Abstract
Purpose of Review
The relationship between cardiovascular health and diet is evolving. Lifestyle modifications including diet changes are the primary approach in managing cardiometabolic risk factors. Thus, understanding different diets and their impact on cardiovascular health is important in guiding primary and secondary prevention of cardiovascular disease (CVD). Yet, there are many barriers and limitations to adopting a heart healthy diet.
Recent Findings
Diets rich in fruits, vegetables, legumes, whole grains, and lean protein sources, with minimization/avoidance of processed foods, trans-fats, and sugar sweetened beverages, are recommended by prevention guidelines. The Mediterranean, DASH, and plant-based diets have all proven cardioprotective in varying degrees and are endorsed by professional healthcare societies, while other emerging diets such as the ketogenic diet and intermittent fasting require more long-term study. The effects of diet on the gut microbiome and on cardiovascular health have opened a new path for precision medicine to improve cardiometabolic risk factors. The effects of certain dietary metabolites, such as trimethylamine N-oxide, on cardiometabolic risk factors, along with the changes in the gut microbiome diversity and gene pathways in relation to CVD management, are being explored.
Summary
In this review, we provide a comprehensive up-to-date overview on established and emerging diets in cardiovascular health. We discuss the effectiveness of various diets and most importantly the approaches to nutritional counseling where traditional and non-traditional approaches are being practiced, helping patients adopt heart healthy diets. We address the limitations to adopting a heart healthy diet regarding food insecurity, poor access, and socioeconomic burden. Lastly, we discuss the need for a multidisciplinary team-based approach, including the role of a nutrition specialist, in implementing culturally-tailored dietary recommendations. Understanding the limitations and finding ways to overcome the barriers in implementing heart-healthy diets will take us miles in the path to CVD prevention and management.
Keywords: cardiovascular disease, diet, guidelines, nutrition, disparities, gut microbiome
Plain Language Summary
The Mediterranean, DASH, and plant-based diets remain the leading heart healthy diets that match cardiology professional society recommendations. Emerging diets like the ketogenic diet and intermittent fasting are effective weight loss diets with unknown heart protective benefits, and require further studies on whether the benefits outweigh the risks. Adopting a heart-healthy diet is a foundational component for cardiovascular disease prevention, but barriers and limitations to adopting heart healthy diets exist and need to be assessed as part of cardiovascular risk assessment.
Introduction
Despite the advances in medical management and evidence-supported dietary interventions for improved heart health, cardiovascular disease (CVD) remains the leading cause of death in the United States.1 Over time, various diets have been studied for their effectiveness in CVD prevention.2 One of the earliest epidemiological studies investigating the link between diet and CVD was the Seven Countries Study led by Ancel Keys between 1958 and 1964, which demonstrated that the myocardial infarction (MI) rate was lower in countries where fruits, vegetables, grains, beans, and fish formed the major part of the diet.3,4 The INTERHEART Study then showed that 90% of MIs were due to preventable factors, with daily consumption of fruits and vegetables, along with regular physical activity, being associated with a 40% reduction in MI [Odds Ratio (OR)=0.60 (95% CI=0.51–0.71)].5 The Prospective Urban Rural Epidemiology (PURE) study conducted in over 130,000 individuals from 18 countries demonstrated that diets rich in fruits, vegetables, and legumes were associated with 19% lower risk of all-cause mortality [Hazard Ratio (HR)=0.81 (95% CI=0.68–0.96)] over 7-years of follow-up.6
Lifestyle changes, including a heart-healthy diet, are the foundation of all CVD prevention guidelines.7–10 The main cardiometabolic risk factors are diabetes, hypertension, dyslipidemia, and excess abdominal fat, which are all affected by dietary changes.11 Unfortunately, there has been a worsening trend in CVD risk factors globally.12 In the United States, the prevalence of type 2 diabetes (T2D) is 37 million adults, or 11% of the adult population, but an estimated 88 million US adults have prediabetes.13 Globally, in 2021, 537 million individuals were affected by T2D, corresponding to 10.5% of the world’s population.14 On a similar trend, the prevalence of controlled blood pressure, which had improved between 1999–2008, did not significantly change from 2007–2014, and then decreased after 2014.15 Globally, between 1975 and 2016, the prevalence of obesity [defined in this study as a Body Mass Index (BMI) ≥25 kg/m2)] has tripled in adults.16 In the US in 2017–2018, the age-adjusted prevalence of obesity (BMI ≥30 kg/m2) among adults was 42% and severe obesity (BMI ≥40 kg/m2) was 9%.17 Increased visceral adiposity is a main driver of excess cardiometabolic risk, and increased abdominal waist circumference has become a CVD risk marker by itself.18
Lifestyle modifications from exercise and dietary interventions have been well studied and proven effective toward CVD prevention and management. A healthy diet is one of the American Heart Association (AHA)’s “Life’s Essential 8” cardiovascular health metrics, which also include favorable levels of physical activity, sleep, nicotine exposure, BMI, blood lipids, blood glucose, and blood pressure.19 However, <1% of US adults met “ideal” criteria for the AHA’s healthy diet metric in 2013–2014.20
Diets rich in fruits, vegetables, legumes, whole grains, and lean protein sources, with minimization/avoidance of processed foods, trans-fats, and sugar sweetened beverages, are recommended by prevention guidelines. The Mediterranean, the Dietary Approaches to Stop Hypertension (DASH), and plant-based diets all have proven cardioprotective in varying degrees and are endorsed by professional healthcare societies, while other emerging diets such as the ketogenic diet and intermittent fasting require more long-term study. The effects of diet on the gut microbiome and on cardiovascular health have opened a new path for precision medicine to improve cardiometabolic risk factors. The effects of certain dietary metabolites, such as trimethylamine N-oxide (TMAO), on cardiometabolic risk factors, along with the changes in the gut microbiome diversity and gene pathways in relation to CVD management, are being explored.
In this review, we will provide a comprehensive up-to-date overview on established and emerging diets for cardiovascular health and CVD prevention. We will address the limitations to adopting a heart healthy diet from food insecurity, poor access, and socioeconomic burden. Lastly, we will discuss the need for additional nutritional education in health professional schools, and the importance of a multidisciplinary team to partner with patients to improve their nutritional knowledge and self-efficacy in adopting heart healthy diets.
Established Heart Healthy Diets
Three major dietary patterns (the Mediterranean diet, the DASH diet, and the healthy plant-based diet) (Figure 1) have the most evidence for CVD prevention and are described below.
Figure 1.
The Heart Healthy Dietary Patterns: Mediterranean Diet, DASH Diet, and Healthy Plant-Based Diet.
