Abstract
Cerebral edema (CE) is a potentially devastating complication of diabetic ketoacidosis (DKA) that almost exclusively occurs in children. Since its first description in 1936, numerous risk factors have been identified; however, there continues to be uncertainty concerning the mechanisms that lead to its development. Currently, the most widely accepted hypothesis posits that CE occurs as a result of ischemia–reperfusion injury, with inflammation and impaired cerebrovascular autoregulation contributing to its pathogenesis. The role of specific aspects of DKA treatment in the development of CE continues to be controversial. This review critically examines the literature on the pathophysiology of CE and attempts to categorize the findings by types of brain injury that contribute to its development: cytotoxic, vasogenic, and osmotic. Utilizing this scheme, we propose a multifactorial pathway for the development of CE in patients with DKA.
Keywords: brain injury, cerebral edema, diabetes mellitus, diabetic ketoacidosis, pediatrics
1 |. BACKGROUND
Diabetic ketoacidosis (DKA) complicated by cerebral edema (CE), a phenomenon first described in 1936,1 is associated with considerable morbidity and mortality.2,3 Clinically apparent or overt CE occurs in approximately 0.3% to 1% of DKA events.4–7 Neuroimaging studies; however, have shown that some degree of CE is present in more than 50% of children with DKA.8 In addition, evidence of neuronal injury, even in the absence of radiographic evidence of CE, has been suggested by the observation of decreased N-acetylaspartate-to-creatine ratios in the basal ganglia on magnetic resonance spectroscopy (MRS).9 In many cases, the injury is largely asymptomatic or associated with only minor mental status changes and is thus considered to be subclinical.8,10–13
Signs and symptoms of clinically apparent CE (Table 1) usually become evident within the first 12 hours of treatment, but can occur before treatment has been initiated4,14–18 or, rarely, as late as 24 to 48 hours after starting treatment.4,19 Overt CE complicating DKA is fatal in 20%–30% of individuals20,21 and, overall, CE accounts for approximately 50%–90% of childhood diabetes-related deaths.4–6 Between 10% and 35% of survivors of CE have residual disabilities ranging from mild neurological impairment to a vegetative state.4–6 The finding of both acute and long-term impairment of cognition and memory function in children with a history of DKA indicates that cerebral injury occurs even without evidence of overt CE.22–30
TABLE 1.
Warning signs and symptoms of clinically apparent cerebral edema
| Signs and symptomsa |
|---|
| Progressively worsening headache or onset of headache after starting DKA treatment |
| Change in mental status (restlessness, irritability, drowsiness, confusion, incontinence) |
| Development of focal neurological signs (cranial nerve palsies, abnormal pupillary responses) |
| Recurrence of emesis |
| Slowing of heart rate (unrelated to sleep or volume resuscitation) |
| Rising blood pressure |
| Irregular respirations with or without hypoxia |
| Appearance of diabetes insipidusb |
Abbreviations: DKA, diabetic ketoacidosis.
Adapted from Wolfsdorf et al. (2018).
A late sign of cerebral herniation with interruption of blood flow to the pituitary gland, indicative of irreversible brain injury.
The interpretation of research to identify the pathophysiologic mechanisms of CE continues to be controversial. Cytotoxic injury, vasogenic processes, and osmotic shifts during DKA treatment have all been implicated. In this article, we summarize the published data on the mechanisms underlying CE and propose a hypothetical multifactorial pathway to explain its development.
2 |. CYTOTOXIC INJURY
Cytotoxic injury is characterized by disruption of cellular metabolism and membrane stability leading to cellular retention of sodium and water and subsequent swelling, ultimately resulting in cell death. It can be caused by hypoxic injury or exposure to toxic substances that promote oxidative stress and mitochondrial dysfunction.31 It has been postulated that cytotoxic injury plays a prominent role early in the development of CE, before starting treatment for DKA, and may contribute to its propagation during treatment. This theory is supported by studies in rats, which have demonstrated significant reductions in the apparent diffusion coefficient (ADC) during conditions simulating untreated DKA, suggesting restricted diffusion of water molecules and indicating cellular swelling.32–35 Hyperglycemia and hyperketonemia independently cause reductions in cortical ADC values.34 The following sections propose several mechanisms whereby DKA can lead to cytotoxic injury.
2.1 |. Metabolic factors and cytotoxic substances
Several studies have found that patients with DKA who develop CE present with more severe hyperglycemia than those without overt CE.13,14,36–39 Hyperglycemia has been suggested to augment cerebral injury via a number of mechanisms, including the production of osmolytes (such as sorbitol), induction of cerebral acidosis, reduction of cerebral blood flow (CBF), and accumulation of lactate and glutamate.40 Cerebral glutamate concentrations have not been examined in patients with DKA and overt CE. In a rat model, however, exposure to hyperglycemia before induction of forebrain ischemia was associated with enhanced neocortical accumulation of extracellular glutamate during ischemia, exaggerating neuronal damage in the forebrain.41 Extrapolating these findings to patients with DKA, it can be postulated that elevated concentrations of cerebral glutamate (or increased sensitivity of the N-methyl-D-aspartate [NMDA] receptor to glutamate), caused by hyperglycemia and hypoxia,42 could contribute to cytotoxic neuronal injury and altered mental status.
Children with DKA have also been found to have elevated plasma concentrations of 3-deoxyglucosone (3-DG), a precursor of advanced glycation end products (AGEs), at baseline and within a few hours of initiating treatment. The time course of the rise in 3-DG concentrations coincided with the expected timing of the progression to subclinical CE. Increases in 3-DG concentrations are caused by hyperglycemia, ketone bodies, and lipid peroxidation and have been associated with generation of reactive oxygen species (ROS) and inactivation of antioxidant enzymes.43 Other studies have also shown increases in lipid peroxidation and reductions in antioxidant vitamins C and E before and during treatment of DKA in children. In addition to cellular damage, lipid peroxidation and ROS have been implicated in endothelial membrane dysfunction at the level of the blood–brain barrier (BBB), suggesting concurrent vasogenic injury (discussed in later sections).44 Finally, activation of the membrane attack complex (C5b-C9), which has been shown to be upregulated in patients with DKA before starting treatment,45 has also been associated with induction of cellular injury.46
2.2 |. Altered membrane transporter activity
Acetoacetate and beta-hydroxybutyrate have been shown to stimulate Na-K-Cl cotransporter activity in rat astrocytes leading to intracellular accumulation of electrolytes and water.32 Several studies have also implicated the Na+/H+ exchanger in propagating neuronal injury in DKA. This exchanger is believed to be activated by cytoplasmic acidification from high concentrations of circulating organic acids13,47 and elevated levels of vasopressin.13,48 During DKA treatment, resolution of acidosis creates an outward chemical gradient for protons, which, together with a direct effect of insulin therapy, has been proposed to enhance the activity of this exchanger.13,47,49 These mechanisms are thought to promote influx of sodium and water into neurons leading to cellular swelling. However, in vivo relationships may be more complex: high intracellular sodium concentrations may lead to activation of other ‘‘corrective’’ pumps that result in net loss of cellular ions and reduction of intracellular volume.50 The role of the Na+/H+ exchanger in development of CE thus remains unclear.
2.3 |. Hypoxic injury
Hypoxic injury has been implicated in the development of CE in DKA since its initial description in 1936.1,51–53 MRS studies in rats with untreated DKA have demonstrated abnormalities in cerebral metabolites typically encountered after a hypoxic insult, including low intracellular pH, elevated lactate,34 and reduced levels of high-energy phosphates.34,54 The distribution of cerebral injury that has been described in some patients also suggests a hypoxia-driven process. In reviewing computed tomography (CT) imaging studies of children with DKA, Muir et al. found focal brain injury in 22% of subjects, affecting primarily the mesial basal ganglia and thalamus, periaqueductal gray matter, and dorsal pontine nuclei. These changes were found exclusively in children whose abnormal neurological signs appeared within a few hours of starting treatment and the authors concluded that they were likely caused by primary metabolic derangements.55 The affected brain regions have a high demand for adenosine triphosphate (ATP) and are, therefore, more susceptible to hypoxic insults, providing further indirect evidence that hypoxia may play a role in the early development of cerebral injury in children with DKA. A combination of hypoperfusion and impaired oxygen utilization are thought to contribute to the development of this injury.
2.3.1 |. Hypoperfusion
Development of CE in DKA has been associated with several factors known to affect cerebral perfusion, including uremia,4,14,37–39,49,56 hyperosmolality,36,38,39 hyperventilation,37 hypocapnia,4,37 and acidosis.13,14,37–39,49 Acidosis,57,58 hyperosmolality,59 and a synergistic combination of the two38 have been implicated in causing altered sensorium in patients with DKA. Elevated blood urea nitrogen (BUN) concentration and hyperosmolality are surrogate biochemical markers for the severity of dehydration. Dehydration leads to contraction of the extracellular space, hemoconcentration, and increased blood viscosity, which may contribute to cerebral hypoperfusion and cytotoxic injury.50,51 In support of this hypothesis, an MRI study in rats with untreated DKA showed a significant negative correlation between ADCs and initial BUN concentrations.33 Hyperventilation, a compensatory mechanism for metabolic acidosis, leads to decreased partial pressure of arterial carbon dioxide (PaCO2) and increased brain extracellular pH, leading to cerebral vasoconstriction.60 In a mouse model, acetoacetate has also been associated with increased production of a potent vasoconstrictor, endothelin-1, from brain vascular endothelial cells.61 All of these factors are thought to contribute to cerebral hypoperfusion in DKA; however, the evidence for its occurrence mainly comes from animal models.
