Abstract
Age-related cognitive decline has been attributed to processing speed differences, as well as differences in executive control and response inhibition. However, recent research has shown that healthy older adults have intact, if not superior, sustained attention abilities compared to younger adults. The present study used a combination of reaction time, thought probes, and pupillometry to measure sustained attention in samples of younger and older adults. The reaction time data revealed that, while slightly slower overall, older adults sustained their attention to the task better than younger adults, and did not show a vigilance decrement. Older adults also reported fewer instances of task-unrelated thoughts and reported feeling more motivated and alert than younger adults, despite finding the task more demanding. Additionally, older adults showed larger, albeit later-peaking, task-evoked pupillary responses, corroborating the behavioral and self-report data. Finally, older adults did not show a shallowing of task-evoked pupillary responses across time, corroborating the finding that their reaction times also did not change across time. The present findings are interpreted in light of processing speed theory, resource-depletion theories of vigilance, and recent neurological theories of cognitive aging.
Keywords: aging, sustained attention, vigilance, mind wandering, pupillometry
Given that aging is associated with declines in attention and inhibitory control (Hasher, Lustig, & Zacks, 2007; Hasher & Zacks, 1988), one would expect older adults to have a relative inability to sustain their attention. However, older adults sometimes show better performance on measures of sustained attention compared to younger adults (see Vallesi, Tronelli, Lomi, & Pezzetta, 2021 for a recent meta-analysis). Sustained attention can also measured via the vigilance decrement, a worsening of performance with time on task, and it is observed across many different tasks, including perceptual discrimination tasks (Jerison & Pickett, 1964; Parasuraman, 1979; Parasuraman & Mouloua, 1987), simple reaction time tasks (Massar, Lim, Sasmita, & Chee, 2016; Massar et al., 2019b; Robison, Unsworth, & Brewer, 2021; Unsworth & Robison, 2016, 2020), n-back tasks (Hopstaken et al., 2015a, 2015b; Hopstaken, Linden, Bakker, Kompier, & Leung, 2016), and go/no-go tasks (McVay & Kane, 2009, 2012a). Parasuraman, Nestor, and Greenwood (1989) showed that older adults had lower discrimination ability overall, compared to younger adults, but older adults did not show steeper vigilance decrements. Deaton and Parasuraman (1993) also showed overall lower discrimination ability among older adults, despite no differences in the vigilance decrement. However, opposite patterns have been observed. For example, Tomporowski and Tinsley (1996) observed significantly better discrimination and a shallower vigilance decrement among older adults compared to younger adults. In a go/no-go task, Staub et al. (2014b) observed that younger adults showed a vigilance decrement across time, but older adults did not, and that older adults also reported being more motivated and reported fewer task-unrelated thoughts (TUTs) than younger adults (see also Brache, Scialfa, & Hudson, 2010; Staub et al., 2014a; Staub, Doignon-Camus, Marques-Carneiro, Bacon, & Bonnefond, 2015). Staub et al. (2014a, 2014b, 2015; 2013) argue that traditional vigilance tasks, which require responses on a rare subset of trials, differ substantially from tasks like the SART, which require frequent responses and withholding of responses on a rare subset of trials. Thus Staub et al. argue that traditional vigilance tasks may overwhelm the available cognitive resources that older adults possess by consuming bottom-up processing capabilities, which may lead to worse performance on such tasks. The SART makes relatively fewer demands on bottom-up processing, but requires top-down control to inhibit incorrect go responses. Further, older adults tend to favor accuracy over speed, and this is a beneficial strategy in the SART (Vallesi et al., 2021). Therefore, age differences in sustained attention - or lack thereof - may be confounded by the bottom-up processing demands of a task.
Mind-wandering, especially during an externally directed task (e.g., reading, having a conversation, driving a car), is often considered a failure of executive control, allowing for internally-directed thoughts to usurp attention away from task goals (McVay & Kane, 2010). However, the aging literature has revealed a robust and somewhat paradoxical pattern indicating that older adults report less mind-wandering than younger adults (Frank, Nara, Zavagnin, Touron, & Kane, 2015; Giambra, 1989, 2000; Jackson & Balota, 2012; Jackson, Weinstein, & Balota, 2013; Krawietz, Tamplin, & Radvansky, 2012; Moran et al., 2021; Shake, Shulley, & Soto-Freita, 2016; Staub et al., 2014b; Zavagnin, Borella, & De Beni, 2014). This seems to contradict the observation that people with relatively poor executive-attention abilities (e.g., working memory capacity, attentional control) tend to report more instances of mind-wandering, especially in attention-demanding situations (Kane et al., 2007, 2016; McVay & Kane, 2009, 2012b, 2012a; Mrazek et al., 2012; Robison, Gath, & Unsworth, 2017; M. K. Robison et al., 2020; Robison & Unsworth, 2015; Rummel & Boywitt, 2014; Unsworth & McMillan, 2013, 2014; Unsworth, McMillan, Brewer, & Spillers, 2012; Unsworth, Robison, & Miller, 2021).
Several hypotheses offer explanations for this perplexing relationship between aging and mind-wandering. One hypothesis is is that mind-wandering is a resource-demanding process (Smallwood & Schooler, 2006). Therefore, compared to younger adults, it may be more taxing for older adults to engage in mind-wandering due to their limited cognitive capacity (Craik & Byrd, 1982). However, McVay, Meier, Touron, and Kane (2013) compared younger and older adults’ sustained attention performance preceding various TUT reports and found that younger and older adults produced similar performance decrements during periods of mind-wandering. If age differences in mind-wandering were driven by cognitive capacity, and cognitive capacity decreases with age, then one would expect older adults to show greater performance decrements during periods of mind-wandering than younger adults.
A second hypothesis posits that older adults either lack the meta-awareness or are more reluctant to report mind-wandering (Einstein & Mcdaniel, 1997; Zavagnin et al., 2014). However, several experiments have challenged this hypothesis: Frank et al. (2015) found that older adults’ TUT reports were just as veridical as younger adults’ in relation to objective mind-wandering-related eye-movement patterns, and Giambra (1973) indicates that older adults report a more positive view of mind-wandering than younger adults. Third, the control failure × current concerns hypothesis suggests that the relationship is driven by age differences in how the testing context cues personally relevant concerns (McVay & Kane, 2010). For older adults, the university laboratory contains few reminders of their current concerns. Whereas, for younger adults, it is more directly tied to their current concerns. However, this hypothesis is contradicted by evidence that older adults still report less mind-wandering than younger adults even when tested outside the laboratory (Diede, Gyurkovics, Nicosia, Diede, & Bugg, 2022; Jackson et al., 2013).
Finally, age-group differences in mind-wandering may be due to dispositional factors such as conscientiousness, task interest, and motivation. Evidence is accumulating to support this hypothesis. For example, Jackson and Balota (2012) proposed that older adults’ increased conscientiousness, task interest, and perceived task difficulty may lead them to be less likely to engage in mind-wandering than younger adults. Further, Krawietz et al. (2012) found that including interest as a covariate eliminated the age difference in mind-wandering. Frank et al. (2015) and Seli et al. (2021) reported that participants’ motivation partially mediated the relationship between age and mind-wandering, and Nicosia and Balota (2021) found that self-reported conscientiousness, interest, and motivation fully mediated the relationship between age and mind-wandering. Thus, it is possible that dispositional factors may explain age differences in mind-wandering, although the physiological mechanisms through which this is achieved remain unclear.