The Mediterranean Diet
The Mediterranean diet, which was inspired by the eating habits of countries that border the Mediterranean sea (ie, Spain, Italy, and Southern Greece) is one that is rich in whole-grains, leafy green vegetables, fruits, legumes, unsalted nuts, herbs, spices, and extra virgin olive oil (EVOO), with a moderate intake of lean proteins such as fish and poultry, moderate alcohol consumption, and limited intake of red meat and sweets. By its very nature, the Mediterranean diet limits or eliminates many of the culprits in the standard American diet that drive CVD – saturated fat, highly processed refined grains and sugars, and red meat.2,10,21 The Mediterranean diet differs from other dietary patterns as there is a greater emphasis on EVOO, nuts, reduced dairy consumption, and eating with family.22 The Mediterranean diet has been found to be favorable as a food pattern, rather than single nutrient supplementation, as it inherently provides anti-oxidants, reduces inflammation of the vascular wall, modulates pro-atherogenic genes, alters gut microbiome, and improves lipid panels by decreasing low density lipoprotein cholesterol (LDL-C) and raising high density lipoprotein cholesterol (HDL-C).21,23–25
Nutritional genomic studies show that greater adherence to a Mediterranean diet blunt the development of an adverse cardiometabolic phenotype in genetically susceptible individuals, with interactions between the Mediterranean diet and polymorphisms of the cyclooxygenase-2, interleukin-6, apolipoprotein A2, cholesteryl ester transfer protein plasma, and transcription factor 7-like 2 genes.26 One study found that the Mediterranean diet plus EVOO prevented increases in levels of pro-atherothrombotic genes such as Cox-2, low-density lipoprotein receptor-related protein, and monocyte chemoattractant protein 1 expression compared to a control diet.27
As mentioned above, the benefits of the Mediterranean diet were first appreciated in the Seven Countries Study (Yugoslavia, Italy, Greece, Finland, the Netherlands, Japan, and the US), led by Ancel Keys in 1958, who found there was a higher rate of mortality from coronary heart disease (CHD) in men from the US and Northern Europe in comparison to regions in Southern Europe.3,28 The countries in southern Europe had higher consumption of olive oil, fruits, vegetables and fish, and lower consumption of meat and animal fats, in comparison to populations in the US and Northern Europe. These populations were also found to be leaner and had active lifestyles, a stark contrast from the US and Northern Europe populations who were mainly sedentary.28 Prospective observational studies also found similar correlations, where persons living in Greece who reported higher adherence to the Mediterranean dietary pattern (per 2 point increment in Mediterranean diet score) were found to have a significantly lower risk of CHD death with an adjusted HR of 0.67 (95% CI=0.47–0.94) over an average of 44 months.29 In another cohort of 15,482 patients who had stable CHD, higher adherence to a Mediterranean-style diet (per 1 unit increase in scores above 12) was independently associated with a 5% reduction in cardiovascular events over 3.7 years (HR=0.95; 95% CI=0.91–0.98).30 Closer adherence to a Mediterranean-style diet has also been shown to be associated with a lower risk of preeclampsia.31
Randomized controlled trials (RCTs) followed these epidemiological studies and have demonstrated the cardiovascular benefits of the Mediterranean style diet. The Lyon Diet Heart Study was a RCT that assessed whether the Mediterranean diet was beneficial in the secondary prevention of CVD among patients after a first MI.32 The authors found a greater than 70% reduction in recurrent non-fatal MI and greater than 50% reduction in mortality in the group adhering to the Mediterranean diet, compared to a prudent diet control group, over a 4-year period.32,33
The Prevención con Dieta Mediterránea (PREDIMED) trial was a RCT that aimed to assess the role of the Mediterranean diet for the primary prevention of CVD among 7,447 patients at elevated cardiovascular risk.34 Participants were randomized to one of three diets: the Mediterranean diet with higher amounts of EVOO, the Mediterranean diet with higher amounts of nuts, or a standard diet that was low in fat, with dietary guidance.34 After 4.8 years, there was approximately a 30% reduction in MI, stroke, or CVD death among those consuming the Mediterranean diet with EVOO (HR=0.69; 95% CI=0.53–0.91) as well as in the group consuming the Mediterranean diet with nuts (HR=0.72; 95% CI=0.54–0.95), compared to the low-fat diet.34 Risk factors for CVD were also shown to benefit with Mediterranean diet intervention, with the incidence of diabetes being 52% lower (95% CI=27–86%) in the two pooled Mediterranean diet groups compared to controls.35 In a biomarkers subgroup analysis, patients following the Mediterranean diet had reduction in N-terminal pro-B-type natriuretic peptide, and those on the Mediterranean diet with EVOO were found to have a reduction in oxidized LDL and lipoprotein(a) plasma concentrations, in comparison to those on the low fat diet.36 Overall the PREDIMED study was one of the groundbreaking trials to show that a Mediterranean diet rich in unsaturated fats and polyphenols can be useful for primary CVD prevention.
Further research has been done to understand why the Mediterranean diet is so valuable in reducing CVD risk. One meta-analysis of observational epidemiologic studies evaluated whether EVOO itself shows benefit in CVD.37 Researchers found that a higher consumption of fats from EVOO had a reduced relative risk (RR) of 0.85 (95% CI=0.77–0.93) for CVD and of 0.83 (95% CI=0.77–0.90) for all-cause mortality. The researchers believed that the anti-inflammatory and antioxidant effects play a role through its abundance of phenolic compounds and vitamin E with the addition of substituting saturated fats from the diet with monounsaturated and polyunsaturated fats.37 Additionally, the anti-inflammatory properties of EVOO play a role in reducing platelet aggregation.21 The emphasis of high fiber foods, such as whole grains and vegetables, in the Mediterranean-style diet provides benefit in the gut microbiome.38 With improvement in the biodiversity of the gut microbiome (as discussed further later in this review), beneficial effects of blood pressure control, reduction in metabolic syndrome and diabetes, lower levels of pro-atherogenic compounds, and ultimately lower rates of CVD have been demonstrated.39 Red meat, which is minimized in the traditional Mediterranean diet, contains the nutrient L-Carnitine, which produces the pro-atherogenic compound γ-butyrobetaine (γBB) as it gets converted to trimethylamine and TMAO by the gut microbes, both of which accelerate atherosclerosis.40 These favorable elements of the Mediterranean dietary pattern are further enhanced by the emphasis of physical activity, which has shown to improve cardiometabolic health and reduce CVD.21
The DASH Diet
The DASH diet was created in 1997 by the National Heart Lung and Blood Institute to prevent and treat hypertension, a known risk factor for CVD.41 The DASH diet is rich in fruits and vegetables, low-fat dairy, whole grain, poultry, fish, nuts, and seeds, while limiting fatty meats, sweets, sugar sweetened beverages, and full-fat dairy products.42 The standard DASH diet restricts salt consumption to 2,300 mg per day. It meets the Dietary Guidelines for Americans’ recommendation of limiting daily salt consumption to less than 2,300 mg. A reduced-sodium version of DASH limits sodium intake to 1,500 mg per day. Significant reduction in cardiometabolic risk is seen with the DASH diet by lowering blood pressure, total cholesterol, and LDL-C, all of which are significant risk factors which are accounted for in the atherosclerotic CVD (ASCVD) risk score and eventually leads to improvement in cardiac function and decreased incidence of diabetes.43–46 One of the main key elements of the DASH diet in lowering blood pressure is the sodium restriction in comparison to other diets.41,42,46
A DASH diet has been shown to reduce blood pressure in persons with or without hypertension, where in an RCT, the DASH diet reduced blood pressure by 5–6 mmHg systolic and 3 mmHg diastolic, while also lowering LDL-C by 11 mg/dL.41 In observational data, a DASH-style dietary pattern has also been associated with a lower risk of CVD, heart failure, and diabetes.47,48 In the Dietary Intervention to Stop Coronary Atherosclerosis in Computed Tomography (DISCO) RCT, 92 individuals, 41% women, with non-obstructive coronary atherosclerosis were randomized to either DASH diet with optimal medical therapy or control group of just optimal medical treatment.49 At a mean of just 67 weeks, a significant reduction in noncalcified plaque in the DASH diet intervention group was found in comparison to the medical treatment alone group.49
The Healthy Plant-Based Diet
A healthy plant-based diet has been shown to be associated with lower cardiovascular risk.50–55 Plant-based diets, a diet consistently of predominantly plants, can be diverse in content, patterns, and even have subsets. The subsets branch into vegetarian (which excludes meat, poultry, seafood, and fish) and vegan (in which all animal food and their products are eliminated from the diet). Other plant-based diet subsets include lacto-vegetarians, who consume dairy products, lacto-ovo-vegetarians, who consume dairy and eggs, pesco-vegetarians or pescatarians, who consume fish, dairy, and eggs, and lastly, semi-vegetarians, who exclude red meats, possibly poultry as well.
These diets are assessed based on the vegetable content of the diet, but do not necessarily account for the sugar, fat, and sodium content, which can decrease the cardioprotective nature of a plant-based diet.51 Indeed, unhealthy plant-based diets (such as those higher in refined grains, potatoes/fries, juices/sweetened beverages, sweets) may have similar CVD risk as animal-based diets.51 The main foundation of a healthy plant-based diet is the whole grains, fruits, vegetables, nuts, and legumes with the low frequency and content of animal food. It has low energy density, with low saturated fats and high fiber content, which triggers early satiety cues and helps with digestion by delaying gastric emptying, with moderate insulinemic and glycemic responses.56,57 Furthermore, studies have shown improved lipid profiles in individuals who follow a plant-based diet due to increased fiber intake, as fat absorption decreases overall.58
Many studies examined this type of diet from different aspects using a pro-vegetarian diet score, which assigns higher scores to plant foods and negative scores to animal foods, or a similar plant-based diet index. In the PREDIMED trial in Spain, vegetarian diets were associated with lower cardiovascular mortality (HR=0.47; 95% CI=0.21–1.04 for highest quintile compared to lowest; p-trend=0.039 across quintiles).59 In two combined observational cohorts of more than 200,000 male and female health professionals in the US, a higher plant-based diet index was inversely associated with incidence of CVD (HR comparing extreme deciles: 0.92; 95% CI=0.83–1.01; p-trend=0.003 across deciles), a relationship which was stronger for a healthy plant-based diet index (HR 0.75; 95% CI=0.68–0.83; p-trend<0.001).51 In another cohort of young and middle-aged adults from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort followed for nearly 32 years, the highest vs lowest quintile of plant-based diet score (time-averaged) was associated with a 52% reduction in incident CVD (HR=0.48; 95% CI=0.28–0.81).60
Furthermore, plant-based diets have low processed meat content or none. Preservatives found in processed meats such as nitrates and sodium contribute to blood pressure elevation, impaired insulin response, and endothelial dysfunction.61 Substantial reductions in cardiometabolic modifiable risk factors, such as blood pressure, LDL-C, blood glucose levels, inflammatory markers, and unhealthy weight patterns, even with similar caloric intake, were seen in plant-based diets in comparison to omnivorous diets.55,61–63 Thus, this type of diet has a great potential in CVD prevention. However, this diet tends to have lower vitamin B12, vitamin D, calcium, zinc, protein, and retinol in comparison to omnivorous diets; for example, those following a vegan diet may require additional supplementation with vitamin B12.64
Reconciling the Healthy Diet Patterns and the Importance of Minimally Processed Food
The Mediterranean, DASH, and healthy plant-based diets have more similarities than differences. Each of these diets emphasize fruits, vegetables, legumes, nuts, and whole grains. Diets rich in fruits and vegetables have been shown to be associated with lower risk of CVD and mortality.6,65 Increased whole grain consumption is also associated with lower risk of CVD and mortality.66 Higher consumption of nuts has also been shown to be associated with lower CVD risk.67
Eating minimally processed whole foods is an important component to all healthy diets, as consuming poor diet quality is one of the leading contributors to cardiometabolic disease globally.68,69 Ultra-processed foods are high in refined carbohydrates, added sugars, saturated and trans fats, sodium, and possibly artificial colors, flavors, and preservatives, and have high energy density and glycemic load, which all contribute to increased cardiometabolic risk factors. They also tend to be highly palatable so that individuals eat more calories in total.70 Furthermore, eating more ultra-processed foods can result in displacement of cardioprotective foods such as fruits and vegetables. In the Framingham Offspring Study, each additional serving a day of ultra-processed foods was associated with a 7% increased risk of incident CVD (HR=1.07; 95% CI=1.03–1.12).70 Similarly, in another cohort from Spain, each additional serving/day of ultra-processed food was associated with 18% higher mortality (HR=1.18; 95% CI=1.05–1.33).71 In controlled feeding trials comparing diets that were matched for total calories, sugar, fat, fiber, and macronutrients, participants allowed ad libitum food intake ate ~500 kcal/day on the ultra-processed diet vs an unprocessed diet, and body weight changes were correlated with dietary differences in energy intake.72
Emerging or Controversial Diets
Additional emerging diet patterns have been studied for their impact on cardiometabolic risk; these are more controversial due to conflicting data regarding their net cardiovascular health benefits or insufficient data to determine their long-term cardiovascular benefits. Two of the more promising ones, the Keto Diet and Time-Restricted Eating (TRE), are discussed below.