Several previously cited studies in rats showed that in addition to reduced ADCs, CBF was also significantly decreased in conditions simulating the metabolic derangements of untreated DKA33,34 and varied directly with PaCO2.33 Hyperglycemia and hyperketonemia independently caused reductions in CBF.34 However, a more recent study by Glaser et al. showed that while experimentally induced hypocapnia led to declines in both CBF and ADC, hyperglycemia and ketosis only exacerbated the reductions in ADC and did not lead to significant further decreases in CBF. These observation suggest that metabolic derangements associated with DKA may make the brain more vulnerable to injury caused by mild reductions in CBF induced by hypocapnia.35
Studies in humans have not convincingly demonstrated cerebral hypoperfusion in DKA. In 1948, Kety et al.62 compared CBF and brain oxygen consumption of adult patients with severe diabetic acidosis with and without coma and found no significant decreases in CBF in either group. Hoffman et al., utilizing transcranial Doppler (TCD) in children, also found no significant changes in mean CBF values between admission and 6, 24, and 48 hours after initiating treatment for DKA. All of the CBF values were within the normal ranges.63
2.3.2 |. Impaired oxygen utilization
While Kety et al. did not show a significant decline in cerebral perfusion in comatose and severely acidotic patients, oxygen consumption was reduced by 48% and 18%, respectively, as compared to reference values of healthy subjects.62 Fein et al. also found impaired oxygen extraction in patients with DKA.10 Young et al. suggested that hemoconcentration may contribute to impaired cerebral oxygenation due to the inability of concentrated blood to efficiently take up oxygen.51 It has also been proposed that hypocapnia may reduce cerebral oxygen consumption in severe DKA.37 Finally, treatment with bicarbonate (discussed in more detail later) has been associated with decreased oxygen delivery to brain cells.64 Graded reductions in cerebral consumption of oxygen in the setting of largely preserved CBF have been associated with declines in the degree of mental alertness in a number of conditions, including diabetic acidosis.65 Taken together, these observations suggest that impaired oxygen utilization may play a more significant role than reduced CBF in contributing to hypoxic injury in patients with DKA.
3 |. VASOGENIC INJURY
In fatal cases of CE complicating DKA, histopathologic examinations have shown absence of tight junction proteins, albumin extravasation, and perivascular changes.66,67 In addition, von Willebrand factor antigen and activity were found to be increased before and during treatment of severe DKA in children.68 Moreover, elevated ADC values have been observed in children undergoing treatment of DKA, indicating increased diffusion of water.69–75 Children with evidence of altered mental status had more significant elevations in their ADC values during DKA treatment compared to those without mental status abnormalities, indicating a more severe degree of cerebral compromise in symptomatic patients.71 Taken together, these findings suggest vasogenic injury, which is characterized by the breakdown of tight junctions between endothelial cells, allowing intravascular proteins and fluid to cross the BBB and penetrate the extracellular brain parenchyma.31 The following sections review mechanisms implicated in its development.
3.1 |. Metabolic and hormonal factors
Hypertonicity from hyperglycemia13,76 and acidosis13 have both been implicated in BBB dysfunction in patients with DKA. Furthermore, acetoacetate and beta-hydroxybutyrate have been shown to stimulate Na-K-Cl cotransporter activity in cerebral microvascular endothelial cells, predisposing them to injury.32 It has also been suggested that vasopressin, which is elevated in patients with DKA at presentation,13 alters the permeability of the BBB. Increased vasopressin release is thought to occur in response to intravascular volume depletion, nausea, and enhanced sensitivity of osmoreceptors to stimulation by hyperglycemia in the absence of insulin.48,77–80 However, no direct correlation has been found between vasopressin levels and development of CE.13
3.2 |. Release of vasoactive substances and induction of inflammation
Hyperglycemia and hyperketonemia have been associated with the release of vasoactive substances, including adhesion molecules and vascular endothelial growth factor, which have a direct effect on the BBB, promoting increased vascular permeability.61,81 In addition, arachidonic acid released into the extracellular milieu during parenchymal injury from cerebral hypoxia has been implicated in inducing capillary permeability in children with DKA, especially following treatment initiation.37
Studies in both animal models82–85 and humans86–101 have also suggested that systemic and cerebrovascular inflammation are associated with the development of CE in DKA. Inflammatory processes are thought to contribute to vasogenic injury through their damaging effects on the BBB.102–105 Table 2 summarizes the literature and the pertinent findings implicating inflammation in the pathogenesis of CE.
TABLE 2.
Inflammatory changes associated with cerebral edema development during diabetic ketoacidosis
| Findings | Clinical implications/associations | |||
|---|---|---|---|---|
| Studies in animals | ||||
| Systemic inflammation | In mice, DKA was associated with higher levels of inflammatory markers, including IL-6, IL-8, IL-10, CCL2, sE-selectin, sICAM-1, and sVCAM-1.82 | Stimulation of brain endothelial cell line with DKA plasma was associated with increased ROS and activation of NF-κB, upregulation of pro-adhesive phenotype, and enhanced leukocyte rolling/adhesion, contributing to cerebrovascular endothelial cell dysfunction.82 | ||
| Neuroinflammation | In rats: • Exposure to hyperglycemia before ischemic event was associated with significantly higher number of myeloperoxidase-positive cells within pial and parenchymal vessels and brain parenchyma.83 • Untreated DKA was associated with increased expression of GFAP, indicating reactive gliosis and activation of microglia; these findings intensified during DKA treatment with insulin/saline.84,85 |
• Pre-ischemic hyperglycemia triggers massive deposition of PMNs in post-ischemic brain, leading to increased PMN adherence to cerebral blood vessels, disruption of the BBB, and PMN migration into brain parenchyma, exacerbating underlying tissue injury.83 • In the setting of cerebral hypoxia, microglia produce several inflammatory cytokines, including IL-1β and TNF-α, ROS, and NO, which could exacerbate BBB dysfunction during reperfusion injury.84,85 |
||
| Studies in humans | ||||
| Systemic inflammation | DKA and hyperketonemia associated with: • Increased activation of peripheral T-lymphocytes86 • Elevated systemic levels of CRP and related markers of cardiovascular risk,87–92 pro-inflammatory cytokines,76,87–90,93,94 and ROS44,88,92 • Reduced levels of antioxidants44 • Complement activation45 |
DKA severity has been shown to correlate with systemic neutrophil activation and degranulation, leading to the release of vasoactive substances, such as proteinase-3, that degrade the BBB.102 | ||
| Neuroinflammation | Fatal cases of DKA associated with: • Increased neuronal expression of cytokines, adhesion molecules, complement peptides,95–97 ROS,98–100 and receptor for AGEs101 • Localization of CCL2, NF-κB, and nitrotyrosine to the perivascular regions of the disrupted BBB66 • Microglial activation and astrocyte reactivity66 |
• Several cytokines and chemokines, including CXCL1 and IL-8, were found to facilitate leukocyte adhesion to cerebrovascular endothelium during DKA.103 • Alterations in the peripheral104 and neuronal105 expression of matrix metalloproteinases in DKA have been implicated in the disruption of the BBB, mediated by the degradation of tight junction proteins. |
||
Abbreviations: AGEs, advanced glycation end products; BBB, blood–brain barrier; CCL2, chemokine (C-C motif) ligand 2; CRP, C-reactive protein; CXCL1, chemokine (C-X-C) motif ligand 1; DKA, diabetic ketoacidosis; GFAP, glial fibrillary acidic protein; IL, interleukin; NF-κB, nuclear factor-kappa B; NO, nitric oxide; PMN, polymorphonuclear lymphocyte; ROS, reactive oxygen species; sE-selectin, soluble endothelial selectin; sICAM-1, soluble intracellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; TNF-α, tumor necrosis factor-alpha.
3.3. |. Impaired cerebrovascular autoregulation
Kety et al. demonstrated reduced cerebrovascular resistance, despite decreased PaCO2 levels, in adult patients with diabetic coma compared to normal controls, leading to higher than normal CBF.62 Utilizing TCD, Hoffman et al. showed a statistically significant increase in Gosling’s pulsatility index (a measure of intracranial pressure) on admission compared to the control value on day 6, as well as decreased cerebral vascular reactivity, suggestive of diminished cerebrovascular tone. Within 24–48 hours of DKA treatment, the pulsatility index decreased and vascular reactivity increased, indicating restoration of CBF regulatory mechanisms. The authors postulated that hyperglycemia and acidosis override the vasoconstrictive effects of hypocapnia, leading to vasoparalysis. As these metabolic derangements improve with treatment, vascular tone in response to low PaCO2 is restored.63
Using TCD and infrared spectroscopy, Roberts et al. showed impaired cerebrovascular autoregulation to tilt-table testing and increased regional cerebral oxygenation, respectively, in children with DKA and impaired mental status within several hours of treatment initiation.106 They later found that children treated for DKA had significantly lower autoregulatory indices during tilt-table testing as compared to children with type 1 diabetes mellitus (T1DM) without DKA.107 Although not statistically significant, the frequency of impaired cerebrovascular autoregulation was higher in patients with DKA who developed CE. The researchers postulated that CE is associated with a temporary loss of cerebrovascular autoregulatory mechanisms, leading to increased CBF and vasogenic injury.
