Abstract
Aims
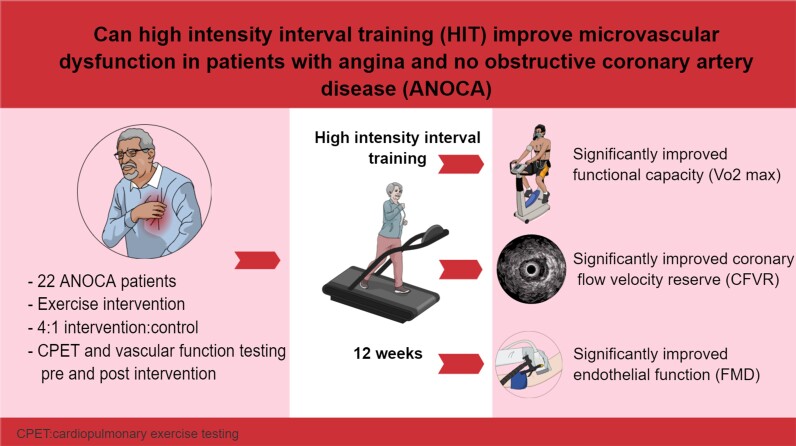
Patients with chest pain and normal coronary angiogram [angina with normal coronary arteries (ANOCA)] constitute a therapeutic problem with considerable functional limitation and reduced quality of life. The aims of the current pilot study were to (i) explore if a structured aerobic high-intensity interval training (HIT) program for 12 weeks was feasible in patients with ANOCA, and (ii) to assess mechanisms related to symptoms in this population.
Methods and results
Sixteen patients with ANOCA underwent a 3-month aerobic HIT program with one-to-one monitored exercise sessions on treadmill in a 4 min × 4 manner, three times a week. Four patients served as controls. Coronary flow velocity reserve (CFVR) transthoracic Doppler, flow-mediated vasodilation (FMD) and VO2max was measured at baseline and after 12 weeks. The average attendance to training sessions was 82.3% ± 10.1 (56–94). CFVR in the training group increased from 2.50 ± 0.48 to 3.04 ± 0.71 (P < 0.001) whereas FMD increased from 4.19 ± 2.42% to 8.28 ± 2.85% (P < 0.001). Improvement in CFVR correlated with the relative improvement in FMD (R = 0.45, P = 0.047). This was associated with an increase in VO2max from 28.75 ± 6.51 mL/kg/min to 31.93 ± 6.46 mL/kg/min (P < 0.001).
Conclusion
A 3-month program of monitored HIT was feasible, with high adherence resulting in improved functional capacity in patients with ANOCA. CFVR improved and this improvement was associated with improved FMD.
ClinicalTrials.gov Identifier
Keywords: ANOCA, Coronary flow velocity reserve, Coronary microvascular dysfunction, Exercise training , Endothelial function, Functional capacity
Graphical Abstract
Graphical abstract.
Introduction
A large part of patients with angina symptoms suggestive of obstructive coronary artery disease presents with angina and no obstructive coronary artery disease [angina with normal coronary arteries (ANOCA)] at selective coronary angiography.1–3 Ischaemia is documented in about 50% of subjects in reported studies (ischaemia with no obstructive coronary artery disease).4
Microvascular disease (MVD) is present in more than 50% of this population.5 It is characterized by impaired coronary flow velocity reserve (CFVR) and is associated with the same risk factors as obstructive coronary artery disease.6 Patients with impaired CFVR and chest pain despite normal coronary angiogram constitute a therapeutic problem with considerable residual morbidity associated with functional limitation, reduced quality of life,7 and increased mortality.8
Exercise training improves both endothelial function and symptoms in patients with obstructive coronary artery disease.9 However, this treatment option is not previously evaluated in patients with ANOCA. Despite the lack of evidence, the authors of the “2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease” suggest adhering to the same exercise recommendations as for long-standing chronic coronary syndrome.10
The aims of the current pilot study were to (i) explore if a structured aerobic high-intensity interval training (HIT) program for 12 weeks was feasible in patients with ANOCA, and (ii) to assess mechanisms related to symptoms in this population.