The Keto and Other Very Low Carb Diets
The ketogenic (or “keto”) diet is generally characterized by intake of very-low carbohydrate (VLC), high fat, and moderate protein. The class ketogenic diet divided total daily energy intake to be 90% from fat, 7% from protein, and only 3% from carbohydrates. Since that time, other formulations of the ketogenic diet have been proposed to improve compliance, as well as other low or very low carbohydrate diets that generally follow similar patterns of high fat low carbs, but do not generate ketosis. VLC diets have been promoted for their effects on weight loss, improvement in insulin resistance, and glycemic control; however, the high fat diet can promote increases in LDL-C, particularly in the subset of individuals who are LDL hyper-responders, suggestive of latent genetic dyslipidemias.73–75 This has limited the enthusiasm of many professional medical societies from endorsing its use.73,75
The ketogenic diet emerged in the early 1920s as an anti-seizure intervention, but with medical innovation and the development of effective anti-seizure medication, the ketogenic diet is no longer used for management of epilepsy.76 Throughout time the ketogenic diet has been revisited by many nutritionists and scientists to explore the ketosis state of the body. It has been found that antioxidants and anti-inflammatory functions of genes were activated by the beta-hydroxybutyrate (BHB) metabolite product of ketosis.75 BHB has been found to reduce reactive oxygen species production mainly by activating the nuclear factor erythroid-derived 2-related factor 2 (Nrf2) which is the major inducer of detoxification genes. Furthermore, it is an endogenous inhibitor of class I and class IIa histone deacetylases which upregulates the transcription of detoxifying genes including catalase, mitochondrial superoxide dismutase and metallothionein 2.77 In an open label non-randomized control study of the nutritional ketosis state of patients with T2D, reductions in diabetes medication use, hemoglobin A1c (HbA1c), and overall weight were seen after 1 year, while patients were being supported in a continuous care model.78 Individuals with insulin resistance or diabetes cannot properly metabolize glucose in the blood, which makes excess carbohydrates intake unfavorable in terms of glycemic control, and as such, following a VLC diet helps improve glycemic control and HbA1c.79,80 Other studies evaluating VLC diets in the management of obesity found it more effective than low fat diets in improvement in cardiometabolic risk factors.80–83 Furthermore, increased satiety due to the high fat and protein content and possible effects of the ketones on the appetite was found in individuals following a ketogenic diet.84
On the other hand, some of the adverse effects that limit adoption of the ketogenic diet are the increase in total cholesterol, LDL-C, non-HDL cholesterol, triglycerides, and total apoB, all of which increase the risk of CVD.73,74,82,85,86 The effect of a ketogenic diet on LDL-C is inconsistent,86 but some patients can have a very dramatic increase in LDL-C in response. This dramatic increase in LDL-C levels can possibly exacerbate development of hyperlipidemia if an underlying genetic predisposition is present.87 Furthermore,a recent meta-analysis found the ketogenic diet conferred unfavorable effects on LDL-C, apoB, and total cholesterol in normal weight adults.88 The potential deleterious effect on lipids needs to be considered carefully before initiating a ketogenic diet, especially for individuals at elevated CVD risk such as those with T2D, and lipid levels should be monitored in patients following a ketogenic diet. Theoretically, a plant-based, ketogenic diet that is low in saturated fats may limit these adverse consequences, but there are no trials examining this. Long-term data of the efficacy and safety of ketogenic diets are still lacking.
Intermittent Fasting/Intermittent Energy Restrictions
Intermittent fasting (IF) (also known as intermittent energy restriction) diets have different forms and patterns with three general goals: prolonged periods of fasting prompting a state of ketosis, decreasing oxidative stress, and feeding and circadian rhythm synchronization.89 The IF strategy holds promise for cardiovascular health, but the evidence is still limited, and lacking long-term studies.90–93 A recent systematic review of RCTs studied the cardiovascular health benefits of IF in comparison to continuous calories restriction; although IF was found to be more effective in weight loss, it was not clinically significant in reduction of cardiometabolic risk factors.93 Benefits on the molecular and cellular level of improved mitochondrial health, DNA repair, autophagy, and promotion of stem cell-based regeneration were found in laboratory mice.94 Reducing oxidative stress is thought to decrease mitochondrial energy production and in return free radical production leading to reduced overall inflammation.95 Alternatively, the reduction in weight and, hence, cardiometabolic risk factors with IF type diets may simply be the result of fewer total calories consumed given the reduced/restricted hours of feeding.
There are two main patterns of IF: alternate day fasting (ADF) and time restricted eating (TRE). TRE limits consumption of calories to a certain window of time each day, usually a 6–10 hour period. The ADF pattern is having a fast day followed by a feast day. A systemic review and meta-analysis on whether ADF helps with weight loss found the cumulative evidence suggests that ADF does reduce weight, BMI, and total cholesterol.96 However, the studies were of short duration (<6 months long). In an RCT studying the effects of ADF in comparison to a calorie restricted diet or control diet on cardiometabolic risk factors, during a 1 year follow-up period of 6 months weight loss and 6 months maintenance, it was found that ADF was not superior to calories restriction in weight loss, weight maintenance, and reduction of cardiometabolic risk factors.97
Regarding TRE, in a study of 139 individuals with unhealthy weight, no significant weight loss difference was found between individuals who adopted TRE with calorie restriction of 1,500 to 1,900 calories in comparison to calorie restriction alone (net difference=−1.8 kg; 95% CI=−4.0 to 0.4; P=0.11).98 On the other hand, several studies have shown that TRE from a 6 to 10 hours window was effective in weight loss, cardiometabolic health enhancement, while maintaining muscle mass.99–101 In one recent study of firefighters who have a shift-work occupation, researchers demonstrated that a 10-hour/day TRE window was feasible with improvement in cardiometabolic risk factors over 12 weeks compared to a standard feeding pattern.102
Another pattern of TRE is one that is focused on aligning diet with the circadian rhythm with the aim to improve insulin sensitivity and weight loss.103 Having feeding in sync with the circadian rhythm is hypothesized to optimize energy metabolism with the active and inactive phases of the circadian rhythm. Studies have shown that when the circadian rhythm is set (whether it be nocturnal or diurnal), TRE during the active phase has more health benefits and improved glucose tolerance with lower insulin levels needed.104 For example, for a nocturnal rhythm, the active phase would be the dark time.