Regional differences in CBF autoregulation have also been reported. Chung et al. showed that impaired cerebrovascular autoregulation was present in 95% of patients on admission and remained abnormal in 50% after DKA resolution. In addition, children with clinical and radiographic evidence of CE had significantly lower mean flow velocity in the basilar artery.108 Glaser et al. observed that during DKA treatment, ADC values (and brain water content) were increased in brain regions supplied by the anterior and middle cerebral arteries, but not in the medulla and occipital cortex, which are supplied by the vertebrobasilar circulation. These observations suggest that CBF autoregulation may vary according to differences in regional vascular supply. Specifically, decreased sympathetic innervation of the vertebrobasilar circulation may contribute to its diminished capacity for CBF autoregulation and vasodilation, leading to diversion of CBF away from the brainstem to other brain regions. This may increase the risk of hypoxic injury or delay recovery from hypoxia in the posterior part of the brain and account for signs of brainstem dysfunction seen in some children with severe DKA-related brain injury without evidence of herniation.69,75
Impaired cerebrovascular autoregulation leading to hyperemia has been at least partially attributed to normalization of PaCO2 levels following prolonged hypocapnia.35,75 Nitric oxide is a potential mediator of this phenomenon. Iori et al. found elevated levels of plasma nitrate (the product of nitric oxide catabolism) and increased mRNA and protein expression of inducible nitric oxide synthase (iNOS) in circulating lymphocytes and monocytes of T1DM patients with ketosis.109 Hoffman et al. also showed increased expression of iNOS in the brains of adolescents with fatal DKA.95 Increased local production of nitric oxide has previously been associated with endothelial dysfunction and hyperemia in ketotic adults with T1DM, implicating its role in the vasodilatory response in these patients.110
3.4 |. Reperfusion injury
Reperfusion of hypoxic brain regions during DKA treatment has been proposed to exacerbate cerebral injury through a variety of mechanisms, including disruption of the BBB.37,40,69,71,72 Increased CBF during treatment is supported by the finding of cerebral hyperemia within several hours of initiating therapy.106,111 Furthermore, several studies have demonstrated elevations in ADCs during treatment compared to the recovery period69 75 and ADCs correlated positively with increased CBF on perfusion MRI.69 In addition, ADC values during treatment directly correlated with initial BUN concentration and respiratory rate.71 As discussed earlier, these parameters are surrogates for dehydration and hypocapnia, which have been associated with cerebral hypoperfusion, suggesting that vasogenic injury may exacerbate the initial hypoxic insult. The distribution of ADC changes during treatment also supports this hypothesis. As discussed previously, the basal ganglia and thalamus have been found to be more susceptible to focal injury in children with DKA and early-onset CE.55 These regions (as well as the frontal white matter) also had the most consistent elevations in ADCs during treatment,69,71–73 suggesting that the initial hypoxic insult associated with metabolic derangements may make those brain regions with higher metabolic demand more vulnerable to reperfusion injury, predisposing them to subsequent development of vasogenic edema.
It is important to point out, however, that the pattern and timing of ADC elevations in relation to DKA presentation, treatment initiation, and recovery period varied across studies. Furthermore, the magnitude of the increases in most cases did not approach the ranges consistent with true vasogenic edema observed in other clinical situations,112 raising doubt as to whether the reported elevations in the ADCs are high enough to be correlated with this process.113 However, most patients in these studies were either asymptomatic or had mild symptoms. Only a minority had clinical or CT evidence of CE requiring intervention and all seemingly recovered without neurological deficits. Thus, it is possible that patients with more severe presentations may demonstrate ADC elevations consistent with true vasogenic edema; however, due to medical instability and ethical considerations, these patients are not suitable candidates for MRI studies.
4 |. TREATMENT-SPECIFIC FACTORS
The role of DKA treatment-specific factors in the development of CE in children remains highly controversial. Several studies have demonstrated that subclinical CE is common during therapy,8,10–13,114 with an estimated prevalence of 54% in one study.8 In earlier studies, its development was attributed to rapid correction of hyperglycemia and decrease in plasma osmolality10,13,114; however, more recent studies using magnetic resonance diffusion-weighted imaging (DWI) suggest a vasogenic process.70,73 Clinically apparent CE usually becomes evident within the first 12 hours of starting treatment,6 potentially implicating therapy-related factors in its development or propagation. Many studies have attempted to elucidate the specific treatment factor(s) that may be responsible for precipitating CE; however, the results have been conflicting and inconclusive. The following sections briefly review the literature investigating the potential effects of intravenous fluid therapy, insulin administration, and use of bicarbonate on the risk of CE development. Table 3 provides an overview of current treatment recommendations for management of DKA.6,115
TABLE 3.
Current treatment guidelines for management of diabetic ketoacidosis
| Treatment guidelinesa | |
|---|---|
| Intravenous fluid therapy | • Initial volume resuscitation with 10–20 mL/kgof 0.9% saline over 30–60 min; additional boluses or faster infusion may be required to ensure adequate tissue perfusion • Subsequent fluid therapy with 0.45% to 0.9% saline (or balanced salt solution), incorporating maintenance requirements and deficit replacement over 24–48 h • Increase in sodium content of fluids is recommended if the measured serum sodium is low and does not rise appropriately as the blood glucose drops |
| Insulin therapy | • Initiation of insulin infusion at 0.05–0.1 units/kg/h at least 1 h after starting fluid replacement therapy • Avoidance of intravenous boluses of insulin |
| Bicarbonate therapy | • Reserved for life-threatening hyperkalemia and severe acidosis with concern for compromised cardiac contractility |
4.1. |. Intravenous fluid therapy
Based on findings in animal models, it has been proposed that rapid correction of hyperglycemia with excess free water leads to an osmotic shift that causes brain cells to swell.116–121 An exaggerated action of vasopressin has been proposed to exacerbate these shifts.48 In humans, several retrospective studies have implicated higher fluid volume, lower fluid tonicity, and rapid correction of hyperglycemia in the development of overt CE.37,39,48,49,56,122–130 One study suggested an inverse relationship between the rate of fluid administration (> 4 L/m2/day) and time to brain herniation.48 The association between administration of excess free water and the development of CE has been linked to the decline (or failure of an appropriate increase) in serum sodium concentration and/or effective plasma osmolality during treatment.48,122,124,127,128 However, several studies have not shown an association between fluid therapy-related factors and these biochemical trends.4,131,132
It is important to highlight several limitations of the studies cited above. The available literature associating fluid therapy-related factors with development of CE is largely retrospective in nature, consisting of case series, case–control, and cohort studies (Table S1). By virtue of their design, these studies were prone to inherent biases (e.g., selection bias), had small sample sizes, frequently lacked appropriate controls, and did not adequately adjust for confounders. Failure to account for potential confounders, such as the degree of dehydration and severity of DKA, precludes accurate interpretation of the findings linking treatment factors to CE development. It is possible that patients with more severe clinical and metabolic derangements on presentation (who were at higher risk of developing CE) were treated more aggressively. A related concern is referral bias in some of the studies that included patients transferred from outside centers to the institutions’ pediatric intensive care units and were thus more likely to have severe DKA.39,56,125
Several prospective studies evaluating the safety of treatment protocols with slower rates of fluid administration delivered over 48 hours and with relatively high sodium concentrations (average of 125 mmol/L) found that serum sodium concentration rose as the glucose concentration fell in most episodes of DKA.122,133,134 There were no deaths or obvious permanent neurologic sequelae, and the authors concluded that their regimen was protective against neardeath experiences and brain herniation. However, it is important to note that in one of the studies, mannitol was administered in 5.5% of cases. It is unclear, therefore, whether the overall good outcomes were related to meticulous monitoring and timely administration of mannitol rather than the fluid regimen per se.134 It must also be noted that none of these studies included a control or comparison treatment group and the sample sizes were insufficient to detect a statistically significant difference in CE occurrence compared to the expected rate of 0.3%–1%.
Previously cited MRI studies have raised doubt about the occurrence of osmotic shifts during DKA treatment by suggesting that therapy-related injury is actually associated with a vasogenic process based on the observation of increased ADC values during therapy (ADC is decreased in osmotic cellular swelling).69–75 In addition, other observational studies have not convincingly demonstrated an association between fluid-related treatment factors (i.e., higher volume, lower tonicity, rapid rate of glucose correction, decline in osmolality, and failure of an appropriate rise in serum sodium concentration) and the development of CE.14,19–21,135–141 Moreover, it has been proposed that insufficient fluid resuscitation may actually be a risk factor for CE due to delayed correction of dehydration, leading to prolongation of compromised cerebral perfusion.142–144
A review of the literature identified three randomized controlled trials (RCTs) assessing the efficacy and safety of different fluid regimens for management of DKA in children. None showed an association between administered fluid volume and development of CE.73,145,146 In one study, children with DKA were randomized to receive fluids at a more rapid rate (n = 8) or a slower rate (n = 10), with all other aspects of treatment identical. DWI showed higher mean brain ADC values during treatment compared to post-recovery in both groups, consistent with vasogenic CE; however, the differences between groups were not statistically significant, leading the authors to conclude that the ADC changes during DKA treatment did not appear to be substantially affected by the rate of fluid administration.73 Bakes et al. investigated the effects of fluid volume on the rate of metabolic normalization in children with DKA. Patients were randomized to receive intravenous fluid at low volume (10 mL/kg initial bolus plus 1.25 x maintenance replacement rate) or high volume (20 mL/kg initial bolus plus 1.5 x maintenance replacement rate) (n = 25 in each group). Higher volume of administered fluids significantly decreased time to metabolic normalization. No cases of CE were identified in either group.145 A major limitation of these RCTs is limited power to detect a statistically significant difference in CE rates.
The Pediatric Emergency Care Applied Research Network (PECARN) FLuid therapies Under Investigation in DKA (FLUID) trial was an RCT that examined the effects of the rate of fluid administration and the sodium chloride content of intravenous fluids on neurologic sequelae in 1389 episodes of DKA in children. Neither the rate of administration nor the sodium chloride content of intravenous fluids significantly influenced neurologic outcomes in children with DKA. In a subgroup analysis of children with severe DKA, rapid rehydration was associated with better performance on measures of short-term memory. It should be noted that children with Glasgow Coma Scale < 11 were excluded from the study after year 2 because these children had already met the primary study outcome and because most clinicians believed that management decisions for these patients should not be determined based on randomization.146 It remains uncertain whether the results of the FLUID trial are generalizable to patients with more profound neurologic compromise at presentation who are at higher risk for overt CE.147
See Table S1 for detailed descriptions of the studies referenced in this section.
4.2 |. Insulin therapy
In an observational study, Edge et al. showed that insulin administration within the first hour and total insulin dose during the first 2 hours of DKA treatment were associated with an increased risk of CE in children after adjusting for age, sex, diabetes status (new or known), and baseline acidosis.49 Hoorn et al. also showed that children with DKA who developed CE received an insulin bolus more often than those who did not develop CE, but did not adjust for severity of DKA.127 Several mechanisms have been proposed to explain the association between insulin administration during treatment of DKA and development of cerebral injury. Early studies in animal models have suggested that insulin therapy, especially when combined with fluids,148 contributes to osmotic shifts associated with rapid correction of hyperglycemia.118,148 It has also been postulated that insulin directly promotes influx of sodium and other electrolytes into brain cells (e.g., through activation of the Na+/H+ exchanger), exacerbating cytotoxic swelling, especially at a time when membrane electrolyte transport is already at its most active in the setting of rapid rehydration.13,47,49 In addition, it has been hypothesized that insulin may have deleterious effects on the BBB61,76; however, no study in humans has been able to directly implicate this mechanism. Despite the observation that CE rarely occurs in the absence of insulin therapy,19 other studies have failed to directly implicate insulin administration in its development,4,13,37,39,131 and the role of insulin in causing CE remains unclear.