Methods
The main inclusion criteria was angina at effort with or without objective signs of ischaemia in patients older than 18 years of age with no obstructive coronary artery disease demonstrated at invasive coronary angiography. This was an open label non-randomized prospective mechanistic pilot trial with a 4:1 intervention to control ratio. The controls were patients who fulfilled the inclusion criteria, but who lived to far away from the training center to participate in the training program.
Ethics
The study was approved by the local ethical committee (2013/98/REK Vest) and performed in accordance with the Helsinki Declaration. All patients had to sign an informed consent form. ClinicalTrials.gov Identifier: NCT02905630.
Test protocols
All patients underwent baseline testing which included blood sampling, echocardiography, functional capacity by VO2max measurement, endothelial function test by flow and nitro-glycerine-mediated vasodilation (NMD), and microvascular function testing by CFVR. Vasoactive drugs were discontinued for at least 24 h prior to the measurement.
Coronary Flow Velocity Reserve
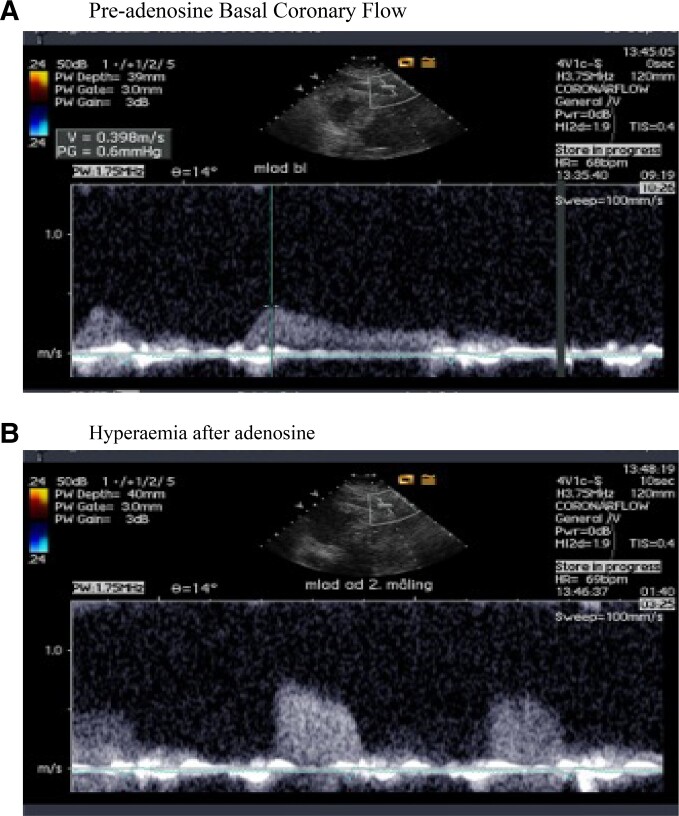
CFVR was assessed using trans-thoracic Doppler. This method has a good reproducibility comparable with other contemporary measures applied in cardiology and is in agreement with myocardial perfusion reserve evaluated with positron emission tomography.11 Blood flow velocities were measured via transthoracic echocardiography in the distal to mid left anterior descending coronary artery using pulsed-wave Doppler with 1.75- to 3.5-MHz frequencies in a sample volume of 1.5 to 5 mm, (VIVID 7, GE medical ultrasound system). Adenosine was administered by intravenous infusion (0.14 mg/kg/min over 2 min) to record spectral Doppler signals during hyperaemia. Peak diastolic flow velocities were measured, and CFVR was calculated as the ratio of hyperaemic to basal peak flow velocities. A predefined cut-off value of <2.5 was used. An example of registration of coronary flow velocity pre and post vasodilatation is illustrated in Figure 1.
Figure 1.
Doppler registrations of coronary flow velocity: (A) during rest and (B) during adenosine infusion.
Flow-mediated endothelial function
Patients were examined with ultrasound detection of brachial artery diameter variations during hyperaemia before and after the intervention period. Patients met fasting for at least 12 h. Two-dimensional brachial artery imaging and measurements were obtained using a 12-MHz linear-array transducer. (VIVID 7, GE medical ultrasound system). Eight- to ten-millimeter straight segments of the brachial artery above the antecubital fossa were examined. Flow-mediated vasodilation (FMD) due to share induced endothelial nitric oxide production was measured after occlusion of the forearm circulation. Following 15 min of rest, new measurements were done after administration of nitro-glycerine sublingually; NMD. We have previously validated this technique with regard to inter- and intra-observer variability.9
Exercise capacity
The patients were evaluated on treadmill (Woodway PPS 55 Med-I) at a speed of 5–5.5 km/h with gradual increasing inclination. A target test duration of ∼8–10 min was aimed for. Gas exchange data was collected continuously with an automated breath-by-breath system (JAEGER, Care Fusion, Germany).