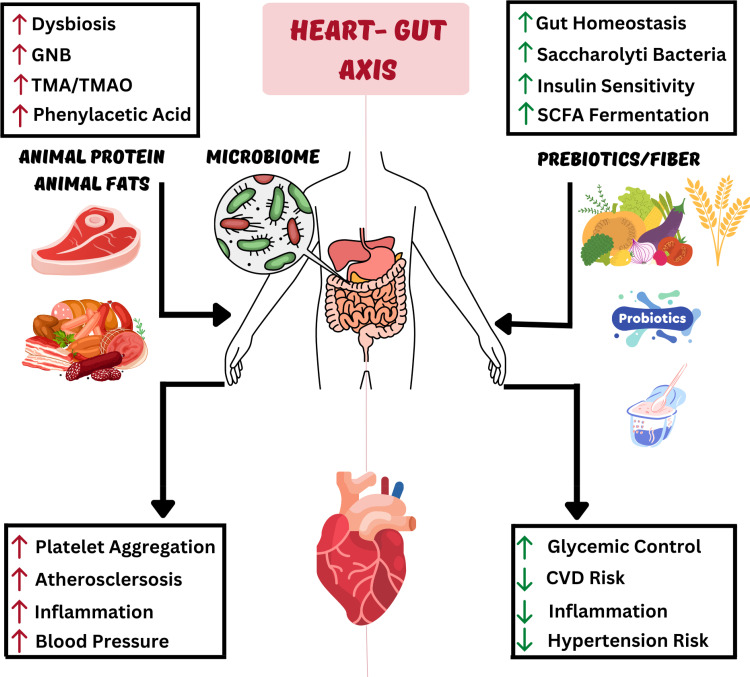
The Gut Microbiome and Heart Health
Lifestyle modifications of diet and exercise have been discussed extensively, but the relationship between cardiometabolic risk factors and the gut microbiome (the heart–gut axis) (Figure 2) is an emerging area of study.39,105 The gut microbiome is affected by dietary intake, antimicrobials, pre-and pro-biotics, and fecal microbial transplant, with potential impact on cardiovascular risk factors. For example, probiotics use has shown benefits in mild reduction in blood pressure, blood glucose, and TMAO levels.106 Higher fiber intake is associated with increased diversity of the gut microbiota,38 and a higher fiber diet is associated with lower risk of hypertension and CVD.107
Figure 2.
The Heart–Gut axis (the role of the Gut Microbiome in Cardiovascular Health).
Individuals with hypertension were found to have altered microbial gut structure, function, and interaction.108 While the autonomic nervous system plays a key role in regulating blood pressure, derangements in the gut microbiome triggers the release of inflammatory mediators and metabolites leading to neuroinflammation and increased blood pressure in return.109 Furthermore, in resistant hypertension, researchers have found altered microbial pathways and metabolites that may drive the increase in blood pressure and be potential targets for new therapeutic approaches in hypertension management.110 Additionally, certain dietary metabolites, such as TMAO, that are found in red meat, poultry, and fish, have been associated with increased risk of CVD.111 TMAO have been linked to kidney fibrosis and loss of function, heart failure, atherosclerosis, and vascular inflammation, as well as thrombosis through platelet hyper-responsiveness.39,111,112 One of the dietary protein metabolites of the gut microbiota is phenylacetic acid, which is metabolized by the liver and converted to phenylacetylglutamine that activates adrenergic receptors and in return increases platelets responsiveness and aggregation.112 Understanding the pathways and interactions of the gut microbiome with diet and medication will help direct precision medicine in CVD management to have specific dietary recommendations, and possibly microbiome targeted therapies.
Cardiology Professional Society Recommendations
The AHA dietary guidance statement to promote cardiometabolic health was most recently released in 2021.113 Diet recommendations were also included in the 2019 American College of Cardiology (ACC)/AHA Primary Prevention of CVD Guideline.7 These guidelines recognize that sedentary lifestyle and increased caloric intake has led to increased excess body weight, which is a modifiable cardiometabolic risk factor. Thus, adjusting energy intake and expenditure to achieve and maintain a healthy body weight was the first recommendation in the dietary guidance.113 These guidelines recognize the importance of healthy dietary patterns, rather than focusing on individual foods or nutrients, and recommend healthy nutrition to begin early in life.113
Additionally the AHA statement recommends eating plenty and a variety of colorful fruits and vegetables;113 “eating the rainbow” is one such approach.114 Leafy green vegetables, other vegetables, whole fruits rather than juice, and legumes can be consumed in all forms such as fresh, frozen, or dried, although canned fruit which often contains added syrups should be avoided.115 The guidelines also recommend choosing whole grain foods and products over refined grains. Whole grains, or products made with 51% whole grains, are rich in fiber which help support healthy weight patterns, improve digestion, and decrease absorption of cholesterol and associated with lower CVD risk.57,107
The AHA statement also recommends eating healthy sources of protein, predominantly plant-based such as legumes and nuts.113 Non-fried fish and seafood intake is recommended given the high omega-3 fatty acid content, especially when it substitutes animal sources of red and processed meat or full-fat dairy products, with ~2 to 3 servings per week of fish being associated with lower CVD incidences.113,116 Other sources of protein include the low-fat or fat-free dairy products which have been shown to have neutral or beneficial effects on CVD risk.113,117 If poultry or meat is desired, the AHA guidelines recommend eating lean and unprocessed forms. Several studies showed increased CVD and mortality incidence with increased intake of red meats.118–121 Increased saturated fats, heme iron content, and l-carnitine metabolites such as TMAO from red meat contribute to atherosclerosis.121,122
The AHA statement recommends using liquid plant oils instead of tropical oils or partially hydrogenated fats.113 The benefits of EVOO, which is high in monounsaturated fat, was reviewed in the Mediterranean diet section. Dietary unsaturated fats, that are found in liquid plant oils such as soybean, corn, safflower and sunflower oils, walnuts, and flax seeds have shown cardiovascular health benefits of reducing LDL-C and total cholesterol levels.123 On the other hand, tropical oils like coconut and palm oils, animal fats like butter and lard, as well as hydrogenated fats raise LDL-C and total cholesterol levels.124,125 Another source of healthy fats is intake of fish that is rich in omega-3 fatty acids, which may have benefits on triglycerides and overall CVD risk, although little effect on LDL-C.123
There had been some conflicting studies about whether linoleic acid (LA), which is found in vegetable oils such as corn, sunflower, and soybean oils, has a harmful effect on cancer and cardiovascular risk. However the purported pro-inflammatory effects of LA and other omega-6 fatty acids have not been conclusively confirmed, with even some studies showing a reduction in inflammation.126 Furthermore, a meta-analysis found higher intakes of LA to be associated with lower (not higher) risk of CVD127 and, along with other evidence, an author of a review concluded that LA intake within the range recommended by AHA has no harms associated with it.128 Another more recent systematic review confirmed the reduction in CHD, CVD, and all-cause mortality with LA, but a slight increased risk in cancer mortality was noted (RR=1.06; 95% CI=1.02–1.11).129 Other meta-analyses have not confirmed an excess cancer risk with higher LA intake.130 Laboratory studies suggest, at higher thresholds, LA does not have tumor-promoting effects and may even be beneficial.131,132 Although a small increase risk in cancer cannot be definitely excluded, there is not sufficient evidence that higher intake of LA substantially increases risk for breast, colorectal, or prostate cancer risk and, thus, should not be specifically avoided.132
The ACC/AHA prevention guidelines discussed reducing added sugars and minimizing sugar-sweetened beverages,7,113 as added sugars have been associated with increased risk of T2D, obesity, and CVD.133,134 Additionally, the AHA guidance is to choose or prepare foods with little or no added salt, and to choose minimally processed foods instead of ultra-processed foods.7,113 As for alcohol intake, the guidelines do not support initiation of alcohol intake for cardio-protection, and to limit alcohol if one does choose to drink.113 Although moderate alcohol intake of 1–2 glasses per day has been associated with low risk of CHD and ischemic stroke,135 the evidence behind this is not strong,135 and some data suggest any alcohol intake, even one glass, is associated with increased risk of atrial fibrillation.136
Approaches to Nutrition Counseling in CV Health
With the vast number of dietary behaviors proven to benefit cardiovascular health, implementation of these patterns can be cumbersome. Studies show that, despite ~80% of CVDs being preventable by adopting a healthier lifestyle, healthy eating behaviors are low, and there are numerous barriers to implementing these recommendations. Healthcare professionals have adopted multiple methods, such as nutrition counseling, culinary medicine, food pharmacies, and inpatient nutrition, all of which have been evaluated as effective means to implement dietary changes to patients.137–139 Nutrition counseling through cognitive or behavioral theory has been shown to be most effective in dietary behaviors, weight, and cardiovascular risk factors as it is a supportive process that helps set priorities, establish goals, and create an individualized plan.138–140 Motivational interviews modeled through behavioral theory, with self-monitoring, meal replacements, and/or meal plans help create a method of self-care. Client-centered counseling techniques in the long-term have been shown to be beneficial in effective chronic disease prevention where one study showed improvement in blood pressure and salt intake.138,140 As nutrition counseling by a dietitian is shown to be beneficial, group counseling is a modality that takes the elements of individual nutrition counseling into a larger population through group therapy or culinary medicine.137,138
Culinary medicine is an evidence-based approach to healthy eating where there is an emphasis on health promotion and illness management through dietary regimens.141 Food pharmacies are designed to increase public access to fruits and vegetables and fully integrate the concept of “food is medicine” into health care systems.142 These centers address financial barriers, knowledge gaps regarding healthy eating, and cooking skills by prescribing fruits and vegetables to patients. Community outreach programs have been successful in dietary changes through education, group counseling, and culinary medicine.143 One such outreach program, “Shop With Your Doc”, has been shown to help implement positive dietary changes by patients gaining knowledge on how to read nutrition labels, read ingredients, and how to choose fresh produce.144 Using surveys, one study found that there was an improvement in fresh vegetable consumption and less waste after produce allocation and educational intervention in low income populations.143 Using the skills from nutrition counseling and applying them to an interactive community model, where patients can gain skills and apply them to their daily routines, may help implement larger scale changes.141,143,144
The outpatient and community programs may offer guidance from a physician, dietitian, and a health coach to assist in not only improved diet, but also improve lifestyle with exercise programs with stress management techniques. Similarly, inpatient dietary guidance can be a method to implement the importance of nutrition when patients may be amenable to lifestyle changes.139,145 Studies support that patients are most vulnerable to lifestyle changes after a life altering event, such as an MI.146 In response to the data supporting the importance of nutrition in treating and preventing CVD, there has been greater pressure from hospital institutions in providing nutritious meals to inpatients.139 Public policies, such as the Healthy Food in Health Care initiative, have been placed to assist hospitals in obtaining local produce and reducing meat products on the menu to improve patient nutrition and decrease environmental impact.147 Despite these efforts to improve hospital menus, the effect on dietary behaviors needs to be further studied.