In the present study, we elected to use the Psychomotor Vigilance Task (PVT) to measure sustained attention because this task has been extensively used with younger adults. The behavioral and physiological correlates of performance are well-understood, and thus offer a clear comparison against which older adults can be evaluated. The PVT is often employed to study the behavioral and physiological correlates of the vigilance decrement in younger adults. It is a simple reaction time (RT) task with an unpredictable stimulus onset time, and thus requires consistent attention both within and across trials. Because the PVT is a simple RT task, it does not require stimulus discrimination, it does not carry speed-accuracy tradeoffs, and it does not require inhibition of prepotent responses, like traditional vigilance tasks or the SART. Therefore, we can dissociate speed and sustained attention without an overriding influence of inhibitory control. Younger adults show a robust vigilance decrement in the PVT as a slowing of RTs across trials (Massar et al., 2019a, 2016; Robison et al., 2021; Unsworth & Robison, 2016, 2020), and it is of interest whether there are age-related differences in this vigilance decrement. In present study, we examined both age-group differences in overall RTs and changes in RT across time. The processing speed theory of cognitive aging (Salthouse, 1996) would predict slower RTs for older adults overall. An open question in the present design is whether older adults will show larger, smaller, or roughly equal vigilance decrements compared to younger adults. Another reason we used the PVT is because young adults report frequent mind-wandering during the PVT, with participants reporting TUTs on about 50% of thought probes (Robison et al., 2021; Unsworth & Robison, 2016). Therefore, we believed it would be a good candidate task to compare younger and older adults in their TUT tendencies to test various hypotheses regarding the age-TUT paradox.
The PVT also produces stereotypical patterns of pupillary responses in young adults. Historically, pupil dilation has been used as a physiological index of mental effort (Beatty, 1982; Beatty & Lucero-Wagoner, 2000). For example, prior work has shown that the pupil dilates when people encode information to be retrieved later (Kahneman & Beatty, 1966; Kahneman & Peavler, 1969), when people experience cognitive conflict (Laeng, Ørbo, Holmlund, & Miozzo, 2011; Wel & Steenbergen, 2018), and when they are trying solve difficult math problems (Hess & Polt, 1964). In the PVT, the magnitude of the pupillary response tends to decline across time (Robison, 2018; Unsworth & Robison, 2016), is larger when participants report being on-task versus off-task (Unsworth & Robison, 2017b), and correlates with individual differences in performance on the task (Unsworth & Robison, 2017b). The magnitude of the pupillary response is also sensitive to motivational incentives (Massar et al., 2019a, 2016). Thus, we can compare the magnitude of pupillary responses, the change in magnitude across trials, and differences in magnitude across attentional states between younger and older adults. Novel to the present study, and rather exploratory in nature, was the question of whether the latency of pupillary responses would differ across age groups. It is possible that this physiological response may not differ in magnitude across younger and older adults, but would differ in the time course with which it occurs. If the pupillary response is an indirect indicator of a neural process, and neural processing is slower among older adults (Salthouse, 1996), then the pupillary response may occur over a later time course than for younger adults. Latency differences would also be predicted by neural response theories of aging (Bartzokis, 2004, 2011; Lu et al., 2011, 2013). However, few studies have specifically examined the latency of pupillary response as they relate to age-related changes in processing speed. In one study, Porter et al. (2010) did not find differences between younger and older adults in the latency of pupillary responses during a visual search task. However in an auditory task, Zekveld, Kramer, and Festen (2011) found that age was correlated with longer peak latencies and larger mean pupillary dilations.
Overall, our goal was to test theories regarding age-related change in processing speed and attention. Specifically, we hoped to answer several questions: First, how do younger and older adults compare in processing speed in a simple RT task? Second, how does the vigilance decrement differ between younger and older adults? Third, what subjective factors might account for age-related differences in both sustained attention and subjective task engagement (i.e., mind-wandering)? Finally, will pupillary measures of effort corroborate the behavioral and subjective differences between younger and older adults? These final analyses were largely exploratory. But processing speed theory predicts that older adults will have significant delays in cognitive processing, and this could be captured by the latency of stimulus-evoked pupillary responses.
Method
Transparency and Openness
All data and analysis code are publicly available on the Open Science Framework (https://osf.io/63wcj/). The design and analysis plan were not preregistered prior to data collection. The data were analyzed and plotted in R using the tidyverse (Wickham, 2017), lmerTest (Kuznetsova, Brockhoff, & Christensen, 2017), cowplot (Wilke, 2020), psych (Revelle, 2018), and lavaan (Rosseel, 2012) packages. The manuscript was written in R Markdown using the papaja package (Aust & Barth, 2018). The sample size for the older adult group was maximized per available funds to compensate participants. The sample size for the younger adult group was selected to match the older adult sample.
Participants
The sample included 60 younger adults and 62 older adults. Younger adults were recruited from the undergraduate human subject pool at Washington University in St. Louis and were compensated with partial course credit. Older adults were recruited from the St. Louis metropolitan area. Washington University maintains a database of older adults who have agreed to be contacted regarding opportunities to participate research studies. Participants in the present study were recruited via phone calls informing them of eligibility. The older adults were compensated with cash ($10/hour). Data for participants’ age, health status, and education levels are listed in Table 1.
Table 1.
Age, health, and education data for each age group
| Younger adults | Older adults | |
|---|---|---|
|
| ||
| Mean age (SD) | 19.88 (1.43) | 75.37 (7.53) |
|
| ||
| Male | 31% | 34% |
| Female | 66% | 66% |
|
| ||
| White | 55% | 95% |
| Black/African American | 3% | 3% |
| Asian | 38% | 0% |
| Hispanic or Latino | 12% | 0% |
| American Indian/Native Alaskan | 2% | 2% |
|
| ||
| “Excellent” health | 43% | 19% |
| “Good” health | 53% | 55% |
| “O.K.” health | 0% | 18% |
| “Fair” health | 0% | 5% |
| “Poor” health | 2% | 0% |
| Health limits activities “not at all” | 92% | 55% |
| Health limits activites “a little” | 5% | 31% |
| Health limits activities “some” | 2% | 11% |
| Health limits activities “A lot” | 0% | 2% |
|
| ||
| Some high school | 0% | 2% |
| High school diploma | 100% | 34% |
| College degree | 0% | 42% |
| Graduate degree | 0% | 23% |
Note. SD = standard deviation
Procedure
The laboratory sessions lasted about two hours. In order, participants completed the following tasks: Psychomotor Vigilance Task (PVT), Raven Advanced Progressive Matrices (Raven, Raven, & Court, 1962), number series (Thurstone, 1938), letter sets (Ekstrom, Dermen, & Harman, 1976), a general knowledge quiz, a synonym quiz, and an antonym quiz (Hambrick, Salthouse, & Meinz, 1999), the color-word Stroop task (Stroop, 1935), the Simon task (Simon, 1990), and the consonant or vowel/odd or even (CVOE) task (Minear & Shah, 2008). The final nine tasks were collected as part of a larger study assessing age-related differences in cognitive abilities. The present examination focuses on the data from the PVT. The data from the other tasks will be analyzed and reported in a separate manuscript examining age-related differences in attentional control, fluid intelligence, and crystallized intelligence. Eye-tracking data were only collected before, during, and immediately after the PVT. Prior to beginning the session, all participants gave written informed consent and completed a demographic questionnaire. The questionnaire asked participants to report their gender, age, handedness, highest level of education, racial/ethnic background, employment status, their occupation/college area of study, domestic arrangement (living alone or with others), self-rated current health, any medical treatment for heart disease, high blood pressure, anxiety, stroke, or depression, as well as an open-ended “other” response option, any prescription medications they believed might affect their memory, any previous brain injuries, and whether they were wearing corrective lenses.
