaccine formulation. Hence, we sought small-molecule compounds that increase immunostimulatory EVs released by antigen-presenting dendritic cells (DCs) for enhancement of vaccine immunogenicity. We previously performed high-throughput screening on a 28K compound library using three THP-1 reporter cell lines with CD63 Turbo-Luciferase, NF-κB, and interferon-sensitive response element (ISRE) reporter constructs, respectively. Because intracellular Ca2+ elevation enhances EV release, we screened 80 hit compounds and identified compound 634 as a Ca2+ influx inducer. 634 enhanced EV release in murine bone marrow-derived dendritic cells (mBMDCs) and increased costimulatory molecule expression on the surface of EVs and the parent cells. EVs isolated from 634-treated mBMDCs induced T cell proliferation in the presence of antigenic peptides. To assess the roles of intracellular Ca2+ elevation in immunostimulatory EV release, we performed structure–activity relationship (SAR) studies of 634. The analogues that retained the ability to induce Ca2+ influx induced more EVs with immunostimulatory properties from mBMDCs than did those that lacked the ability to induce Ca2+ influx. The levels of Ca2+ induction of synthesized analogues correlated with the numbers of EVs released and costimulatory molecule expression on the parent cells. Collectively, our study presents that a small molecule, 634, enhances the release of EVs with immunostimulatory potency via induction of Ca2+ influx. This agent is a novel tool for EV-based immune studies and vaccine development.
Introduction
Extracellular vesicles (EVs) act as carriers of cell-type-specific molecules, including those involved in innate immune responses, such as cytokines, chemokines, adhesion molecules, lipids, nucleic acids, coding and non-coding RNAs (including microRNAs), and DNA fragments.1−5 EV cargo can convey specific intercellular communications and mediate immune responses to microbial pathogens and tumors.6−8 Thus, EVs are a potential tool for vaccine adjuvant strategies.9,10
EVs derived from dendritic cells (DCs) may present on their surface the major histocompatibility (MHC) class I and II molecules and B7 costimulatory molecules, such as B7.1 (CD80) and B7.2 (CD86), which directly engage and activate CD4+ or CD8+ T cells.11−14 EVs also act as antigen-transferring/delivering tools. EVs from tumor cells can contribute to immunotherapy via delivering the tumor antigens to antigen-presenting cells.15 Circulating EVs from individuals who received mRNA-based vaccination for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were loaded with SARS-CoV-2 spike protein and induced spike protein-specific T cell responses and antibodies.16 EVs released from antigen-pulsed DCs or engineered EVs equipped with antigens can serve as artificial antigen-presenting particles.10,17,18 Thus, EVs are recognized as a next-generation vaccine platform because they function as cargo that transfers antigens and adjuvants and could be a promising strategy for enhancing vaccine efficacy.9,10,19
Intracellular Ca2+ influx is associated with both EV secretion and immune responses.20−27 Calcium signaling plays multiple roles in the activation, migration, and maturation of DCs that contribute to T cell priming and activation.20,21 Intracellular Ca2+ increment leads to plasma membrane EV biogenesis.22−25 Recent reports indicate that calcium ionophore ionomycin (ION) and A23187 enhance EV release22,25,26 and induce maturation and activation of antigen-presenting cells (APCs).27 However, these compounds are often toxic for in vivo utilization.28 Hence, we hypothesized that small-molecule compounds that can increase intracellular Ca2+ levels without cytotoxicity could enhance immune modulatory EV release from APCs.
To identify small molecules that increase EV release from APCs, we performed three independent high-throughput screenings (HTSs) on a 28K compound library with extensive chemical space diversity purchased from Maybridge (Leeds, United Kingdom).29 This library consists of two subset libraries that are representative of the diversity of the different compound collections, including the entire Maybridge Screening collection of more than 53,000 compounds and representative of the diverse collection of 550,000 compounds. We utilized a human monocytic leukemia THP-1 reporter cell line engineered with a fusion construct of EV-associated tetraspanin (CD63)-linked Turbo-luciferase (Tluc) (CD63 Tluc-CD9 EmGFP THP-1 reporter cells)30 and two additional THP-1 reporter cell lines for NF-κB and interferon-stimulated response element (ISRE) activation. Eighty hit compounds were identified after validation by in vitro functional screenings using murine bone marrow-derived dendritic cells (mBMDCs), in vivo immunization studies, and assessment from a medicinal chemistry perspective.29
Several reports indicate that manipulation of intracellular Ca2+ levels increases the number of EVs released.22−25 Thus, we screened the 80 hit compounds for the ability to induce Ca2+ influx and identified another hit compound ethyl 2-(benzo[c][1,2,5]thiadiazole-4-sulfonamido)-4,5-dimethylthiophene-3-carboxylate (hereafter designated as 634) that triggers Ca2+ influx in mBMDCs. Compound 634 enhanced the number of EVs released and also costimulatory molecule expression on EVs. Purified EVs from 634-treated mBMDCs promoted antigen-specific T cell proliferation. Moreover, focused structure–activity relationship (SAR) studies on 634 suggested that an increase in intracellular Ca2+ is closely associated with the immunostimulatory potency of EVs released by 634-treated mBMDCs.
Results
Compound 634 Induces Ca2+ Influx
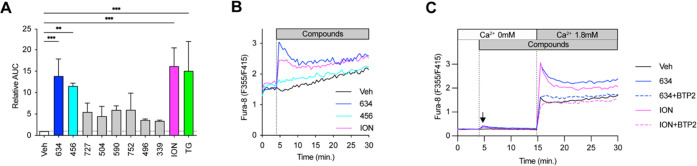
Our previous work demonstrated that small-molecule Ca2+ channel activators used as a coadjuvant enhance vaccine adjuvant activity.31,32 Triggering Ca2+ influx rapidly increases intracellular Ca2+ concentration, which enhances plasma membrane EV biogenesis.22,23 Thus, we postulated that small-molecule compounds that increase intracellular Ca2+ in APCs would enhance the release of immune stimulatory EVs. Eighty hit compounds selected by our triple HTSs were further analyzed by a ratiometric Ca2+ indicator assay in a human monocytic cell line (THP-1 cells) (Figure S1A). Ionomycin (ION) and thapsigargin (TG), a calcium ionophore and an inhibitor of sarco/endoplasmic reticulum Ca2+-ATPase, respectively, were used as positive controls.31 In a validation assay, compounds 634 and 456 significantly increased intracellular Ca2+ compared to the vehicle control (Veh, 0.5% DMSO) (Figures 1A and S1A,B). The top two hits, 634 and 456, were further assayed for Ca2+ influx in primary mBMDCs using a ratiometric Ca2+ indicator. Compound 634 induced Ca2+ influx in mBMDCs, and the increase of intracellular Ca2+ was comparable to that of ION (1 μM), while 456 failed to elevate intracellular Ca2+ levels (Figure 1B). Compound 634 was nontoxic to mBMDC at 10 μM, which triggered Ca2+ influx. Thus, we selected compound 634 for further characterization.
Figure 1.
Compound 634 increases intracellular Ca2+ levels in mBMDCs. (A) Intracellular Ca2+ influx levels of the top eight compounds. THP-1 cells were loaded with the ratiometric Ca2+ indicator, Fura-2, and treated with ION (1 μM), TG (1 μM), or test compounds (5 μM). The time–response pattern of intracellular Ca2+ levels was recorded for 25 min. Area under the curve (AUC) of OD340/380 ratios corresponds to the intracellular Ca2+ kinetics, and the baseline-subtracted AUC was calculated by GraphPad Prism. Data presented are relative AUC to Veh (1.74 at 1st exp. and 1.06 ± 0.05 at 2nd exp. were set as 1, respectively) and mean ± SD of pooled data from three experiments showing similar results. **p < 0.01, ***p < 0.001 by one-way ANOVA with Dunnett’s posthoc test compared to Veh. (B) Ca2+ mobilization by compounds 456 and 634 in mBMDCs. mBMDCs were loaded with Fura-8 and treated with ION (1 μM), 634 or 456 (10 μM) for 25 min. The dashed line indicates the timing of compounds added. The data shown are representative of three independent experiments showing similar results. (C) Ca2+ add-back assay. Fura-8-loaded mBMDC were treated with ION, compound 634, ION plus BTP2, or 634 plus BTP2 for 10 min in the absence of extracellular Ca2+, and then Ca2+ (final 1.8 mM) was added. ION, 634, and BTP2 were added at final concentrations of 1, 10, and 5 μM, respectively. The data presented are averages of duplicates and representatives of two independent experiments showing similar results.