Training protocol
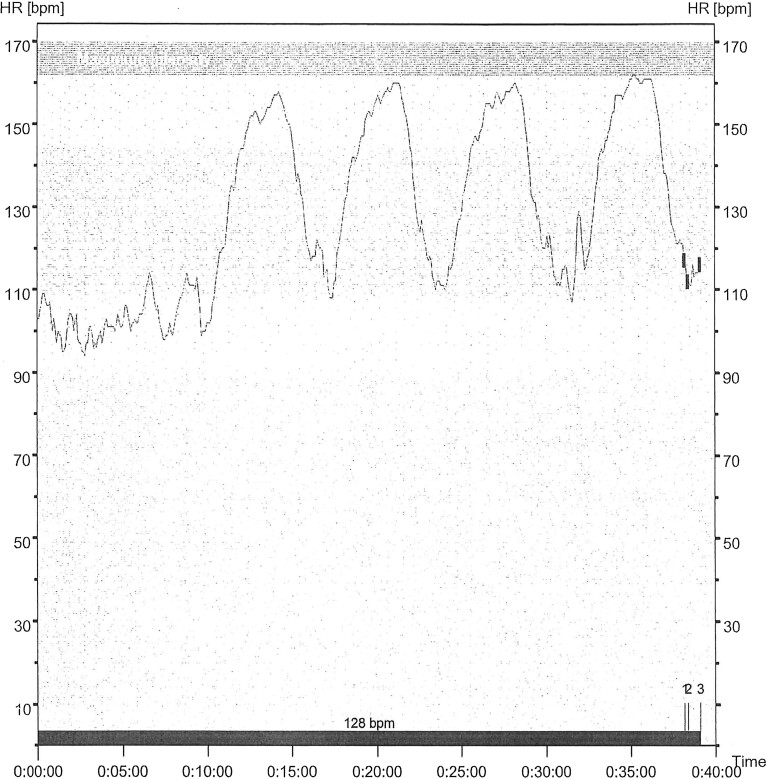
The training group participated in a specially designed aerobic HIT program. Participants exercised on treadmills, first a 10 min of warm-up at 60–70% of maximal heart rate, followed by four 4-min intervals at 80–90% of maximal heart rate. Intervals were interrupted by three minutes of active recovery at 60–70% of maximal heart rate. The training sessions were repeated three times a week over a twelve-week period. Patients were divided into groups of maximum five. Intensity control during workouts was assessed with heart rate monitors (Figure 2) by physical therapists specialized in cardiac rehabilitation.
Figure 2.
Typical heart rate diagram during an HIT session.
Statistics
Continuous variables were presented by mean ± standard deviation (SD) or median with first and third quartiles, categorical variables were presented using count and percentage. Group comparisons of baseline clinical data were carried out using Mann–Whitney U test for continuous variables and Fisher exact test for categorical variables. Significance of differences between baseline and follow-up were assessed using Wilcoxon signed-rank test. Differences in changes of physiological parameters before and after intervention between the groups were evaluated using Mann–Whitney U test. Strength of association between characteristics was evaluated using Spearman (ρ) correlation coefficient. All tests were two-sided, with statistical significance defined as P < 0.05.
Results
Twenty-two patients were included, 18 in the training group and four as controls. Two patients in the training group had to quit because of health problems unrelated to the exercise-training program. In total, 16 patients participated in the training group and four in the control group.
Except for baseline pretest relative change NMD (HIT 9.2 vs. Control 12.1 with P < 0.023) and BMI (HIT 28 vs. Control 26 with P < 0.050), there were no statistical differences in baseline characteristics between the groups (Table 1). Pretest CFVR was lower than suggested cut-off value for normal CFVR (<2.5) in both groups (HIT; 2.50 ± 0.48 and Control; 2.43 ± 0.59).
Table 1.
