Abstract
Background
The goal to eliminate the parasitic disease of poverty schistosomiasis as a public health problem is aligned with the 2030 United Nations agenda for sustainable development goals, including universal health coverage (UHC). Current control strategies focus on school-aged children, systematically neglecting adults. We aimed at providing evidence for the need of shifting the paradigm of schistosomiasis control programs from targeted to generalized approaches as key element for both the elimination of schistosomiasis as a public health problem and the promotion of UHC.
Methods
In a cross-sectional study performed between March 2020 and January 2021 at three primary health care centers in Andina, Tsiroanomandidy and Ankazomborona in Madagascar, we determined prevalence and risk factors for schistosomiasis by a semi-quantitative PCR assay from specimens collected from 1482 adult participants. Univariable and multivariable logistic regression were performed to evaluate odd ratios.
Results
The highest prevalence of S. mansoni, S. haematobium and co-infection of both species was 59.5%, 61.3% and 3.3%, in Andina and Ankazomborona respectively. Higher prevalence was observed among males (52.4%) and main contributors to the family income (68.1%). Not working as a farmer and higher age were found to be protective factors for infection.
Conclusions
Our findings provide evidence that adults are a high-risk group for schistosomiasis. Our data suggests that, for ensuring basic health as a human right, current public health strategies for schistosomiasis prevention and control need to be re-addressed towards more context specific, holistic and integrated approaches.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40249-023-01094-z.
Keywords: Schistosomiasis, Schistosoma haematobium, Schistosoma mansoni, Universal health coverage, Madagascar
Background
Access to health care is a human right and the urge of achieving universal health coverage (UHC) is emphasized by the United Nations (UN) in the sustainable development goals (SDGs) [1, 2]. The control of infectious diseases of poverty, and particularly those characterized by long term consequences, have been suggested as indicators towards UHC [3–5].
Schistosomiasis is a waterborne neglected disease of poverty prevalent in tropical areas but not limited to them [6]. It is caused by different species of the trematode schistosome, of which S. mansoni and S. haematobium are the most frequent worldwide [7]. The Neglected Tropical Diseases (NTDs) roadmap, released by the World Health Organization (WHO) in 2021 [8], targets the elimination of the disease as a public health problem by 2030 in all endemic countries. Progress on morbidity control has been made through vertical control strategies, especially by preventive chemotherapy through mass drug administration (MDA) with praziquantel in school aged children [9–11]. However, it has been reported that the implementation of vertical control programs can exacerbate health inequalities amplifying disparities based on sex, gender, socio-economic status and age [3, 12–14], hampering the achievement of UHC. Even though adults in high-risk areas and with occupational risk are theoretically eligible to receive annual MDA [15], campaigns including adults are rare [16]. In 2019, the WHO reported a global coverage rate of 67.2% in school aged children, while it was only 17.7% in adults [17]. The limited availability of praziquantel on the market alongside with the low MDA uptake of adults because of occupational duties, fear of side effects and understanding schistosomiasis as a pediatric disease, exclude de facto adults from national programs [16]. As a consequence, untreated adults do not only serve as infection reservoir for the community, but are also under risk to develop severe chronic forms of the disease [16].
Chronic intestinal schistosomiasis caused by S. mansoni can lead to hepato-splenomegaly and portal hypertension, chronic urogenital schistosomiasis caused by S. haematobium carries high risk of squamous bladder cancer and manifestations like genital schistosomiasis [7]. Both forms may present symptoms like chronic pain, fatigue and morbidities like anemia and undernutrition, resulting in a great loss of quality-adjusted life-years (QALY) [18, 19], impaired work capacity of adults [20] and finally reduced economic productivity [7]. Adapted guidelines for the diagnosis and treatment of chronic forms of schistosomiasis are often missing in endemic countries generating a lack of services aggravated by the low knowledge among the health-care workers of signs, symptoms and management of chronic forms of the disease, such as female genital schistosomiasis [8, 21]. Preventive chemotherapy at early stages of life, early diagnosis and treatment can prevent long-term consequences and increase QALY [22]. Diagnostic services are still limited in low resource settings because of a lack of affordable and easy to implement diagnostic tools [23]. Moreover, the health seeking behaviors for schistosomiasis care show several barriers as for example social stigma or opening hours of health services that often overlap with the working hours of the users limiting their accessibility [24, 25]. Even though treatment of chronic forms in adults might be available, it can be linked to out-of-pocket expenses as health interventions and transport costs to the health care centers are frequently not affordable for the affected populations [26]. Further, patients, especially women, with morbidities of chronic genital schistosomiasis, like infertility and vaginal discharge, fear marginalization when accessing health services as the symptoms resemble those of sexually transmitted diseases [27].
The life cycle of schistosome infection [7] justifies the adoption of a holistic approach combining MDA with health education programs and improved water, sanitation and hygiene (WASH) [28] measures in a One Health-oriented [29] approach to tackle the disease at its sources [8, 10]. Infection control measures should be encouraged in low resource settings to control morbidity, prevent chronic forms of the disease and meet the ambitious goal of the NTD roadmap [8]. However, several infection control interventions have so far proven to be ineffective for schistosomiasis prevention and control due to contextual barriers that demand the synergistic implementation of multiple measures in order to impact on the transmission of the disease [7, 30].
This study is based on data collected in Madagascar, a country highly endemic for schistosomiasis where both, S. mansoni and S. haematobium exist in distinct geographic areas [31, 32]. With the exception of few surveys conducted among adults the most of the existing data in the country refers to school-aged children with prevalence ranging between 15.2% and 87.7% [33–36].