Furthermore, we used the Ca2+ add-back assay in mBMDCs to seek the mechanism of intracellular Ca2+ increase by 634. The store-operated Ca2+ entry (SOCE) is a major mechanism for Ca2+ import from extracellular to intracellular space in immune cells.33 SOCE is initiated by Ca2+ release from the endoplasmic reticulum (ER) stores and mediated by the interaction of the plasma membrane protein Orai and the ER membrane protein Stim. In the absence of extracellular Ca2+, 634 induced a small increase (black arrow in Figure 1C), which indicates a release of Ca2+ from the endoplasmic reticulum, similar to that of positive control for a SOCE inducer, ION (Figure 1C). When extracellular Ca2+ was replenished, 634 and ION treatment resulted in a sharp increase in Ca2+ via the SOCE. To confirm that 634-induced Ca2+ influx was mediated by SOCE, we applied two SOCE inhibitors, BTP2 (also known as YM-58483, N-[4-[3,5-bis(trifluoromethyl)pyazol-1-yl]phenyl]-4-methylthiadiazole-5-carboxamide) and AnCoA4 (3-(6-methoxy-1,3-benzodioxol-5-yl)-8,8-dimethylpyrano[2,3-f]chromen-4-one, Orai1 channel inhibitor).34,35 Both compounds inhibited Ca2+ entry induced by 634 (Figures 1C and S2). These results indicated that Orai1-mediated SOCE predominantly contributes to the increase of intracellular Ca2+ levels induced by 634.
An RNA-seq experiment was performed to assess the mechanism of action of 634. The 5 h treatment time was chosen to assess early cellular responses and avoid possible indirect effects due to compound stimulation. Differential expression analysis at the gene level (R-limma, www.r-project.org) comparing 634 against the vehicle control in mBMDCs showed that among 103 genes whose expression was modulated, 7 genes related to calcium signaling were affected by 634 (5 genes upregulated and 2 downregulated) at Benjamini-Hochberg FDR≤0.05 and fold-change >2 (86 genes upregulated, 17 genes downregulated) (Table S1). To validate the RNA-seq data, the expression of the genes affected by 634 was further analyzed by reverse transcriptase-quantitative PCR (RT-qPCR) using ION as a positive control (Figure S3). The expression of five out of seven genes in 634-treated cells, 5-hydroxytryptamine (serotonin) receptor 7 (Htr7), cadherin 1 (Cdh1), protein phosphatase 1E (Ppm1e), cadherin-related family member 1 (Cdhr1), and histamine receptor H1 (Hrh1), showed trends similar to the cells exposed to ION, implying that 634 acts via Ca2+ signaling pathways.
634 Increases EV Release
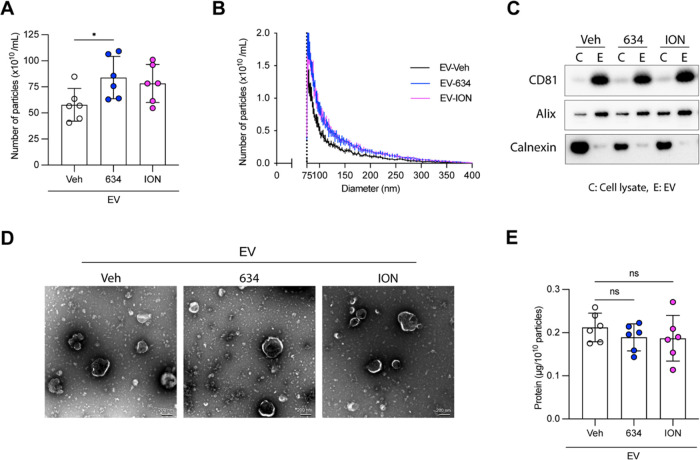
To confirm that 634 increased the number of EVs released in the culture supernatant, we measured the numbers of EVs from 634 (10 μM)-treated mBMDCs using microfluidic resistive pulse sensing (MRPS) with a Spectradyne nCS1 instrument. ION (1 μM) was used as a positive control.25,36 The EVs were isolated using a multistep differential ultracentrifugation protocol after 48 h treatment.29 The 48 h treatment time was chosen because EVs released by the vehicle-treated cells were detected only after 48 h based on the kinetics of EV secretion using CD63 Tluc reporter cells (data not shown). Compound 634 significantly increased the number of EVs released in the culture supernatant compared to that of the Veh control (0.5% DMSO) by 45% (p < 0.05, Figure 2A).
Figure 2.
Compound 634 enhances EV release. mBMDCs were incubated with compound 634 (10 μM), ION (1 μM), or Veh (0.5% DMSO) for 48 h. EVs were isolated from the culture supernatant by differential ultracentrifugation, and final pellets were resuspended with 50 μL of PBS (designated as EV634, EVION, and EVVeh). (A) Results were analyzed by MRPS, and the EV number was calculated per mL. Data shown are means ± SDs of EVs from six experiments using different mBMDC batches. *p < 0.05 by one-way ANOVA with Dunnett’s posthoc test vs Veh. (B) Size distributions of EV634, EVION, and EVVeh were measured by MRPS. Data shown are means ± SEMs of EVs from six different mBMDC batches. (C) Immunoblots of EVs (designated as “E”) and parental cell lysates (designated as “C”). The proteins (2 μg/well) were separated by 4–12% NuPAGE gel. Blots were probed with anti-CD81, anti-Alix, or anti-Calnexin antibodies. The images shown are representative of two independent experiments showing similar results. (D) Morphological examination of EV634, EVION, and EVVeh by TEM. Scale bars represent 200 nm. (E) Ratios of total protein amount to particles of EV634, EVION, and EVVeh were calculated per 1010 EV particles. The results were measured using the Micro BCA Assay kit. Experiments were repeated six times using individual mBMDC batches. Data shown are means ± SDs of data from six measurements. n.s., not significant by one-way ANOVA with Dunnett’s posthoc test vs Veh.
Since the heterogeneity of EVs often makes them difficult to obtain as relatively pure preparations and to characterize properly, the International Society for Extracellular Vesicles proposed Minimal Information for Studies of Extracellular Vesicles (“MISEV”) 2018 guidelines recommend the characterization of purified EVs by global quantification, e.g., particle number, total protein amount, and by single vesicle analysis.37 Thus, we characterized EV preparations (EVVeh, EV634, and EVION) according to MISEV2018 guidelines. The sizes of the majority populations of EVVeh, EV634, and EVION were less than 200 nm and thus characterized as small EVs (<200 nm), suggesting that 634 did not affect the heterogeneity of EVs (Figure 2B). EVs were further confirmed by their specific protein composition showing enrichment of the EV markers, CD81 and Alix, and reduced amounts of a protein, calnexin, not associated with small EVs, as shown by immunoblots (Figure 2C and S4). The single vesicles of EVVeh, EV634, and EVION were morphologically similar by transmission electron microscopy (Figure 2D). The total protein amounts of EVVeh, EV634, and EVION were measured by the Micro BCA Assay kit to evaluate protein contamination in EV preparations. Total protein amounts of these preparations were comparable, indicating that the purity of EVs was similar among the EVVeh, EV634, and EVION (Figure 2E). Collectively, these data indicate that 634 increased the number of EVs released by mBMDCs and whose properties were verified according to the MISEV2018 guidelines.
