Abstract
Introduction:
Patients with clinical T4M0 breast cancer are recommended to undergo neoadjuvant chemotherapy, modified radical mastectomy, and postmastectomy radiotherapy. Here we determine if BREAST-Q scores differ by decision to pursue reconstruction or timing of reconstruction.
Methods:
Retrospective, single institutional study of cT4 breast cancer patients from 2014-2021 without evidence of distant metastatic disease undergoing mastectomy with or without reconstruction.. BREAST-Q was administered as routine care preoperatively, and 6 months, 1 year, and 2 years postoperatively. Satisfaction and quality of life domains were compared between mastectomy with no reconstruction (NR), immediate reconstruction (IR), and delayed reconstruction (DR) groups.
Results:
144 patients were eligible:71(49%) had NR;36 (25%) had DR;37(26%) had IR. Patients undergoing reconstruction were younger and more likely to elect contralateral prophylactic mastectomy. Timing of reconstruction was not associated with significant differences in satisfaction with breasts (SATBR) at any timepoint. For patients having DR, breast satisfaction increased over time following reconstructive surgery. Physical well-being of the chest (PWB-CHEST) did not significantly differ between IR, DR, or NR, at any timepoint. Patients undergoing DR experienced improvement in PWB-CHEST from preoperative scores; patients with IR and NR experienced PWB-CHEST decline over time. Psychosocial well-being (PSWB) did not significantly differ across time or by subgroup.
Conclusions:
Patients with T4 breast cancer who elected reconstruction had no difference in patient-reported outcomes based on timing of reconstruction. In the DR cohort, SATBR significantly improved following reconstructive surgery. These data can help inform breast reconstructive decision making for patients facing the choice between DR, IR, and NR.
INTRODUCTION
Clinical stage T4 breast cancer is defined as any breast tumor, regardless of size, with direct extension into the chest wall, breast skin resulting in edema or erythema, or inflammatory breast cancer (IBC).1 Clinical T4 breast cancer is thought to represent a unique subset of breast cancer associated with a more aggressive clinical nature and poorer outcomes compared to earlier-stage breast cancer, so trimodality therapy—neoadjuvant chemotherapy, modified radical mastectomy, and postmastectomy radiotherapy (PMRT)—is currently standard of care for clinical stage T4 breast cancer with no evidence of distant metastatic disease.2–4 For these patients, however, the impact of breast reconstruction, immediate or delayed, on satisfaction and quality of life after mastectomy is not well understood.
Many patients with T4 breast cancer do not undergo reconstruction. For patients with clinical stage T4d (inflammatory breast carcinoma) breast cancer, reconstruction has also been associated with a higher risk of complications and delays in radiotherapy.5 For T4d patients, therefore, an initial flat closure without reconstruction is the standard of care.4–7 Even for non-IBC T4a-c patients, most patients elect to postpone or forego reconstruction to decrease risk for complications and potential delays in adjuvant therapy.5,8 Ultimately, among women with T4 breast cancer who underwent an initial flat closure, less than 20% choose to have reconstruction.5 Multiple studies have shown that breast reconstruction significantly improves long-term patient satisfaction and quality of life, although PMRT has been independently associated with lower breast satisfaction among women undergoing implant-based reconstruction and autologous reconstruction.9,10 However these studies focused primarily on non-T4 patients, so the breast reconstruction experience of T4 patients is lacking in current literature.
In this study, we utilize the BREAST-Q, a validated patient-reported outcome measure (PROM) for satisfaction and quality of life after breast surgery,11–13 to determine whether patient satisfaction and quality of life differs among T4 patients based on their decision to pursue reconstruction and the timing of reconstruction.
METHODS
Following approval from the Memorial Sloan Kettering Cancer Center institutional review board, we performed a retrospective cohort study of patients diagnosed with clinical stage T4 breast cancer without evidence of distant metastatic disease between 2014-2021 who underwent mastectomy with IR, DR, or NR. BREAST-Q is routinely requested for all patients undergoing mastectomy at our intuition. For this cohort, all patients were eligible and requested to complete the BREAST-Q at baseline and at 6 months, 1 year, and 2 years postoperatively. The BREAST-Q was routinely administered via an electronic portal as well as offered in clinic using an institutionally approved electronic tablet. Patients did not have to have complete BREAST-Q questionnaires at all time points and for all domains to be included in the study. All available data at each time point and domain were included when completed.