Our study aims to provide prevalence estimates, describe risk associations of schistosomiasis in adults in the country and motivate a shift in the paradigm of schistosomiasis control programs from targeted to broader approaches as key element for both the elimination of schistosomiasis as a public health problem and the promotion of UHC.
Materials and methods
Study design, area and population
The planning, organization, implementation and analysis of this cross-sectional study was conducted by eight interdisciplinary collaborating institutes: the Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany; the German Centre for Infection Research, Hamburg-Borstel-Lübeck-Riems, Germany; the Centre d’Infectiologie Charles Mérieux, University of Antananarivo, Madagascar; University of Fianarantsoa Andrainjato, Madagascar; University Clinical Research Centre, Bamako, Mali; Leiden University Medical Centre, Netherlands; Johannes Gutenberg University Mainz, Germany.
This study was conducted at three Primary Health Care Centers (PHCCs) in Madagascar: Andina (20°30′58.8″S 47°09′05.2″E) and Tsiroanomandidy (18°46′21″S 46°02′57″E) in the centre, Ankazomborona (16°06′50″S 46°45′24″E) in the north western part of the island (Fig. 1). On the basis of the infrastructure and population size, Andina can be described as rural area, Ankazomborona and Tsiroanomandidy as peri-urban and urban areas, respectively [37], even though this classification cannot be aligned to the standard definition of the UN statistical commission due to the limited available data of the study sites [38].
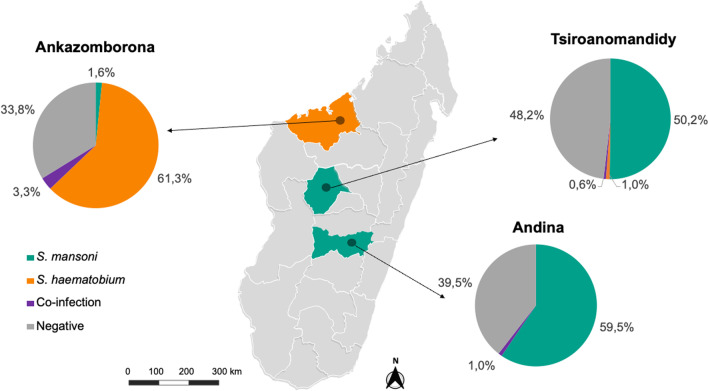
Fig. 1.
Map of Madagascar showing the crude prevalence of Schistosoma species and co-infection status based on PCR at the study sites, n = 1482. Map was adapted using a template from https://yourfreetemplates.com
Information sessions about the study were organized in the catchment areas of the included PHCCs. If interested, individuals were asked to attend PHCCs to assess their eligibility for the study (18 or older, not pregnant and willing to comply with protocol requirements). If eligible, an informed consent was signed in case of enrolment.
Data collection and management
Data collection took place between March 2020 and January 2021. Background characteristics of the participant were collected by means of a case report form (CRF). Completed CRFs were checked for missing entries manually by local study nurses and doctors following standard operating procedures. CRF data were fed into the database REDCap® (Vanderbilt University, Nashville, USA) via double data entry by two independent operators. Quality control of data processing and data validation was undertaken at regular intervals during the course of data entry.
Sample collection and management
For this study a sample of 9 ml of venous blood was collected from each participant. Blood samples were centrifuged at 1600 × g for 10 min and two aliquots of 1 ml each of serum was produced by qualified laboratory technicians. Samples were stored according to required quality standards at – 20 °C and transferred to long-term storage at – 80 °C in Madagascar. At the end of sample collection, one of the serum aliquots was shipped to Hamburg on dry ice and stored at – 80 °C until the analysis was performed.
Sample analysis
DNA was extracted with QIAamp MinElute ccfDNA Mini Kit from 1 ml serum according to the manufacturer’s instructions (Qiagen, Hilden, Germany). Extracted DNA was stored at – 20 ℃.
The semi quantitative standardized polymerase-chain reaction (qPCR) analysis was based on the previously published protocol by Frickmann et al. [39] with described sensitivity of 94.9% and specificity of 98.4%. The primers used for the amplification were: S. mansoni—Forward Primer: 5′ CAA CCG TTC TAT GAA AAT CGT TGT 3′, S. mansoni—Reverse Primer: 5′ CCA CGC TCT CGC AAA TAA TCT 3′, S. mansoni—Probe: ‘Fam-TCC GAA ACC ACT GGA CGG ATT TTT ATG AT-BHQ1′, S. haematobium—Forward Primer: 5′ GAT CTC ACC TAT CAG ACG AAA C 3′, S. haematobium—Reverse Primer: 5′ TCA CAA CGA TAC GAC CAA C 3′, S. haematobium—Probe: 5′ Joe-TGT TGG AAG ZGC CTG TTT CGC AA-BHQ1 3′ all synthesized by Biomers.net, Ulm, Germany. Primers and a probe were added for the detection of Phocid herpesvirus (PhHV) DNA as internal positive control (PhHV—Forward Primer: 5′ GGG CGA ATC ACA GAT TGA ATC 3′, PhHV—Reverse Primer: 5′ GCG GTT CCA AAC GTA CCA A 3′, PhHV—Probe: 5′ Cy5.5-TTT TTA TGT GTC CGC CAC CA-BBQ 3′).