Task
Each trial began with a row of black fixation crosses centered on a gray background. This fixation screen appeared for 2 seconds. Then, a row of blue zeros (00.000) appeared at the center of the screen. After a random time interval sampled from a uniform distribution ranging from 2–10 s, the zeros began counting forward like a stopwatch. The participants’ task was to press the spacebar as soon as they noticed this change occurring. After the participant pressed the spacebar, the numbers turned red and paused for 1 second, revealing the RT for that trial (e.g., 00.378). Then, after a 1-second blank intertrial interval, the next trial began. There were 160 trials in total. For statistical analyses, the trials were divided into 5 blocks of 32 trials. But this division was not apparent to participants, and there was no division between blocks present in the procedure. Participants completed the tasks individually in a well-lit room. The task took about 30 minutes to complete. Each block took about 5 minutes. The remaining time comprised instruction screens and practice trials.
Thought probes
The thought probe screens asked participants to “Please characterize your current conscious experience.” There were 5 response options: 1) I am totally focused on the current task, 2) I am thinking about my performance on the task or how long it is taking, 3) I am distracted by sights/sounds/temperature or by physical sensations (hungry/thirsty), 4) I am daydreaming/my mind is wandering about things unrelated to the task, and 5) I am not very alert/my mind is blank or I’m drowsy. Participants pressed the key corresponding to the response that best matched their immediately preceding thoughts. Response 1 was scored as on-task, response 2 as task-related interference, response 3 as external distraction, response 4 as mind-wandering, and response 5 as mind-blanking (Unsworth & Robison, 2016). Thought probes appeared on 30 randomly-sampled trials.
Self-report questionnaires
After the PVT, participants were asked to rate, on a scale from 1–9, how motivated they felt to perform well on the task (1 = completely unmotivated, 9 = completely motivated). They were also asked to rate, on a scale from 1–9, how drowsy they felt (1 = very alert, 9 = very drowsy). Participants then completed the NASA-Task Load Index (TLX), a measure of workload (Hart & Staveland, 1988). Participants were asked to make ratings regarding mental demand, physical demand, temporal demand (i.e., task pacing), perceptions of performance, effort level, and frustration level on the PVT. These ratings were made on a scale from −10 to 10 in 0.5-unit increments.
Pupillometry
Pupil data were collected via an SR Systems EyeLink eye-tracker at 1000 Hz. Prior to beginning the task, participants’ left eyes were manually calibrated to the eye-tracker using the built-in calibration procedure for pupil size and gaze position. The EyeLink reports pupil size in arbitrary units. Data were normalized within participants in several ways. First, the data from the fixation screen were extracted for each trial. Average pupil size across the 2-second window was computed and then standardized within individuals. Then, the data were epoched to include the window from 100 ms before stimulus onset to 1,200 ms after stimulus onset. Data were normalized within this window, then averaged into 50-ms-wide windows. All values were then subtracted from the average of the 100-ms prestimulus window to compute a task-evoked pupillary response (TEPR) on each trial for each participant.
Data analysis
Data were screened for outliers by eliminating any data point outside +/− 3 SDs of each group mean.
Results
Descriptive statistics for all measures are listed in Table 2.
Table 2.
Descriptive statistics for each dependent measure by age group
| Age Group | Measure | Mean | SD | Range | Skew | Kurtosis |
|---|---|---|---|---|---|---|
|
| ||||||
| Younger adults | Average reaction time | 364.39 | 49.41 | [274.64, 510.10] | 0.57 | 0.40 |
| Vigilance decrement | 13.74 | 10.88 | [−7.65, 57.92] | 1.34 | 3.23 | |
| Motivation | 6.43 | 1.57 | [1.00, 9.00] | −0.85 | 1.21 | |
| Drowsiness | 6.12 | 1.76 | [1.00, 9.00] | −1.08 | 0.96 | |
| TUT proportion | 0.50 | 0.23 | [0.03, 0.93] | 0.08 | −0.97 | |
| Mean pretrial pupil size | 893.25 | 168.53 | [620.93, 1,352.16] | 0.64 | −0.29 | |
| SD pretrial pupil size | 100.91 | 29.36 | [62.05, 206.99] | 1.39 | 2.16 | |
| TEPR magnitude | 1.59 | 0.46 | [0.47, 2.52] | −0.36 | −0.16 | |
| TEPR latency | 615.88 | 57.61 | [516.67, 800.00] | 1.12 | 1.87 | |
| Older adults | Average reaction time | 384.52 | 55.00 | [286.27, 596.36] | 1.10 | 2.27 |
|
| ||||||
| Vigilance decrement | 4.18 | 11.98 | [−45.78, 39.08] | −0.55 | 3.98 | |
| Motivation | 7.85 | 2.19 | [1.00, 9.00] | −2.33 | 4.11 | |
| Drowsiness | 3.69 | 2.37 | [1.00, 8.00] | 0.42 | −1.30 | |
| TUT proportion | 0.23 | 0.23 | [0.00, 0.87] | 0.96 | 0.09 | |
| Mean pretrial pupil size | 580.53 | 173.41 | [273.02, 1,016.71] | 0.52 | −0.54 | |
| SD pretrial pupil size | 43.18 | 22.20 | [14.53, 95.31] | 0.77 | −0.46 | |
| TEPR magnitude | 2.04 | 0.42 | [0.62, 2.98] | −0.98 | 1.31 | |
| TEPR latency | 744.71 | 82.78 | [600.00, 950.00] | 0.31 | −0.61 | |
Note. SD = standard deviation, TEPR = task-evoked pupillary response.
Behavioral results
For most analyses of both the behavioral and pupillary data, we used linear mixed effects models to examine age-group differences, time-on-task effects, and their interactions. Unless otherwise noted, the linear mixed effect models set participant as a random effect with both the intercept and the slope (e.g., the effect of block) to vary across participants.