Baseline characteristics
| Characteristica | HIT group | Controls | P-value |
|---|---|---|---|
| (n = 16) | (n = 4) | ||
| Age, years | 60 (56–66) | 62 (60–66) | 0.394 |
| Male, n (%) | 8 (50) | 1 (25) | 0.591 |
| Current smoker, n (%) | 1 (6) | 0 (0) | 1.000 |
| BMI | 28 (28–32) | 26 (24–27) | 0.050 |
| Systolic blood pressure (mmHg) | 136 (124–144) | 156 (146–171) | 0.098 |
| SR, n (%) | 16 (100) | 4 (100) | 1.000 |
| Hypertension, n (%) | 7 (44) | 3 (75) | 0.582 |
| Medications, n (%) | |||
| Acetylcysteine | 7 (44) | 3 (75) | 0.582 |
| Betablocker | 3 (19) | 3 (75) | 0.061 |
| ACE-inhibitor | 1 (6) | 0 (0) | 1.000 |
| Angiotensin receptor blocker | 5 (31) | 2 (50) | 0.587 |
| Statin | 8 (50) | 3 (75) | 0.591 |
| Nitrates | 1 (6) | 1 (25) | 0.368 |
| Other characteristics at baseline | |||
| Pretesting VO2max (mL/kg/min) | 25 (23–33) | 27 (24–30) | 0.670 |
| Pretest coronary rest velocity | 0.2 (0.2–0.3) | 0.3 (0.2–0.4) | 0.537 |
| Pretest coronary flow reserve (CFR) (max/rest) | 2.5 (2.2–2.7) | 2.4 (2.1–2.8) | 0.892 |
| Pretest baseline diameter brachial artery | 3.9 (3.5–4.5) | 3.5 (3.2–3.9) | 0.344 |
| Pretest absolute change flow-mediated dilation(FMD) | 0.2 (0.1–0.2) | 0.2 (0.1–0.2) | 0.810 |
| Pretest relative change FMD | 4 (2.0–5.7) | 4.8 (3.9–5.2) | 0.887 |
| Pretest absolute change nitric oxide mediated dilation(NMD) | 0.4 (0.3–0.4) | 0.4 (0.4–0.5) | 0.235 |
| Pretest relative change NMD | 9.2 (8.6–10.1) | 12.1 (11.6–13.0) | 0.023 |
n = 20 patients.
Continuous data are median with first and third quartiles; SR, sinus rhythm; BMI, body mass index, CFVR, coronary flow velocity reserve; FMD, flow-mediated vasodilation relative change; NMD, nitric oxide mediated dilation.
No history/observation of arrhythmias, diabetes, vascular disease or previous TIA/cerebral insult.
The average attendance to training sessions was 82.3% ± 10.1 (56–94). There were no adverse events related to the aerobic HIT sessions reported other than expected angina and fatigue.
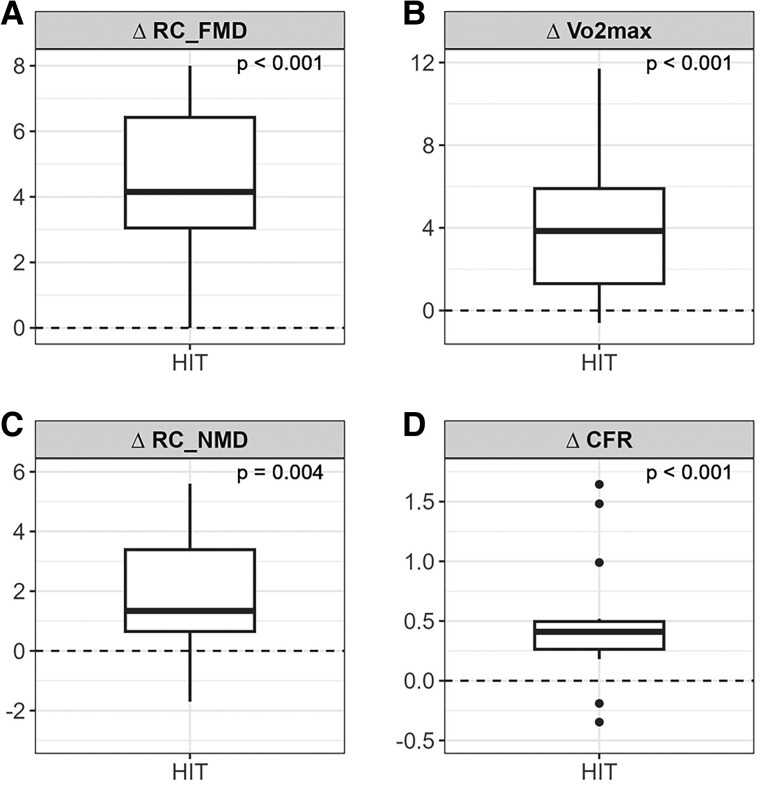
After 12 weeks, exercise capacity expressed as VO2max (mL/kg/min) increased in both groups, but it was only statistically significant in the aerobic HIT group (28.75 ± 6.51 to 31.93 ± 6.46, P < 0.001, Table 2, Figure 3). The between-group difference was significant (P = 0.022, Table 2).