The qPCRs were performed in a total reaction volume of 25 µl containing 12.5 µl of HotStarTaq Mastermix (Qiagen, Hilden, Germany), 1.75 × 10−8 mol MgCl2, 1.25 × 10−13 mol of each Schistosoma primer, 6.25 × 10−14 mol of the Schistosoma probes, 1.0 × 10−12 mol of the PhHV primer, 1.25 × 10−12 mol of the PhHV probe, 1.425 µg of the PhHV DNA template, 4 × 10−5 mg bovine serum albumin and 5 µl DNA eluate. In each run two positive controls (S. mansoni and S. haematobium DNA) and a negative control (H2O) were included. The qPCR was conducted using Corbett RotorGene 6000 (Qiagen, Redwood City, USA) with following steps: 15 min at 95 °C followed by 50 cycles of 15 s at 95 °C and 60 seconds at 60 °C with an initial touchdown from 65 to 60 °C in the first 11 cycles. The readout resulted from the RotorGene 6000 Software v.7.87 (Qiagen, Hilden, Germany). Results with a clean sigmoid curve within the PCR cycles were considered positive [39].
Statistical analysis
All analyses were conducted using R® v.4.1.2 (R Foundation for statistical computing, Vienna, Austria). Continuous variables were described using median and interquartile ranges (IQR). Categorical variables were presented using frequencies and percentages.
To estimate the prevalence of schistosome infection among the study population, relative frequencies of positive test results along with exact 95% confidence intervals (CIs) were determined.
For the risk factor analyses, the study population was divided into two distinct groups according to the areas of endemicity based on the species-specific PCR results. Test result distribution among individuals with various risk factors (study site, sex, age, education level, ever been treated with praziquantel, working as a farmer and being the main contributor to the family income) was described using frequencies and percentages. Univariable and multivariable logistic regression were performed to derive unadjusted and adjusted odds ratios (OR) and 95% CIs.
Ethical consideration
Ethical clearance was obtained from the National Ethics Committee of Madagascar (ref. no. N°23-MSANP/CERBM, 05/03/2018) and the Ethics Committee of the Hamburg State Medical Chamber in Germany (ref. no. PV7019-4419-BO-ff, 29/10/2019).
All participants were informed about the aims of the study and its procedures in the local language (Malagasy). Study participation was voluntary and informed consent for the participation was obtained from the individual participant by signature or, in case of illiteracy, through a thumbprint in the presence of an independent witness. In all cases, participants had the right to refuse participation and withdraw the informed consent at any time without giving reasons. No monetary incentives were released to participate in the study and exclusively travel costs were reimbursed, calculated on the basis of the distance of the participants’ households from the PHCC.
Results
Study population
A total of 1482 adults were included in the study. Missing data are reported in the sections profession (0.8%) and praziquantel treatment (4.6%). All study sites were equally represented (ranging between 488 and 498 individuals across sites). The sex was balanced with highest proportion of females in Tsiroanomandidy (60.2%, Table 1), 54.5% females in Ankazomborona and 47.6% females in Andina.
Table 1.
Study population characteristics
| Characteristics | Andina n (%) | Tsiroanomandidy n (%) | Ankazomborona n (%) |
|---|---|---|---|
| Total | 496 (33.5) | 498 (33.6) | 488 (32.9) |
| Female sex | 236 (47.6) | 300 (60.2) | 266 (54.5) |
| Age (years)1 | 38.5 (27.0–50.0) | 36.0 (25.0–49.8) | 28.0 (21.0–40.0) |
| Age group (years)1 | |||
| 18–29 | 155 (31.2) | 181 (36.3) | 255 (52.3) |
| 30–39 | 101 (20.4) | 92 (18.5) | 97 (19.9) |
| 40–49 | 104 (21.0) | 100 (20.1) | 68 (13.9) |
| 50 + | 136 (27.4) | 125 (25.1) | 68 (13.9) |
| Education level | |||
| Secondary school or higher | 134 (27.0) | 280 (56.2) | 211 (43.2) |
| Primary school | 313 (63.1) | 160 (32.1) | 152 (31.2) |
| Never gone to school | 49 (9.9) | 58 (11.7) | 125 (25.6) |
| Never treated with praziquantel | 353 (71.2) | 142 (30.9) | 118 (25.8) |
| Working as a farmer | 471 (95.0) | 314 (63.1) | 370 (76.4) |
| Main contribution to family income | |||
| Yes | 338 (68.1) | 311 (62.4) | 257 (52.7) |
| Of whom female | 140 (41.4) | 152 (48.9) | 101 (39.3) |
| No | 158 (31.9) | 187 (37.6) | 231 (47.3) |
| Of whom female | 96 (60.8) | 148 (79.1) | 165 (71.4) |
Stratified by study sites (n = 1482)
1Median age and interquartile range
Participants’ age ranged from 18 to 84 years. Lowest median age of 28 years was described in participants from Ankazomborona. The majority of participants were aged between 18 and 29 years across all sites.
Most of the participants reported an education level of secondary school or higher in Tsiroanomandidy and in Ankazomborona. In Andina the majority had only completed primary school.
More than two thirds of the population has never been treated with praziquantel in Andina while it was less than a third at the other two study sites.
Farming was the most common occupation at all sites. However, the distribution was not equal: in Andina almost everyone was a farmer, while there were less in Ankazomborona and rarely in Tsiroanomandidy.
At all sites most of participants were the main contributor to the family income. In the group of the main contributors a higher percentage were males, while a higher percentage of females stated that they were not the main contributor.