Complete BREAST-Q data were obtained from an institutional database. Domains of the BREAST-Q examined included satisfaction with breasts (SATBR), physical well-being of the chest (PWB-CHEST) and psychosocial well-being (PSWB). Each domain was scored on a scale of 0-100. Higher scores on a 0-100 scale indicated superior satisfaction. SATBR is validated for patients who undergo reconstruction after mastectomy, but not for those who elect flat closure. A minimum 3-point difference was considered clinically important for PWB-CHEST and 4-point difference was considered clinically important for SATBR and PSWB.14 We compared median SATBR, PWB-CHEST, and PSWB scores between mastectomy with IR, DR, and NR groups at baseline, 6 months, 1 year, and 2 years postoperatively. Postoperative timing was defined as after reconstruction in patients undergoing delayed reconstruction, or from the index operation (mastectomy) if no reconstruction or immediate reconstruction were performed.
Clinicopathologic and treatment data were collected from an institutional tumor registry and from medical records. Demographic and clinicopathologic data including age at surgery, race/ethnicity, body mass index (BMI: kg/m2), clinical and pathologic tumor and nodal stage, performance of contralateral prophylactic mastectomy (CPM), and receipt of radiotherapy and chemotherapy were collected. Characteristics were compared between NR, IR, and DR, using Fishers’ exact test for categorical variables and the Kruskal-Wallis test for continuous variables. Multiple comparison correction was done using the Benjamini Hochberg procedure. Univariate analysis was performed to compare patient and tumor characteristics among the groups.
Available BREAST-Q data at all time points were included in the analysis. Median (interquartile range [IQR]) scores were reported for the NR, IR, and DR cohorts at baseline, 6 months, 1 year, and 2 years. Mean preoperative scores were also reported for the NR, IR, and DR cohorts, and the difference from baseline mean scores were reported at 6 months, 1 year and 2 years. Boxplots were created to assess trends in scores over time for each group.
RESULTS
144 patients with T4 breast cancer without evidence of distant metastatic disease were included in the study. Seventy-one (49.3%) underwent mastectomy without reconstruction (NR) and 73 (50.7%) underwent mastectomy with reconstruction. Of those who elected reconstruction, 36 (49.3%) had delayed reconstruction (DR) and 37 (50.7%) had immediate reconstruction (IR). In those who elected delayed reconstruction; 12 (25%) had reconstruction 6-12 months after initial surgery, 7 (14.6%) had reconstruction 12-18 months after initial surgery, 7 (14.6%) had reconstruction 18-24 months after initial surgery, and 3 (6.2%) had reconstruction greater than 24 months after initial surgery. All patients in the IR group had tissue expanders placed at the initial surgery. Following the initial surgery, 13 of 37 (35.1%) went on to have autologous reconstruction and 24 of 37 (64.9%) had implant reconstruction. In the delayed reconstruction group, all patients had autologous reconstruction.
Most patients identified as non-Hispanic (82%) and were White (67%); 17% self-identified as Black/African American and 10% as Asian. Race and ethnicity were similar across NR, IR, and DR cohorts. The median age was 52 years (range 24-88) and the median BMI was 28.2 (range 17.3-54.7). Patients undergoing reconstruction were younger than patients who elected NR (median 49 versus 55 years, p < 0.001). All patients undergoing IR or DR received neoadjuvant chemotherapy, and 2 patients in the NR cohort declined chemotherapy. All patients received PMRT except 1 patient in the immediate reconstruction cohort who refused radiation therapy. Patients undergoing immediate reconstruction were more likely to elect CPM as compared to the DR and NR cohorts, (27% versus 8.3% versus 11%, p = 0.058). Patients with IBC were less likely to undergo IR as compared to DR and NR (5.4% versus 47% versus 61, p < 0.001)(Table 1).
TABLE 1.