Reaction times (RTs)
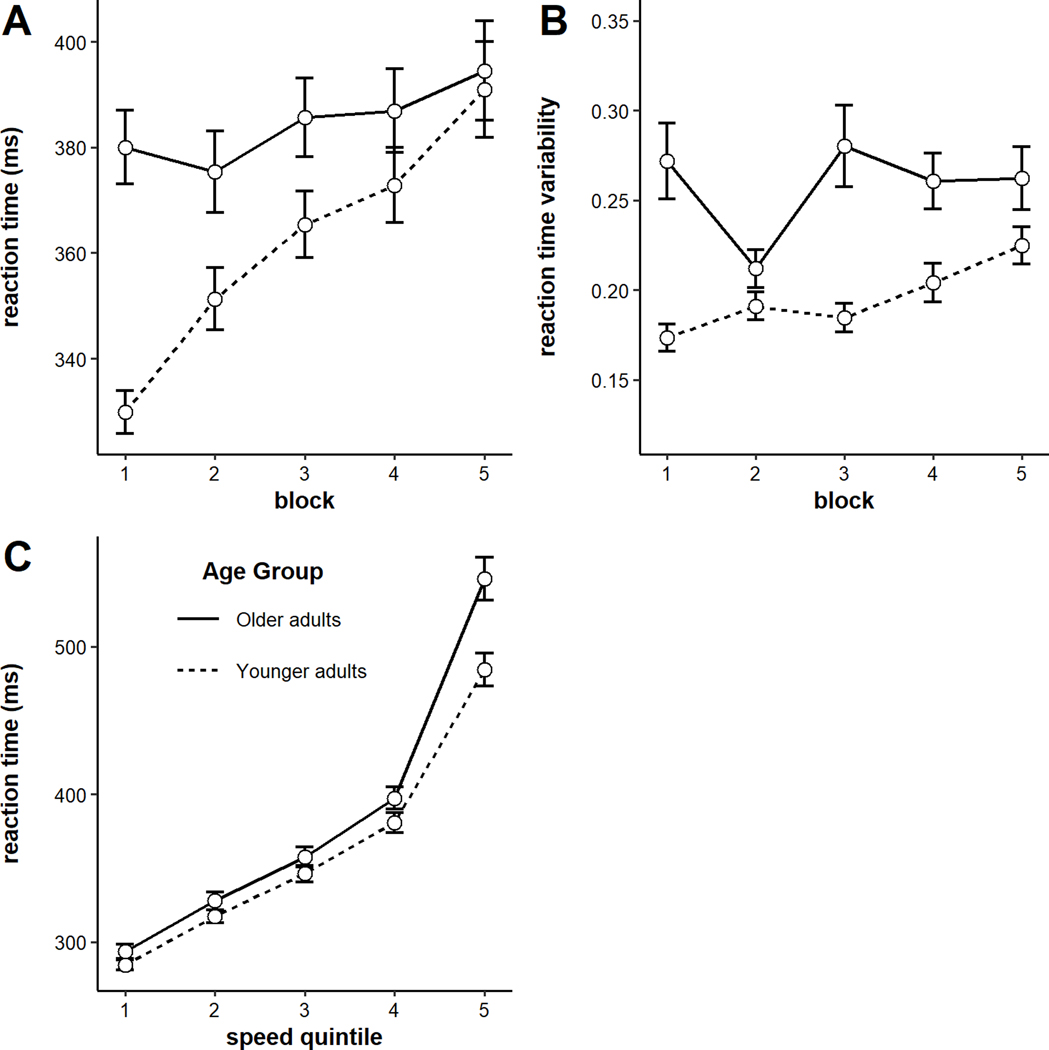
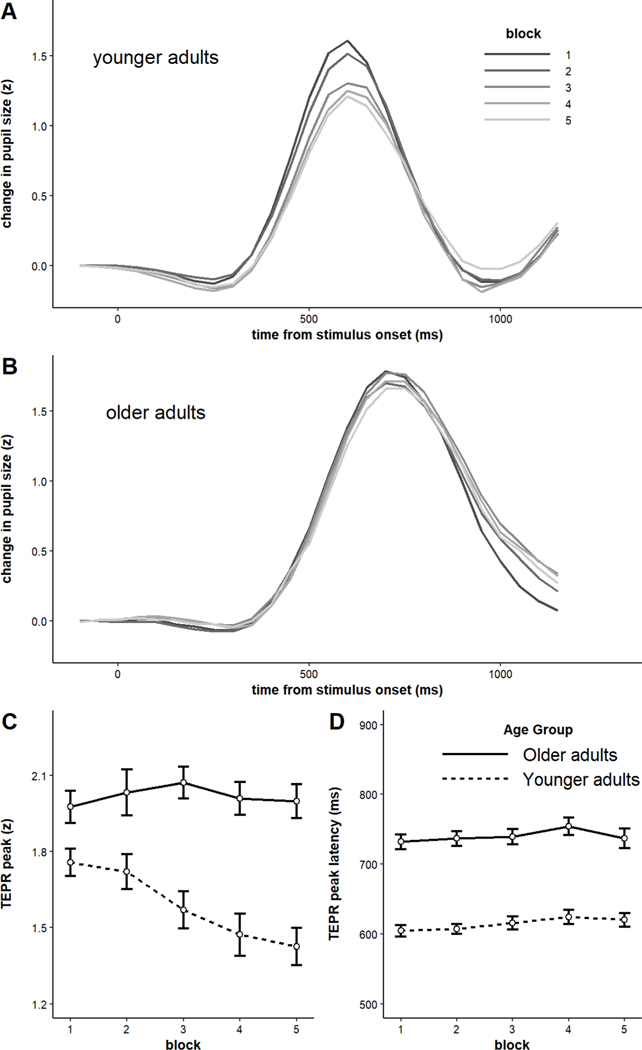
The model on RTs indicated a significant main effect of block, such that RTs increased across blocks (b = 9.00, SE = 1.29, p < .001), but a non-significant main effect of age group (b = 23.89, SE = 14.73, p = 0.11). Older adults’ RTs were slightly but not significantly slower overall (see Figure 1A). The main effects were qualified by a significant block x age group interaction, such that older adults exhibited significantly shallower vigilance decrements than younger adults (b = −5.86, SE = 1.29, p < .001). Examining the RT data for each age group individually revealed that, while younger adults showed significant slowing of RTs across time (b = 14.86, SE = 1.78, p < .001), older adults did not (b = 3.10, SE = 1.88, p = 0.10).
Figure 1.
A) Reaction times by block and age group, and B) Reaction times by speed quintile and age group. Error bars represent +/− one standard error.
Next we analyzed group differences in two additional measures: intraindividual variability in RTs and RTs by speed quintile. To analyze intraindividual variability, we computed each participant’s coefficient of variation (standard deviation/mean; CoV) of RTs in each block. The model revealed a significant main effect of block, such that intraindividual variability in RTs increased across time (b = 0.007, SE = 0.003, p = 0.02), and a significant main effect of age (b = 0.03, SE = 0.007, p < .001), such that older adults showed more intraindividual variability in RT than younger adults (see Figure 1B). The block x age group interaction was not significant (b = −0.005, SE = 0.003, p = 0.10).