Table 2.
Changes in physiological parameters
| Measurement | Number | Baseline | Follow-up | Difference | P-value | P-value § |
|---|---|---|---|---|---|---|
| CFR | ||||||
| HIT | 16 | 2.50 ± 0.48 | 3.04 ± 0.71 | 0.57 ± 0.61 | <0.001 | |
| Control | 4 | 2.43 ± 0.59 | 2.74 ± 0.48 | 0.31 ± 0.94 | 0.625 | 0.211 |
| FMD delta/RC % | ||||||
| HI T | 16 | 4.19 ± 2.42 | 8.28 ± 2.85 | 4.38 ± 2.30 | <0.001 | |
| Control | 4 | 4.32 ± 2.17 | 4.62 ± 2.13 | 0.31 ± 1.44 | 0.875 | 0.005 |
| FMD delta/AC | ||||||
| HIT | 16 | 0.16 ± 0.09 | 0.31 ± 0.13 | 0.16 ± 0.12 | 0.001 | |
| Control | 4 | 0.15 ± 0.07 | 0.17 ± 0.08 | 0.02 ± 0.05 | 0.461 | 0.042 |
| Peak VO2 [mL/kg/min] | ||||||
| HIT | 16 | 28.75 ± 6.51 | 31.93 ± 6.46 | 3.81 ± 3.12 | <0.001 | |
| Control | 4 | 26.52 ± 4.10 | 27.17 ± 3.51 | 0.65 ± 0.98 | 0.250 | 0.022 |
| NMD delta/RC % | ||||||
| HIT | 15 | 9.73 ± 2.33 | 11.33 ± 2.49 | 1.96 ± 2.03 | 0.004 | |
| Control | 4 | 12.43 ± 1.88 | 9.95 ± 2.95 | −2.48 ± 4.54 | 0.625 | 0.028 |
| FMD baseline (mm) | ||||||
| HIT | 16 | 4.03 ± 0.86 | 3.92 ± 0.87 | 0.11 ± 0.15 | 0.011 | 0.042 |
| Control | 4 | 3.59 ± 0.51 | 3.65 ± 0.56 | 0.06 ± 0.22 | 0.625 | |
Data are means ± SD.
CFVR, coronary flow velocity reserve; HIT, high-intensity interval training; FMD, flow-mediated vasodilatation; RC, relative change; AC, absolute change; NMD, nitric oxide mediated dilatation.
P-value denotes comparison from baseline to follow-up within group.
P§: P-value denotes comparison between groups.
Figure 3.
Changes in physiological parameters after 12 weeks of intervention: (A) Relative change in flow mediated vasodilation (%), (B) Change in VO2max (ml/kg/min), (C) Relative change in nitro mediated vasodilation (%), (D) Change in coronary flow reserve (ratio hyperaemic to basal peak flow velocities).
CFVR increased in both groups, but the increase was only statistically significant in the aerobic HIT group (from 2.50 ± 0.48 to 3.04 ± 0.71, P < 0.001, Figure 3). There was a numerical difference between groups, but this was not statistically significant. Improvement in CFVR correlated with the relative improvement in FMD (ρ = 0.45, P = 0.047).
FMD expressed in relative change (%) increased in both groups, but the increase was only statistically significant in the aerobic HIT group (4.19 ± 2.42 to 8.28 ± 2.85, P < 0.001, Figure 3). The between-group differences for relative changes was significant (P = 0.005).