4.3 |. Bicarbonate therapy
Studies in animal models have found associations with bicarbonate administration and development of CE,64,149 especially when given concomitantly with insulin, presumably due to rapid systemic alkalinization.149 Several mechanisms for the detrimental effect of bicarbonate on the central nervous system (CNS) have been proposed. Rapid correction of acidosis shifts the oxygen-hemoglobin dissociation curve to the left, increasing hemoglobin’s affinity for oxygen and making it less available to neurons, thus exacerbating cerebral hypoxia.150 In adults with uncontrolled diabetes, bicarbonate administration resulted in a significant leftward shift of the oxyhemoglobin dissociation curve.151 In a study of 10 children with DKA, however, this shift was not significantly different between children treated with bicarbonate and those who did not receive bicarbonate.152 Bicarbonate administration has also been shown to induce paradoxical acidosis in the CNS during treatment of DKA.64,153 However, while in dogs, the resultant CNS acidosis was associated with decreased oxygen delivery to brain cells, cerebral hypoxia, and lactic acidosis,64 it did not lead to any discernible adverse clinical outcomes in children.153
Although a few observational studies in children found potential associations between the use of bicarbonate and development of CE,4,39 many others did not show a significant relationship.14,19,21,49,56,127,131,153 Due to other potential risks (especially hypokalemia) and unclear benefit, bicarbonate is not recommended for routine use in the treatment of DKA.6
5 |. DISCUSSION
Although uncommon, clinically apparent CE is associated with high rates of mortality and morbidity. A review of the literature has identified a number of proposed mechanisms for its development; however, no consensus has been reached due to the inherent difficulties in studying this life-threatening complication in children. Moreover, the reason for its higher prevalence in children as compared to adults is not understood.
Based on the metabolic findings, cerebral perfusion data, and ADC values observed at the onset and during treatment of DKA both in animal models and children, the most widely accepted current hypothesis is that CE is the result of ischemia–reperfusion injury.40 The effect of rising PaCO2 levels after prolonged hypocapnia leading to impaired cerebrovascular autoregulation and abnormal vasodilation,75 as well as disruption of the BBB from inflammation, exacerbate the secondary vasogenic injury.146 This is an appealing model that may account for a proportion of CE cases. Based on our review of the literature, we offer several comments.
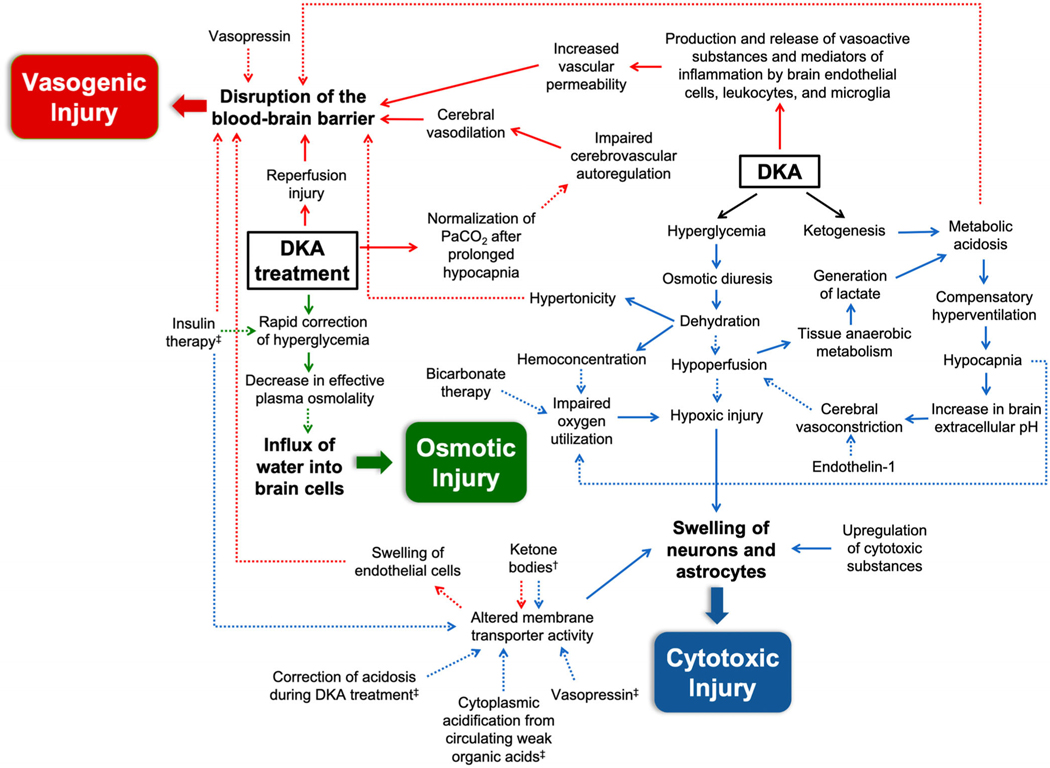
First, we have organized this review into three main categories describing the different types of cerebral injury that are hypothesized to occur in patients with DKA: cytotoxic, vasogenic, and osmotic. As opposed to a sequential process, we propose a model where these processes overlap and where each can be exacerbated by certain patient- or treatment-related factors. Most often, this results in subclinical cerebral injury, associated with mild symptoms and alterations in neurologic function. Uncommonly, when the degree of injury surpasses the threshold for compensatory mechanisms, for unclear reasons, clinically apparent CE occurs. Within each category, several contributory processes have been proposed. Under the rubric of cytotoxic injury, metabolic factors and upregulation of neurotoxic substances, altered activity of certain membrane transporters, and hypoxic injury all likely play some role in driving the disruption of cellular metabolism and membrane stability, culminating in cellular swelling and subsequent death. Concurrent with cytotoxic injury, breakdown of the BBB occurs as a result of a combination of metabolic and hormonal factors, inflammation, alterations in cerebrovascular autoregulation, and reperfusion injury. Osmotic shifts and other therapy-related factors during treatment may further exacerbate cerebral injury. However, both subclinical12,13,154 and clinically apparent CE4,14–18 have been described before starting therapy, suggesting that reperfusion injury and treatment-related factors are not necessary components of the pathophysiologic pathway leading to CE development, but may play a role in propagating cerebral injury in a subset of predisposed patients (i.e., those with more severe dehydration and acidosis at presentation). Figure 1 shows a proposed multifactorial pathway leading to the development of the different types of cerebral injury in patients with DKA. It is likely that some combination of cytotoxic, vasogenic, and osmotic injury ultimately leads to overt CE in predisposed individuals.
FIGURE 1.
A hypothetical schema of the pathways that contribute to the development of cerebral injury in children with diabetic ketoacidosis. Solid arrows indicate pathophysiologic mechanisms that have been observed in humans. Dashed arrows represent hypothesized mechanisms or those that have only been shown in animal studies. The blue arrows signify factors that lead to cytotoxic injury, including upregulation of cytotoxic substances, altered membrane transporter activity, and hypoxic injury. The red arrows depict mechanisms that predispose towards vasogenic injury, characterized by the disruption of the blood–brain barrier. The green arrows denote treatment-related effects that may cause osmotic injury. A combination of these processes causes cerebral edema in high-risk children. †Factors associated with activation of Na-K-Cl cotransporter in astrocytes and endothelial cells. ‡Factors associated with activation of Na+/H+ exchanger in neurons. Abbreviations: DKA, diabetic ketoacidosis
Second, we have avoided using the term ‘‘ischemic’’ when describing cerebral injury that is thought to occur secondary to impaired cerebral perfusion/oxygenation and have instead referred to it as ‘‘hypoxic’’. Cerebral ischemia is defined as insufficient blood flow to provide adequate oxygenation to brain tissue, whereas cerebral hypoxia refers more broadly to reduced supply of oxygen to the brain, which can be secondary to ischemia or another process, such as impaired oxygen utilization. As mentioned previously, studies in humans that have investigated cerebral perfusion during DKA have not shown significantly reduced CBF approaching the threshold for true ischemia.62,63 The only studies that have demonstrated significantly decreased CBF were those performed in rat models with streptozotocin-induced metabolic derangements simulating DKA.33–35 In this model, the mechanism of pancreatic injury is different and its onset typically more acute, leading to a more rapid induction of severe hyperglycemia and associated metabolic derangements. In T1DM, development of autoimmunity is usually an insidious process and it takes months to years to reach a critical mass of beta cell destruction and insulinopenia associated with onset of symptoms. During this process, there is a more gradual development of hyperglycemia. It can be postulated that this chronic progression of biochemical abnormalities has different effects on brain microstructure, neuronal metabolism, and cerebral perfusion and oxygen utilization than an acute metabolic derangement, which may, at least partially, explain the discrepancy in the CBF and ADC findings between studies in rats and those conducted in humans. In addition, while significant reductions in perfusion have not been demonstrated in patients with DKA, impaired oxygen extraction has been implicated.10,37,51,62,64 For these reasons, we believe that the term ‘‘hypoxic’’ is a more appropriate description of the events that may contribute to early development of CE.