To examine RTs by speed quintile, we first rank-ordered individuals’ RTs from shortest to longest. Then, we binned RTs into five quintiles by speed (fastest 20% to slowest 20% of trials). The model indicated a significant quintile x age interaction (b = 6.94, SE = 0.48, p < .001), such that larger age differences were observed at the longer RT quintiles. This suggests that when younger and older adults are at their fastest, they are roughly equivalent. But, in the instances when younger and older adults responded slowly, the older adults responded particularly slowly (see Figure 1C). An additional way to perform this analysis is by entering the fastest quintile and Age into a model predicting the slowest RTs (e.g., Salthouse, 1993). Doing so revealed significant main effects of both the fastest RTs (b = 1.70, SE = 0.24, t = 7.22, p = < .001) and Age (b = −49.03, SE = 15.27, t = −3.21, p = .002; R2 = 38%). This again indicates that age differences in RTs are largest when younger and older adults are at their slowest.
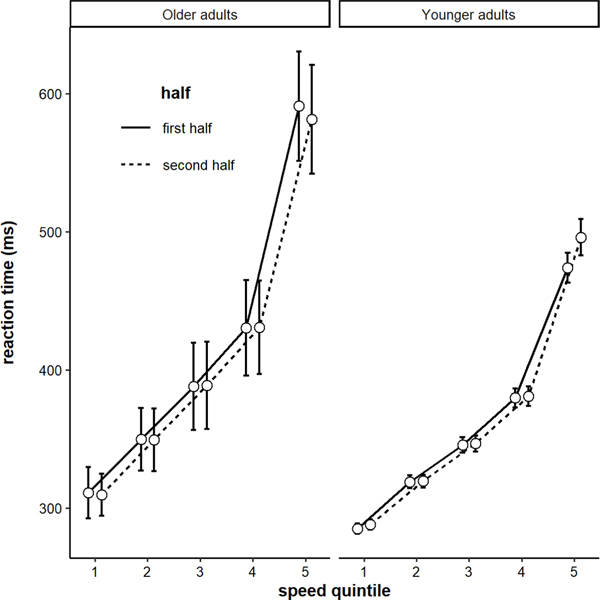
As a final analysis on RTs, we examined how distributions of RTs shifted across blocks for each age group. Specifically, why were younger adults’ RTs changing across blocks? Were only their slow RTs getting longer, or were all quintiles getting longer about equally? To analyze this, we submitted RTs to a model with fixed effects of block, quintile, age group, and their interactions. There was a significant 3-way interaction among block, quintile, and age (b = −1.82, SE = 0.35, p < .001). This pattern is plotted in Figure 2. Older adults’ RT distributions did not shift, on average. But younger adults slowest RTs tended to increase across the task. In other words, the vigilance decrement was present primarily in the slow end of the distribution.
Figure 2.
Reaction times by speed quintile for the first half and second half of the task by age group. Error bars represent +/− one standard error of the mean.
Pupillary results
There were two sets of pupillary measures. The first was pretrial pupil diameter, which was measured as the average pupil diameter over the 2-second fixation screen preceding each trial, and the second was TEPRs, which were computed as a change in pupil diameter in response to stimulus onset on each trial.
Pretrial pupil size
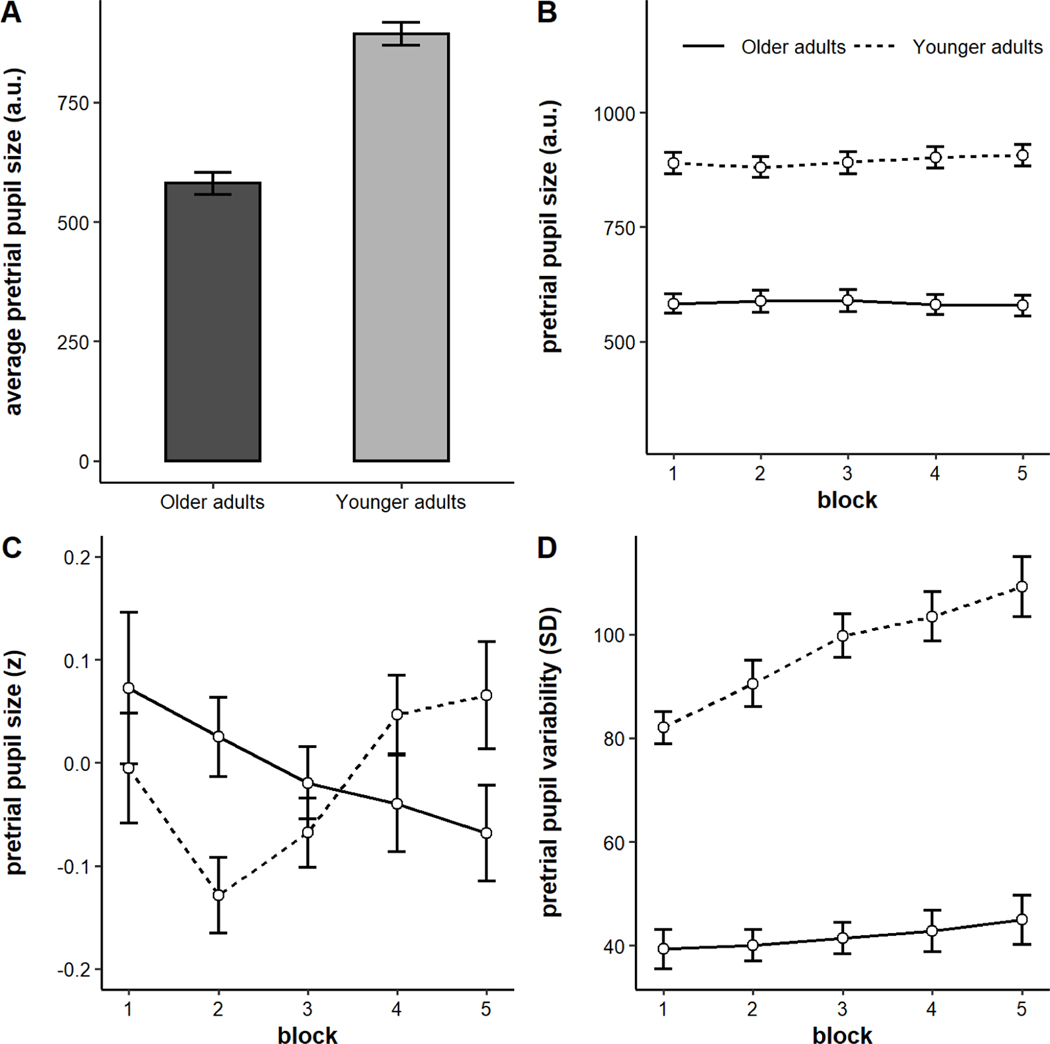
Consistent with prior work, older adults had significantly smaller pupil sizes than younger adults (Figure 6A, M older = 580.53 a.u., M younger = 893.25 a.u., t(102) = −9.30, p < .001; Bak, Yoo, Yang, & Hwang, 2017; Birren, Casperson, & Botwinick, 1950; Winn, Whitaker, Elliott, & Phillips, 1994). We also analyzed pretrial pupil dynamics across blocks. Pretrial pupil size is plotted by block and age group in arbitrary units in Figure 6B and in individually-standardized units in Figure 6C. Younger and older adults showed different patterns across blocks, confirmed by the presence of a block x age group interaction (b = −0.03, SE = 0.01, p = 0.003). Whereas older adults tended to show a small but significant monotonic decrease in pretrial pupil size across blocks (b = −0.03, SE = 0.02, p = 0.03), younger adults actually tended to show a small but significant increase (b = 0.03, SE = 0.01, p = 0.04). Although the effect in younger adults appeared curvilinear, the quadratic effect did not reach significance (b = 0.02, SE = 0.01, p = 0.06).