Prevalence of schistosome infection in study area
Of importance, the S. mansoni infection showed dominant prevalence of 59.5% (95% CI: 55.0–63.8) in the rural area Andina and 50.2% (95% CI: 45.7–54.7) in the urban area Tsiroanomandidy in the central area of Madagascar and 1.6% (95% CI: 0.7–3.2) in peri-urban area Ankazomborona in the north-western part (Fig. 1). Mono-infections with S. haematobium were not found in Andina, while they were identified in 1.0% (95% CI: 0.3–2.3) of the participants in Tsiroanomandidy and 61.3% (95% CI: 56.8–65.6) in Ankazomborona. The prevalence of co-infections was generally low with 1.0% (95% CI: 0.3–2.3) in Andina, 0.6% (95% CI: 0.1–1.8) in Tsiroanomandidy and highest in Ankazomborona with 3.3% (95% CI: 1.9–5.3). Additional background information is listed in the Additional file 1: Table S1, specifying place of birth and residence of co-infected participants (n = 24). Endemicity of place of birth and place of home differed in six participants (25%), who were all participants in Ankazomborona.
Risk factors for schistosome infection
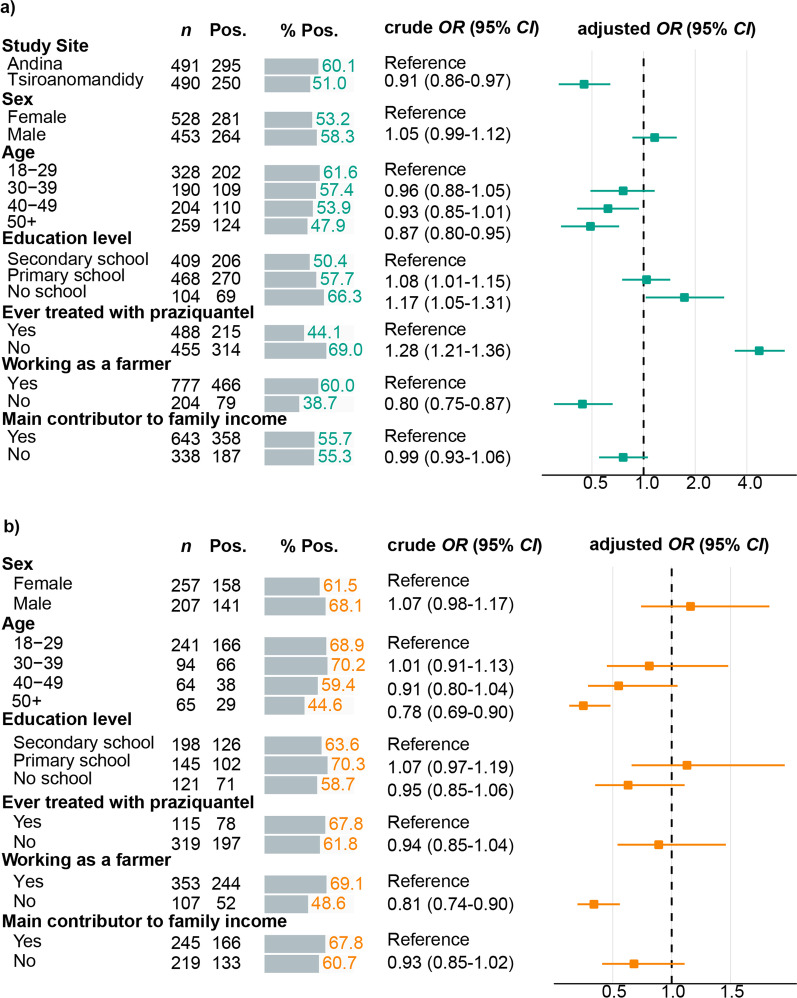
For the risk factor analysis co-infected participants (n = 24) and participants positive for the non-endemic species (S. mansoni area: n = 5, S. haematobium area: n = 8) were excluded yielding n = 981 participants from S. mansoni and n = 464 participants from S. haematobium endemic regions evaluable for risk factor assessments. Associations between risk factors and S. mansoni and S. haematobium infection status from logistic regression models are shown in Fig. 2. Adults attending the PHCC Tsiroanomandidy had lower odds of infection with S. mansoni when compared to adults attending the PHCC in Andina (adjusted OR = 0.45, 95% CI: 0.32–0.64). Higher infection rates of S. mansoni (adjusted OR = 1.16, 95% CI: 0.86–1.56) and S. haematobium (adjusted OR = 1.16, 95% CI: 0.74–1.83) were more likely to be observed in males than in females. Age groups older than 18–29 years, especially the 50 + age group, tended to have lower odds of infection with S. mansoni (adjusted OR = 0.49, 95% CI: 0.33–0.72) and S. haematobium (adjusted OR = 0.25, 95% CI: 0.13–0.48). Illiteracy increased the odds of infection with S. mansoni (adjusted OR = 1.73, 95% CI: 1.03–2.95) while infection rates with S. haematobium varied across education levels being lowest in participants with no school education (58.7%). Odds of infection also varied among both species regarding the previous treatment of praziquantel. Participants, who have never been treated with praziquantel before, tended to have higher odds of infection with S. mansoni (adjusted OR = 4.72, 95% CI: 3.40–6.65), while chances of infection with S. haematobium were lower (adjusted OR = 0.89, 95% CI: 0.54–1.46). If not working as a farmer participants had lower odds of infection with S. mansoni (adjusted OR = 0.44, 95% CI: 0.30–0.66) and S. haematobium (adjusted OR = 0.34, 95% CI: 0.20–0.56). Similar S. mansoni positivity rates were observed between being main contributor to the family income and not being main contributor to the family (adjusted OR = 0.76, 95% CI: 0.55–1.06). Odds of infection with S. haematobium were lower when not being main contributor to the family income (adjusted OR = 0.68, 95% CI: 0.41–1.11).