Young children are dependent on adult caretakers for consistent provision of fluids and rely on parents and healthcare professionals to recognize symptoms of diabetes. They are at highest risk for protracted hyperglycemia and more severe dehydration and acidosis. In addition, children have significantly higher oxygen requirements compared to adults (5.1 versus 3.3 mL/100 g/min)155 and may be particularly susceptible to even mild hypoxic injury, especially when complicated by impaired oxygen extraction.4,50,55 This is further compounded by the fact that diabetes itself may be associated with increased brain energy requirement.156 In light of these factors, in children, even slight reductions in cerebral metabolic rate during hypoxia may be detrimental to neuronal integrity during DKA, leading to cytotoxic injury. Compared to young adults, children and adolescents with fatal DKA have also been shown to have higher expression of certain immune markers, such as C5b-C9,96 and this may also mediate cellular injury.46
6 |. CONCLUSIONS
This comprehensive review of the literature and our proposed pathway highlight a complex interplay between cytotoxic, vasogenic, and osmotic processes in the development of brain injury and CE in children with DKA. Standard management with mannitol and controlled hyperventilation,21,157,158 if initiated in a timely fashion, may reduce rates of mortality and morbidity.19,131 However, improved understanding of the underlying mechanisms of CE and its early identification may ultimately help guide therapeutic interventions aimed at specific molecular targets, with the aim of preventing fatalities and irreversible neurologic sequelae (Table 4).32,33,44,47,54,85,159,160 The role of genetic factors in susceptibility to CE is another area of interest, but without data.
TABLE 4.
Possible molecular targets for treatment of cerebral edema
| Proposed target | Proposed therapeutic approach | Evidence from studies |
|---|---|---|
| Na+/H+ exchanger | Selective amiloride analogues47 | In juvenile mice, associated with reduced cellular swelling and decreased water content during DKA therapy159–160 |
| Na-K-Cl cotransporter | Loop diuretic bumetanide | In rats, associated with reduction of cytotoxic edema and prevention of reperfusion injury32–33–54 |
| Ca-activated K channel | Inhibitor TRAM-34 | In juvenile rats, associated with reduced microglial activation and reactive gliosis during DKA therapy85 |
| Oxidative stress | Antioxidant vitamins C and E44 | No studies-to-date |
| NMDA receptor | NMDA receptor antagonist | No studies-to-date |
Abbreviations: DKA, diabetic ketoacidosis; NMDA, N-methyl-D-aspartate.
Optimal fluid strategies for treatment of severe DKA and neurologic compromise at presentation should continue to be investigated. More importantly, prompt recognition of diabetes symptoms and initiation of management before the onset of DKA is imperative to prevent CE. Large-scale awareness campaigns have been effective in decreasing the incidence of DKA; however, the results have not been consistently replicated. Thus, it is important to design both national and community-level initiatives with widespread influence that target medical providers, teachers, and parents.161
Supplementary Material
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: 5T32DK007699-39
Footnotes
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13152.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Dillon ES, Riggs HE, Dyer WW. Cerebral lesions in uncomplicated fatal diabetic acidosis. Am J Med Sci. 1936;192(3):360–365. [Google Scholar]
- 2.Scibilia J, Finegold D, Dorman J, Becker D, Drash A. Why do children with diabetes die? Acta Endocrinol Suppl. 1986;279:326–333. [DOI] [PubMed] [Google Scholar]
- 3.Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990–96. Arch Dis Child. 1999;81(4):318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glaser N, Barnett P, McCaslin I, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric emergency medicine collaborative research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344(4):264–269. [DOI] [PubMed] [Google Scholar]
- 5.Dunger DB, Sperling MA, Acerini CL, et al. European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society consensus statement on diabetic ketoacidosis in children and adolescents. Pediatrics. 2004;113(2):e133–e140. [DOI] [PubMed] [Google Scholar]
- 6.Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD clinical practice consensus guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27): 155–177. [DOI] [PubMed] [Google Scholar]
- 7.Patel A, Singh D, Bhatt P, Thakkar B, Akingbola OA, Incidence SSK. Trends, and outcomes of cerebral Edema among children with Diabetic ketoacidosis in the United States. Clin Pediatr (Phila). 2016;55 (10):943–951. [DOI] [PubMed] [Google Scholar]
- 8.Glaser NS, Wootton-Gorges SL, Buonocore MH, et al. Frequency of sub-clinical cerebral edema in children with diabetic ketoacidosis. Pediatr Diabetes. 2006;7(2):75–80. [DOI] [PubMed] [Google Scholar]
- 9.Wootton-Gorges SL, Buonocore MH, Kuppermann N, et al. Cerebral proton magnetic resonance spectroscopy in children with diabetic ketoacidosis. AJNR Am J Neuroradiol. 2007;28(5):895–899. [PMC free article] [PubMed] [Google Scholar]
- 10.Fein IA, Rachow EC, Sprung CL, Grodman R. Relation of colloid osmotic pressure to arterial hypoxemia and cerebral edema during crystalloid volume loading of patients with diabetic ketoacidosis. Ann Intern Med. 1982;96(5):570–575. [DOI] [PubMed] [Google Scholar]
- 11.Krane EJ, Rockoff MA, Wallman JK, Wolfsdorf JI. Subclinical brain swelling in children during treatment of diabetic ketoacidosis. N Engl J Med. 1985;312(18):1147–1151. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman WH, Steinhart CM, el Gammal T, Steele S, Cuadrado AR, Morse PK. Cranial CT in children and adolescents with diabetic ketoacidosis. AJNR Am J Neuroradiol. 1988;9(4):733–739. [PMC free article] [PubMed] [Google Scholar]
- 13.Durr JA, Hoffman WH, Sklar AH, el Gammal T, Steinhart CM. Correlates of brain edema in uncontrolled IDDM. Diabetes. 1992;41(5): 627–632. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence SE, Cummings EA, Gaboury I, Daneman D. Populationbased study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. J Pediatr. 2005;146(5):688–692. [DOI] [PubMed] [Google Scholar]
- 15.Deeb LC. Development of fatal cerebral oedema during outpatient therapy for diabetic ketoacidosis. Pract Diabetes Int. 1989;6(5): 212–213. [Google Scholar]
- 16.Glasgow AM. Devastating cerebral edema in diabetic ketoacidosis before therapy. Diabetes Care. 1991;14(1):77–78. [DOI] [PubMed] [Google Scholar]
- 17.Couch RM, Acott PD, Wong GW. Early onset fatal cerebral edema in diabetic ketoacidosis. Diabetes Care. 1991;14(1):78–79. [DOI] [PubMed] [Google Scholar]
- 18.Fiordalisi I, Harris GD, Gilliland MG. Prehospital cardiac arrest in diabetic ketoacidemia: why brain swelling may lead to death before treatment. J Diabetes Complications. 2002;16(3):214–219. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbloom AL. Intracerebral crises during treatment of diabetic ketoacidosis. Diabetes Care. 1990;13(1):22–33. [DOI] [PubMed] [Google Scholar]
- 20.Edge JA, Hawkins MM, Winter DL, Dunger DB. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child. 2001;85(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcin JP, Glaser N, Barnett P, et al. Factors associated with adverse outcomes in children with diabetic ketoacidosis-related cerebral edema. J Pediatr. 2002;141(6):793–797. [DOI] [PubMed] [Google Scholar]
- 22.Ghetti S, Lee JK, Sims CE, Demaster DM, Glaser NS. Diabetic ketoacidosis and memory dysfunction in children with type 1 diabetes. J Pediatr. 2010;156(1):109–114. [DOI] [PubMed] [Google Scholar]
- 23.Shehata G, Eltayeb A. Cognitive function and event-related potentials in children with type 1 diabetes mellitus. J Child Neurol. 2010;25 (4):469–474. [DOI] [PubMed] [Google Scholar]
- 24.Nadebaum C, Scratch SE, Northam EA, Cameron FJ, Diabetic K. Brain injury study G. clinical utility of mental state screening as a predictor of intellectual outcomes 6 months after diagnosis of type 1 diabetes. Pediatr Diabetes. 2012;13(8):632–637. [DOI] [PubMed] [Google Scholar]
- 25.Cameron FJ, Scratch SE, Nadebaum C, et al. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care. 2014; 37(6):1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jessup AB, Grimley MB, Meyer E, et al. Effects of Diabetic ketoacidosis on visual and verbal neurocognitive function in Young patients presenting with new-onset type 1 diabetes. J Clin Res Pediatr Endocrinol. 2015;7(3):203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cato MA, Mauras N, Mazaika P, et al. Longitudinal evaluation of cognitive functioning in Young children with type 1 diabetes over 18 months. J Int Neuropsychol Soc. 2016;22(3):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenkovich K, Bischoff A, Doty T, et al. Clinical presentation and memory function in youth with type 1 diabetes. Pediatr Diabetes. 2016;17(7):492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aye T, Mazaika PK, Mauras N, et al. Impact of early diabetic ketoacidosis on the developing brain. Diabetes Care. 2019;42(3):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cameron FJ, Northam EA, Ryan CM. The effect of type 1 diabetes on the developing brain. Lancet Child Adolesc Health. 2019;3(6): 427–436. [DOI] [PubMed] [Google Scholar]