Figure 6.
A) Average pretrial pupil size by age group, B) Pretrial pupil size by block and age group in arbitrary units, C) Intraindividually-standardized pupil size by block and age group, D) Intraindividual variability (standard deviation) in pretrial pupil size by block and age group. Error bars represent +/− one standard error.
Interestingly, younger and older adults also showed differential patterns of intraindividual variability dynamics. The model on variability (standard deviation of pretrial pupil size in arbitrary units) revealed a significant main effect of block (b = 4.06, SE = 0.64, p < .001), such that intraindividual variability increased across time, and a significant main effect of age group, such that older adults exhibited less variability in pretrial pupil size than younger adults (b = −27.62, SE = 2.78, p < .001). These main effects were qualified by a significant block x age group interaction (b = −2.66, SE = 0.64, p < .001). Whereas younger adults showed a significant increase in variability across blocks (b = 6.72, SE = 1.15, p < .001), this effect was much smaller in older adults (b = 1.40, SE = 0.65, p = 0.04).1 This pattern is shown in Figure 6D.
Task-evoked pupillary responses (TEPRs)
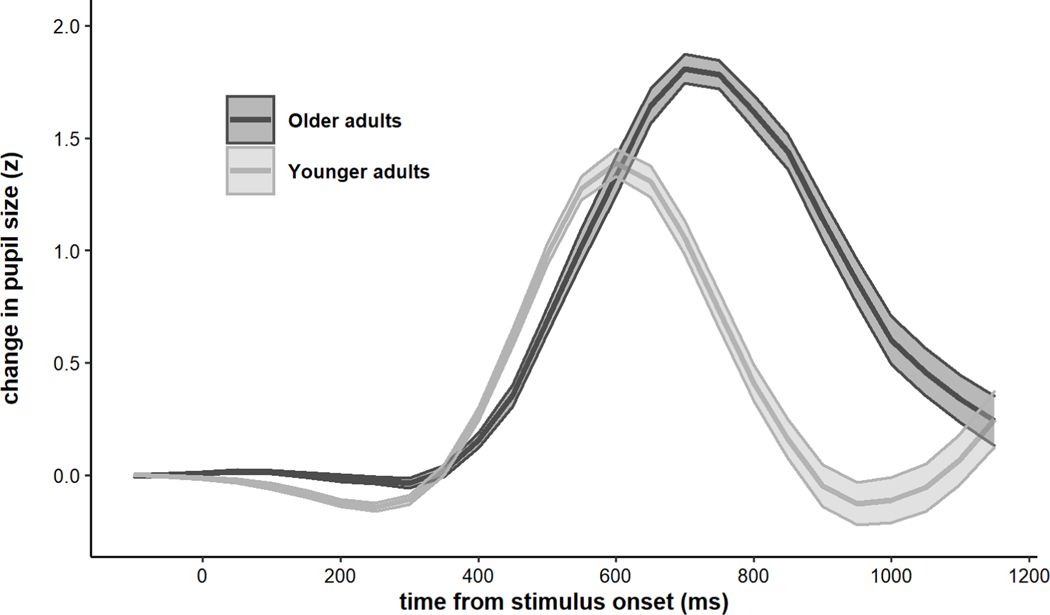
The grand-averaged TEPRs for each age group are shown in Figure 7. Magnitude of TEPRs was computed by taking the peak of the response on each trial and averaging those values within each participant. Similarly, latency was computed by taking the timepoint at which the TEPR peaked in each trial and averaging those values within each participant. Older adults had significantly larger TEPRs in terms of magnitude (M older = 2.04 standardized units, M younger = 1.59 standardized units, t(117) = 5.59, p < .001), but their TEPRs peaked significantly later than younger adults M older = 744.71 ms, M younger = 615.88 ms, t(114) = 9.73, p < .001; see Figure 7).
Figure 7.
Average task-evoked pupillary responses by age group. Older adults exhibited larger but later-peaking pupillary responses. Shaded error bars represent +/− one standard error.
Our next analysis compared the time-on-task dynamics in TEPRs (both peak and latency). The averages are plotted by block and age group in Figure 8. Regarding TEPR magnitude, there was a significant main effect of age group (b = 0.21, SE = 0.04, p < .001), a significant main effect of block (b = −0.04, SE = 0.01, p < .001), and a significant block x age group interaction (b = 0.21, SE = 0.04, p < .001). Although younger adults showed a significant reduction in TEPR magnitude across blocks (b = −0.09, SE = 0.02, p < .001), older adults did not (b = 0.002, SE = 0.01, p = 0.89). This is consistent with the fact that younger adults showed significant slowing of RTs across blocks, but older adults did not. Regarding latency, the model revealed a significant main effect of age group, such that older adults’ TEPRs peaked later than younger adults’ (b = 61.08, SE = 6.88, p < .001), a significant main effect of block, such that TEPRs tended to peak later as the task progressed (b = 4.28, SE = 1.59, p = 0.009), but no significant block x age group interaction (b = −0.63, SE = 1.59, p = 0.69).
Figure 8.
A) Average task-evoked pupillary responses by block among younger adults, B) Average task-evoked pupillary response by block among older adults, C) Peak of task-evoked response by block and age group, D) Latency of task-evoked response by block and age group. Error bars represent +/− one standard error.