EV634 Displays an Enhanced Expression of Costimulatory Molecules on the EV Surface
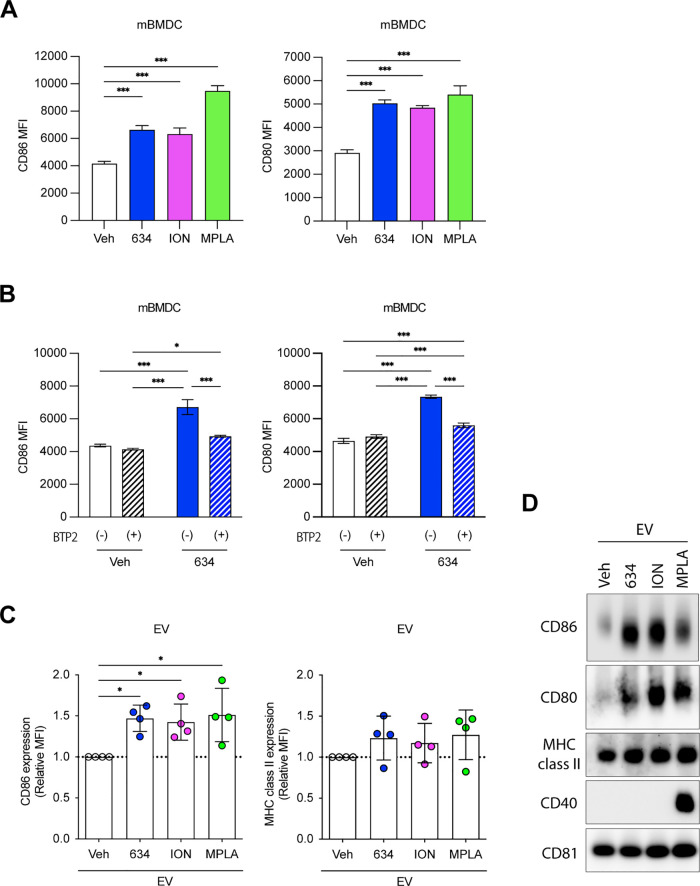
T cell activation requires antigens displayed on APC interacting with costimulatory and MHC molecules.13 We and others reported that calcium signaling regulates APC function.20,21 In the context of EVs, costimulatory molecules such as CD86 and MHC class II are expressed on EVs from parent DCs.13 Thus, we hypothesized that 634 increases the expression of costimulatory molecules and MHC class II on mBMDCs that are subsequently transferred to the surface of released EVs. To test this notion, mBMDCs were treated with Veh (0.5% DMSO), 634 (10 μM), ION (1 μM), or monophosphoryl lipid A (MPLA, 1 μg/mL) overnight, and the expression levels of CD86, CD80, MHC class II, and CD40 on mBMDCs were analyzed by flow cytometry (Figures 3A and S5). The TLR4 ligand, MPLA, was used as a positive control.38634 and ION, as well as MPLA, increased CD86 and CD80 expression on mBMDCs (Figure 3A). To test whether intracellular Ca2+ increase induced by 634 led to the enhanced expression of CD86 and CD80, BTP2 was used to inhibit SOCE-mediated Ca2+ influx. Incubation with BTP2 significantly suppressed CD86 and CD80 expression, indicating that SOCE-mediated Ca2+ influx contributes to costimulatory molecule expression enhanced by 634 (Figure 3B). We next evaluated costimulatory molecules on EVs using single-particle high-resolution flow cytometry, with an Amnis CellStream39 (Figure 3C), and by immunoblots (Figures 3D and S6). Higher levels of CD86 expression were detected on EV634 and EVION, similar to those on EVMPLA in comparison to EVveh (p < 0.05). This upregulation was also confirmed by immunoblots of isolated EVs (Figures 3D and S6). The immunoblots showed that CD86 and CD80 expression was higher on EV634 than that on EVVeh. These results highlighted that higher costimulatory molecule expression on EV634 mirrored the increased expression in parental mBMDCs treated with 634.
Figure 3.
Compound 634 enhances costimulatory molecule expression on mBMDC and EV634. (A and B) After incubation with 634 (10 μM), ION (1 μM), MPLA (1 μg/mL), Veh (0.5% DMSO), BTP2 (5 μM), or 634 plus BTP2 for 20 h, mBMDCs were stained with a cocktail of anti-CD11c, anti-CD86, anti-CD80, anti-MHC class II, and anti-CD40 and analyzed by flow cytometry. The mean fluorescence intensity (MFI) is shown. Data shown are means ± SDs of triplicates representative of two independent experiments. (A) ***p < 0.001 by one-way ANOVA with Dunnett’s posthoc test vs Veh. (B) *p < 0.05, ***p < 0.001 by two-way ANOVA with Tukey’s posthoc test. (C) EVVeh, EV634, EVION, and EVMPLA were stained with a cocktail of vFRed, anti-CD86, and anti-MHC class II and analyzed by flow cytometry. Relative MFI to EVVeh is shown. CD86: 58.4 ± 0.4 at 1st batch, 49.2 ± 0.2 at 2nd exp., 56.0 ± 0.5 at 3rd exp., and 63.1 ± 3.7 at 4th exp. and MHC class II: 416.5 ± 57.3 at 1st exp., 556.5 ± 44.5 at 2nd exp., 711.5 ± 16.3 at 3rd exp., and 559.0 ± 14.1 at 4th exp. were set as 1, respectively. Each dot represents a data set from individual mBMDC batches. Data shown are means ± SDs of EVs from four different mBMDC batches. *p < 0.05 by one-way ANOVA with Dunnett’s posthoc test vs EVVeh. (D) Immunoblot of EVs. The protein (2 μg/well) was separated and probed with anti-CD86, anti-CD80, anti-MHC class II, anti-CD40, or anti-CD81 antibodies. The images shown are representative of two independent experiments with similar results.
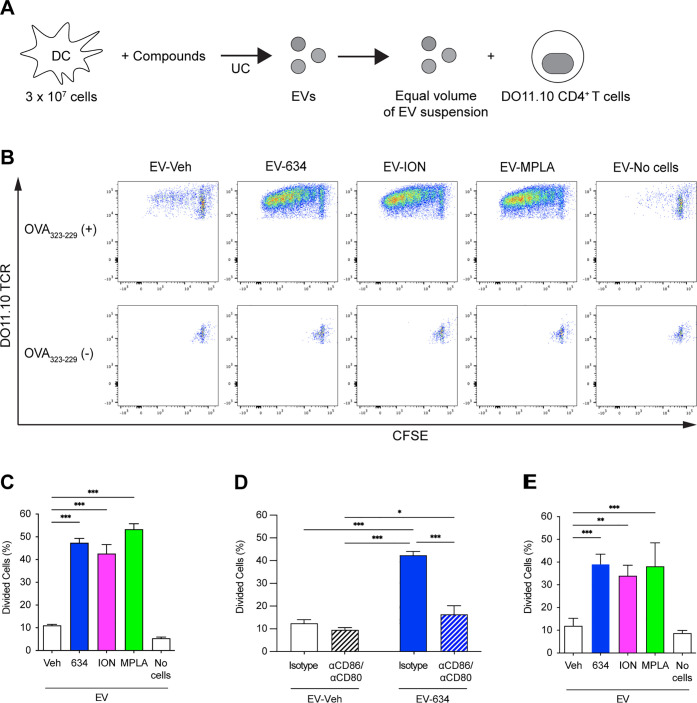
EVs Derived from mBMDCs Treated with 634 Stimulate DO11.10 T Cell Proliferation
The above data demonstrates that EV634 carried the costimulatory molecules CD86 and CD80 that are needed to prime naïve T cells during antigen presentation.13 To evaluate whether EV634 promotes T cell proliferation, we employed CD4+ T cells expressing ovalbumin (OVA)-specific T cell receptors (TCR) from DO11.10 mice that proliferate upon the engagement of TCR and OVA MHC class II peptides (OVA323–339)40,41 (Figure 4A). Carboxyfluorescein succinimidyl ester (CFSE)-labeled DO11.10 CD4+ T cells were cocultured with EV634 in the presence of OVA323–339. We used MPLA (1 μg/mL) as a positive control.38 EVs isolated from the medium without mBMDCs (EVNo cells) served as negative controls. The amounts of EVs added to the T cell culture were normalized by the volumes of the culture supernatants and parent cell numbers. The proliferation of DO11.10 CD4+ T cells, as shown by CFSE dilution and IL-2 release into the culture supernatant, was monitored to evaluate the antigen-presenting function42 (Figures 4B, 4C, and S7A). In the presence of OVA323–339, EV634 induced significantly higher T cell proliferation and IL-2 release, equivalent to that of EVMPLA or EVION (p < 0.001). In contrast, no T cell proliferation was detected by particles isolated from the medium (EVNo cells) or in the absence of OVA323–339 (Figure 4B). These data indicate that EV634 stimulates T cell proliferation in a TCR-dependent manner.
Figure 4.