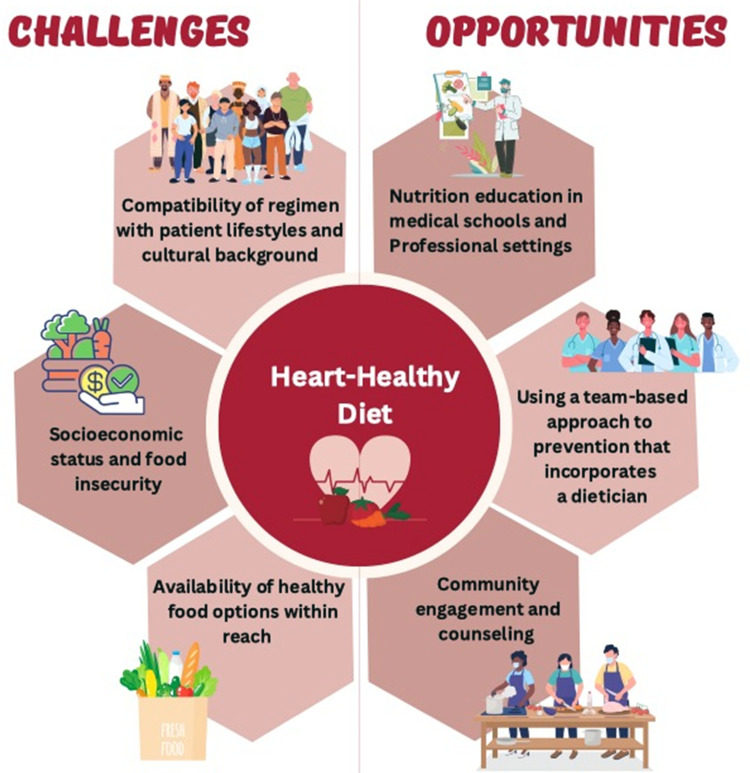
Limitations and Disparities to Implementing Dietary Changes
There are many challenges, but also many opportunities, to implementing healthy heart eating patterns (Figure 3).
Figure 3.
Challenges and Opportunities for Implementing a Heart Healthy Diet.
Some of the main challenges and barriers to behavior or diet change have to do with the compatibility of regimen with patient lifestyles, including social and cultural factors of socioeconomic status, lack of social support, and certain cultural values, all of which have to be taken into consideration when recommending dietary changes. All diet recommendations need to be considered in the context of social determinants of health and societal barriers that drive health inequities.148–151 Underserved populations, ethnic minorities, gender, and sexual minorities have been found to have a higher incidence of CVD and suffer from greater mortality.151,152 These same groups are also faced with social barriers that make it difficult to adhere to preferable dietary patterns to improve their cardiovascular health. Additionally, individuals of lower income have been shown to be less likely counseled on diet modifications for CVD prevention in a representative US sample.153
“Food deserts” (or grocery areas that lack fresh produce), “food swamps” (with excess of ultra-processed poor quality foods), and food insecurity all are contributors to obesity and chronic disease.150,154,155 Recent studies have shown that food insecurity is a growing problem in the United States.156 As a result of the COVID-19 pandemic, food insecurity has tripled, with an even higher prevalence among US adults with an income below the poverty level.156 In comparison to high and middle income families, persons of low socioeconomic class are more likely to under consume fruits, vegetables, and whole grains and instead consume highly processed meats and beverages.143 The fluctuating nature of eating food insecurity causes has been found to increase insulin resistance, increase blood pressure, and lead to poor weight management, which ultimately increase CVD risk.152,156 These groups of patients may also not be able to access health care or seek treatment, which could lead to undiagnosed cardiovascular risk factors; this trend has been seen in gender and sexual minorities.152 Food insecurity thus may pose as a strong barrier to adhering to lifestyle interventions, thus social and political support to provide access is needed.
Opportunities remain for improvement. Community engagement, such as through faith-based organizations, has been demonstrated to be successful at promoting fruit and vegetable consumption, along with other preventive strategies.157 Culturally relevant and language appropriate resources are needed to assist patients from diverse backgrounds in making healthier choices. Family meals eaten together can be an important factor for cardiovascular health promotion, especially with parental modeling of healthy behaviors such as intake of vegetables and fruits; companionship at mealtimes that establishes a positive atmosphere around meals has been associated with improved dietary quality.158
Physicians and other healthcare professionals can be the greatest advocates for their patients and can help provide counseling and information to access foods. However, medical schools are lacking in nutrition education and on average provide 20 hours of nutrition education to medical students.159 Additionally, there is little emphasis on counseling and teaching patients about diet and how to make foods on a budget while maintaining cultural appropriateness. In recent years, programs have been placed where students engage in community outreach and culinary medicine practices.137 These programs help to improve confidence in the knowledge of nutrition and their ability to provide counseling. Improving the nutritional education of physicians and other healthcare professionals, working as part of interdisciplinary teams, can help improve partnerships with their patients in improving nutritional quality and dietary behaviors with the aim to reduce CVD.159
Conclusions
The AHA highlights diet as one of “Life’s Essential Eight” to help promote cardiovascular health and avoid disease. The Mediterranean diet remains one of the most effective and recommended balanced diets for cardiovascular health promotion, followed by the DASH diet for hypertension prevention and management, and healthy plant-based diet with careful consideration to its sugar and salt content as well as nutrient deficiencies. Other emerging diets that are widely adopted for weight loss like the keto or VLC diets remain controversial on whether the benefits of weight loss outweigh potential adverse changes to the lipid profile for example, and whether IF patterns have additional cardiometabolic benefits beyond simply the reduction of total calorie intake. The effects of diet on the gut microbiome and the importance of the gut–heart axis may be the future of our cardiovascular dietary recommendations where precision nutrition guides precision prevention.
Funding Statement
Dr. Michos is funded by the Amato Fund for Women’s Cardiovascular Health at Johns Hopkins University and by an American Heart Association Award, Number: 946222. Dr. Gulati is funded by the Anita Dann Friedman Chair in Women’s Cardiovascular Medicine and Research, and by the Department of Defense (Award Number: W81XWH-17-2-0030).
Abbreviations
ACC, American College of Cardiology; AHA, American Heart Association; ADP, Alternate Day Fasting; BMI, Body Mass Index; CVD, Cardiovascular Disease; DASH, Dietary Approaches to Stop Hypertension; HDL-C, High Density Lipoprotein Cholesterol; IF, Intermittent Fasting; LDL-C, Low Density Lipoprotein Cholesterol; MI, Myocardial Infarction; RCT, Randomized Controlled Trial; T2D, Type 2 Diabetes; TRE, Time Restricted Eating; TMAO, Trimethylamine-N-oxide; US, United States; VLC, Very Low Carbohydrate.
