Abstract
To reduce the time needed for clamping or circulatory arrest (or both) during substitution of a prosthesis for the thoracic aorta, we developed an expandable device that can be used with any commercially available prosthetic graft to enable sutureless aortic anastomosis. Improvements upon a previous version of the device include the use of nickel-titanium alloy (Nitinol) instead of stainless steel. This, together with an improved wire-looping design, now enables continuous control of diameter, even when the device is in contact with blood. A further improvement consists of 4 metallic hooks on the outer surface, which enable firm fixation to the aortic stump.
In March 2001, a 47-year-old man was admitted to our institution for evaluation of left upper-lobe bronchogenic adenocarcinoma that had infiltrated the distal aortic arch and upper descending aorta. Re-staging of the neoplasm ruled out distant metastasis. We resected the infiltrated aortic wall en bloc with the upper lobe. The expandable device enabled the distal anastomosis of the aortic prosthesis to be performed easily, in less than 3 minutes. The main advantages of this device are an easier, quicker anastomosis and the absolute prevention of suture-line hemorrhage (no suture line). The expandable device overcomes the drawbacks of the intraluminal ringed prosthesis used in the past. (Tex Heart Inst J 2002;29:56–9)
Key words: Anastomosis, surgical/methods; aorta, thoracic/surgery; blood vessel prosthesis implantation/methods; lung neoplasms/surgery; male; middle age; neoplasm invasiveness; prosthesis design; vascular neoplasms/surgery; vascular surgical procedures/methods
Substitution of a prosthesis for the thoracic aorta is a technically demanding surgical procedure that is still associated with considerable morbidity and mortality, even in centers with much experience. 1 Spinal cord injury 2 is the most feared complication in descending aorta surgery. Embolic or ischemic lesions of the central nervous system (CNS) are common complications of prosthetic replacement of the aortic arch. Although the pathogenesis of spinal cord and other CNS injuries is multifactorial, the literature agrees that the duration of aortic clamping and circulatory arrest is a major factor in the incidence of these postoperative complications. In order to reduce the time needed for clamping or circulatory arrest, we developed an expandable device 3 that can be used with any commercially available prosthetic graft to enable sutureless aortic anastomosis.
The Device
This device consists of expandable loops of nickel-titanium (Nitinol) wire sewn to a Dacron cuff (Fig. 1). A removable guide expands and tightens the Nitinol loops in such a way that the final diameter of the device can be varied from 13 to 34 mm in diameter (the device has been prepared in 3 sizes, each of which has an expansion range of 8 or 9 mm), while the cylindrical shape is maintained.

Fig. 1 The device relies on a number of metallic wires, arranged in circular loops and sewn to a Dacron cuff. Traction on the wires by an actuating guide expands and tightens the loops to create a rigid cylindrical ring with a variable and controllable diameter. Two different versions of this device (Types I and II) are generally used for the 1st and the 2nd anastomosis, respectively.
Type I: Before clamping, the expandable device (a) is sutured to one end of a vascular prosthesis (b) in such a way that the device, once retracted (c, d), can be inserted into the vascular stump (e) and then expanded to the final diameter.
Type II: A different arrangement of the metallic wires enables the device to be used as a double-anastomosis ring, with both ends activated by the same guide. This version is better used for the 2nd anastomosis; when used with the type II device, the prosthesis can be cut in position, precisely to the desired length.
With both types, external ligature is required for hemostasis and device stabilization. After blood flow is re-established, the activating guides are folded as close as possible to the prosthesis and then cut; the acute fold of the wires within the guides' polypropylene conduits prevents their sliding backwards.
Both types I and II have been prepared in 3 sizes, yielding the following internal-diameter ranges: SMALL, from 13 to 22 mm; MEDIUM, from 20 to 28 mm; and LARGE, from 25 to 34 mm.
(Modified from Nazari S, Luzzana F, Banfi C, Mourad Z, Salvi S, Gaspari A, Nazari-Coerezza F. Expandable prosthesis for sutureless anastomosis in thoracic aorta prosthetic substitution. Eur J Cardiothorac Surg 1996;10:1003–9. © 1996 Springer-Verlag GmbH & Co. With permission from Elsevier Science.)