There was also a statistically significant increase in NMD, expressed in relative change (%) in the aerobic HIT group only (9.73 ± 2.33 to 11.33 ± 2.49, P = 0.004, Figure 3). The between-group differences for relative changes in NMD was significant (P = 0.028).
Discussion
The findings in this ET trial in patients with ANOCA were that the increased functional capacity after 12 weeks of aerobic HIT was associated with improved CFVR and that this improvement correlated with the improvement in FMD. Both FMD and NMD improved after HIT. This improvement in vasodilator response found after HIT is thus not only explained by improved endothelial function but also involves improvement in vascular smooth muscle response. This may indicate a therapeutic role for exercise in the treatment of patients with ANOCA, targeting the often associated microvascular dysfunction as evidenced by impaired CFVR.
Despite the positive effect of exercise training in patients with CAD,12 reduced physical activity is one of the major avoidance behaviours in patients with angina, including the large proportion with ANOCA.13 However, in the current study, a HIT program was feasible. Additionally the participants had a high adherence to ET session. This is probably related to the sense of confidence and being able to master the training as revealed in a sub study on quality of life in this population.14 Participants in the current study did physical exercise on a treadmill overseen by experienced physiotherapists giving guidance and emotional support to obtain adequate heart rate during the HIT sessions (Figure 2). The participants valued being tested, as well as the possibility of getting a diagnosis, and they talked about being “seen” and “selected” when they were asked to participate in the project, which implied testing and training.
The findings of improved functional capacity and endothelial function are in accordance with previous research on the effect of exercise training in patients with cardiovascular disease.15,16 These beneficial effects of aerobic HIT are extended with a modest positive effect on a pathological low CFVR (<2.5)7–9 in the present study. This is consistent with the positive effects of exercise training on myocardial perfusion evaluated by SPECT in a group of patients with microvascular angina.20 However, the finding of an increased CFVR after a structured HIT program in patients with ANOCA is as far as we know, not previously reported.
MVD is characterised with impaired coronary blood flow response to exercise. This is partly due to diminished vasodilator and contractile responses of coronary resistance arterioles.21 Smooth muscle cells modulation from a contractile to a synthetic phenotype is an early key event in atherogenesis. This conversion to a new phenotype is characterized by increased secretion of proteins and molecules that promote vascular degeneration through chronic inflammation and extracellular matrix degradation.22,23 In experimental studies, a reversal of this process with improved arteriolar contractile responsiveness and a younger smooth muscle phenotype induced by exercise training24 has been associated with improved endothelial function.25
These findings are consistent with our findings in this human study. The beneficial effects of HIT including improved functional capacity, endothelial function, vascular smooth muscle response, and myocardial arteriolar vasomotor response indicate that aerobic HIT might be a therapeutic option in ANOCA. However, these results must first be confirmed in an adequately powered RCT. The sample size needed to show a significant difference in CFVR can be calculated using a two-way ANOVA model. For two groups (HIT and control), two study time points (baseline and 12 weeks), medium effect size (Cohen’s f of 0.25), power of 0.8, significance level α of 0.05 and equal number of subjects in each group, total required sample size would be n = 128. For a large effect size (Cohen’s f of 0.4), the total sample size is reduced to n = 52.
Limitations
The registrations of CFVR, FMD, and peak VO2 were performed at baseline and after 12 weeks of intervention. The lack of blinding of the interpretation of the measurements adequately is a limitation in this study.
Flow velocity in the brachial artery was not recorded during FMD. There was no direct evaluation of the relationship between improvement in physiological parameters and changes in the degree of angina. However, in a qualitative exploration in the same group of patients quality of life was considerably improved after the HIT program.14
Conclusion
A 3-month program of monitored HIT was feasible, with high adherence resulting in improved functional capacity in patients with ANOCA. CFVR improved and this improvement was associated with improved flow-mediated vasodilatation.
Lead author biography
 A.I.L. is Professor of Medicine at the University of Bergen and Head of Cardiovascular Interventions and Research at Stavanger University Hospital, Stavanger. He is an interventional cardiologist and pacemaker cardiologist with research interests in coronary circulation, acute myocardial infarction, sudden cardiac arrest, heart failure, and exercise training in cardiology. Research Fellow University of Calgary 2001. Former Board Member of the Norwegian Society of cardiology 2003–2013. Advisory Board Member in the Norwegian registry for invasive cardiology and the Norwegian registry for sudden cardiac arrest. C.S. shared first authorship is Consultant Cardiologist at the Department of Cardiology, Stavanger University Hospital and Research Fellow at University of Bergen.