EV634 enhances T cell proliferation in the presence of antigenic peptides. (A, B) CFSE-labeled CD4+ T cells isolated from splenocytes of OVA TCR transgenic strain, DO11.10; splenocytes were treated with an equal volume (7 μL out of 50 μL) of the suspensions of EVVeh, EV634, EVION, or EVMPLA in the presence or absence of OVA323-339 peptide for 5 days. EVNo cells were used as a negative control. (C–E) T cell proliferation was determined by CFSE dilution using flow cytometry. Percentages of divided T cells induced by EVs from the same volumes of the culture supernatants and the number of parent cells. Data shown are means ± SDs of triplicates representative of two independent experiments. (C) In the presence of the OVA323–339 peptide, T cells were treated with an equal volume of the EV suspensions (7 μL out of 50 μL). (D) In the presence of the OVA323-339 peptide, T cells were treated with an equal volume (7 μL out of 50 μL) of the suspensions of EVveh or EV634 in the presence of anti-CD86 and anti-CD80 antibodies or isotype controls for 5 days. Data shown are means ± SDs of triplicates representative of two independent experiments. (E) In the presence of the OVA323–339 peptide, T cells were treated with an equal particle number (3.13 × 109). *p < 0.05, **p < 0.01, and ***p < 0.001 by one-way ANOVA with Dunnett’s posthoc test vs EVVeh (C and E), and by two-way ANOVA with Tukey’s posthoc test (D).
CD86 and CD80 on EV634 Are Required for EV634-Enhanced T Cell Proliferation
The above data indicate that EV634 enhanced TCR-mediated T cell proliferation in the absence of APC. Because 634 enhanced the expression of costimulatory molecules on EV634 (Figure 3C, D), we hypothesized that CD86 and CD80 on EV634 contribute to the EV634 function. We used neutralizing antibodies for CD86 and CD80 to block the engagement of these molecules on EV to T cells during antigen presentation. The incubation with neutralizing antibodies significantly decreased T cell proliferation induced by EV634 to baseline levels (EVveh) (Figure 4D). These data indicate that the engagement of CD86 and CD80 on EV634 and T cells is required for TCR-dependent T cell activation.
In the above studies, the dosage of EVs was normalized by the volumes of culture supernatants and parent cell numbers.38 By this approach, we could not distinguish whether the induction of T cell proliferation was attributable to the increased EV number or to the immunostimulatory qualities of EVs. To address this question, an equal number of EVs (109/mL) was cultured with CFSE-labeled DO11.10 CD4+ T cells. EV634 maintained higher levels of T cell proliferation and IL-2 release compared to EVveh (p < 0.001) when equal numbers of EVs were used (Figures 4E and S7B). These data suggest that the higher particle number and the enhanced function of each EV634 particle both contributed to enhanced T cell proliferation. Collectively, 634 induced a higher number of EVs equipped with TCR-dependent T cell activation capacity, and both CD86 and CD80 on EV634 were associated with the T cell activation by EV634.
Contaminants from the Culture Medium Have No Impact on EV634 Function
It is possible that the EV preparation may be contaminated with the components of the culture medium, namely, 634, in the EV isolation process, referred to here as carryover 634. To estimate the concentration of carryover 634, we measured the level of 634 in an EV preparation using liquid chromatography–mass spectrometry and estimated the concentration to be 32 nM (Figure S8). To test whether carryover 634 in the EV preparation contributed to T cell proliferation, T cells were cultured with 634 alone (0.1, 1, and 10 μM) or 634 plus EVveh (Figure S9). Neither treatment with 634 alone nor cotreatment with 634 and EVveh affected T cell proliferation at as high as 10 μM, which is 300 times higher concentration than the estimated concentration of carryover 634 (32 nM). Other potential contaminants in the EV preparation are the high-molecular-weight complexes, i.e., aggregated proteins and other cellular components.43 To exclude these contaminants, we isolated EVs using the combination of ultracentrifugation and size-exclusion chromatography (SEC) and used them for the T cell proliferation assay. The combination method improves the purity of EVs by removal of non-EV-bound soluble proteins.44,45 EV particles concentrated by ultracentrifugation were further separated by SEC into two fractions: SEC-EV and SEC-contaminants (Figure S10A). SEC-EV634 induced significantly higher T cell proliferation compared to SEC-contaminants634 or EVveh, suggesting that the contaminants had a minimal impact on T cell proliferation (Figure S10B). These results indicate that EV634 contributes to T cell activation and that carryover 634 and the high-molecular-weight complexes in the EV preparation did not influence the EV634 function.
Structure–Activity Relationship (SAR) Studies of 634
We demonstrated that EV634 could prime naïve T cells in a TCR-dependent manner. To confirm that Ca2+ influx is associated with immunostimulatory EV functions, we added to the mBMDC culture chelators for extracellular or intracellular calcium (EGTA and BAPTA-AM, respectively). However, these chelators were toxic and alone enhanced particle release from dead and dying cells. We, therefore, utilized focused SAR studies to obtain 634 analogues that had lost the ability to induce Ca2+ influx. We hypothesized that the potency is mediated by the chelation effects likely due to the resulting carboxylic acid functionality obtained by intracellular hydrolysis of the ester bond assisted by the presence of hydrogen-bonding atoms such as oxygen of the sulfonamide and nitrogen of the benzothiadiazole group.
Since compound 634 was not commercially available for bulk purchase, we first undertook its synthesis. Starting with ethyl 2-amino-4,5-dimethylthiophene-3-carboxylate with benzo[c][1,2,5]thiadiazole-4-sulfonyl chloride and pyridine as the base, compound 634 was obtained in good yields (44.5%). Next, using compound 634 as a common synthon, we first de-esterified the ethyl ester to obtain the free carboxylic acid analogue 2H013. Then, the acid was converted to acid chloride with thionyl chloride to obtain an advanced reactive intermediate that was reacted with several different alcohol reagents to obtain ester-modified analogues. These included analogues with alkyl esters of varying chain lengths, such as 2G179a (methyl ester), 2G176 (propyl ester), and 2G179b (hexyl ester), and branched alkyl analogues, including 2G179c (isopropyl ester), 2G179d (isopentyl ester), 2G179h (tert-butyl ester), and 2G179e (cyclohexyl ester). Additionally, we also prepared analogues bearing phenolic (2G179g) and benzylic (2G179f) ester functionalities. An ethylamide analogue of 634 (2E241) was synthesized to probe the necessity of the ester functional group for Ca2+ mobilization. The sulfonamide group was also altered by N-methylation to obtain compound 2F186 (Figure 5).
Figure 5.