Fig. 2.
Risk factor analysis for PCR positivity of participants enrolled at (a) Schistosoma mansoni (n = 981) and (b) S. haematobium (n = 464) endemic study sites. Positivity rates, univariable and multivariable logistic regression with effect estimates in terms of crude and adjusted ORs and 95% CI. Variables that were controlled for in the multivariable regression, included: study site (when applicable), sex, age groups, education level, ever treated with praziquantel, working as a farmer and being main contributor to the family income. CI confidence interval, n sample size, OR odds ratio, PCR polymerase chain reaction, Pos. positivity frequencies, % Pos. positivity percentages
Discussion
This cross-sectional study reports findings of elevated prevalence of schistosome infections in adults with S. mansoni in central and S. haematobium in north-west of Madagascar. Our data show an alarming gap for the accomplishment of UHC in endemic countries like Madagascar as adults are at risk of infection with schistosomiasis, but excluded by structured control programs for the disease.
To our knowledge this is the first study describing prevalence of both endemic species in Malagasy adults with a highly sensitive and specific serum PCR. With prevalence of around 60% in a S. mansoni and a S. haematobium endemic area we found similar prevalence as previous studies on different age groups in the country, indicating that beside school-aged children, also adults carry a high burden of infection in Madagascar. The geographic distribution of schistosome species here described, is also aligned with the current literature [31, 40] and seems to be associated with the distribution of the different species of freshwater snails [41] in the country.
Based on our findings, our study areas can be classified by WHO definition as high prevalence (> 50%) areas with need for interventions [10]. However, we show that less than half of the infected study population was never treated with praziquantel suggesting that they could have been a carrier of the infection for longer with a higher likelihood of developing the chronic form of the disease and serving as reservoir for the community [16]. Interestingly, in the S. mansoni endemic population, never having been treated with praziquantel, represents a risk factor for adult infections. Since it is extensively described that recovering from treatment does not represent a protective factor for further infections, we can speculate that exposure to treatment and/or MDA campaigns can raise awareness for the disease and induce less risky behaviors towards the infection. However, our findings show that treatment with praziquantel did not lower the odds of infection in S. haematobium areas. This supports the concept that many risk factors depend on the geographical and cultural contexts which need to be taken into account when planning interventions.
Our study provides new insights into the distribution of schistosomiasis among Malagasy adults, which will be essential for the shift of schistosomiasis control programs from targeted to broader groups of intervention as key element for the elimination of schistosomiasis and the promotion of UHC. As an example, the frequency of infection in our study population significantly decreased with increasing age, meaning that in these areas, control strategies should also target adults especially within the young working population of Madagascar. It is concerning to notice that young adults for whom productivity is supposed to be highest, are highly infested with a parasite notably known to weaken individuals productivity, creating disabilities and perpetuating the vicious cycle of poverty [7, 25, 42, 43].
Further, we observed higher prevalence of schistosome spp. in the rural (Andina) and peri-urban (Ankazomborona) areas than in the urban area (Tsiroanomandidy) and higher odds of infection with S. mansoni in Andina compared to Tsiroanomandidy. This is aligned with country data showing that rural populations of Madagascar are six times more likely to live without clean drinking water and twice as likely to have no access to sanitation in comparison to urban areas, increasing the risk to have contact with infested water [44]. Also, we observed a lower level of education in the rural area: while 56% of the participants in Tsiroanomandidy (urban) and 43% of the participants in Ankazomborona (peri-urban) reported a secondary school or higher education, only 27% of the participants in Andina (rural) had a secondary school or higher education. In the risk factor analysis we saw that a low level of education was associated with a higher frequency of schistosome infection in the S. mansoni group, which was previously explained by a higher likelihood of health illiteracy [45]. However, we could not observe the same in the S. haematobium group.
Very interestingly, our data show how two main characteristics identified as risk factors in the S. mansoni area (no school education and never been treated with praziquantel before) could not be described in the S. haematobium area. This might be due to the different cultural contexts, but in line with Wiegand, et al. [46], it can also be suggested that measurements for the two species should differ in order to prevent a neglect of diverting transmission and risk factors of the schistosome spp.
Interestingly, we also detected co-infections with the two endemic species and mono-infections with the non-endemic species (Figure 1). Even though the number of co-infected individuals was not particularly high, our data suggests that further investigations towards the migration history might reveal further risk factors of coinfections in Madagascar. This shows the importance also in diagnostics to promote tools that can allow the detection of both species with the same sample in order not to miss the presence of non-endemic species, which can further delay the diagnosis and treatment of schistosomiasis and lead to more complicated chronic forms of the disease especially in our global world, where mobility is becoming more frequent even in traditionally settled communities.