Improvements upon a previous version 3 of the device include the use of nickel-titanium alloy (Nitinol) instead of stainless steel. This alloy is expected to be compatible with magnetic resonance imaging during postoperative examination, even though study of the matter is not yet complete. Moreover, Nitinol appears to have mechanical qualities that are better suited to the device's working mechanism, and this, together with an improvement in the wire-looping design, now enables continuous control of diameter—even when the device is in contact with blood. Lack of control under that circumstance significantly hampered clinical use of the previous version. A further improvement consists of 4 metallic hooks on the outer surface, which enable firm fixation of the device to the aortic stump or graft.
Case Report
In March 2001, a 47-year-old man was admitted to our institution for evaluation of left upper-lobe bronchogenic adenocarcinoma that had infiltrated the distal aortic arch and upper descending aorta. Two years earlier, he had undergone exploratory thoracotomy at another hospital for this same malignancy, which at that time was judged to be unresectable due to infiltration of the descending aorta and the distal arch at the level of the left subclavian artery. Chemotherapy had substantially reduced the pulmonary mass, but the mass enlarged during the subsequent 2 years, and the patient underwent another exploratory thoracotomy at a 2nd hospital.
Upon his admission to our institution, re-staging of the neoplasm ruled out distant metastasis, and the patient underwent anterolateral thoracotomy. The hilar elements of the upper lobe were prepared and divided at the anterior (vein 1st, then bronchus, then artery), which left the lobe attached only to the aorta. After administering 7,500 IU of heparin intravenously, we established a partial (2 L/min) left bypass (left upper pulmonary vein–left femoral artery, a Biomedicus pump, and heparinized circuit) without the use of an oxygenator. The infiltrated aortic wall was resected en bloc with the upper lobe (Fig. 2, inset). A 28-mm Albograft prosthesis (Biomateriali Srl; Brindisi, Italy)—prepared earlier by sewing the cuff containing the expandable device into 1 end—was then positioned in the stump of the descending aorta and fixed with external ligatures (Fig. 2). The proximal anastomosis was then carried out with Prolene 4-0 running suture. A Dacron graft (8 mm) was used for connecting the left subclavian artery to the aortic prosthesis. The total clamping time was 24 minutes, a significant part of it spent on cleansing of the tracheobronchial angle and periaortic region after removal of the upper lobe and aortic wall; yet the expandable device enabled the distal aortic anastomosis to be performed easily, in less than 3 minutes.
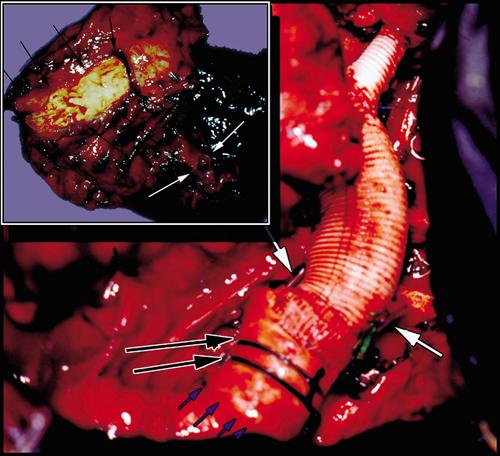
Fig. 2 Operative view of the prosthesis in place. Small blue arrows outline the limit of the expandable end and long black arrows indicate the 2 external ligatures used to stabilize the prosthesis and to achieve hemostasis; white arrows mark the limits of the activating wires, cut and folded within their polypropylene sheaths to prevent the wires from sliding backwards. Inset: Operative specimen including the left upper lobe (white arrows = bronchial stump) resected en bloc with the aortic wall (black arrows).
The postoperative course was complicated by basal right pneumonia and suboptimal left residual lobe ventilation. A chest radiograph shows the shape of the device's wires (Fig. 3). The patient was discharged after 2 weeks and underwent radiotherapy of the mediastinum thereafter.

Fig. 3 Postoperative chest radiograph showing the metallic loops of the expandable end (short arrows) and the cut and folded activating wires (larger arrows).
Commentary
Patients with bronchogenic cancer involving the vascular structures of the mediastinum are rarely surgical candidates. However, there have been reports (particularly in the Japanese literature 4–6) of occasional long-term survival after radical resection of an infiltrated thoracic aorta 7 or superior vena cava. Because of our patient's young age, together with the tumor's relatively slow growth, we considered this an acceptable case for radical surgery.