- 31.Leinonen V, Vanninen R, Rauramaa T. Raised intracranial pressure and brain edema. Handb Clin Neurol. 2017;145:25–37. [DOI] [PubMed] [Google Scholar]
- 32.Lam TI, Anderson SE, Glaser N, O’Donnell ME. Bumetanide reduces cerebral edema formation in rats with diabetic ketoacidosis. Diabetes. 2005;54(2):510–516. [DOI] [PubMed] [Google Scholar]
- 33.Yuen N, Anderson SE, Glaser N, Tancredi DJ, O’Donnell ME. Cerebral blood flow and cerebral edema in rats with diabetic ketoacidosis. Diabetes. 2008;57(10):2588–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glaser N, Ngo C, Anderson S, Yuen N, Trifu A, O’Donnell M. Effects of hyperglycemia and effects of ketosis on cerebral perfusion, cerebral water distribution, and cerebral metabolism. Diabetes. 2012;61 (7):1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaser N, Bundros A, Anderson S, et al. Brain cell swelling during hypocapnia increases with hyperglycemia or ketosis. Pediatr Diabetes. 2014;15(7):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coma at the onset of young insulin-dependent diabetes in Japan. The results of a nationwide survey. Japan and Pittsburgh childhood diabetes research groups. Diabetes. 1985;34(12):1241–1246. [PubMed] [Google Scholar]
- 37.Mahoney CP, Vlcek BW, DelAguila M. Risk factors for developing brain herniation during diabetic ketoacidosis. Pediatr Neurol. 1999; 21(4):721–727. [DOI] [PubMed] [Google Scholar]
- 38.Nyenwe EA, Razavi LN, Kitabchi AE, Khan AN, Wan JY. Acidosis: the prime determinant of depressed sensorium in diabetic ketoacidosis. Diabetes Care. 2010;33(8):1837–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaneva NY, Konstantinova MM, Iliev DI. Risk factors for cerebral oedema in children and adolescents with diabetic ketoacidosis. Biotechnol Biotechnol Equipment. 2016;30(6):1142–1147. [Google Scholar]
- 40.Glaser N. Cerebral injury and cerebral edema in children with diabetic ketoacidosis: could cerebral ischemia and reperfusion injury be involved? Pediatr Diabetes. 2009;10(8):534–541. [DOI] [PubMed] [Google Scholar]
- 41.Li PA, Shuaib A, Miyashita H, He QP, Siesjo BK, Warner DS. Hyperglycemia enhances extracellular glutamate accumulation in rats subjected to forebrain ischemia. Stroke. 2000;31(1):183–192. [DOI] [PubMed] [Google Scholar]
- 42.Collins RC, Dobkin BH, Choi DW. Selective vulnerability of the brain: new insights into the pathophysiology of stroke. Ann Intern Med. 1989;110(12):992–1000. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman WH, Kappler F, Passmore GG, Mehta R. Diabetic ketoacidosis and its treatment increase plasma 3-deoxyglucosone. Clin Biochem. 2003;36(4):269–273. [DOI] [PubMed] [Google Scholar]
- 44.Lee DM, Hoffman WH, Carl GF, Khichi M, Cornwell PE. Lipid peroxidation and antioxidant vitamins prior to, during, and after correction of diabetic ketoacidosis. J Diabetes Complications. 2002;16(4): 294–300. [DOI] [PubMed] [Google Scholar]
- 45.Jerath RS, Burek CL, Hoffman WH, Passmore GG. Complement activation in diabetic ketoacidosis and its treatment. Clin Immunol. 2005; 116(1):11–17. [DOI] [PubMed] [Google Scholar]
- 46.Rus HG, Niculescu FI, Shin ML. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol Rev. 2001;180:49–55. [DOI] [PubMed] [Google Scholar]
- 47.Van der Meulen JA, Klip A, Grinstein S. Possible mechanism for cerebral oedema in diabetic ketoacidosis. Lancet. 1987;2(8554):306–308. [DOI] [PubMed] [Google Scholar]
- 48.Duck SC, Wyatt DT. Factors associated with brain herniation in the treatment of diabetic ketoacidosis. J Pediatr. 1988;113(1 Pt 1): 10–14. [DOI] [PubMed] [Google Scholar]
- 49.Edge JA, Jakes RW, Roy Y, et al. The UKcase-control study of cerebral oedema complicating diabetic ketoacidosis in children. Diabetologia. 2006;49(9):2002–2009. [DOI] [PubMed] [Google Scholar]
- 50.Muir A. Cerebral Edema in diabetic ketoacidosis: a look beyond rehydration. J Clin Endocrinol Metabol. 2000;85(2):509–513. [Google Scholar]
- 51.Young E, Bradley RF. Cerebral edema with irreversible coma in severe diabetic ketoacidosis. N Engl J Med. 1967;276(12):665–669. [DOI] [PubMed] [Google Scholar]
- 52.Hayes TM, Woods CJ. Unexpected death during treatment of uncomplicated diabetic ketoacidosis. Br Med J. 1968;4(5622):32–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammond P, Wallis S. Cerebral oedema in diabetic ketoacidosis. BMJ. 1992;305(6847):203–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glaser N, Yuen N, Anderson SE, Tancredi DJ, O’Donnell ME. Cerebral metabolic alterations in rats with diabetic ketoacidosis: effects of treatment with insulin and intravenous fluids and effects of bumetanide. Diabetes. 2010;59(3):702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muir AB, Quisling RG, Yang MC, Rosenbloom AL. Cerebral edema in childhood diabetic ketoacidosis: natural history, radiographic findings, and early identification. Diabetes Care. 2004;27(7):1541–1546. [DOI] [PubMed] [Google Scholar]
- 56.Tiwari LK, Jayashree M, Singhi S. Risk factors for cerebral edema in diabetic ketoacidosis in a developing country: role of fluid refractory shock. Pediatr Crit Care Med. 2012;13(2):e91–e96. [DOI] [PubMed] [Google Scholar]
- 57.Rosival V. The influence of blood hydrogen ion concentration on the level of consciousness in diabetic ketoacidosis. Ann Clin Res. 1987; 19(1):23–25. [PubMed] [Google Scholar]
- 58.Edge JA, Roy Y, Bergomi A, et al. Conscious level in children with diabetic ketoacidosis is related to severity of acidosis and not to blood glucose concentration. Pediatr Diabetes. 2006;7(1):11–15. [DOI] [PubMed] [Google Scholar]
- 59.Fulop M, Rosenblatt A, Kreitzer SM, Gerstenhaber B. Hyperosmolar nature of diabetic coma. Diabetes. 1975;24(6):594–599. [DOI] [PubMed] [Google Scholar]
- 60.Brian JE Jr. Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88(5):1365–1386. [DOI] [PubMed] [Google Scholar]
- 61.Isales CM, Min L, Hoffman WH. Acetoacetate and beta-hydroxybutyrate differentially regulate endothelin-1 and vascular endothelial growth factor in mouse brain microvascular endothelial cells. J Diabetes Complications. 1999;13(2):91–97. [DOI] [PubMed] [Google Scholar]
- 62.Kety SS, Polis BD, Nadler CS, Schmidt CF. The blood flow and oxygen consumption of the human brain in diabetic acidosis and coma.J Clin Invest. 1948;27(4):500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffman WH, Pluta RM, Fisher AQ, Wagner MB, Yanovski JA. Transcranial Doppler ultrasound assessment of intracranial hemodynamics in children with diabetic ketoacidosis. J Clin Ultrasound. 1995;23(9):517–523. [DOI] [PubMed] [Google Scholar]
- 64.Bureau MA, Begin R, Berthiaume Y, Shapcott D, Khoury K, Gagnon N. Cerebral hypoxia from bicarbonate infusion in diabetic acidosis. J Pediatr. 1980;96(6):968–973. [DOI] [PubMed] [Google Scholar]
- 65.Clarke DD, Sokoloff L. Cerebral metabolic rate in various physiological states. In: Siegel GJAB, Albers RW, et al. , eds. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th ed. Philadelphia: Lippincott-Raven; 1999. [Google Scholar]
- 66.Hoffman WH, Stamatovic SM, Andjelkovic AV. Inflammatory mediators and blood brain barrier disruption in fatal brain edema of diabetic ketoacidosis. Brain Res. 2009;1254:138–148. [DOI] [PubMed] [Google Scholar]
- 67.Haringhuizen A, Tjan DH, Grool A, van Vugt R, van Zante AR. Fatal cerebral oedema in adult diabetic ketoacidosis. Neth J Med. 2010;68 (1):35–37. [PubMed] [Google Scholar]
- 68.Carl GF, Hoffman WH, Passmore GG, et al. Diabetic ketoacidosis promotes a prothrombotic state. Endocr Res. 2003;29(1):73–82. [DOI] [PubMed] [Google Scholar]
- 69.Glaser NS, Wootton-Gorges SL, Marcin JP, et al. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr. 2004; 145(2):164–171. [DOI] [PubMed] [Google Scholar]
- 70.Figueroa RE, Hoffman WH, Momin Z, Pancholy A, Passmore GG, Allison J. Study of subclinical cerebral edema in diabetic ketoacidosis by magnetic resonance imaging T2 relaxometry and apparent diffusion coefficient maps. Endocr Res. 2005;31(4):345–355. [DOI] [PubMed] [Google Scholar]
- 71.Glaser NS, Marcin JP, Wootton-Gorges SL, et al. Correlation of clinical and biochemical findings with diabetic ketoacidosis-related cerebral edema in children using magnetic resonance diffusion-weighted imaging. J Pediatr. 2008;153(4):541–546. [DOI] [PubMed] [Google Scholar]
- 72.Vavilala MS, Marro KI, Richards TL, et al. Change in mean transit time, apparent diffusion coefficient, and cerebral blood volume during pediatric diabetic ketoacidosis treatment. Pediatr Crit Care Med. 2011;12(6):e344–e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glaser NS, Wootton-Gorges SL, Buonocore MH, et al. Subclinical cerebral edema in children with diabetic ketoacidosis randomized to 2 different rehydration protocols. Pediatrics. 2013;131(1): e73–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dervan L, Poliakov A, Friedman SD, et al. Change in fractional anisotropy during treatment of diabetic ketoacidosis in children. Pediatr Res. 2014;75(1–1):62–66. [DOI] [PubMed] [Google Scholar]