A.I.L. is Professor of Medicine at the University of Bergen and Head of Cardiovascular Interventions and Research at Stavanger University Hospital, Stavanger. He is an interventional cardiologist and pacemaker cardiologist with research interests in coronary circulation, acute myocardial infarction, sudden cardiac arrest, heart failure, and exercise training in cardiology. Research Fellow University of Calgary 2001. Former Board Member of the Norwegian Society of cardiology 2003–2013. Advisory Board Member in the Norwegian registry for invasive cardiology and the Norwegian registry for sudden cardiac arrest. C.S. shared first authorship is Consultant Cardiologist at the Department of Cardiology, Stavanger University Hospital and Research Fellow at University of Bergen.
Contributor Information
Alf Inge Larsen, Department of Cardiology, Stavanger University Hospital, Gerd-Ragna Bloch Thorsens gate 8, 4011 Stavanger, Norway; Institute of Clinical Science, University of Bergen, Jonas Lies veg 87, 5021 Bergen, Norway.
Charlotte Sæland, Department of Cardiology, Stavanger University Hospital, Gerd-Ragna Bloch Thorsens gate 8, 4011 Stavanger, Norway.
Johnny Vegsundvåg, Department of Medicine, Ålesund Hospital, Åsehaugen 5, 6017 Ålesund, Norway.
Mette Storebø Skadberg, Department of Cardiology, Stavanger University Hospital, Gerd-Ragna Bloch Thorsens gate 8, 4011 Stavanger, Norway.
Jorunn Nilsen, Department of Research, Stavanger University Hospital, Gerd-Ragna Bloch Thorsens gate 8, 4011 Stavanger, Norway.
Noreen Butt, Department of Cardiology, Stavanger University Hospital, Gerd-Ragna Bloch Thorsens gate 8, 4011 Stavanger, Norway.
Anastasia Ushakova, Department of Research, Stavanger University Hospital, Gerd-Ragna Bloch Thorsens gate 8, 4011 Stavanger, Norway.
Torstein Valborgland, Department of Cardiology, Stavanger University Hospital, Gerd-Ragna Bloch Thorsens gate 8, 4011 Stavanger, Norway.
Peter Scott Munk, Kristiansand Hjertepraksis, Vestre Strandgate 42, 4612 Kristiansand, Norway.
Kjetil Isaksen, Department of Cardiology, Stavanger University Hospital, Gerd-Ragna Bloch Thorsens gate 8, 4011 Stavanger, Norway.
Author contributions
A.I.L. had the conceptual idea. A.I.L., K.I., P.S.M., and M.S.S. designed the study. K.I. and J.N. organized the training and testing protocol. N.B. and T.V. performed the echocardiographic examination. C.S., J.V., M.S.S., and A.I.L. performed the coronary flow Doppler registrations. K.I. and M.S.S. performed the functional tests. P.S.M. and C.S. performed the FMD measurements. C.S., A.U., and A.I.L. performed the statistical analyses. A.U. confirmed the statistical methods used. All authors read and approved the manuscript. A.I.L., K.I., and C.S. drafted the manuscript. Thanks to Anne Haugland and Marit Christiansen for contribution during the training period.
Data availability
Data are available on request.