Patient, tumor, and treatment characteristics in the delayed, immediate, and no reconstruction cohorts
| Reconstruction | |||||
|---|---|---|---|---|---|
| Characteristic | Overall, n = 144 | Delayed, n = 36 | Immediate, n = 37 | None, n = 71 | p-value1 |
| Median Age, years | 51 | 51 | 47 | 55 | < 0.001 |
| Median BMI | 28.2 | 30.0 | 27.7 | 26.4 | 0.13 |
| Race, n(%) | > 0.9 | ||||
| Asian-Far East/Indian Subcont | 15 (10%) | 3 (8.3%) | 4 (11%) | 8 (11%) | |
| Black or African American | 24 (17%) | 6 (17%) | 8 (22%) | 10 (14%) | |
| Other | 3 (2.1%) | 1 (2.8%) | 0 (0%) | 2 (2.8%) | |
| White | 96 (67%) | 25 (69%) | 24 (65%) | 47 (66%) | |
| Unknown | 6 (4.2%) | 1 (2.8%) | 1 (2.7%) | 4 (5.6%) | |
| Ethnicity, n (%) | 0.5 | ||||
| Hispanic or Latino | 14 (9.7%) | 4 (11%) | 2 (5.4%) | 8 (11%) | |
| Non-Hispanic | 118 (82%) | 31 (86%) | 32 (86%) | 55 (77%) | |
| Unknown | 12 (8.3%) | 1 (2.8%) | 3 (8.1%) | 8 (11%) | |
| Inflammatory | < 0.001 | ||||
| No | 82 (57%) | 19 (53%) | 35 (95%) | 28 (39%) | |
| Yes | 62 (43%) | 17 (47%) | 2 (5.4%) | 43 (61%) | |
| Clinical N, n (%) | 0.3 | ||||
| N0 | 17 (12%) | 5 (14%) | 8 (22%) | 4 (5.6%) | |
| N1 | 98 (68%) | 24 (67%) | 23 (62%) | 51 (72%) | |
| N2 | 12 (8.3%) | 2 (5.6%) | 3 (8.1%) | 7 (9.9%) | |
| N3 | 17 (12%) | 5 (14%) | 3 (8.1%) | 9 (13%) | |
| Pathologic T, n (%) | 0.019 | ||||
| T0 | 48 (33%) | 21 (58%) | 7 (19%) | 20 (28%) | |
| T1 | 39 (27%) | 4 (11%) | 15 (41%) | 20 (28%) | |
| T2 | 27 (19%) | 4 (11%) | 9 (24%) | 14 (20%) | |
| T3 | 7 (4.9%) | 2 (5.6%) | 1 (2.7%) | 4 (5.6%) | |
| T4 | 23 (16%) | 5 (14%) | 5 (14%) | 13 (18%) | |
| Pathologic, n (%) | 0.4 | ||||
| N0 | 65 (45%) | 20 (56%) | 16 (43%) | 29 (41%) | |
| N1 | 37 (26%) | 8 (22%) | 12 (32%) | 17 (24%) | |
| N2 | 25 (17%) | 7 (19%) | 5 (14%) | 13 (18%) | |
| N3 | 17 (12%) | 1 (2.8%) | 4 (11%) | 12 (17%) | |
| CPM, n (%) | 0.058 | ||||
| Yes | 21 (15%) | 3 (8.3%) | 10 (27%) | 8 (11%) | |
| No | 123 (85%) | 33 (92%) | 27 (73%) | 63 (89%) | |
| Radiation, n (%) | 0.5 | ||||
| Yes | 143 (99%) | 36 (100%) | 36 (97%) | 71 (100%) | |
| No | 1 (0.7%) | 0 (0%) | 1 (2.7%) | 0 (0%) | |
| Chemotherapy, n (%) | 0.8 | ||||
| Neoadjuvant | 141 (98%) | 36 (100%) | 37 (100%) | 68 (96%) | |
| Adjuvant | 1 (0.7%) | 0 (0%) | 0 (0%) | 1 (1.4%) | |
| None | 2 (1.4%) | 0 (0%) | 0 (0%) | 2 (2.8%) | |
| Reconstruction Type, n (%) | |||||
| TE/Implant | 0 (0%) | 24 (64.9%) | NA | ||
| Autologous | 36 (100%) | 13 (35.1%) | NA | ||
Statistical tests performed: Kruskal-Wallis test and Fisher’s exact test
Abbreviations: BMI body mass index, Subcont subcontinent, CPM contralateral prophylactic mastectomy, NA not available
Among patients undergoing reconstruction after mastectomy, there were no statistically significant differences in SATBR between delayed and immediate reconstruction at any time point (Fig. 1). Six months after reconstruction, SATBR median scores were 57 versus 58 for DR versus IR, respectively (p = 0.2); 2 years after reconstruction, SATBR median scores were 64 versus 58 for DR versus IR, respectively (p = 0.3)(Table 2). When examining the trend in SATBR over time, patients who elected DR had an increase in median SATBR score over time following reconstructive surgery, with a median preoperative score of 46 compared to a 2-year median postoperative score of 64. In patients electing immediate reconstruction, SATBR scores remained similar over time (Table 2) with no statistically significant change in mean SATBR score at 2 years (p = 0.87)(Table 3). Delayed reconstruction patients had a statistically significant increase in mean SATBR scores after reconstruction (2 years post-op – pre-op: +18.6, p < 0.001)(Table 3).
Fig. 1.