- 75.Glaser NS, Wootton-Gorges SL, Kim I, et al. Regional brain water content and distribution during Diabetic ketoacidosis. J Pediatr. 2017;180:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vavilala MS, Richards TL, Roberts JS, et al. Change in blood-brain barrier permeability during pediatric diabetic ketoacidosis treatment. Pediatr Crit Care Med. 2010;11(3):332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vokes TP, Aycinena PR, Robertson GL. Effect of insulin on osmoregulation of vasopressin. Am J Physiol. 1987;252(4 Pt 1): E538–E548. [DOI] [PubMed] [Google Scholar]
- 78.Tulassay T, Rascher W, Korner A, Miltenyi M. Atrial natriuretic peptide and other vasoactive hormones during treatment of severe diabetic ketoacidosis in children. J Pediatr. 1987;111(3):329–334. [DOI] [PubMed] [Google Scholar]
- 79.Zerbe RL, Vinicor F, Robertson GL. Plasma vasopressin in uncontrolled diabetes mellitus. Diabetes. 1979;28(5):503–508. [DOI] [PubMed] [Google Scholar]
- 80.Charlton JA, Thompson CJ, Baylis PH. Possible mechanisms responsible for the rise in plasma vasopressin associated with diabetic ketoacidosis in the rat. J Endocrinol. 1988;116(3):343–348. [DOI] [PubMed] [Google Scholar]
- 81.Hoffman WH, Cheng C, Passmore GG, Carroll JE, Hess D. Acetoacetate increases expression of intercellular adhesion molecule-1 (ICAM-1) in human brain microvascular endothelial cells. Neurosci Lett. 2002;334(2):71–74. [DOI] [PubMed] [Google Scholar]
- 82.Close TE, Cepinskas G, Omatsu T, et al. Diabetic ketoacidosis elicits systemic inflammation associated with cerebrovascular endothelial cell dysfunction. Microcirculation. 2013;20(6):534–543. [DOI] [PubMed] [Google Scholar]
- 83.Lin B, Ginsberg MD, Busto R, Li L. Hyperglycemia triggers massive neutrophil deposition in brain following transient ischemia in rats. Neurosci Lett. 2000;278(1–2):1–4. [DOI] [PubMed] [Google Scholar]
- 84.Lo W, O’Donnell M, Tancredi D, Orgain M, Glaser N. Diabetic ketoacidosis in juvenile rats is associated with reactive gliosis and activation of microglia in the hippocampus. Pediatr Diabetes. 2016; 17(2):127–139. [DOI] [PubMed] [Google Scholar]
- 85.Glaser N, Little C, Lo W, et al. Treatment with the KCa3.1 inhibitor TRAM-34 during diabetic ketoacidosis reduces inflammatory changes in the brain. Pediatr Diabetes. 2017;18(5):356–366. [DOI] [PubMed] [Google Scholar]
- 86.Hoffman WH, Helman SW, Passmore G. Acute activation of peripheral lymphocytes during treatment of diabetic ketoacidosis. J Diabetes Complications. 2001;15(3):144–149. [DOI] [PubMed] [Google Scholar]
- 87.Gogos CA, Giali S, Paliogianni F, Dimitracopoulos G, Bassaris HP, Vagenakis AG. Interleukin-6 and C-reactive protein as early markers of sepsis in patients with diabetic ketoacidosis or hyperosmosis. Diabetologia. 2001;44(8):1011–1014. [DOI] [PubMed] [Google Scholar]
- 88.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004; 53(8):2079–2086. [DOI] [PubMed] [Google Scholar]
- 89.Karavanaki K, Karanika E, Georga S, et al. Cytokine response to diabetic ketoacidosis (DKA) in children with type 1 diabetes (T1DM). Endocr J. 2011;58(12):1045–1053. [DOI] [PubMed] [Google Scholar]
- 90.Dalton RR, Hoffman WH, Passmore GG, Martin SL. Plasma C-reactive protein levels in severe diabetic ketoacidosis. Ann Clin Lab Sci. 2003;33(4):435–442. [PubMed] [Google Scholar]
- 91.Lindroos-Jokinen K, Keltanen T, Vanhala T, Valonen T, Sajantila A. Postmortem measurement of C-reactive protein and interpretation of results in ketoacidosis. Leg Med. 2012;14(3):140–146. [DOI] [PubMed] [Google Scholar]
- 92.Ma SG, Jin Y, Xu W, Hu W, Bai F, Wu XJ. Increased serum levels of ischemia-modified albumin and C-reactive protein in type 1 diabetes patients with ketoacidosis. Endocrine. 2012;42(3):570–576. [DOI] [PubMed] [Google Scholar]
- 93.Hoffman WH, Burek CL, Waller JL, Fisher LE, Khichi M, Mellick LB. Cytokine response to diabetic ketoacidosis and its treatment. Clin Immunol. 2003;108(3):175–181. [DOI] [PubMed] [Google Scholar]
- 94.Jain SK, Kannan K, Lim G, Matthews-Greer J, McVie R, Bocchini JA Jr. Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. 2003;26(7):2139–2143. [DOI] [PubMed] [Google Scholar]
- 95.Hoffman WH, Casanova MF, Cudrici CD, et al. Neuroinflammatory response of the choroid plexus epithelium in fatal diabetic ketoacidosis. Exp Mol Pathol. 2007;83(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoffman WH, Artlett CM, Boodhoo D, et al. Markers of immunemediated inflammation in the brains of young adults and adolescents with type 1 diabetes and fatal diabetic ketoacidosis. Is there a difference? Exp Mol Pathol. 2017;102(3):505–514. [DOI] [PubMed] [Google Scholar]
- 97.Hoffman WH, Cudrici CD, Zafranskaia E, Rus H. Complement activation in diabetic ketoacidosis brains. Exp Mol Pathol. 2006;80(3): 283–288. [DOI] [PubMed] [Google Scholar]
- 98.Hoffman WH, Siedlak SL, Wang Y, Castellani RJ, Smith MA. Oxidative damage is present in the fatal brain edema of diabetic ketoacidosis. Brain Res. 2011;1369:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoffman WH, Andjelkovic AV, Zhang W, Passmore GG, Insulin SAA. IGF-1 receptors, nitrotyrosin and cerebral neuronal deficits in two young patients with diabetic ketoacidosis and fatal brain edema. Brain Res. 2010;1343:168–177. [DOI] [PubMed] [Google Scholar]
- 100.Hoffman WH, Shacka JJ, Andjelkovic AV. Autophagy in the brains of young patients with poorly controlled T1DM and fatal diabetic ketoacidosis. Exp Mol Pathol. 2012;93(2):273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoffman WH, Artlett CM, Zhang W, et al. Receptor for advanced glycation end products and neuronal deficit in the fatal brain edema of diabetic ketoacidosis. Brain Res. 2008;1238:154–162. [DOI] [PubMed] [Google Scholar]
- 102.Woo MM, Patterson EK, Clarson C, et al. Elevated leukocyte Azurophilic enzymes in human Diabetic ketoacidosis plasma degrade cerebrovascular endothelial Junctional proteins. Crit Care Med. 2016;44(9):e846–e853. [DOI] [PubMed] [Google Scholar]
- 103.Omatsu T, Cepinskas G, Clarson C, et al. CXCL1/CXCL8 (GROalpha/IL-8) in human diabetic ketoacidosis plasma facilitates leukocyte recruitment to cerebrovascular endothelium in vitro. Am J Physiol Endocrinol Metab. 2014;306(9):E1077–E1084. [DOI] [PubMed] [Google Scholar]
- 104.Garro A, Chodobski A, Szmydynger-Chodobska J, et al. Circulating matrix metalloproteinases in children with diabetic ketoacidosis. Pediatr Diabetes. 2017;18(2):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoffman WH, Cudrici CD, Boodhoo D, Tatomir A, Rus V, Rus H. Intracerebral matrix metalloproteinase 9 in fatal diabetic ketoacidosis. Exp Mol Pathol. 2019;108:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roberts JS, Vavilala MS, Schenkman KA, Shaw D, Martin LD, Lam AM. Cerebral hyperemia and impaired cerebral autoregulation associated with diabetic ketoacidosis in critically ill children. Crit Care Med. 2006;34(8):2217–2223. [DOI] [PubMed] [Google Scholar]
- 107.Ma L, Roberts JS, Pihoker C, et al. Transcranial Doppler-based assessment of cerebral autoregulation in critically ill children during diabetic ketoacidosis treatment. Pediatr Crit Care Med. 2014;15(8): 742–749. [DOI] [PubMed] [Google Scholar]
- 108.Chung M, Maa T, O’Brien N. 986: prolonged abnormal cerebral autoregulation in children with moderate to severe diabetic ketoacidosis. Crit Care Med. 2013;41(12):A247–A248. [Google Scholar]
- 109.Iori E, Calo L, Valbusa D, et al. Diabetic ketosis activates lymphomonocyte-inducible nitric oxide synthase. Diabet Med. 2002; 19(9):777–783. [DOI] [PubMed] [Google Scholar]
- 110.Avogaro A, Calo L, Piarulli F, et al. Effect of acute ketosis on the endothelial function of type 1 diabetic patients: the role of nitric oxide. Diabetes. 1999;48(2):391–397. [DOI] [PubMed] [Google Scholar]
- 111.Glaser NS, Tancredi DJ, Marcin JP, et al. Cerebral hyperemia measured with near infrared spectroscopy during treatment of diabetic ketoacidosis in children. J Pediatr. 2013;163(4):1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sener RN, Diffusion MRI. Apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput Med Imaging Graph. 2001;25(4): 299–326. [DOI] [PubMed] [Google Scholar]
- 113.Tasker RC, Acerini CL. Cerebral edema in children with diabetic ketoacidosis: vasogenic rather than cellular? Pediatr Diabetes. 2014; 15(4):261–270. [DOI] [PubMed] [Google Scholar]
- 114.Clements RS Jr, Blumenthal SA, Morrison AD, Winegard AI. Increased cerebrospinal fluid pressure during therapy for diabetic acidosis. Trans Assoc Am Physicians. 1971;84:102–112. [PubMed] [Google Scholar]
- 115.Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6(1):40. [DOI] [PubMed] [Google Scholar]
- 116.Clements RS Jr, Prockop LD, Winegrad AI. Acute cerebral oedema during treatment of hyperglycaemia. An experimentsl model. Lancet. 1968;2(7564):384–386. [DOI] [PubMed] [Google Scholar]
- 117.Prockop LD. Hyperglycemia, polyol accumulation, and increased intracranial pressure. Arch Neurol. 1971;25(2):126–140. [DOI] [PubMed] [Google Scholar]