Previous control strategies by the WHO already recommend preventive treatment for adults with occupations, which bring them in steady contact with infested water, like farming, fishing and irrigation work [15] even though the alignment of national strategies is still delayed. Our data confirms the importance of occupational exposure as not working as a farmer, represented a strong protective factor for schistosome infection. As in Madagascar rice farming is the main occupation of the population [47], more tailored studies to deeply investigate the specific risk factors associated to farming are urgent in order to adapt infection control strategies. The higher prevalence of both species in men than in women could be attributed to the fact that in our study population men were more often the main contributors to the family income and thus engaging more in occupations like fishing or farming [48]. But in fact, in areas where farming or fishing is mostly done by women, higher prevalence of schistosomiasis was reported in women [42]. This shows that in shifting societies, occupational risk for schistosome infection should be addressed in a gender-neutral manner and accounting for the actual occupational risk of the specific communities.
This study has strengths and limitations. As a main strength, it assessed the schistosome infection status through a PCR methodology highly sensitive and specific allowing to distinguish the presence of two different schistosome species simultaneously [49–51]. Further, our study assessed the infection status of adults for the first time in rural, peri-urban and urban settings in Madagascar. The limitation of the applied methodology is that the test results may stay positive for a certain time following treatment [49, 52]. However, given that our sampling areas show a of high risk of transmission, we cannot exclude re-infections shortly after treatment. Moreover, the sampling approach through community workers did not guarantee the representativeness of the study population. Additional risk factors such as daily water activities, access to sanitation and hygiene behavior [53] could not have been explored in the frame of this study due to the structure of the investigation tool.
Conclusions
Our study provides evidence of a high prevalence of schistosomiasis in adult populations of Madagascar. Diagnosis and treatment of the disease are a clear unmet medical need hampering the goal of schistosomiasis elimination as public health problem in the country by 2030. As our data show that prevalence of schistosomiasis can differ by schistosome species, geographic location, age, sex and other factors it requires targeted context specific, holistic and integrated control strategies to reduce morbidity and work towards guaranteeing the essential human right of health in all individuals. A shift in schistosomiasis control strategies is urgent to reinforce fragile health systems, positively impacting the fight against other diseases and health seeking behaviors of populations left behind from the UHC goal.
Supplementary Information
Additional file 1: Table S1. Mobility history of co-infected participants.
Acknowledgements
We are grateful to all the participants, and the field team including drivers and IT staff who contributed to the success of the study. We thank the German Centre for Infection research (DZIF) of the German Federal Ministry of Education and Research, which funded the project. A special thanks to all the country authorities who allowed the implementation of this study and were supportive of its activities.
Abbreviations
- CI
Confidence interval
- CRF
Case report form
- DNA
Deoxyribonucleic acid
- IQR
Interquartile range
- MDA
Mass drug administration
- NTD
Neglected tropical disease
- OR
Odds ratio
- PHCC
Primary health care centre
- PhHV
Phocid Herpes virus
- QALY
Quality-adjusted life-years
- qPCR
Quantitative polymerase chain reaction
- SDGs
Sustainable development goals
- WASH
Water, sanitation and hygiene
- WHO
World Health Organization
- UHC
Universal health coverage
- UN
United Nations
Author contributions
DF and SKG contributed to the conceptualization of the manuscript. The main writers of the manuscript were SKG and DF. TR, RAR, ARR, ZTR, RMR, PRS, NR, RR, PK and DF contributed to the field implementation, data and sample collection. AZ and HvT performed the laboratory testing. AJ, PR and COD contributed to data management and cleaning. SKG performed data analysis with the support of PR and under the supervision of EL. NGS, DF, RR, RAR, GvD, ET and JM guaranteed the required funding for the study. All authors revised and approved the manuscript before submission. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was partly funded by the German Centre for Infection research (DZIF) through the projects SCHISDIMA (project number: TI 03.907) and NAMASTE (project number: 8008803819).
Availability of data and materials
Data and materials can be made available upon specific request.
Declarations
Ethics approval and consent to participate
Ethical clearance was obtained from the National Ethics Committee of Madagascar (ref. no. N°23-MSANP/CERBM, 05/03/2018) and the Ethics Committee of the Hamburg State Medical Chamber in Germany (ref. no. PV7019-4419-BO-ff, 29/10/2019). All participants were informed about the aims of the study and its procedures in the local language (Malagasy). Study participation was voluntary and informed consent for the participation was obtained from the participant by signature or, in case of illiteracy, through a thumbprint in the presence of an independent witness.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.United Nations. Transforming our world: The 2030 Agenda for sustainable development. 2015.
- 2.Universal Health Coverage. https://www.who.int/news-room/fact-sheets/detail/universal-health-coverage-(uhc). Accessed 3 Dec 2022.
- 3.Dean L, Ozano K, Adekeye O, Dixon R, Fung EG, Gyapong M, et al. Neglected Tropical Diseases as a “litmus test” for Universal Health Coverage? Understanding who is left behind and why in Mass Drug Administration: lessons from four country contexts. PLoS Negl Trop Dis. 2019;13:e0007847. doi: 10.1371/journal.pntd.0007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzpatrick C, Engels D. Leaving no one behind: a neglected tropical disease indicator and tracers for the Sustainable Development Goals. Int Health. 2016;8(Suppl 1):i15–i18. doi: 10.1093/inthealth/ihw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Investing to Overcome the Global Impact of Neglected Tropical Diseases. Geneva; 2015.
- 6.Boissier J, Grech-Angelini S, Webster BL, Allienne J-F, Huyse T, Mas-Coma S, et al. Outbreak of urogenital schistosomiasis in Corsica (France): an epidemiological case study. Lancet Infect Dis. 2016;16:971–979. doi: 10.1016/S1473-3099(16)00175-4. [DOI] [PubMed] [Google Scholar]
- 7.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou X-N. Schistosomiasis Nat Rev Dis Primers. 2018;4:1–19. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. Geneva; 2021.