Disclosure
Unrelated to this work, Dr. Michos has served on advisory boards for Amgen, Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Esperion, Novartis, Novo Nordisk, and Pfizer. Dr. Gulati has served on advisory boards for Novartis and Bayer. The authors report no other conflicts of interest in this work.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. 2022;145(8):e153–e639. [DOI] [PubMed] [Google Scholar]
- 2.Fischer NM, Pallazola VA, Xun H, Cainzos-Achirica M, Michos ED. The evolution of the heart-healthy diet for vascular health: a walk through time. Vasc Med. 2020;25(2):184–193. [DOI] [PubMed] [Google Scholar]
- 3.Menotti A, Puddu PE. How the Seven Countries Study contributed to the definition and development of the Mediterranean diet concept: a 50-year journey. Nutr Metab Cardiovasc Dis. 2015;25(3):245–252. [DOI] [PubMed] [Google Scholar]
- 4.Delarue J. Mediterranean Diet and cardiovascular health: an historical perspective. Br J Nutr. 2021;1–14. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. [DOI] [PubMed] [Google Scholar]
- 6.Miller V, Mente A, Dehghan M, et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390(10107):2037–2049. [DOI] [PubMed] [Google Scholar]
- 7.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42(34):3227–3337.34458905 [Google Scholar]
- 9.Ferraro RA, Fischer NM, Xun H, Michos ED. Nutrition and physical activity recommendations from the United States and European cardiovascular guidelines: a comparative review. Curr Opin Cardiol. 2020;35(5):508–516. [DOI] [PubMed] [Google Scholar]
- 10.Belardo D, Michos ED, Blankstein R, et al. Practical, Evidence-Based Approaches to Nutritional Modifications to Reduce Atherosclerotic Cardiovascular Disease: an American Society For Preventive Cardiology Clinical Practice Statement. Am J Prev Cardiol. 2022;10:100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badimon L, Chagas P, Chiva-Blanch G. Diet and Cardiovascular Disease: effects of Foods and Nutrients in Classical and Emerging Cardiovascular Risk Factors. Curr Med Chem. 2019;26(19):3639–3651. [DOI] [PubMed] [Google Scholar]
- 12.Roth GA, Mensah GA, Johnson CO, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths — selected Counties and Indian Reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 2020;69:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Diabetes Foundation. IDF Diabetes Atlas 10th Edition; 2021. Available from: https://diabetesatlas.org/. Accessed December 8, 2022.
- 15.Muntner P, Hardy ST, Fine LJ, et al. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324(12):1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Obesity and overweight. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:. Accessed March 24, 2022.
- 17.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief. 2020;3(360):1–8. [PubMed] [Google Scholar]
- 18.Powell-Wiley TM, Poirier P, Burke LE, et al. Obesity and Cardiovascular Disease: a Scientific Statement From the American Heart Association. Circulation. 2021;143(21):e984–e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s Essential 8: updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: a Presidential Advisory From the American Heart Association. Circulation. 2022;146(5):e18–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 21.Gantenbein KV, Kanaka-Gantenbein C. Mediterranean Diet as an Antioxidant: the Impact on Metabolic Health and Overall Wellbeing. Nutrients. 2021;13(6):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Torre-Moral A, Fabregues S, Bach-Faig A, et al. Family Meals, Conviviality, and the Mediterranean Diet among Families with Adolescents. Int J Environ Res Public Health. 2021;18(5):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Gonzalez MA, Salas-Salvado J, Estruch R, et al. Benefits of the Mediterranean Diet: insights From the PREDIMED Study. Prog Cardiovasc Dis. 2015;58(1):50–60. [DOI] [PubMed] [Google Scholar]
- 24.Estruch R, Martinez-Gonzalez MA, Corella D, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Int Med. 2006;145(1):1–11. [DOI] [PubMed] [Google Scholar]
- 25.Richardson LA, Izuora K, Basu A. Mediterranean Diet and Its Association with Cardiovascular Disease Risk Factors: a Scoping Review. Int J Environ Res Public Health. 2022;19(19):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ros E, Martinez-Gonzalez MA, Estruch R, et al. Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Adv Nutr. 2014;5(3):330S–336S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llorente-Cortes V, Estruch R, Mena MP, et al. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis. 2010;208(2):442–450. [DOI] [PubMed] [Google Scholar]
- 28.Keys A, Menotti A, Aravanis C, et al. The seven countries study: 2289 deaths in 15 years. Prev Med. 1984;13(2):141–154. [DOI] [PubMed] [Google Scholar]
- 29.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. [DOI] [PubMed] [Google Scholar]
- 30.Stewart RA, Wallentin L, Benatar J, et al. Dietary patterns and the risk of major adverse cardiovascular events in a global study of high-risk patients with stable coronary heart disease. Eur Heart J. 2016;37(25):1993–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minhas AS, Hong X, Wang G, et al. Mediterranean-Style Diet and Risk of Preeclampsia by Race in the Boston Birth Cohort. J Am Heart Assoc. 2022;11(9):e022589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–785. [DOI] [PubMed] [Google Scholar]
- 33.Kris-Etherton P, Eckel RH, Howard BV, et al. AHA Science Advisory: Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Cholesterol Education Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease. Circulation. 2001;103(13):1823–1825. [DOI] [PubMed] [Google Scholar]
- 34.Estruch R, Ros E, Salas-Salvado J, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018;378(25):e34. [DOI] [PubMed] [Google Scholar]
- 35.Salas-Salvado J, Bullo M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fito M, Estruch R, Salas-Salvado J, et al. Effect of the Mediterranean diet on heart failure biomarkers: a randomized sample from the PREDIMED trial. Eur J Heart Fail. 2014;16(5):543–550. [DOI] [PubMed] [Google Scholar]
- 37.Xia M, Zhong Y, Peng Y, Qian C. Olive oil consumption and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Front Nutr. 2022;9:1041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cronin P, Joyce SA, O’Toole PW, O’Connor EM. Dietary Fibre Modulates the Gut Microbiota. Nutrients. 2021;13(5):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witkowski M, Weeks TL, Hazen SL. Gut Microbiota and Cardiovascular Disease. Circ Res. 2020;127(4):553–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koeth RA, Levison BS, Culley MK, et al. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20(5):799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. [DOI] [PubMed] [Google Scholar]
- 42.Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47(2):296–308. [DOI] [PubMed] [Google Scholar]
- 43.Wickman BE, Enkhmaa B, Ridberg R, et al. Dietary Management of Heart Failure: DASH Diet and Precision Nutrition Perspectives. Nutrients. 2021;13(12):4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 45.Kucharska A, Gajewska D, Kiedrowski M, et al. The impact of individualised nutritional therapy according to DASH diet on blood pressure, body mass, and selected biochemical parameters in overweight/obese patients with primary arterial hypertension: a prospective randomised study. Kardiol Pol. 2018;76(1):158–165. [DOI] [PubMed] [Google Scholar]
- 46.Filippou C, Tatakis F, Polyzos D, et al. Overview of salt restriction in the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet for blood pressure reduction. Rev Cardiovasc Med. 2022;23(1):36. [DOI] [PubMed] [Google Scholar]
- 47.Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH Dietary Pattern and Cardiometabolic Outcomes: an Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients. 2019;11(2):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campos CL, Wood A, Burke GL, Bahrami H, Bertoni AG. Dietary Approaches to Stop Hypertension Diet Concordance and Incident Heart Failure: the Multi-Ethnic Study of Atherosclerosis. Am J Prev Med. 2019;56(6):819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henzel J, Kepka C, Kruk M, et al. High-Risk Coronary Plaque Regression After Intensive Lifestyle Intervention in Nonobstructive Coronary Disease: a Randomized Study. JACC Cardiovasc Imaging. 2021;14(6):1192–1202. [DOI] [PubMed] [Google Scholar]
- 50.Satija A, Hu FB. Plant-based diets and cardiovascular health. Trends Cardiovasc Med. 2018;28(7):437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satija A, Bhupathiraju SN, Spiegelman D, et al. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J Am Coll Cardiol. 2017;70(4):411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jabri A, Kumar A, Verghese E, et al. Meta-analysis of Effect of Vegetarian Diet on Ischemic Heart Disease and All-cause Mortality. Am J Prev Cardiol. 2021;7:100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez-Donoso C, Martinez-Gonzalez MA, Martinez JA, et al. A Provegetarian Food Pattern Emphasizing Preference for Healthy Plant-Derived Foods Reduces the Risk of Overweight/Obesity in the SUN Cohort. Nutrients. 2019;11(7):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orlich MJ, Singh PN, Sabate J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173(13):1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumoto S, Beeson WL, Shavlik DJ, et al. Association between vegetarian diets and cardiovascular risk factors in non-Hispanic white participants of the Adventist Health Study-2. J Nutr Sci. 2019;8:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith CE, Tucker KL. Health benefits of cereal fibre: a review of clinical trials. Nutr Res Rev. 2011;24(1):118–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59(5):129–139. [DOI] [PubMed] [Google Scholar]