References
- 1. Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, Jørgensen E, Kelbæk H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33::734–744. [DOI] [PubMed] [Google Scholar]
- 2. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;11:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaw J, Anderson T. Coronary endothelial dysfunction in non-obstructive coronary artery disease: risk, pathogenesis, diagnosis and therapy. Vasc Med 2016;21:146–155. [DOI] [PubMed] [Google Scholar]
- 4. Beltrame JF, Tavella R, Jones D, Zeitz C. Management of ischaemia with non-obstructive coronary arteries (INOCA). BMJ 2021;26:375. [DOI] [PubMed] [Google Scholar]
- 5. Ford TJ, Yii E, Sidik N, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, McCartney P, Corcoran D, Collison D, Rush C, Stanley B, McConnachie A, Sattar N, Touyz RM, Oldroyd KG, Berry C. Ischemia and no obstructive coronary artery disease: prevalence and correlates of coronary vasomotion disorders. Circ Cardiovasc Interv 2019;12:e008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;22:830–840. [DOI] [PubMed] [Google Scholar]
- 7. Kaski JC. Pathophysiology and management of patients with chest pain and normal coronary arteriograms (cardiac syndrome X). Circulation 2004;10:568–572. [DOI] [PubMed] [Google Scholar]
- 8. Marks DS, Gudapati S, Prisant LM, Weir B, diDonato-Gonzalez C, Waller JL, Houghton JL. Mortality in patients with microvascular disease. J Clin Hypertens 2004;6:304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Munk PS, Staal EM, Butt N, Isaksen K, Larsen AI. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation: a randomized controlled trial evaluating the relationship to endothelial function and inflammation. Am Heart J 2009;158:734–741. [DOI] [PubMed] [Google Scholar]
- 10. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos-Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M; ESC Scientific Document Group . 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. [DOI] [PubMed] [Google Scholar]
- 11. Shelton ME, Senneff MJ, Ludbrook PA, Sobel BE, Bergmann SR. Concordance of nutritive myocardial perfusion reserve and flow velocity reserve in conductance vessels in patients with chest pain with angiographically normal coronary arteries. J Nucl Med 1993;34:717–722. [PubMed] [Google Scholar]
- 12. Wise FM, Patrick JM. Resistance exercise in cardiac rehabilitation. Clin Rehabil 2011;25:1059–1065. [DOI] [PubMed] [Google Scholar]
- 13. Amundsen BH, Wisløff U, Slørdahl SA. Trening ved hjerte-og karsykdommer. Tidsskr Nor Laegeforen 2007;15:446–448. [PubMed] [Google Scholar]
- 14. Røysland IØ, Friberg F, Støre Brinchmann B, Nordeide Svello S, Valborgland T, Larsen AI. Confronting one's vulnerability—patients with chest pain participating in a high-intensity exercise programme. J Clin Nurs 2017;26:2006–2015. [DOI] [PubMed] [Google Scholar]
- 15. Kissel CK, Nikoletou D. Cardiac rehabilitation and exercise prescription in symptomatic patients with non-obstructive coronary artery disease-a systematic review. Curr Treat Options Cardiovasc Med 2018;18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kolmos M, Krawcyk RS, Kruuse C. Effect of high-intensity training on endothelial function in patients with cardiovascular and cerebrovascular disease: a systematic review. SAGE Open Med 2016;4:2050312116682253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group . 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 18. Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol 2015;12:48–62. [DOI] [PubMed] [Google Scholar]
- 19. Gould KL, Bui L, Kitkungvan D, Patel MB. Reliability and reproducibility of absolute myocardial blood flow: does it depend on the PET/CT technology, the vasodilator, and/or the software? Curr Cardiol Rep 2021;23:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szot W, Zając J, Kubinyi A, Kostkiewicz M. The effects of cardiac rehabilitation on overall physical capacity and myocardial perfusion in women with microvascular angina. Kardiol Pol 2016;74:431–438. [DOI] [PubMed] [Google Scholar]
- 21. Hanna MA, Taylor CR, Chen B, La HS, Maraj JJ, Kilar CR, Behnke BJ, Delp MD, Muller-Delp JM. Structural remodeling of coronary resistance arteries: effects of age and exercise training. J Appl Physiol (1985) 2014;117:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang M, Khazan B, Lakatta EG. Central arterial aging and angiotensin II signaling. Curr Hypertens Rev 2010;6:266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin H, Pickering JG. Cellular senescence and vascular disease: novel routes to better understanding and therapy. Can J Cardiol 2016;32:612–623. [DOI] [PubMed] [Google Scholar]
- 24. Muller-Delp JM, Hotta K, Chen B, Behnke BJ, Maraj JJ, Delp MD, Lucero TR, Bramy JA, Alarcon DB, Morgan HE, Cowan MR, Haynes AD. Effects of age and exercise training on coronary microvascular smooth muscle phenotype and function. J Appl Physiol (1985) 2018;124:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hotta K, Chen B, Behnke BJ, Ghosh P, Stabley JN, Bramy JA, Sepulveda JL, Delp MD, Muller-Delp JM. Exercise training reverses age-induced diastolic dysfunction and restores coronary microvascular function. J Physiol 2017;595:3703–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request.