- 9.Status of schistosomiasis endemic countries 2021. https://apps.who.int/neglected_diseases/ntddata/sch/sch.html. Accessed 22 Dec 2022.
- 10.World Health Organization. WHO guideline on control and elimination of human schistosomiasis. Geneva; 2022. [PubMed]
- 11.Kokaliaris C, Garba A, Matuska M, Bronzan RN, Colley DG, Dorkenoo AM, et al. Effect of preventive chemotherapy with praziquantel on schistosomiasis among school-aged children in sub-Saharan Africa: a spatiotemporal modelling study. Lancet Infect Dis. 2022;22:136–149. doi: 10.1016/S1473-3099(21)00090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fusco D, Martínez-Pérez GZ, Remkes A, De Pascali AM, Ortalli M, Varani S, et al. A sex and gender perspective for neglected zoonotic diseases. Front Microbiol. 2022;13. [DOI] [PMC free article] [PubMed]
- 13.Rilkoff H, Tukahebwa EM, Fleming FM, Leslie J, Cole DC. Exploring gender dimensions of treatment programmes for neglected tropical diseases in Uganda. PLoS Negl Trop Dis. 2013;7:e2312. doi: 10.1371/journal.pntd.0002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohn DA, Kelly MP, Bhandari K, Zoerhoff KL, Batcho WE, Drabo F, et al. Gender equity in mass drug administration for neglected tropical diseases: data from 16 countries. Int Health. 2019;11:370–378. doi: 10.1093/inthealth/ihz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schistosomiasis. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis. Accessed 15 Nov 2022.
- 16.Faust CL, Osakunor DNM, Downs JA, Kayuni S, Stothard JR, Lamberton PHL, et al. Schistosomiasis control: leave no age group behind. Trends Parasitol. 2020;36:582–591. doi: 10.1016/j.pt.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Schistosomiasis and soil-transmitted helminthiases: numbers of people treated in 2019. Geneva; 2019.
- 18.Fürst T, Silué KD, Ouattara M, N’Goran DN, Adiossan LG, N’Guessan Y, et al. Schistosomiasis, soil-transmitted helminthiasis, and sociodemographic factors influence quality of life of adults in Côte d’Ivoire. PLoS Negl Trop Dis. 2012;6:e1855. doi: 10.1371/journal.pntd.0001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nascimento GL, Domingues ALC, de Ximenes RAA, Itria A, Cruz LN, de Oliveira MRF. Quality of life and quality-adjusted life years of chronic schistosomiasis mansoni patients in Brazil in 2015. Trans R Soc Trop Med Hyg. 2018;112:238–244. doi: 10.1093/trstmh/try038. [DOI] [PubMed] [Google Scholar]
- 20.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. The Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 21.Christinet V, Lazdins-Helds JK, Stothard JR, Reinhard-Rupp J. Female genital schistosomiasis (FGS): from case reports to a call for concerted action against this neglected gynaecological disease. Int J Parasitol. 2016;46:395–404. doi: 10.1016/j.ijpara.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 22.King CH, Olbrych SK, Soon M, Singer ME, Carter J, Colley DG. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl Trop Dis. 2011;5:e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amoah AS, Hoekstra PT, Casacuberta-Partal M, Coffeng LE, Corstjens PLAM, Greco B, et al. Sensitive diagnostic tools and targeted drug administration strategies are needed to eliminate schistosomiasis. Lancet Infect Dis. 2020;20:e165–e172. doi: 10.1016/S1473-3099(20)30254-1. [DOI] [PubMed] [Google Scholar]
- 24.Dawkins B, Renwick C, Ensor T, Shinkins B, Jayne D, Meads D. What factors affect patients’ ability to access healthcare? An overview of systematic reviews. Tropical Med Int Health. 2021;26:1177–1188. doi: 10.1111/tmi.13651. [DOI] [PubMed] [Google Scholar]
- 25.Disability. https://www.who.int/news-room/fact-sheets/detail/disability-and-health. Accessed 2 Oct 2022.
- 26.Evans DB, Hsu J, Boerma T. Universal health coverage and universal access. Bull World Health Organ. 2013;91:546–546A. doi: 10.2471/BLT.13.125450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orish VN, Morhe EKS, Azanu W, Alhassan RK, Gyapong M. The parasitology of female genital schistosomiasis. Curr Res Parasitol Vector Borne Dis. 2022;2:100093. doi: 10.1016/j.crpvbd.2022.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Water, Sanitation and Hygiene (WASH). https://www.unicef.org/wash. Accessed 3 Dec 2022.
- 29.One health. https://www.who.int/health-topics/one-health#tab=tab_1. Accessed 3 Dec 2022.
- 30.World Health Organization. Towards universal coverage for preventive chemotherapy for Neglected Tropical Diseases: guidance for assessing “who is being left behind and why.” Geneva; 2017.