- 58.Najjar RS, Feresin RG. Plant-Based Diets in the Reduction of Body Fat: physiological Effects and Biochemical Insights. Nutrients. 2019;11(11):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez-Gonzalez MA, Sanchez-Tainta A, Corella D, et al. A provegetarian food pattern and reduction in total mortality in the Prevencion con Dieta Mediterranea (PREDIMED) study. Am J Clin Nutr. 2014;100 Suppl 1:320S–328S. [DOI] [PubMed] [Google Scholar]
- 60.Choi Y, Larson N, Steffen LM, et al. Plant-Centered Diet and Risk of Incident Cardiovascular Disease During Young to Middle Adulthood. J Am Heart Assoc. 2021;10(16):e020718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokoyama Y, Nishimura K, Barnard ND, et al. Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med. 2014;174(4):577–587. [DOI] [PubMed] [Google Scholar]
- 62.Shah B, Newman JD, Woolf K, et al. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association-Recommended Diet in Coronary Artery Disease Trial. J Am Heart Assoc. 2018;7(23):e011367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rizzo NS, Sabate J, Jaceldo-Siegl K, Fraser GE. Vegetarian dietary patterns are associated with a lower risk of metabolic syndrome: the adventist health study 2. Diabetes Care. 2011;34(5):1225–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Craig WJ, Mangels AR, Fresan U, et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients. 2021;13(11):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang DD, Li Y, Bhupathiraju SN, et al. Fruit and Vegetable Intake and Mortality: results From 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation. 2021;143(17):1642–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aune D, Keum N, Giovannucci E, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aune D, Keum N, Giovannucci E, et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016;14(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juul F, Deierlein AL, Vaidean G, Quatromoni PA, Parekh N. Ultra-processed Foods and Cardiometabolic Health Outcomes: from Evidence to Practice. Curr Atheroscler Rep. 2022;24(11):849–860. [DOI] [PubMed] [Google Scholar]
- 69.Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Juul F, Vaidean G, Lin Y, Deierlein AL, Parekh N. Ultra-Processed Foods and Incident Cardiovascular Disease in the Framingham Offspring Study. J Am Coll Cardiol. 2021;77(12):1520–1531. [DOI] [PubMed] [Google Scholar]
- 71.Rico-Campa A, Martinez-Gonzalez MA, Alvarez-Alvarez I, et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ. 2019;365:l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall KD, Ayuketah A, Brychta R, et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: an Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019;30(1):67–77 e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirkpatrick CF, Bolick JP, Kris-Etherton PM, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019;13(5):689–711 e681. [DOI] [PubMed] [Google Scholar]
- 74.Kirkpatrick CF, Willard KE, Maki KC. Keto is Trending: implications for Body Weight and Lipid Management. Curr Cardiol Rep. 2022;24(9):1093–1100. [DOI] [PubMed] [Google Scholar]
- 75.O’Neill B, Raggi P. The ketogenic diet: pros and cons. Atherosclerosis. 2020;292:119–126. [DOI] [PubMed] [Google Scholar]
- 76.D’Andrea Meira I, Romao TT. Ketogenic Diet and Epilepsy: what We Know So Far. Front Neurosci. 2019;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: new Perspectives for Neuroprotection in Alzheimer’s Disease. Antioxidants. 2018;7(5):w365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: an Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018;9(2):583–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tinguely D, Gross J, Kosinski C. Efficacy of Ketogenic Diets on Type 2 Diabetes: a Systematic Review. Curr Diab Rep. 2021;21(9):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi YJ, Jeon SM, Shin S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: a Meta-Analysis of Randomized Controlled Trials. Nutrients. 2020;12(7):r45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bueno NB, de Melo IS, de Oliveira SL. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110(7):1178–1187. [DOI] [PubMed] [Google Scholar]
- 82.Mansoor N, Vinknes KJ, Veierod MB, Retterstol K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115(3):466–479. [DOI] [PubMed] [Google Scholar]
- 83.Sackner-Bernstein J, Kanter D, Kaul S. Dietary Intervention for Overweight and Obese Adults: comparison of Low-Carbohydrate and Low-Fat Diets. A Meta-Analysis. PLoS One. 2015;10(10):e0139817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr. 2008;87(1):44–55. [DOI] [PubMed] [Google Scholar]
- 85.Buren J, Ericsson M, Damasceno NRT, Sjodin A, Ketogenic Low-Carbohydrate A. High-Fat Diet Increases LDL Cholesterol in Healthy, Young, Normal-Weight Women: a Randomized Controlled Feeding Trial. Nutrients. 2021;13(3):45645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Retterstol K, Svendsen M, Narverud I, Holven KB. Effect of low carbohydrate high fat diet on LDL cholesterol and gene expression in normal-weight, young adults: a randomized controlled study. Atherosclerosis. 2018;279:52–61. [DOI] [PubMed] [Google Scholar]
- 87.Goldberg IJ, Ibrahim N, Bredefeld C, et al. Ketogenic diets, not for everyone. J Clin Lipidol. 2021;15(1):61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joo M, Moon S, Lee YS, Kim MG. Effects of very low-carbohydrate ketogenic diets on lipid profiles in normal-weight (body mass index < 25 kg/m2) adults: a meta-analysis. Nutr Rev. 2023;nuad017. doi: 10.1093/nutrit/nuad017 [DOI] [PubMed] [Google Scholar]
- 89.Dong TA, Sandesara PB, Dhindsa DS, et al. Intermittent Fasting: a Heart Healthy Dietary Pattern? Am J Med. 2020;133(8):901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tinsley GM, Horne BD. Intermittent fasting and cardiovascular disease: current evidence and unresolved questions. Future Cardiol. 2018;14(1):47–54. [DOI] [PubMed] [Google Scholar]
- 91.Crupi AN, Haase J, Brandhorst S, Longo VD. Periodic and Intermittent Fasting in Diabetes and Cardiovascular Disease. Curr Diab Rep. 2020;20(12):83. [DOI] [PubMed] [Google Scholar]
- 92.Varady KA, Cienfuegos S, Ezpeleta M, Gabel K. Cardiometabolic Benefits of Intermittent Fasting. Annu Rev Nutr. 2021;41:333–361. [DOI] [PubMed] [Google Scholar]
- 93.Allaf M, Elghazaly H, Mohamed OG, et al. Intermittent fasting for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2021;1(1):CD013496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wegman MP, Guo MH, Bennion DM, et al. Practicality of intermittent fasting in humans and its effect on oxidative stress and genes related to aging and metabolism. Rejuvenation Res. 2015;18(2):162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park J, Seo YG, Paek YJ, Song HJ, Park KH, Noh HM. Effect of alternate-day fasting on obesity and cardiometabolic risk: a systematic review and meta-analysis. Metabolism. 2020;111:154336. [DOI] [PubMed] [Google Scholar]
- 97.Trepanowski JF, Kroeger CM, Barnosky A, et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: a Randomized Clinical Trial. JAMA Intern Med. 2017;177(7):930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu D, Huang Y, Huang C, et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N Engl J Med. 2022;386(16):1495–1504. [DOI] [PubMed] [Google Scholar]
- 99.Moro T, Tinsley G, Bianco A, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schroder JD, Falqueto H, Manica A, et al. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J Transl Med. 2021;19(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020;31(1):92–104 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manoogian ENC, Zadourian A, Lo HC, et al. Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: the Healthy Heroes randomized control trial. Cell Metab. 2022;34(10):1442–1456 e1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Longo VD, Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016;23(6):1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Goede P, Foppen E, Ritsema W, Korpel NL, Yi CX, Kalsbeek A. Time-Restricted Feeding Improves Glucose Tolerance in Rats, but Only When in Line With the Circadian Timing System. Front Endocrinol. 2019;10:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanchez-Rodriguez E, Egea-Zorrilla A, Plaza-Diaz J, et al. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients. 2020;12(3):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smits LP, Kootte RS, Levin E, et al. Effect of Vegan Fecal Microbiota Transplantation on Carnitine- and Choline-Derived Trimethylamine-N-Oxide Production and Vascular Inflammation in Patients With Metabolic Syndrome. J Am Heart Assoc. 2018;7(7):e008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434–445. [DOI] [PubMed] [Google Scholar]
- 108.Guo Y, Li X, Wang Z, Yu B. Gut Microbiota Dysbiosis in Human Hypertension: a Systematic Review of Observational Studies. Front Cardiovasc Med. 2021;8:650227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richards EM, Li J, Stevens BR, Pepine CJ, Raizada MK, Microbiome G. Neuroinflammation in Hypertension. Circ Res. 2022;130(3):401–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakai M, Ribeiro RV, Stevens BR, et al. Essential Hypertension Is Associated With Changes in Gut Microbial Metabolic Pathways: a Multisite Analysis of Ambulatory Blood Pressure. Hypertension. 2021;78(3):804–815. [DOI] [PubMed] [Google Scholar]