- 118.Arieff AI, Kleeman CR. Studies on mechanisms of cerebral edema in diabetic comas. Effects of hyperglycemia and rapid lowering of plasma glucose in normal rabbits. J Clin Invest. 1973;52(3):571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arieff AI, Kleeman CR. Cerebral edema in diabetic comas. II. Effects of hyperosmolality, hyperglycemia and insulin in diabetic rabbits. J Clin Endocrinol Metab. 1974;38(6):1057–1067. [DOI] [PubMed] [Google Scholar]
- 120.Harris GD, Lohr JW, Fiordalisi I, Acara M. Brain osmoregulation during extreme and moderate dehydration in a rat model of severe DKA. Life Sci. 1993;53(3):185–191. [DOI] [PubMed] [Google Scholar]
- 121.Silver SM, Clark EC, Schroeder BM, Sterns RH. Pathogenesis of cerebral edema after treatment of diabetic ketoacidosis. Kidney Int. 1997;51(4):1237–1244. [DOI] [PubMed] [Google Scholar]
- 122.Harris GD, Fiordalisi I, Harris WL, Mosovich LL, Finberg L. Minimizing the risk of brain herniation during treatment of diabetic ketoacidemia: a retrospective and prospective study. J Pediatr. 1990;117(1 Pt 1):22–31. [DOI] [PubMed] [Google Scholar]
- 123.Edge JA, Dunger DB. Variations in the management of diabetic ketoacidosis in children. Diabet Med. 1994;11(10):984–986. [DOI] [PubMed] [Google Scholar]
- 124.Duck SC, Weldon VV, Pagliara AS, Haymond MW. Cerebral edema complicating therapy for diabetic ketoacidosis. Diabetes. 1976;25(2): 111–115. [DOI] [PubMed] [Google Scholar]
- 125.Agarwal N, Dave C, Patel R, Shukla R, Kapoor R, Bajpai A. Factors associated with cerebral Edema at admission in Indian children with diabetic ketoacidosis. Indian Pediatr. 2020;57(4):310–313. [PubMed] [Google Scholar]
- 126.Vlcek BW. Risk factors for cerebral edema associated with diabetic ketoacidosis. Ann Neurol. 1986;20(3):407. [Google Scholar]
- 127.Hoorn EJ, Carlotti AP, Costa LA, et al. Preventing a drop in effective plasma osmolality to minimize the likelihood of cerebral edema during treatment of children with diabetic ketoacidosis. J Pediatr. 2007; 150(5):467–473. [DOI] [PubMed] [Google Scholar]
- 128.Durward A, Ferguson LP, Taylor D, Murdoch IA, Tibby SM. The temporal relationship between glucose-corrected serum sodium and neurological status in severe diabetic ketoacidosis. Arch Dis Child. 2011;96(1):50–57. [DOI] [PubMed] [Google Scholar]
- 129.Toledo JD, Modesto V, Peinador M, et al. Sodium concentration in rehydration fluids for children with ketoacidotic diabetes: effect on serum sodium concentration. J Pediatr. 2009;154(6):895–900. [DOI] [PubMed] [Google Scholar]
- 130.Akcan N, Uysalol M, Kandemir I, et al. Evaluation of the efficacy and safety of 3 different management protocols in pediatric diabetic ketoacidosis. Pediatr Emerg Care. 2019;1–6. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 131.Bello FA, Sotos JF. Cerebral oedema in diabetic ketoacidosis in children. Lancet. 1990;336(8706):64. [DOI] [PubMed] [Google Scholar]
- 132.Hale PM, Rezvani I, Braunstein AW, Lipman TH, Martinez N, Garibaldi L. Factors predicting cerebral edema in young children with diabetic ketoacidosis and new onset type I diabetes. Acta Paediatr. 1997;86(6):626–631. [DOI] [PubMed] [Google Scholar]
- 133.Harris GD, Fiordalisi I. Physiologic management of diabetic ketoacidemia. A 5-year prospective pediatric experience in 231 episodes. Arch Pediatr Adolesc Med. 1994;148(10):1046–1052. [DOI] [PubMed] [Google Scholar]
- 134.Fiordalisi I, Novotny WE, Holbert D, Finberg L, Harris GD. Critical care management G. an 18-yr prospective study of pediatric diabetic ketoacidosis: an approach to minimizing the risk of brain herniation during treatment. Pediatr Diabetes. 2007;8(3):142–149. [DOI] [PubMed] [Google Scholar]
- 135.Rosenbloom AL, Riley WJ, Weber FT, Malone JI, Donnelly WH. Cerebral edema complicating diabetic ketoacidosis in childhood. J Pediatr. 1980;96(3 Pt 1):357–361. [DOI] [PubMed] [Google Scholar]
- 136.Mel JM, Werther GA. Incidence and outcome of diabetic cerebral oedema in childhood: are there predictors? J Paediatr Child Health. 1995;31(1):17–20. [DOI] [PubMed] [Google Scholar]
- 137.Felner EI, White PC. Improving management of diabetic ketoacidosis in children. Pediatrics. 2001;108(3):735–740. [DOI] [PubMed] [Google Scholar]
- 138.Savas-Erdeve S, Berberoglu M, Oygar P, et al. Efficiency of fluid treatments with different sodium concentration in children with type 1 diabetic ketoacidosis. J Clin Res Pediatr Endocrinol. 2011;3(3): 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hsia DS, Tarai SG, Alimi A, Coss-Bu JA, Haymond MW. Fluid management in pediatric patients with DKA and rates of suspected clinical cerebral edema. Pediatr Diabetes. 2015;16(5):338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hanas R, Lindgren F, Lindblad B. Diabetic ketoacidosis and cerebral oedema in Sweden—a 2-year paediatric population study. Diabet Med. 2007;24(10):1080–1085. [DOI] [PubMed] [Google Scholar]
- 141.Smedman L, Escobar R, Hesser U, Persson B. Sub-clinical cerebral oedema does not occur regularly during treatment for diabetic ketoacidosis. Acta Paediatr. 1997;86(11):1172–1176. [DOI] [PubMed] [Google Scholar]
- 142.Jayashree M, Singhi S. Diabetic ketoacidosis: predictors of outcome in a pediatric intensive care unit of a developing country. Pediatr Crit Care Med. 2004;5(5):427–433. [DOI] [PubMed] [Google Scholar]
- 143.White PC, Dickson BA. Low morbidity and mortality in children with diabetic ketoacidosis treated with isotonic fluids. J Pediatr. 2013; 163(3):761–766. [DOI] [PubMed] [Google Scholar]
- 144.Turan C, Yurtseven A, Basa EG, Goksen D, Saz EU. The effects of prehospital care on outcome in pediatric diabetic ketoacidosis. J Clin Res Pediatr Endocrinol. 2020;12(2):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bakes K, Haukoos JS, Deakyne SJ, et al. Effect of volume of fluid resuscitation on metabolic normalization in children presenting in Diabetic ketoacidosis: a randomized controlled trial. J Emerg Med. 2016;50(4):551–559. [DOI] [PubMed] [Google Scholar]
- 146.Kuppermann N, Ghetti S, Schunk JE, et al. Clinical trial of fluid infusion rates for Pediatric Diabetic ketoacidosis. N Engl J Med. 2018; 378(24):2275–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Glaser N, Kuppermann N. Fluid treatment for children with diabetic ketoacidosis: how do the results of the pediatric emergency care applied research network fluid therapies under investigation in diabetic ketoacidosis (FLUID) trial change our perspective? Pediatr Diabetes. 2019;20(1):10–14. [DOI] [PubMed] [Google Scholar]
- 148.Tornheim PA. Regional localization of cerebral edema following fluid and insulin therapy in streptozotocin-diabetic rats. Diabetes. 1981; 30(9):762–766. [DOI] [PubMed] [Google Scholar]
- 149.Rose KL, Pin CL, Wang R, Fraser DD. Combined insulin and bicarbonate therapy elicits cerebral edema in a juvenile mouse model of diabetic ketoacidosis. Pediatr Res. 2007;61(3):301–306. [DOI] [PubMed] [Google Scholar]
- 150.Schade DS, Eaton RP, Alberti KGMM, Johnston DG. Bicarbonate administration. Diabetic Coma: Ketoacidotic and Hyperosmolar. Albuquerque: University of New Mexico Press; 1981:171–183. [Google Scholar]
- 151.Alberti KG, Emerson PM, Darley JH, Hockaday TD. 2,3-Diphosphoglycerate and tissue oxygenation in uncontrolled diabetes mellitus. Lancet. 1972;2(7774):391–395. [DOI] [PubMed] [Google Scholar]
- 152.Munk P, Freedman MH, Levison H, Ehrlich RM. Effect of bicarbonate on oxygen transport in juvenile diabetic ketoacidosis. J Pediatr. 1974;84(4):510–514. [DOI] [PubMed] [Google Scholar]
- 153.Assal JP, Aoki TT, Manzano FM, Kozak GP. Metabolic effects of sodium bicarbonate in management of diabetic ketoacidosis. Diabetes. 1974;23(5):405–411. [DOI] [PubMed] [Google Scholar]
- 154.Agarwal HS. Subclinical cerebral edema in diabetic ketoacidosis in children. Clin Case Rep. 2019;7(2):264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jones MD Jr. Energy metabolism in the developing brain. Semin Perinatol. 1979;3(2):121–129. [PubMed] [Google Scholar]
- 156.Mans AM, DeJoseph MR, Davis DW, Hawkins RA. Brain energy metabolism in streptozotocin-diabetes. Biochem J. 1988;249(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tasker RC, Lutman D, Peters MJ. Hyperventilation in severe diabetic ketoacidosis. Pediatr Crit Care Med. 2005;6(4):405–411. [DOI] [PubMed] [Google Scholar]
- 158.Marcin JP, Glaser N, Kuppermann N. Ventilation in pediatric diabetic ketoacidosis—not too much, but not too little. Pediatr Crit Care Med. 2005;6(4):489–490. [DOI] [PubMed] [Google Scholar]
- 159.Rose K, Drysdale TA, Fraser DD. Sodium hydrogen exchanger (NHE) antagonist methyl isobutyl amiloride inhibits cerebral edema associated with diabetic ketoacidosis (DKA-CE). FASEB J. 2007;21(6): A964-A964. [Google Scholar]
- 160.Rose KL, Watson AJ, Drysdale TA, et al. Simulated diabetic ketoacidosis therapy in vitro elicits brain cell swelling via sodiumhydrogen exchange and anion transport. Am J Physiol Endocrinol Metab. 2015;309(4):E370–E379. [DOI] [PubMed] [Google Scholar]
- 161.Watts W, Edge JA. How can cerebral edema during treatment of diabetic ketoacidosis be avoided? Pediatr Diabetes. 2014;15(4):271–276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.