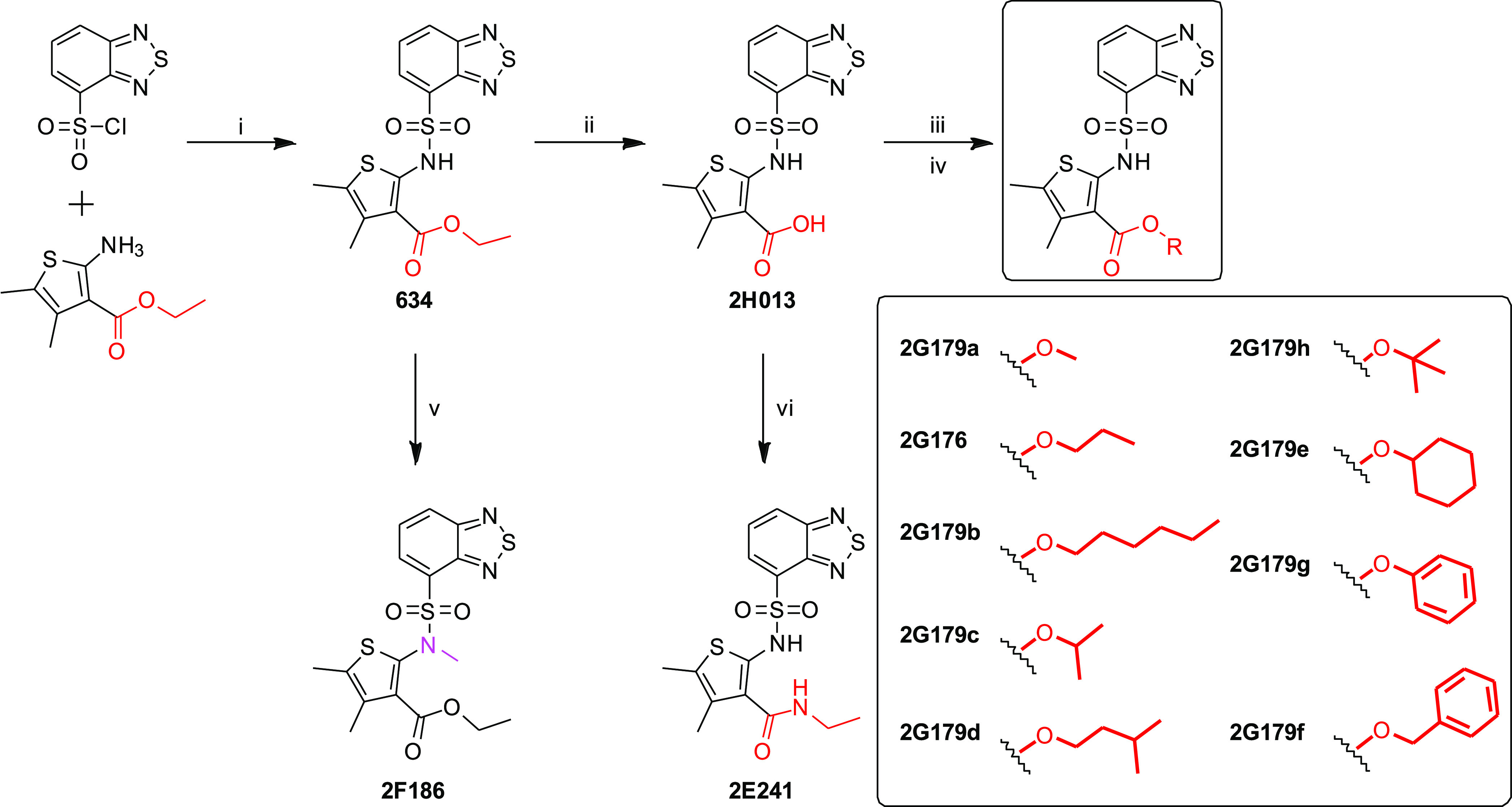
Focused SAR studies on 634. Syntheses of twelve 634 analogues using a modification of the ester site of 634.
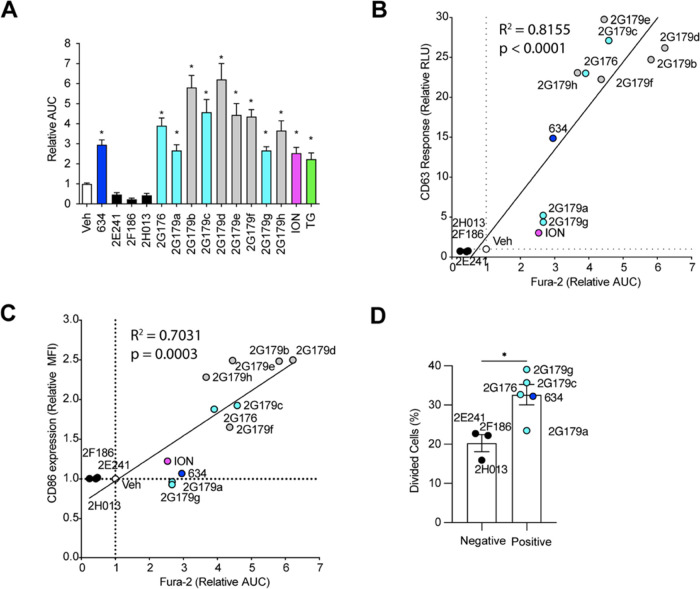
Ca2+ Influx Inducing 634 Analogues Release EVs That Promote T Cell Proliferation
To examine the properties of the 634 analogues, we incubated mBMDCs with 12 of the compounds and followed intracellular Ca2+ levels (Figures 6A and S11) by the Fura-2 assay. Three analogues, 2E241 (amide analogue), 2F186 (N-methyl sulfonamide analogue), and 2H013 (carboxylic analogue), did not induce Ca2+ influx in mBMDCs according to the Fura-2 assay. The other nine analogues each statistically increased intracellular Ca2+ levels compared to Veh.
Figure 6.
Correlation between intracellular Ca2+ influx and T cell proliferation by EVs from mBMDCs treated with 634 analogues. (A) Intracellular Ca2+ levels in mBMDCs were monitored following 634 analogue treatment. mBMDCs were loaded with Fura-2 and treated with 634, its SAR analogues (10 μM), Veh (0.5% DMSO), ION (1 μM), or TG (1 μM). Data are presented as the normalized AUC (Veh was 1.00 ± 0.15). Data presented are means ± SDs of pooled two independent experiments performed in triplicate, showing similar results. *p < 0.05 by one-way ANOVA with Dunnett’s posthoc test compared to Veh. (B) Correlation analysis between intracellular Ca2+ induction (AUC) and the CD63 Tluc-CD9 EmGFP THP-1 reporter responses. Relative Luminescence activity to Veh (792.7 ± 84.58 at 1st exp. and 353.3 ± 29.14 at 2nd exp. were set as 1, respectively) is shown. (C) Correlation analysis between intracellular Ca2+ induction (AUC) and CD86 expression (MFI). mBMDCs were incubated with Veh, ION (1 μM), 634, and its analogues (10 μM) for 24 h. MFI was normalized to Veh (1.00 ± 0.02). The fitted regression line is shown. Pearson correlation analysis for 634 and its SAR analogues was performed by Graphpad Prism 9. (D) CFSE-labeled DO11.10 CD4+ T cells were cultured with an equal volume of EV suspension (7 μL out of 50 μL) in the presence of the OVA323–339 peptide for 5 days. The dosage of EVs was normalized by the volumes of culture supernatants and parent cell numbers. Percentages of divided T cells relative to the original population are calculated. Each dot shown is the average of three independent experiments performed in triplicates, and data shown are means ± SEMs of negative compounds (2E241, 2F186, and 2H013) or five positive compounds (634, 2G176, 2G179a, 2G179c, and 2G179g), respectively. *p < 0.05 by the Mann–Whitney U test.
EV release and costimulatory molecule expression by mBMDCs treated with the 634 analogues were screened using CD63 Tluc reporter cells30 and by FACS in mBMDCs, respectively. Three analogues that lost the ability of Ca2+ influx, 2E241, 2F186, and 2H013, did not increase luciferase activities in the culture supernatant of CD63 Tluc reporter cells nor enhance costimulatory expression on mBMDC. Furthermore, the levels of intracellular Ca2+ increase induced by the 634 analogues positively correlated with CD63 Tluc reporter cell responses and costimulatory molecule expression (p < 0.001, Figure 6B, C).
To confirm that Ca2+ influx is associated with immunostimulatory EV functions, we performed DO11.10 CD4+ T cell proliferation assays using EVs released by mBMDCs treated with 634 analogues. Due to the lower cell viability in exosome-depleted FBS medium, we excluded five agents (2G179b, 2G179d, 2G179e, 2G179f, and 2G179h) highlighted in gray in Figure 6A–C (Figures S12A,B).46 EVs were isolated from 48 h culture supernatants of mBMDCs treated with Ca2+ influx-positive (634, 2G176, 2G179a, 2G179c, and 2G179g) and -negative (2E241, 2F186, and 2H013) compounds. When T cell proliferation was expressed as relative values of divided cells (EVveh = 1), EVs from mBMDCs treated with Ca2+ influx-positive compounds elicited significantly higher T cell proliferation than did Ca2+ influx-negative compounds (Figures 6D and S13). These results imply that the analogues that retain the ability to increase intracellular Ca2+ level induced higher immunostimulatory EV release than analogues that lost this ability and that ester and sulfonamide functional groups are necessary for the immunostimulatory function of EVs.
Conclusions
Utilizing three parallel HTSs, we previously selected 80 hit compounds that enhance EV release with NF-κB activity and ISRE activity.29 In this study, the 80 hit compounds were screened for Ca2+ influx, and compound 634 was identified as a Ca2+ influx inducer mediated by SOCE. Compound 634 enhanced the number of EVs released by mBMDCs. This agent also increased costimulatory molecule expression on parental mBMDCs in a SOCE-dependent manner. EVs released from 634-treated mBMDCs also had increased CD86 and CD80 expression compared to control EVs. The 634-induced EVs markedly enhanced T cell proliferation in a TCR-dependent manner. Engagement of CD86 and CD80 on EV634 to T cells was required for the EV function. The SAR studies suggest that 634 analogues bearing the ester functional group retained the ability to induce Ca2+ influx and induced immunostimulatory EV release from mBMDCs compared to the amide, carboxylic acid, and N-methyl sulfonamide analogues, all of which lost the ability to induce Ca2+ influx. This agent and its analogues should be useful tools for the development of effective EV-based vaccines.