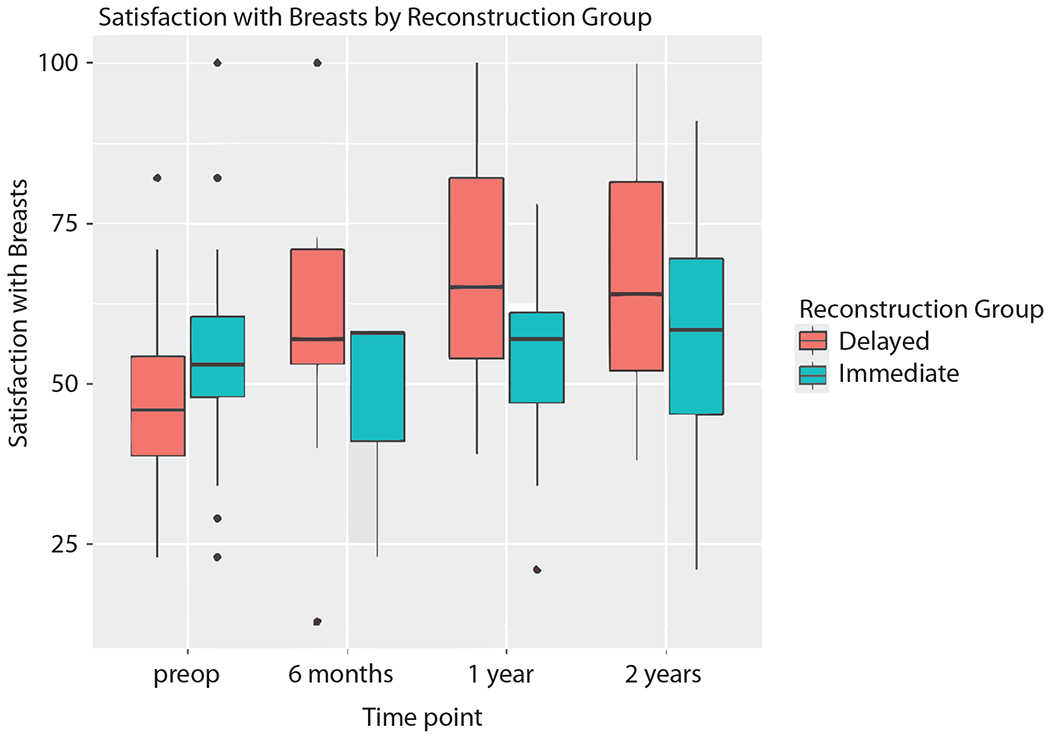
Satisfaction with breasts scores in the delayed reconstruction and immediate reconstruction cohorts.
TABLE 2.
Median (IQR) SATBR, PWB-CHEST, and PSWB scores by type of reconstruction
| Delayed Reconstruction | Immediate Reconstruction | No Reconstruction | p-value1 | |
|---|---|---|---|---|
| SATBR scores, median (IQR) | ||||
| Preoperative | 46 (39, 54) | 53 (48, 60) | 0.2 | |
| 6 month* | 57 (53, 71) | 58 (41, 58) | 0.2 | |
| 1 year* | 65 (54, 82) | 57 (47, 61) | 0.079 | |
| 2 year* | 64 (52, 82) | 58 (45, 70) | 0.3 | |
| PWB-CHEST scores, median (IQR) | ||||
| Preoperative | 66 (56, 84) | 74 (68, 100) | 78 (61, 98) | 0.092 |
| 6 month* | 60 (55, 74) | 72 (50, 78) | 60 (50, 68) | 0.3 |
| 1 year* | 72 (64, 80) | 68 (55, 76) | 68 (63, 76) | 0.5 |
| 2 year* | 76 (76, 80) | 66 (51, 80) | 64 (55, 80) | 0.4 |
| PSWB scores, median (IQR) | ||||
| Preoperative | 54 (48, 68) | 60 (48, 70) | 64 (50, 70) | 0.8 |
| 6 month* | 62 (50, 74) | 56 (50, 63) | 61 (53, 66) | 0.9 |
| 1 year* | 66 (49, 86) | 53 (48, 69) | 61 (48, 80) | 0.4 |
| 2 year* | 64 (53, 88) | 74 (56, 83) | 58 (50, 84) | 0.9 |
Statistical tests performed: Kruskal-Wallis test and Wilcoxon rank sum test
indicates time from reconstruction if preformed
Abbreviations: IQR interquartile range, SATBR satisfaction with breasts, PWB-CHEST physical well-being of the chest, PSWB psychosocial well-being
TABLE 3.