- 31.Deka MA. Predictive risk mapping of schistosomiasis in madagascar using ecological niche modeling and precision mapping. Trop Med Infect Dis. 2022;7:15. doi: 10.3390/tropicalmed7020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté L-A, Garba A, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz NG, Rakotozandrindrainy R, Heriniaina JN, Randriamampionona N, Hahn A, Hogan B, et al. Schistosoma mansoni in schoolchildren in a Madagascan highland school assessed by PCR and sedimentation microscopy and Bayesian estimation of sensitivities and specificities. Acta Trop. 2014;134:89–94. doi: 10.1016/j.actatropica.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Rasoamanamihaja CF, Rahetilahy AM, Ranjatoarivony B, Dhanani N, Andriamaro L, Andrianarisoa SH, et al. Baseline prevalence and intensity of schistosomiasis at sentinel sites in Madagascar: informing a national control strategy. Parasit Vectors. 2016;9:50. doi: 10.1186/s13071-016-1337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spencer SA, Linder C, Penney JMS, Russell HJ, Hyde K, Sheehy C, et al. Five-year follow-up on the prevalence and intensity of infections of Schistosoma mansoni in a hard-to-reach district of Madagascar. Am J Trop Med Hyg. 2021;104:1841–1850. doi: 10.4269/ajtmh.20-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson KE, Grewal EP, Pritt BS, Lloyd M, Stephano AM, Fardine M, et al. Schistosomiasis prevalence and low-cost diagnostics in rural Northwestern Madagascar: a pilot study. J Global Health Reports. 2021;5:e2021034. doi: 10.29392/001c.22123. [DOI] [Google Scholar]
- 37.Madagascar en chiffres. https://www.instat.mg/madagascar-en-chiffres. Accessed 22 Mar 2022.
- 38.UN Statistical Commission. A recommendation on the method to delineate cities, urban and rural areas for international statistical comparisons. 2020.
- 39.Frickmann H, Lunardon L-M, Hahn A, Loderstädt U, Lindner AK, Becker SL, et al. Evaluation of a duplex real-time PCR in human serum for simultaneous detection and differentiation of Schistosoma mansoni and Schistosoma haematobium infections—cross-sectional study. Travel Med Infect Dis. 2021;41:102035. doi: 10.1016/j.tmaid.2021.102035. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann T, Carsjens I, Rakotozandrindrainy R, Girmann M, Randriamampionona N, Maïga-Ascofaré O, et al. Serology- and blood-PCR-based screening for schistosomiasis in pregnant women in madagascar—a cross-sectional study and test comparison approach. Pathogens. 2021;10:722. doi: 10.3390/pathogens10060722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stothard JR, Brémond P, Andriamaro L, Sellin B, Sellin E, Rollinson D. Bulinus species on Madagascar: molecular evolution, genetic markers and compatibility with Schistosoma haematobium. Parasitology. 2001;123(Suppl):S261–275. doi: 10.1017/S003118200100806X. [DOI] [PubMed] [Google Scholar]
- 42.Bakuza JS, Denwood MJ, Nkwengulila G, Mable BK. Estimating the prevalence and intensity of Schistosoma mansoni infection among rural communities in Western Tanzania: the influence of sampling strategy and statistical approach. PLoS Negl Trop Dis. 2017;11:e0005937. doi: 10.1371/journal.pntd.0005937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bassa FK, Eze IC, Assaré RK, Essé C, Koné S, Acka F, et al. Prevalence of Schistosoma mono- and co-infections with multiple common parasites and associated risk factors and morbidity profile among adults in the Taabo health and demographic surveillance system, South-Central Côte d’Ivoire. Infect Dis Poverty. 2022;11:3. doi: 10.1186/s40249-021-00925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unicef. Water, Sanitation and Hygiene (WASH) Sectoral and OR+ (Thematic) Report 2018. Madagascar; 2019.
- 45.Raghupathi V, Raghupathi W. The influence of education on health: an empirical assessment of OECD countries for the period 1995–2015. Arch Public Health. 2020;78:20. doi: 10.1186/s13690-020-00402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiegand RE, Fleming FM, de Vlas SJ, Odiere MR, Kinunghi S, King CH, et al. Defining elimination as a public health problem for schistosomiasis control programmes: beyond prevalence of heavy-intensity infections. Lancet Glob Health. 2022;10:e1355–e1359. doi: 10.1016/S2214-109X(22)00287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.The Commune Census of Madagascar in 2001. http://www.ilo.cornell.edu/ilo/data.html. Accessed 15 Sep 2022.
- 48.Ayabina D, Clark J, Bayley H, Lamberton P, Turner J, Hollingsworth T. Gender-related differences in prevalence, intensity and associated risk factors of Schistosoma infections in Africa: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15:e0009083. doi: 10.1371/journal.pntd.0009083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuss A, Mazigo HD, Mueller A. Evaluation of serum-based real-time PCR to detect Schistosoma mansoni infection before and after treatment. Infect Dis Poverty. 2020;9:74. doi: 10.1186/s40249-020-00698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann Trop Med Parasitol. 2008;102:625–633. doi: 10.1179/136485908X337490. [DOI] [PubMed] [Google Scholar]
- 51.Cnops L, Soentjens P, Clerinx J, Van Esbroeck M. A Schistosoma haematobium-specific real-time PCR for Diagnosis of urogenital schistosomiasis in serum samples of international travelers and migrants. PLoS Negl Trop Dis. 2013;7:e2413. doi: 10.1371/journal.pntd.0002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wichmann D, Panning M, Quack T, Kramme S, Burchard G-D, Grevelding C, et al. Diagnosing schistosomiasis by detection of cell-free parasite DNA in human plasma. PLoS Negl Trop Dis. 2009;3:e422. doi: 10.1371/journal.pntd.0000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grimes JET, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The relationship between water, sanitation and schistosomiasis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8:e3296. doi: 10.1371/journal.pntd.0003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Mobility history of co-infected participants.
Data Availability Statement
Data and materials can be made available upon specific request.