Materials and Methods
Reagents
Detailed information for the reagents is shown in Table S2.
Compounds Synthesis
Compound 634 and 12 analogues were synthesized in our laboratory as described in the Supporting Information and Figure 5. These compounds were dissolved in DMSO (#D2438, MilliporeSigma, Temecula, CA) to obtain stock solutions (2 mM).
Animals
Wild-type BALB/c and C57BL/6 mice and DO11.10 mice were purchased from the Jackson Laboratory. All animal experiments were approved by the Institutional Animal Care and Use Committee for UC San Diego.
Generation of mBMDCs
mBMDCs were prepared from bone marrow cells harvested from femurs of BALB/c mice as previously described.47,48 mBMDCs were washed with RPMI 1640 medium and incubated with the compound or vehicle (0.5% DMSO) in RPMI 1640 supplemented with exosome-depleted FBS in a T182 flask (7.5 × 105 cells/mL, total 40 mL) for 46–48 h. The culture supernatants were used for EV isolation.
Cell Lines
THP-1 cells were cultured in RPMI 1640 medium supplemented with 10% dialyzed FBS supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 μM 2-mercaptoethanol. The CD63 Tluc reporter cell line30 were cultured in RPMI 1640 medium supplemented with 10% dialyzed FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 1 × MEM nonessential amino acids, and 5 μg of blastidin. Both cell types were maintained in humidified conditions with 5% CO2 at 37 °C.
Calcium Influx Assay
Ca2+ influx was measured using Fura-2 or Fura-8 reagents. mBMDCs or THP-1 cells were loaded with ratiometric Ca2+ indicator, Fura-2-AM (4 μM) or Fura-8-AM (4 μM) in HBSS assay buffer [1× HBSS, 10 mM HEPES (pH 7.4), 1.8 mM CaCl2, 0.8 mM MgCl2, and 0.1% BSA], containing 0.04% Pluronic F127 at 37 °C for 40 min and at RT for additional 20 min. OD340/380 (emission) and OD510 nm (excitation) were read for Fura-2 by a fluorescence plate reader (Tecan2000, #30016056, TECAN, San Jose, CA). For Fura-8, OD 355/415 nm (excitation) and OD540 (emission) were read. Data were presented as OD ratios for 340/380 or 355/415 as a representative of changes in the intracellular Ca2+ level. The baseline-subtracted AUC of 340/380 ratios was calculated using GraphPad Prism (version 9, GraphPad Software, San Diego, CA) (Figure S14).
Ca2+ Add-Back Assay
mBMDCs were loaded with Fura-8-AM in Ca2+-depleted HBSS assay buffer [1× HBSS, 10 mM HEPES (pH 7.4), 0.8 mM MgCl2, and 0.1% BSA] containing 0.04% Pluronic F127 at 37 °C for 40 min and at RT for additional 30 min. OD 355/415 nm (excitation) and OD540 (emission) were read. Data were presented as OD ratios for 340/380 or 355/415. Cells were treated with test compounds in the absence of Ca2+ first for 10 min and then CaCl2 was added up to 1.8 mM.
EV Isolation by Differential Ultracentrifugation
EVs were isolated as described previously with minor modifications.30 Conditioned culture medium (40 mL) was spun at 300g for 10 min, at 2000g for another 10 min, and at 10,000g for 30 min. Next, 30 mL of supernatant was transferred to 31.5 polypropylene UC tubes and spun at 100,000gavg for 3 h in an SW28 rotor (K-factor: 2554) by a Beckman Optima XL-90 ultracentrifuge (Beckman Coulter Life Sciences, Brea, CA). The supernatant was aspirated (leaving ∼50 μL), and the pellet was resuspended in 30 mL cold-filtered PBS. The resuspended pellet was spun under the same conditions as the previous spin, followed by another round of gentle aspiration and resuspension to a final volume of 50 μL in cold 0.02 μm filtered PBS. All centrifugation steps were performed at 4 °C, and the resulting samples were stored at −80 °C until use. All relevant data was submitted to the EV-TRACK knowledgebase (EV TRACK ID: EV220366, https://evtrack.org/index.php).49
Measurement of EV Concentrations
EV particle numbers and size distributions were determined by the MRPS technique with an nCS1 particle analyzer utilizing C-400 cartridges (Spectradyne, Signal Hill, CA). EV samples were diluted 200-fold in 1% Tween20-filtered PBS. All results were analyzed using the nCS1 Data Analyzer (Spectradyne). The setting of the peak filters was “transit time (μs) from 0 to 100, symmetry from 0.2 to 4.0, and signal-to-noise ratio (S/N) of at least 10”. Particles below 75 nm were cut off to exclude the background particles from the diluent, 1% Tween20-filtered PBS (Figure S15), and particles with diameters from 75 to 400 nm were measured.
Immunoblotting
Immunoblotting was performed using anti-CD81, anti-Alix, anti-Calnexin, anti-CD86, anti-CD80, anti-MHC class II, and anti-CD40 antibodies as primary antibodies as previously described by us.30 mBMDCs were lysed with PhosphoSafe extraction reagent supplemented with protease inhibitors. The total protein in the samples was quantitated by the Micro BCA Assay kit. Two micrograms of protein of cell or EV lysates was mixed with 4× NuPAGE sample buffer under reducing conditions with dithiothreitol (DTT) for Alix, Calnexin, CD86, CD80, MHC class II, and CD40 or nonreducing conditions (without DTT) for CD81. Samples were also denatured at 95 °C for 5 min prior to loading. After fractionation on NuPAGE 4–12% Bis-Tris gels, proteins were blotted onto Immobilon-P PVDF membranes and blocked for 1 h in 5% BSA-TBS-T at RT. The blots were then incubated with primary antibodies (Abs), anti-CD81, anti-Alix, anti-Calnexin, anti-CD86, anti-CD80, anti-MHC class II, and anti-CD40 Abs (1:1000 dilution), overnight at 4 °C with gentle agitation. After washing, the membranes were incubated with the corresponding secondary antibody for 30 min at RT with gentle agitation. Blots were developed with ProSignal Dura ECL and visualized using a ChemiDoc Imaging System. AccuRuler Prestained Protein Ladder was used for the molecular weight markers. Details for antibodies are shown in Table S3.
Transmission Electron Microscopy
For the morphological characterization of EVs, negative stain transmission electron microscopy was performed as previously described.30 Formvar-carbon-coated copper grids (400 mesh, Ted Pella, Redding, CA) were placed on 10 μL drops of each sample solution displayed on a parafilm sheet. After allowing the material to adhere to the grids for 5 min, grids were washed three times by rinsing with more than 200 μL drops of milli-Q water before being left for 1 min on 2% (wt/vol) uranyl acetate in water. The excess solution was removed with 11 μm Whatman filter paper, and grids were left to dry for 20 min before viewing. Grids were examined using an FEI Tecnai Spirit G2 BioTWIN transmission electroscope equipped with a bottom-mount Eagle 4k (16 megapixels) camera (FEI, Hillsboro, OR).
Costimulatory Molecule Expression Analysis
Costimulatory molecule expression on mBMDC was measured by the flow cytometry assay as described previously.29 mBMDCs (106 cells/mL) were incubated with 10 μM compound, 1 μM Ionomycin, and 1 μg/mL MPLA for 20–24 h. DMSO (0.5%) was used as the vehicle. Cells were incubated with antimouse CD16/32 antibodies for blocking FcR and stained with anti- CD11c, anti-CD40, anti-CD80, anti-CD86, or anti-MHC class II antibodies for 30 min at 4 °C. Cells were stained with 4′,6-diamino-2-phenylindole (DAPI) for 10 min at RT. Data were acquired using MACSQuant Analyzer 10 (Miltenyi Biotec, Germany) and analyzed with FlowJo (version 10.8.1, Becton Dickinson, Ashland, OR). The gating strategy is shown in Figure S16.