Summary of BREAST-Q scores prior to reconstruction and at 6 months, 1 year, and 2 years after reconstruction
| Preoperative Mean (SE) | Difference in Mean from Preop to 6 months (SE) (n) | p-value* | Difference in Mean from Preop to 1 year (SE) (n) | p-value* | Difference in Mean from Preop to 2 year (SE) (n) | p-value* | |
|---|---|---|---|---|---|---|---|
| Delayed Reconstruction | |||||||
| SATBR | 48.5 (4.0) | 16.3 (3.6) (13) | < 0.001 | 18.0 (3.8) (12) | < 0.001 | 18.6 (4.3) (10) | < 0.001 |
| PWB-CHEST | 66.8 (3.1) | −2.24(3.3) (15) | 0.59 | 4.53 (3.5) (12) | 0.30 | 6.05 (4.0) (10) | 0.28 |
| PSWB | 61.5 (3.9) | 5.05 (3.7) (14) | 0.31 | 9.7 (4.0) (11) | 0.11 | 7.98 (4.6) (9) | 0.28 |
| Immediate Reconstruction | |||||||
| SATBR | 55.4 (4.2) | −4.39 (7.2) (4) | 0.87 | 2.07 (5.9) (7) | 0.87 | 1.81 (5.2) (10) | 0.87 |
| PWB-CHEST | 76.4 (4.1) | −11.44 (4.3) (16) | 0.03 | −11.73 (4.2) (18) | 0.03 | −8.58 (4.6) (11) | 0.14 |
| PSWB | 63.5 (4.1) | 0.81 (6.8) (14) | 0.91 | −2.72 (5.4) (7) | 0.74 | 4.81 (4.7) (10) | 0.74 |
| No Reconstruction | |||||||
| PWB-CHEST | 73.3 (3.4) | −13.0 (3.8) (11) | 0.008 | −5.85 (3.7) (10) | 0.18 | −9.43 (5.0) (4) | 0.13 |
| PSWB | 65.4 (2.9) | −4.8 (2.8) (11) | 0.45 | −3.79 (2.7) (10) | 0.45 | −4.47 (3.6) (4) | 0.45 |
adjusted for multiple comparison correction
Abbreviations: Preop preoperative, SATBR satisfaction with breasts, PWB-CHEST physical well-being of the chest, PSWB psychosocial well-being, SE standard error
PWB-CHEST did not significantly differ between IR, DR, or NR at any time point (Fig. 2). Patients undergoing DR were noted to have a trend in improving PWB-CHEST scores from preoperative score to 2-year post-operative scores (median scores 66 versus 76), while patients with IR and NR experienced a decline in PWB-CHEST over time (median scores 74 versus 66 and 78 versus 64, respectively), although these were not found to be statistically significant. A statistically significant decline in mean PWB-CHEST scores was noted in the IR group at both 6 months and 1 year after surgery (−11.44 and −11.73, respectively, p = 0.03). Additionally, PSWB did not significantly differ between IR, DR, or NR at any time point (Fig. 3) and, over time, there was no significant change in PSWB (Table 2).
Fig 2.
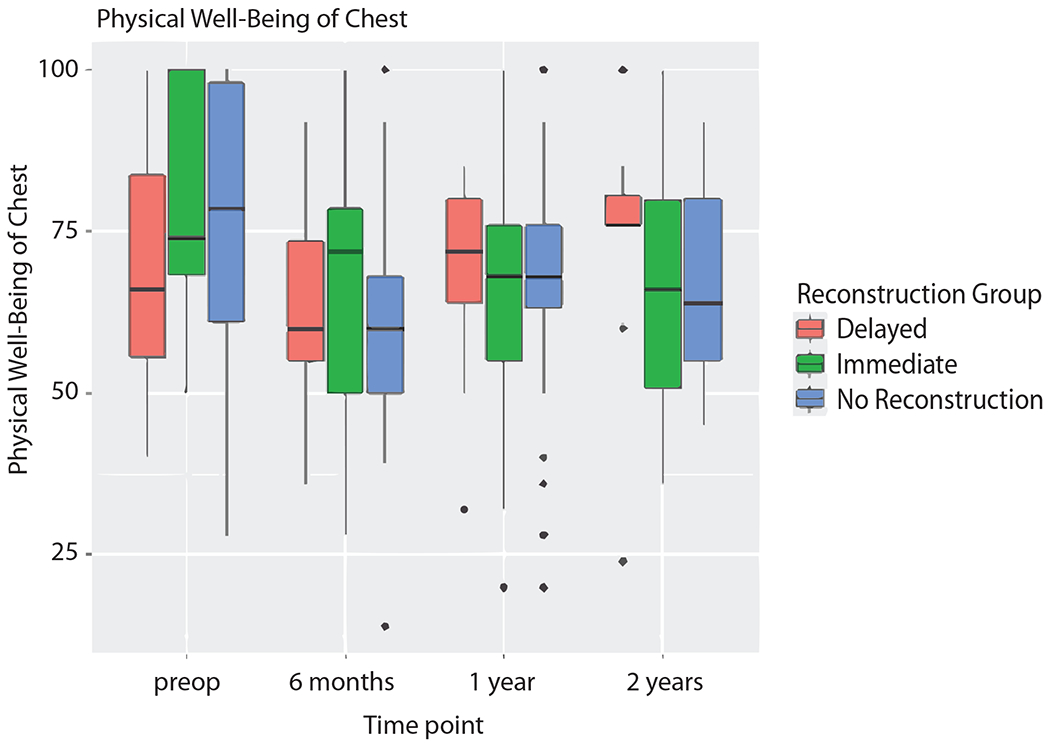
Physical well-being of the chest scores in the no reconstruction, delayed reconstruction, and immediate reconstruction cohorts.