- 111.Lee Y, Nemet I, Wang Z, et al. Longitudinal Plasma Measures of Trimethylamine N-Oxide and Risk of Atherosclerotic Cardiovascular Disease Events in Community-Based Older Adults. J Am Heart Assoc. 2021;10(17):e020646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duttaroy AK. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: a Review. Nutrients. 2021;13(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lichtenstein AH, Appel LJ, Vadiveloo M, et al. 2021 Dietary Guidance to Improve Cardiovascular Health: a Scientific Statement From the American Heart Association. Circulation. 2021;144(23):e472–e487. [DOI] [PubMed] [Google Scholar]
- 114.Minich DM. A Review of the Science of Colorful, Plant-Based Food and Practical Strategies for “Eating the Rainbow”. J Nutr Metab. 2019;2019:2125070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kwok CS, Gulati M, Michos ED, et al. Dietary components and risk of cardiovascular disease and all-cause mortality: a review of evidence from meta-analyses. Eur J Prev Cardiol. 2019;26(13):1415–1429. [DOI] [PubMed] [Google Scholar]
- 116.Jiang L, Wang J, Xiong K, Xu L, Zhang B, Ma A. Intake of Fish and Marine n-3 Polyunsaturated Fatty Acids and Risk of Cardiovascular Disease Mortality: a Meta-Analysis of Prospective Cohort Studies. Nutrients. 2021;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bhupathi V, Mazariegos M, Cruz Rodriguez JB, Deoker A. Dairy Intake and Risk of Cardiovascular Disease. Curr Cardiol Rep. 2020;22(3):11. [DOI] [PubMed] [Google Scholar]
- 118.Papier K, Knuppel A, Syam N, Jebb SA, Key TJ. Meat consumption and risk of ischemic heart disease: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2021;1–12. [DOI] [PubMed] [Google Scholar]
- 119.Orlich MJ, Sabate J, Mashchak A, et al. Ultra-processed food intake and animal-based food intake and mortality in the Adventist Health Study-2. Am J Clin Nutr. 2022;115(6):1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhong VW, Van Horn L, Greenland P, et al. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake With Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern Med. 2020;180(4):503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang M, Wang Z, Lee Y, et al. Dietary Meat, Trimethylamine N-Oxide-Related Metabolites, and Incident Cardiovascular Disease Among Older Adults: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2022;42(9):e273–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sacks FM, Katan M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am J Med. 2002;113:13S–24S. [DOI] [PubMed] [Google Scholar]
- 124.Khaw KT, Sharp SJ, Finikarides L, et al. Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open. 2018;8(3):e020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Neelakantan N, Seah JYH, van Dam RM. The Effect of Coconut Oil Consumption on Cardiovascular Risk Factors: a Systematic Review and Meta-Analysis of Clinical Trials. Circulation. 2020;141(10):803–814. [DOI] [PubMed] [Google Scholar]
- 126.Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2018;132:41–48. [DOI] [PubMed] [Google Scholar]
- 127.Marklund M, Wu JHY, Imamura F, et al. Biomarkers of Dietary Omega-6 Fatty Acids and Incident Cardiovascular Disease and Mortality. Circulation. 2019;139(21):2422–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanders TAB. Omega-6 Fatty Acids and Cardiovascular Disease. Circulation. 2019;139(21):2437–2439. [DOI] [PubMed] [Google Scholar]
- 129.Naghshi S, Aune D, Beyene J, Mobarak S, Asadi M, Sadeghi O. Dietary intake and biomarkers of alpha linolenic acid and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of cohort studies. BMJ. 2021;375:n2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou Y, Wang T, Zhai S, Li W, Meng Q. Linoleic acid and breast cancer risk: a meta-analysis. Public Health Nutr. 2016;19(8):1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu X, Yu H, Ma Q, Shen S, Das UN. Linoleic acid suppresses colorectal cancer cell growth by inducing oxidant stress and mitochondrial dysfunction. Lipids Health Dis. 2010;9:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zock PL, Katan MB. Linoleic acid intake and cancer risk: a review and meta-analysis. Am J Clin Nutr. 1998;68(1):142–153. [DOI] [PubMed] [Google Scholar]
- 133.Rippe JM, Angelopoulos TJ. Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: current Understanding. Nutrients. 2016;8(11):526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174(4):516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hoek AG, Oort S, Mukamal KJ, Beulens JWJ. Alcohol Consumption and Cardiovascular Disease Risk: placing New Data in Context. Curr Atheroscler Rep. 2022;24(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marcus GM, Vittinghoff E, Whitman IR, et al. Acute Consumption of Alcohol and Discrete Atrial Fibrillation Events. An Intern Med. 2021;174(11):1503–1509. [DOI] [PubMed] [Google Scholar]
- 137.Hashimi H, Boggs K, Harada CN. Cooking demonstrations to teach nutrition counseling and social determinants of health. Educ Health. 2020;33(2):74–78. [DOI] [PubMed] [Google Scholar]
- 138.Spahn JM, Reeves RS, Keim KS, et al. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J Am Diet Assoc. 2010;110(6):879–891. [DOI] [PubMed] [Google Scholar]
- 139.Aggarwal M, Grady A, Desai D, et al. Successful Implementation of Healthful Nutrition Initiatives into Hospitals. Am J Med. 2020;133(1):19–25. [DOI] [PubMed] [Google Scholar]
- 140.Matsuzaki K, Fukushima N, Saito Y, et al. The Effects of Long-Term Nutrition Counseling According to the Behavioral Modification Stages in Patients with Cardiovascular Disease. Nutrients. 2021;13(2):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.LeBlanc-Morales N. Culinary Medicine: patient Education for Therapeutic Lifestyle Changes. Crit Care Nurs Clin North Am. 2019;31(1):109–123. [DOI] [PubMed] [Google Scholar]
- 142.Donohue JA, Severson T, Martin LP. The food pharmacy: theory, implementation, and opportunities. Am J Prev Cardiol. 2021;5:100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Metcalfe JJ, McCaffrey J, Schumacher M, Kownacki C, Prescott MP. Community-based nutrition education and hands-on cooking intervention increases farmers’ market use and vegetable servings. Public Health Nutr. 2022;25(9):2601–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.O’Connor M. DOCTOR’S ORDERS ‘Shop With Your Doc’ program improves food choices. Hosp Health Netw. 2017;91(2):13. [PubMed] [Google Scholar]
- 145.Petersen MR, Freeman AM, Madrid M, Aggarwal M. Strategies for Incorporating Lifestyle Medicine in Everyday Hospital Practice. Am J Lifestyle Med. 2021;15(5):531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Steca P, Monzani D, Greco A, et al. Stability and change of lifestyle profiles in cardiovascular patients after their first acute coronary event. PLoS One. 2017;12(8):e0183905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moran A, Krepp EM, Johnson Curtis C, Lederer A. An Intervention to Increase Availability of Healthy Foods and Beverages in New York City Hospitals: the Healthy Hospital Food Initiative, 2010-2014. Prev Chronic Dis. 2016;13:E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fischer NM, Duffy EY, Michos ED. Protecting Our Youth: support Policy to Combat Health Disparities Fueled by Targeted Food Advertising. J Am Heart Assoc. 2021;10(1):e018900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ramesh G, Belardo D, Gulati M, Ostfeld RJ, Michos ED. Agricultural policy and societal factors influence patients’ ability to follow a healthy diet. Am J Prev Cardiol. 2021;8:100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cooksey-Stowers K, Schwartz MB, Brownell KD. Food Swamps Predict Obesity Rates Better Than Food Deserts in the United States. Int J Environ Res Public Health. 2017;14(11):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mannoh I, Hussien M, Commodore-Mensah Y, Michos ED. Impact of social determinants of health on cardiovascular disease prevention. Curr Opin Cardiol. 2021;36(5):572–579. [DOI] [PubMed] [Google Scholar]
- 152.Caceres BA, Bynon M, Doan D, Makarem N, McClain AC, VanKim N. Diet, Food Insecurity, and CVD Risk in Sexual and Gender Minority Adults. Current Atheroscler Rep. 2022;24(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shahu A, Okunrintemi V, Tibuakuu M, et al. Income disparity and utilization of cardiovascular preventive care services among U.S. adults. Am J Prev Cardiol. 2021;8:100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Decker D, Flynn M. Food Insecurity and Chronic Disease: addressing Food Access as a Healthcare Issue. R I Med J. 2018;101(4):28–30. [PubMed] [Google Scholar]
- 155.Dhurandhar EJ. The food-insecurity obesity paradox: a resource scarcity hypothesis. Physiol Behav. 2016;162:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu Y, Eicher-Miller HA. Food Insecurity and Cardiovascular Disease Risk. Curr Atheroscler Rep. 2021;23(6):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Allicock M, Johnson LS, Leone L, et al. Promoting fruit and vegetable consumption among members of black churches, Michigan and North Carolina, 2008-2010. Prev Chronic Dis. 2013;10:E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vedanthan R, Bansilal S, Soto AV, et al. Family-Based Approaches to Cardiovascular Health Promotion. J Am Coll Cardiol. 2016;67(14):1725–1737. [DOI] [PubMed] [Google Scholar]
- 159.Van Horn L, Lenders CM, Pratt CA, et al. Advancing Nutrition Education, Training, and Research for Medical Students, Residents, Fellows, Attending Physicians, and Other Clinicians: building Competencies and Interdisciplinary Coordination. Adv Nutr. 2019;10(6):1181–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]