High-Resolution Single EV Analysis by Imaging Flow Cytometry
EVs were characterized using a commercially available reagent, vFC assay kit (#CBS, Cellarcus Biosciences, La Jolla, CA), using an Amnis CellStream Flow Cytometer equipped with 488 and 642 nm lasers (Luminex, Austin, TX) according to the manufacturer’s instructions39 with some modifications. In brief, samples were diluted 1:64 in vesicle staining buffer (Cellarcus Biosciences) and stained with an antibody cocktail of vFRed, anti-CD86, and anti-MHC class II Abs for 1 h at room temperature. Samples were diluted 1:200 in vesicle staining buffer before acquisition. Data were acquired using the Cellstream instrument with FSC and SSC turned off and all other lasers set to 100% of the maximum power. Each sample was run for 20 s at a sample volumetric flow rate of 3.66 μL/min. Relative MFI to EVVeh is shown. Data were analyzed using FlowJo. Details for antibodies and flow cytometer configurations are shown in Table S3 and Figure S17.
Antigen-Specific T Cell Proliferation Assay and ELISA
Transgenic OVA323-329 specific CD4+ cells were isolated from DO11.10 mice splenocytes using the EasySep Mouse CD4+ T cell isolation kit (negative selection). CFSE (4 μM)-labeled DO.11.10 CD4+ T cells were cocultured with an equal volume or equal number (3.13 × 109 or 3.99 × 109 EV particles) of EVs in the presence of the OVA323–339 peptide.40 In the assays with neutralization of CD86 and CD80, isotype control antibodies (rat IgG2a or American hamster IgG) or anti-CD86 and anti-CD80 antibodies (1.25 μg/mL) were added. IL-2 in the supernatant was tested by the Mouse IL-2 DuoSet ELISA kit. Cells were stained with antimouse DO11.10 clonotypic TCR antibody, and antigen-specific CD4+ T cell proliferation was evaluated by CFSE dilution using a MACSQuant flow cytometer (Meltyni Biotec, San Diego, CA). Cell proliferation was quantitated by the percentage of divided cells relative to the original population42 (Figure S18). Data were analyzed using FlowJo (version 10.8.1, FlowJo, Ashland, OR).
CD63 Tluc-CD9 EmGFP THP-1 Reporter Cell Assay
The reporter cell assay was carried out as described previously.29 CD63 Tluc-CD9 EmGFP THP-1 reporter cells (designated as CD63 Tluc reporter cells) were incubated with 10 μM test compounds, ION (1 μM), or 0.5% DMSO (negative control) in RPMI 1640 supplemented with exosome-depleted FBS at 5 × 104 cells/200 μL/well in a 96-well plate for 48 h at 37 °C. Subsequently, the plate was centrifuged, the supernatant was transferred, and chemiluminescence was measured by the TurboLuc Luciferase One-Step Glow Assay kit.
Cell Viability Assay
The cell viability was measured by the MTT assay as described previously.29 mBMDCs (2 × 106 cells/200 μL/well in RPMI 1640 supplemented with dialyzed 10% FBS or 1.5 × 106 cells/200 μL/well in RPMI1640 supplemented with 10% exosome-depleted FBS) were treated with 10 μM of each test compound in 96-well plates. After 46–48 h of compound treatment, MTT (0.5 mg/mL) was added to each well. The cells were lysed after overnight incubation, and absorbance values at 570 and 650 nm were read.
Statistical Analysis
To compare multiple groups, one-way ANOVA with Dunnett’s posthoc test or two-way ANOVA with Tukey’s posthoc test was used. To compare two groups, the two-tailed Mann–Whitney test was used. Prism 9 software (GraphPad Software, San Diego, CA) was used. P values smaller than 0.05 were considered statistically significant.
Acknowledgments
The authors thank M. Corr (UC, San Diego) for the scientific consultation, J. Jin and R. Cozza (UC, San Diego) for experimental support, JB Vasquez (Spectradyne LLC, Signal Hill, CA) for technical support for nCS1, and G. Castillon and the UC San Diego – Cellular and Molecular Medicine Electron Microscopy Core (UCSD-CMM-EM Core, RRID:SCR_022039, supported by NIH S10OD023527) for equipment access and technical assistance.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.3c00134.
Additional data on cytokine levels, expression of costimulatory molecules, and cell viability; original data of the Ca2+ influx assay and immunoblotting; compound data of 1H NMR, 13C NMR, HRMS, and LC–MS analyses; and additional information about biological experiments (PDF)
Author Contributions
Y.S., D.A.C., and T.H. designed the study. Y.S., F.S.-K., S.Y., T.S., F.S.L., H.K., and Marina P. performed experiments. N.M.S., M.M.B., M.C., and H.B.C. synthesized and characterized the compounds and contributed to writing the manuscript. Y.S., M.P., and K.M. performed statistical analysis. Y.S., D.A.C., and T.H. interpreted data and wrote the manuscript. All authors contributed to discussion and had opportunities to revise the manuscript.
This study was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases under contracts 75N93019C00042 and HHSN272201800048C (Subcontract No. PG19-61078-01) (Principal Investigator DAC). This publication includes data generated at the UC San Diego IGM Genomics Center utilizing an Illumina NovaSeq 6000 system that was purchased with funding from a National Institutes of Health SIG grant (#S10 OD026929)
The authors declare no competing financial interest.
Supplementary Material
References
- Cossetti C.; Iraci N.; Mercer T. R.; Leonardi T.; Alpi E.; Drago D.; et al. Extracellular Vesicles from Neural Stem Cells Transfer IFN-γ via Ifngr1 to Activate Stat1 Signaling in Target Cells. Mol. Cell 2014, 56, 193–204. 10.1016/j.molcel.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez-Mó M.; Siljander P.R.-M.; Andreu Z.; Zavec A. B.; Borràs F. E.; Buzas E. I.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J.; Wurdinger T.; Rijn S.; van Meijer D.; Gainche L.; Sena-Esteves M.; et al. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H.; Ekström K.; Bossios A.; Sjöstrand M.; Lee J. J.; Lötvall J. O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Latifkar A.; Hur Y. H.; Sanchez J. C.; Cerione R. A.; Antonyak M. A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132, jcs222406 10.1242/jcs.222406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Sun X.; Zhao J.; Yang Y.; Cai X.; Xu J.; Cao P. Exosomes: A Novel Strategy for Treatment and Prevention of Diseases. Front. Pharmacol. 2017, 8, 300. 10.3389/fphar.2017.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J. H.; Soares R. P.; Ribeiro K.; Andrade A. C.; Batista W. L.; Torrecilhas A. C. Extracellular Vesicles: Role in Inflammatory Responses and Potential Uses in Vaccination in Cancer and Infectious Diseases. J. Immunol. Res. 2015, 2015, 832057 10.1155/2015/832057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R.; LeBleu V. S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos P.; Almeida F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021, 12, 711565 10.3389/fimmu.2021.711565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabanovic B.; Piva F.; Cecati M.; Giulietti M. Promising Extracellular Vesicle-Based Vaccines against Viruses, Including SARS-CoV-2. Biology 2021, 10, 94. 10.3390/biology10020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admyre C.; Johansson S. M.; Paulie S.; Gabrielsson S. Direct exosome stimulation of peripheral humanT cells detected by ELISPOT. Eur. J. Immunol. 2006, 36, 1772–1781. 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- Schorey J. S.; Cheng Y.; Singh P. P.; Smith V. L. Exosomes and other extracellular vesicles in host–pathogen interactions. Embo Rep. 2015, 16, 24–43. 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbergh M. F.; Stoorvogel W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. 10.1146/annurev-immunol-041015-055700. [DOI] [PubMed] [Google Scholar]