Fig 3.
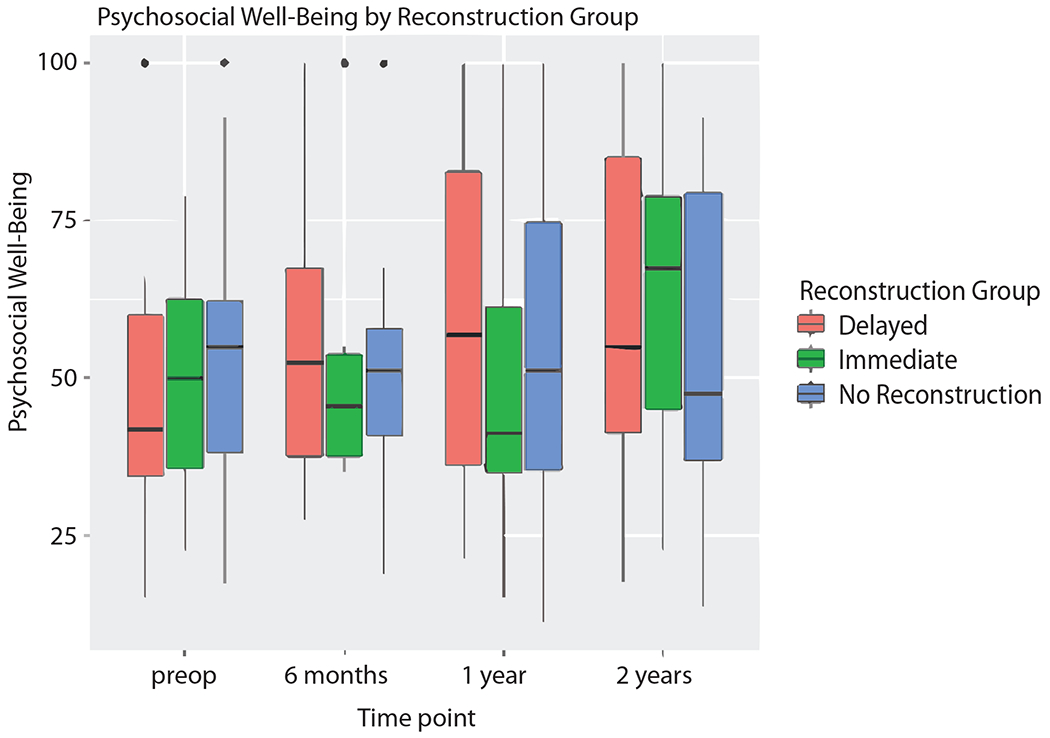
Psychosocial well-being scores in the no reconstruction, delayed reconstruction, and immediate reconstruction cohorts.
DISCUSSION
In this preliminary study evaluating BREAST-Q scores among clinical T4 breast cancer patients undergoing mastectomy with NR, DR, or IR, neither the decision to pursue reconstruction nor the timing of reconstruction was associated with superior patient-reported outcomes. However, patients who underwent delayed reconstruction did experience significantly improved breast satisfaction after surgery.
Although cT4 breast cancer is a locally advanced and often aggressive presentation of breast cancer, as therapy continues to improve and patients are living longer, patients’ satisfaction with outcomes becomes an even more important consideration. Immediate reconstruction compared to delayed reconstruction was not associated with superior outcomes for breast satisfaction at any time point in this study. However, when examined longitudinally, patients in the DR cohort experienced a significant improvement in breast satisfaction scores at both 6 months and 2 years postoperatively, compared to their preoperative baseline scores. Patients who underwent IR did not experience a significant change in breast satisfaction scores at any postoperative timepoint compared to their preoperative baseline scores. Similarly, in a cohort of 175 patients from the Mastectomy Reconstruction Outcomes Consortium (MROC), Billig et al. reported patients undergoing PMRT with both delayed and immediate autologous reconstruction had equivalent satisfaction outcomes determined by BREAST-Q scores taken at 1 and 2 years postoperatively.15 These data suggest that patients who desire reconstruction can expect an improvement in their breast satisfaction following DR to, ultimately, a level similar to those who elect IR.