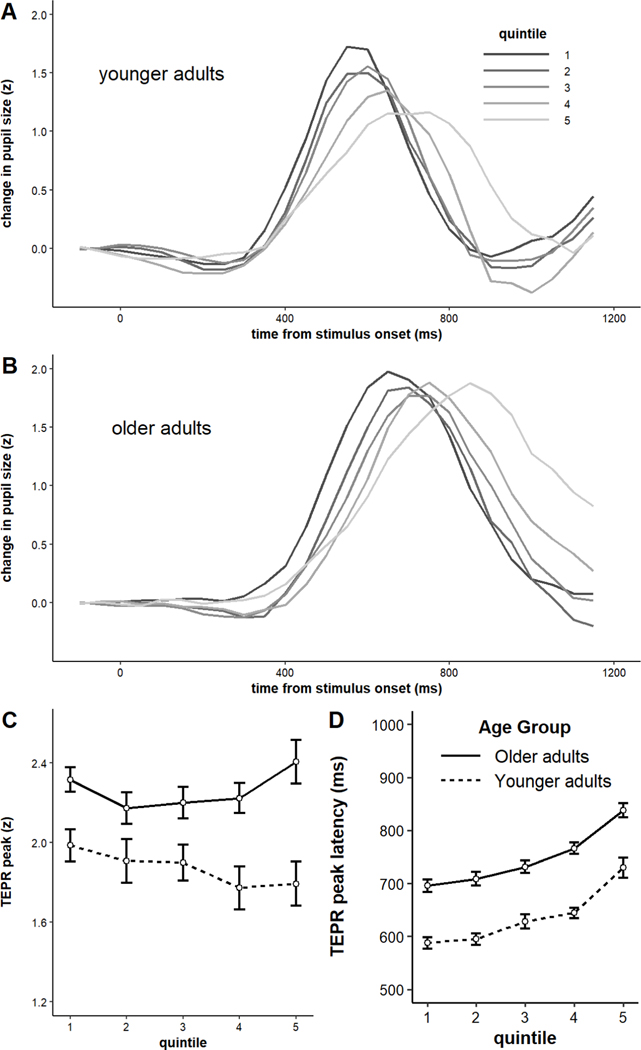
Next, we examined how the TEPRs differed on particularly fast trials vs. particularly slow trials for younger and older adults. This was a novel, albeit exploratory, analysis. But it allowed us to assess whether faster RTs were accompanied by larger and/or earlier-peaking TEPRS? Is the pattern preserved across age groups, or different? The TEPR waveforms are plotted by speed quintile in Figure 9. Younger adults’ TEPRs seemed to be both shallower and later-peaking on slower trials. Older adults, although showing longer latencies on slower trials, did not appear to have shallower TEPRs on slower trials. To examine this statistically, we repeated the analysis performed above on peak and latency by block, but with speed quintile as the independent variable rather than block. The model on TEPR magnitude revealed a small, non-significant main effect of quintile (b = −0.01, SE = 0.02, p = 0.44), a significant main effect of age (b = 0.20, SE = 0.04, p < .001), and a small and marginally significant quintile x age group interaction (b = 0.04, SE = 0.02, p = 0.05). Running the model separately on younger and older adults revealed that whereas older adults show a non-significant positive effect of quintile on TEPR magnitudes (b = 0.02, SE = 0.03, p = 0.36), younger adults showed a small and non-significant negative effect (b = −0.05, SE = 0.03, p = 0.07) (see Figure 9C). The model on latencies revealed a main effect of age (b = 53.31, SE = 6.18, p < .001) and a main effect of quintile (b = 34.46, SE = 2.67, p < .001), such that TEPR latencies increased for slower RTs. There was not a significant age group x quintile interaction on latency (b = 0.70, SE = 2.67, p = 0.79), so both younger and older adults showed roughly equal effects (see Figure 9D). To our knowledge, these results are the first to demonstrate significant changes in both TEPR magnitude and latency when comparing fast and slow trials with identical demands.
Figure 9.
A) Average task-evoked pupillary responses by speed quintile among younger adults, B) Average task-evoked pupillary response by block among older adults, C) Peak of task-evoked response by speed quintile and age group, D) Latency of task-evoked response by speed quintile and age group. Error bars represent +/− one standard error.
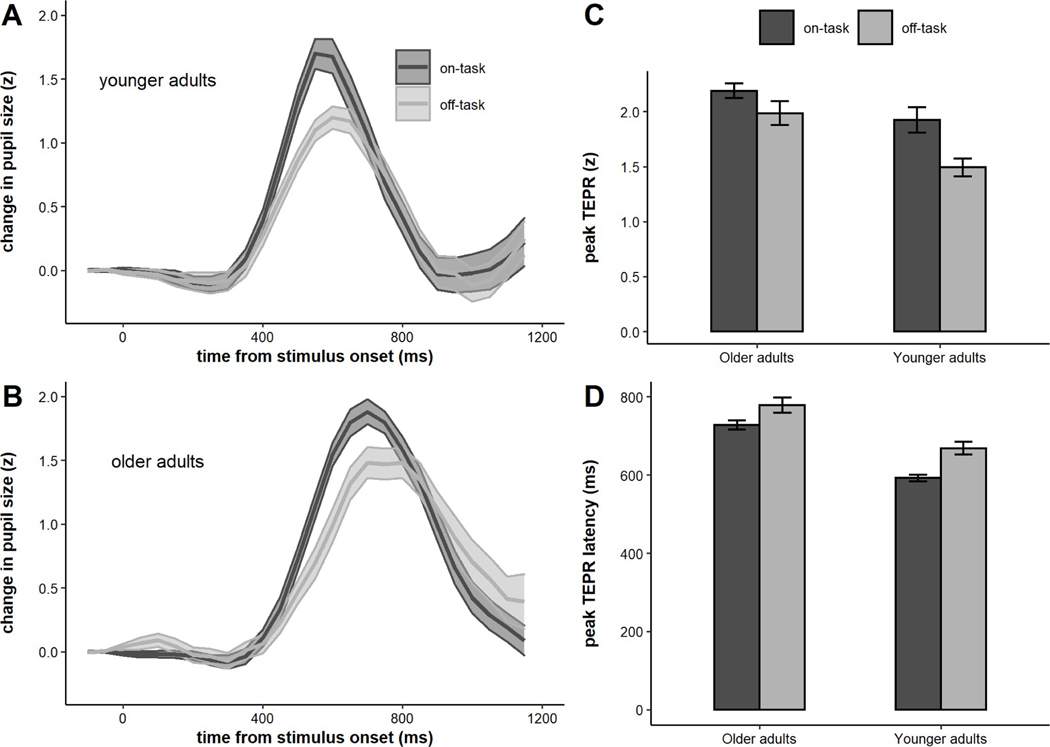
Our final set of analyses involving TEPRs separately examined TEPRs during trials preceding on-task and TUT reports (i.e., mind-wandering, external distraction, mind-blanking). The respective waveforms for younger and older adults are plotted in Figure 10. We submitted the average peak and latency of the TEPR for each participant to a linear mixed model with fixed effects for age group and report (on vs. off). There was a significant difference for on- vs. off-task reports on peaks (b = 0.16, SE = 0.05, p < .001), but there was no significant report x age group interaction (b = −0.06, SE = 0.05, p = 0.22). Thus, both age groups showed shallowed task-evoked responses on trials where they reported being off-task, and the magnitude of this effect did not differ across age groups. There was also a significant difference between the latency of the TEPRs for on- and off-task trials (b = −32.24, SE = 5.69, p < .001), yet no age group x report interaction (b = 7.06, SE = 5.69, p = 0.22). On-task trials were accompanied by significantly earlier-peaking TEPRs in both age groups, and the magnitude of this effect did not differ across age groups. This pattern replicates prior studies showing significant differences in TEPR magnitude during on- and off-task attentional states (Unsworth & Robison, 2016, 2017b, 2018). However, to our knowledge it is the first to show significant changes in TEPR latency during off-task attentional states.
Figure 10.
A) Average task-evoked pupillary responses for on- and off-task trials for older adults, B) Average task-evoked pupillary response for on- and off-task trials older adults, C) Peaks of task-evoked response for on- and off-task trials by age group, D) Latencies of task-evoked responses for on- and off-task trials by age group. Error bars represent +/− one standard error.