- Anand P. K. Exosomal membrane molecules are potent immune response modulators. Commun. Integr. Biol. 2010, 3, 405–408. 10.4161/cib.3.5.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T.; Hayashi T.; Pan W.-W.; Fujita Y.; Holt M. V.; Qin J.; et al. The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell 2016, 167, 1525–1539.e17. 10.1016/j.cell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal S.; Perincheri S.; Fleming T.; Poulson C.; Tiffany B.; Bremner R. M.; Mohanakumar T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021, 207, 2405–2410. 10.4049/jimmunol.2100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M. Antigen Presentation by MHC-Dressed Cells. Front. Immunol. 2015, 5, 672 10.3389/fimmu.2014.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecalvo A.; Shufesky W. J.; Stolz D. B.; Sullivan M. G.; Wang Z.; Divito S. J.; et al. Exosomes As a Short-Range Mechanism to Spread Alloantigen between Dendritic Cells during T Cell Allorecognition. J. Immunol. 2008, 180, 3081–3090. 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- Jesus S.; Soares E.; Cruz M. T.; Borges O. Exosomes as adjuvants for the recombinant hepatitis B antigen: First report. Eur. J. Pharm. Biopharm. 2018, 133, 1–11. 10.1016/j.ejpb.2018.09.029. [DOI] [PubMed] [Google Scholar]
- Rao A.; Hogan P. G. Calcium signaling in cells of the immune and hematopoietic systems. Immunol. Rev. 2009, 231, 5–9. 10.1111/j.1600-065x.2009.00823.x. [DOI] [PubMed] [Google Scholar]
- Vig M.; Kinet J-P. Calcium signaling in immune cells. Nat Immunol. 2009, 10, 21–27. 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina A.; Furlán M.; Vidal M.; Colombo M. I. Exosome Release Is Regulated by a Calcium-dependent Mechanism in K562 Cells. J. Biol. Chem. 2003, 278, 20083–20090. 10.1074/jbc.m301642200. [DOI] [PubMed] [Google Scholar]
- Ambattu L. A.; Ramesan S.; Dekiwadia C.; Hanssen E.; Li H.; Yeo L. Y. High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism. Commun. Biol. 2020, 3, 553 10.1038/s42003-020-01277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.; Azimi I.; Monteith G.; Bebawy M. Ca2+ mediates extracellular vesicle biogenesis through alternate pathways in malignancy. J. Extracell. Vesicles 2020, 9, 1734326 10.1080/20013078.2020.1734326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger S. W.; Woo S. S.; Sun Z.; Martin TFJ. A Ca2+-stimulated exosome release pathway in cancer cells is regulated by Munc13-4. J. Cell Biol. 2018, 217, 2877–2890. 10.1083/jcb.201710132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer-Albers E.-M.; Bretz N.; Tenzer S.; Winterstein C.; Möbius W.; Berger H.; et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons?. Proteomics: Clin. Appl. 2007, 1, 1446–1461. 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- Czerniecki B. J.; Carter C.; Rivoltini L.; Koski G. K.; Kim H. I.; Weng D. E.; et al. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J. Immunol. 1997, 159, 3823–37. 10.4049/jimmunol.159.8.3823. [DOI] [PubMed] [Google Scholar]
- Smith K. J.; Hall S. M. Central demyelination induced in vivo by the calcium ionophore ionomycin. Brain. 1994, 117, 1351–1356. 10.1093/brain/117.6.1351. [DOI] [PubMed] [Google Scholar]
- Shukla N. M.; Sato-Kaneko F.; Yao S.; Pu M.; Chan M.; Lao F. S.; et al. A Triple High Throughput Screening for Extracellular Vesicle Inducing Agents With Immunostimulatory Activity. Front. Pharmacol. 2022, 13, 869649 10.3389/fphar.2022.869649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpigelman J.; Lao F. S.; Yao S.; Li C.; Saito T.; Sato-Kaneko F.; et al. Generation and Application of a Reporter Cell Line for the Quantitative Screen of Extracellular Vesicle Release. Front. Pharmacol. 2021, 12, 668609 10.3389/fphar.2021.668609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T.; Shukla N. M.; Sato-Kaneko F.; Sako Y.; Hosoya T.; Yao S.; et al. Small Molecule Calcium Channel Activator Potentiates Adjuvant Activity. ACS Chem. Biol. 2022, 17, 217–229. 10.1021/acschembio.1c00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T.; Sako Y.; Sato-Kaneko F.; Hosoya T.; Yao S.; Lao F. S.; et al. Small Molecule Potentiator of Adjuvant Activity Enhancing Survival to Influenza Viral Challenge. Front Immunol. 2021, 12, 701445 10.3389/fimmu.2021.701445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B.; Putney J. W. Store-Operated Calcium Channels. Physiol. Rev. 2005, 85, 757–810. 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Ishikawa J.; Ohga K.; Yoshino T.; Takezawa R.; Ichikawa A.; Kubota H.; Yamada T. A Pyrazole Derivative, YM-58483, Potently Inhibits Store-Operated Sustained Ca2+ Influx and IL-2 Production in T Lymphocytes. J. Immunol. 2003, 170, 4441–4449. 10.4049/jimmunol.170.9.4441. [DOI] [PubMed] [Google Scholar]
- Sadaghiani A. M.; Lee S. M.; Odegaard J. I.; Leveson-Gower D. B.; McPherson O. M.; Novick P.; et al. Identification of Orai1 Channel Inhibitors by Using Minimal Functional Domains to Screen Small Molecule Microarrays. Chem Biol. 2014, 21, 1278–1292. 10.1016/j.chembiol.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Durcin M.; Fleury A.; Taillebois E.; Hilairet G.; Krupova Z.; Henry C.; et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1305677 10.1080/20013078.2017.1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C.; Witwer K. W.; Aikawa E.; Alcaraz M. J.; Anderson J. D.; Andriantsitohaina R.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi K. R.; Gehrmann U.; Jordö E. D.; Karlsson MCI.; Gabrielsson S. Antigen-loaded exosomes alone induce Th1-type memory through a B cell–dependent mechanism. Blood 2009, 113, 2673–2683. 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- Crooks E. T.; Almanza F.; D’Addabbo A.; Duggan E.; Zhang J.; Wagh K.; et al. Engineering well-expressed, V2-immunofocusing HIV-1 envelope glycoprotein membrane trimers for use in heterologous prime-boost vaccine regimens. Plos Pathog. 2021, 17, e1009807 10.1371/journal.ppat.1009807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D.-H.; Paz P.; Villaflor G.; Rivas A.; Mehta-Damani A.; Angevin E.; et al. Exosomes as a Tumor Vaccine: Enhancing Potency Through Direct Loading of Antigenic Peptides. J. Immunother. 2003, 26, 440–450. 10.1097/00002371-200309000-00007. [DOI] [PubMed] [Google Scholar]
- Näslund T. I.; Gehrmann U.; Qazi K. R.; Karlsson MCI.; Gabrielsson S. Dendritic Cell–Derived Exosomes Need To Activate Both T and B Cells To Induce Antitumor Immunity. J. Immunol. 2013, 190, 2712–2719. 10.4049/jimmunol.1203082. [DOI] [PubMed] [Google Scholar]
- Roederer M. Interpretation of cellular proliferation data: Avoid the panglossian. Cytometry, Part A 2011, 79A, 95–101. 10.1002/cyto.a.21010. [DOI] [PubMed] [Google Scholar]
- Jeppesen D. K.; Fenix A. M.; Franklin J. L.; Higginbotham J. N.; Zhang Q.; Zimmerman L. J.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan K.; Martin K.; FitzGerald S. P.; O’Sullivan J.; Wu Y.; Blanco A.; et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. 2020, 10, 1039 10.1038/s41598-020-57497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R.; Zhao L.; Kong G.; Liu X.; Zhu S.; Zhang S.; Min L. Combination of Size-Exclusion Chromatography and Ultracentrifugation Improves the Proteomic Profiling of Plasma-Derived Small Extracellular Vesicles. Biol. Proced. Online 2020, 22, 12 10.1186/s12575-020-00125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E.; Zhang S.; Witwer K. W.; Mattson M. P. Extracellular vesicle–depleted fetal bovine and human sera have reduced capacity to support cell growth. J. Extracell. Vesicles 2015, 4, 26373. 10.3402/jev.v4.26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M. B.; Kukutsch N.; Ogilvie ALJ.; Rößner S.; Koch F.; Romani N.; Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223, 77–92. 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Datta S. K.; Redecke V.; Prilliman K. R.; Takabayashi K.; Corr M.; Tallant T.; et al. A Subset of Toll-Like Receptor Ligands Induces Cross-presentation by Bone Marrow-Derived Dendritic Cells. J. Immunol. 2003, 170, 4102–4110. 10.4049/jimmunol.170.8.4102. [DOI] [PubMed] [Google Scholar]
- Van Deun J.; Deun J. V.; Mestdagh P.; Agostinis P.; Akay Ö.; Anand S.; et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods. 2017, 14, 228–232. 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.