Prior studies have demonstrated increased complication rates in T4 patients pursuing IR,5 as well as some delays to postoperative therapy,16,17 suggesting that patients should be counseled regarding this risk when discussing timing of reconstruction. The risk of complications and delays in adjuvant therapy was prohibitively high among IBC patients (46%), and the standard recommendation for this group remains NR at the time of initial surgery.5 Over the past few years, there have been an increasing number of women who elect to forego reconstruction and “go flat”. A recent study utilizing an online survey of 931 women who elected NR reported a mean scaled satisfaction score of 3.72 (out of 5), suggesting that many women are satisfied with the decision to avoid reconstruction.18 A limitation of our study is that the BREAST-Q was not designed to assess breast satisfaction in women who elected no reconstruction. Therefore, this group was not included in analyses regarding breast satisfaction. Further efforts to assess satisfaction for the NR patients can help to inform shared decision making for clinical T4 breast cancer patients.
PWB-CHEST scores did not differ between the NR, DR, and IR cohorts at any time point. However, when assessing mean scores over time, both the NR and IR groups had decreased mean scores compared to preoperative baseline at 6 months. As all patients in the NR and IR groups received PMRT immediately following surgery, this decline likely reflects the impact of radiotherapy on PWB-CHEST. Similarly, in a study of 3268 patients undergoing mastectomy with reconstruction, patients who received postoperative radiation had poorer PWB-CHEST scores up to 3 years after surgery. This study suggests that the untoward effects of radiotherapy on the PWB-CHEST are not mitigated by reconstruction.19 In the group that elected delayed reconstruction, PWB-CHEST did not significantly change following surgery. Importantly, this group underwent PMRT prior to reconstructive surgery further supporting the association between radiation and PWB-CHEST.
Recently, 2 studies have demonstrated the safety of preoperative radiotherapy followed by skin-sparing mastectomy with autologous reconstruction.20,21 A clinical trial examining the feasibility and safety of neoadjuvant radiotherapy followed by conventional modified radical mastectomy with autologous reconstruction among women with non-metastatic clinical T4 breast cancer is ongoing.22 The impact of this novel approach on patient-reported outcomes will be an important secondary aim of this trial to help inform shared decision making.
The present study is, to our knowledge, the first to assess if BREAST-Q scores differ among patients with T4 breast cancer undergoing mastectomy with and without reconstruction. Its limitations include the retrospective design and the potential for selection bias as patients pursued the reconstruction of their choice in discussion with their physician. Furthermore, the breast satisfaction domain of the BREAST-Q is only validated for women with reconstruction. Tools to better assess satisfaction among women without reconstruction are needed. As there are a growing number of patients who do not desire reconstruction,18 and as there is not yet a tool designed to specifically assess satisfaction among this cohort of patients, it is feasible that we are under-representing the patient experience in those who do not pursue reconstruction, as the BREAST-Q is not validated in those without reconstruction.
Conclusions
In patients with T4 breast cancer, timing of reconstruction was not associated with differences in outcomes for SATBR, PWB-CHEST, or PSWB at any time point. In the DR cohort, there was improvement in SATBR through 2 years postoperatively. These data can help to inform breast reconstructive decision making for patients facing the choice between DR, IR, and NR. Novel strategies to improve outcomes for these patients are needed.
Synopsis:
Aim:
Do PROMs differ among clinical T4 patients undergoing mastectomy with and without reconstruction?
Findings:
Neither reconstruction nor timing of reconstruction were associated with superior outcomes for breast satisfaction, physical well-being of the chest, or psychosocial well-being at any timepoint.
ACKNOWLEDGMENTS
The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center, and this study was presented in poster format at the 23rd Annual Meeting of the American Society of Breast Surgeons, Las Vegas, NV, April 6-10, 2022. Dr. Monica Morrow has received honoraria from Exact Sciences and Roche. All other authors have no conflict of interest disclosures to report.
REFERENCES
- 1.Amin MB, Edge SB, Greene FL, et al. eds. American Joint Committee on Cancer (AJCC) Staging Manual. 8th ed. New York: Springer; 2017. [Google Scholar]
- 2.Murphy BL, Hoskin TL, Boughey JC, et al. Contemporary operative management of T4 breast cancer. Surgery. 2016;160(4):1059–1069. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN). 2022 NCCN Clinical Practice Guidelines In Oncology: Breast Cancer v2.2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed June 13, 2022).