The availability of our device for a quick and easy aortic anastomosis greatly simplified that part of the procedure. A cancer case, in comparison with the much more common aneurysm cases, poses some additional surgical problems, such as a somewhat longer clamping time due to the necessity of complete removal of the aortic wall. More importantly, the proximity of the tumor to the left carotid artery precluded our use of the device for the proximal anastomosis, since that would have required circulatory arrest. Were this an aneurysm extending to the same aortic arch level, the device could also be used effectively at the proximal end of the graft, provided that the end with the expandable ring could reach healthy vascular wall within the aneurysmal wall (Fig. 4). 8

Fig. 4 The expandable end also can be positioned against aneurysmal wall, provided that its distal end reaches healthy vascular wall. Thrombosis of the tract between the ligature and the prosthesis will soon move the effective anastomosis line (*) to the point where a standard suture line would be.
(Reprinted with permission from the Society of Thoracic Surgeons. From Nazari S, Salvi S, Aluffi A, Visconti E, Rescigno G, Buniva P. Prosthesis for aortic arch substitution. Ann Thorac Surg 1997;64:1339–44. © 1997 The Society of Thoracic Surgeons.)
The main advantages of this device are an easier, quicker anastomosis and the absolute prevention of suture-line hemorrhage (no suture line). The expandable ring overcomes the previously described drawbacks 3 of the intraluminal ringed prosthesis 9,10 used in the past.
Footnotes
One of the authors (SN) holds international patents on the device described herein. This device is not yet commercially available.
Address for reprints: Stefano Nazari, MD, Residenza Parco 152, 20080 Basiglio – Milano 3, Italy
References
- 1.LeMaire SA, Miller CC 3rd, Conklin LD, Schmittling ZC, Koksoy C, Coselli JS. A new predictive model for adverse outcomes after elective thoracoabdominal aortic aneurysm repair. Ann Thorac Su rg 2001;71:1233–8. [DOI] [PubMed]
- 2.Coselli JS, LeMaire SA, Schmittling ZC, Koksoy C. Cerebrospinal fluid drainage in thoracoabdominal aortic surgery. Semin Vasc Surg 2000;13:308–14. [PubMed]
- 3.Nazari S, Luzzana F, Banfi C, Mourad Z, Salvi S, Gaspari A, Nazari-Coerezza F. Expandable prosthesis for sutureless anastomosis in thoracic aorta prosthetic substitution. Eur J Cardiothor Surg 1996;10:1003–9. [DOI] [PubMed]
- 4.Shimokawa S, Watanabe S, Sakasegawa K. Combined resection of T4 lung cancer with invasion of the descending thoracic aorta. Ann Thorac Surg 2000;69:971–2. [DOI] [PubMed]
- 5.Nishimura Y, Iwata S, Adachi A, Hayashida R, Hayashi A, Yo R, et al. Surgical indication in lung cancer and evaluation of extended combined resection [in Japanese]. Kyobu Geka 1988;41:797–800. [PubMed]
- 6.Kodama K, Doi D, Higashiyama M, Yokouchi H, Kabuto T, Kobayashi T, Hiraishi T. Combined resection of the descending aorta for lung cancer with aortic invasion [in Japanese]. Kyobu Geka 1995;48:367–71. [PubMed]
- 7.Klepetko W, Wisser W, Birsan T, Mares P, Taghavi S, Kupilik N, Wolner E. T4 lung tumors with infiltration of the thoracic aorta: is an operation reasonable? Ann Thorac Surg 1999;67:340–4. [DOI] [PubMed]
- 8.Nazari S, Salvi S, Aluffi A, Visconti E, Rescigno G, Buniva P. Prosthesis for aortic arch substitution. Ann Thorac Surg 1997;64:1339–44. [DOI] [PubMed]
- 9.Lemole GM, Strong MD, Spagna PM, Karmilowicz NP. Improved results for dissecting aneurysms. Intraluminal sutureless prosthesis. J Thorac Cardiovasc Surg 1982;83:249–55. [PubMed]
- 10.Lemole GM. Aortic replacement with sutureless intraluminal grafts. Tex Heart Inst J 1990;17:302–9. [PMC free article] [PubMed]