- 4.Wilson RE. Surgical management of locally advanced and recurrent breast cancer. Cancer. 1984;53(3 Suppl):752–757. [DOI] [PubMed] [Google Scholar]
- 5.Pawloski KR, Barrio AV, Gemignani ML, et al. Reconstruction in Women with T4 Breast Cancer after Neoadjuvant Chemotherapy: When Is It Safe? J Am Coll Surg. 2021;233(2):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22(3):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson FM, Bondy M, Yang W, et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60(6):351–375. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Chen H, Wu K, Ding A, Zhang P, Zhang M. Post-mastectomy immediate breast reconstruction is oncologically safe in well-selected T4 locally advanced breast cancer: a large population-based study and matched case-control analysis. Breast Cancer Res Treat. 2019;176(2):337–347. [DOI] [PubMed] [Google Scholar]
- 9.Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL. The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg. 2012;129(2):293–302. [DOI] [PubMed] [Google Scholar]
- 10.Klassen AF, Dominici L, Fuzesi S, et al. Development and Validation of the BREAST-Q Breast-Conserving Therapy Module. Ann Surg Oncol. 2020;27(7):2238–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagsi R, Momoh AO, Qi J, et al. Impact of Radiotherapy on Complications and Patient-Reported Outcomes After Breast Reconstruction. J Natl Cancer Inst. 2018;110(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson JA, Allen RJ Jr., Polanco T, et al. Long-term Patient-reported Outcomes Following Postmastectomy Breast Reconstruction: An 8-year Examination of 3268 Patients. Ann Surg. 2019;270(3):473–483. [DOI] [PubMed] [Google Scholar]
- 13.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. [DOI] [PubMed] [Google Scholar]
- 14.Voineskos SH, Klassen AF, Cano SJ, Pusic AL, Gibbons CJ. Giving Meaning to Differences in BREAST-Q Scores: Minimal Important Difference for Breast Reconstruction Patients. Plast Reconstr Surg. 2020;145(1):11e–20e. [DOI] [PubMed] [Google Scholar]
- 15.Billig J, Jagsi R, Qi J, et al. Should Immediate Autologous Breast Reconstruction Be Considered in Women Who Require Postmastectomy Radiation Therapy? A Prospective Analysis of Outcomes. Plast Reconstr Surg. 2017;139(6):1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakhlis F, Regan MM, Chun YS, et al. Patterns of breast reconstruction in patients diagnosed with inflammatory breast cancer: The Dana-Farber Cancer Institute’s Inflammatory Breast Cancer Program experience. Breast J. 2020;26(3):384–390. [DOI] [PubMed] [Google Scholar]
- 17.Newman LA, Kuerer HM, Hunt KK, et al. Feasibility of immediate breast reconstruction for locally advanced breast cancer. Ann Surg Oncol. 1999;6(7):671–675. [DOI] [PubMed] [Google Scholar]
- 18.Baker JL, Dizon DS, Wenziger CM, et al. “Going Flat” After Mastectomy: Patient-Reported Outcomes by Online Survey. Ann Surg Oncol. 2021;28(5):2493–2505. [DOI] [PubMed] [Google Scholar]
- 19.Hwang ES, Locklear TD, Rushing CN, et al. Patient-Reported Outcomes After Choice for Contralateral Prophylactic Mastectomy. J Clin Oncol. 2016;34(13):1518–1527. [DOI] [PubMed] [Google Scholar]
- 20.Singh P A Prospective Clinical Trial of Immediate Breast Reconstruction Following Pre-mastectomy Radiotherapy for Operable Breast Cancer. Quickshot presentation, 23rd Annual Meeting of the American Society of Breast Surgeons, April 6-10, 2022, Las Vegas, NV. [Google Scholar]
- 21.Thiruchelvam PTR, Leff DR, Godden AR, et al. Primary radiotherapy and deep inferior epigastric perforator flap reconstruction for patients with breast cancer (PRADA): a multicentre, prospective, non-randomised, feasibility study. Lancet Oncol. 2022;23(5):682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A Study of an Alternative Treatment Approach (Preoperative Radiotherapy, Then Mastectomy, Then Immediate Reconstruction Surgery) in People With T4 Breast Cancer. ClinicalTrials.gov Identifier: NCT05412225. Updated June 13, 2022. https://www.clinicaltrials.gov/ct2/show/NCT05412225 (Accessed June 25, 2022).