Thought probe responses
Next we analyzed thought probe responses by age group and block. On-task responses significantly decreased across blocks (b = −0.07, SE = 0.007, p < .001), and older adults reported more on-task thoughts than younger adults (b = 0.12, SE = 0.02, p < .001). The block x age group interaction was not significant (b = 0.004, SE = 0.007, p = 0.60). There was no significant age group difference in reports of task-related interference (b = 0.02, SE = 0.02, p = 0.28), and these reports did not significantly change across blocks (b = −0.009, SE = 0.008, p = 0.24). The block x age group interaction was modest in size but did not reach significance (b = 0.01, SE = 0.008, p = 0.07). Reports of external distraction were rare, and there was no difference between age groups (b = −0.02, SE = 0.010, p = 0.12), but they did significantly increase across time (b = 0.01, SE = 0.004, p = 0.003). There was no block x age group interaction (b = 0.00004, SE = 0.004, p = 0.99). Mind-wandering significantly increased across blocks (b = 0.02, SE = 0.005, p < .001), and older adults reported significantly less mind-wandering than younger adults (b = −0.05, SE = 0.01, p < .001). However there was not a significant block x age group interaction on mind-wandering (b = 0.001, SE = 0.005, p = 0.84). Finally, the model on mind-blanking reports indicated a significant main effect of block (b = 0.05, SE = 0.005, p < .001), such that mind-blanking increased across time, a significant main effect of age, such that older adults reported significantly less mind-blanking than younger adults (b = −0.06, SE = 0.01, p < .001), and also a significant block x age group interaction, such that younger adults showed a steeper increase in mind-blanking across time than older adults (b = −0.02, SE = 0.005, p < .001).
Post-task self-report scales
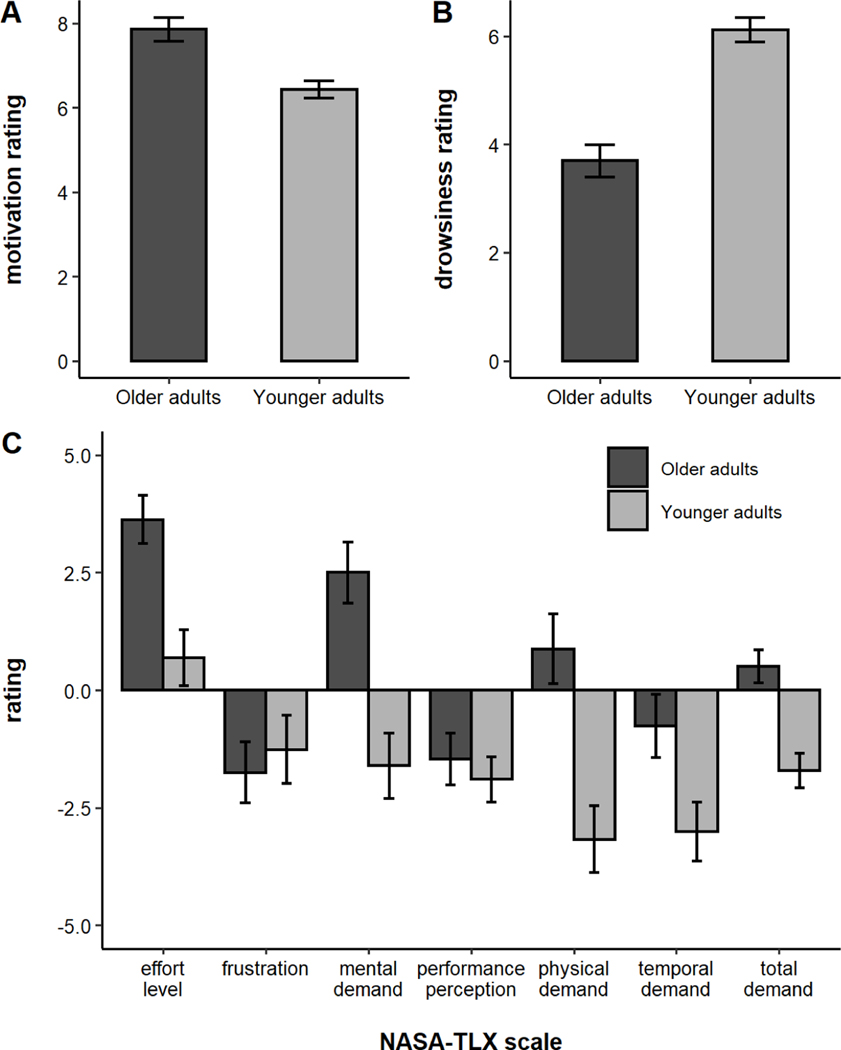
The average self-report ratings are plotted by age group in Figure 3. Older adults reported being significantly more motivated than younger adults (t(120) = 4.11, p < .001) and significantly less drowsy (t(120) = −6.39, p < .001). Overall, older adults rated the PVT as more demanding (t(120) = 4.40, p < .001). On the subscales, older adults rated the PVT as more mentally demanding (t(120) = 4.31, p < .001), more physically demanding (t(120) = 3.93, p < .001), more temporally demanding (t(120) = 2.43, p = 0.02), and requiring more effort (t(120) = 3.74, p < .001). younger and older adults did not differ on how frustrating they found the task (t(120) = −0.50, p = 0.62), nor on how well they felt they performed on the task (t(120) = 0.59, p = 0.56).
Figure 3.
A) Motivation ratings, B) Drowsiness ratings, and C) NASA-TLX workload ratings by age group. Error bars represent +/− one standard error.
Mediation of age-related differences in sustained attention
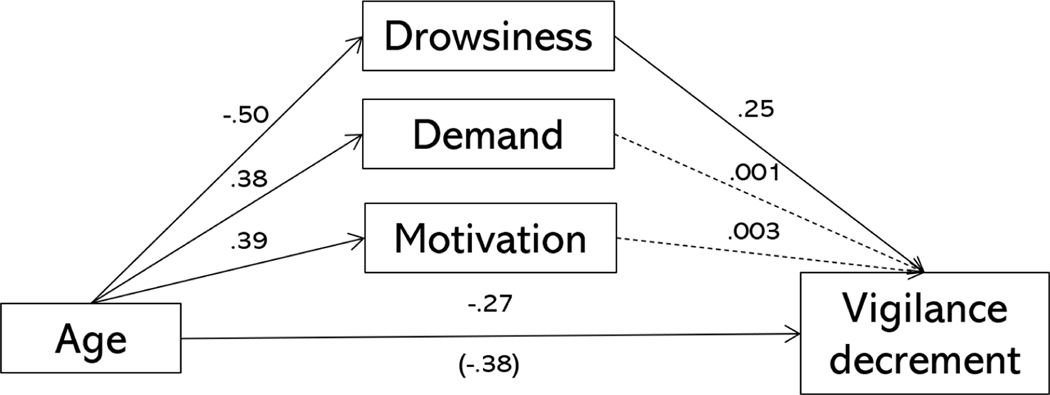
Older adults showed no vigilance decrement in the current study, which is also rather paradoxical given age-related differences in attention. To examine whether this was due to differences in motivation, drowsiness, and perceptions of task demand, we estimated individual-level vigilance decrements using a linear mixed effect model. Then, we used these estimates in a model where age was set to have indirect effects on the vigilance decrement via self-reports of motivation, drowsiness, and task demand. In this case, there were no significant indirect effects (motivation: b = 0.001, p = 0.97, 95% CI = [−0.07, 0.07]; drowsiness: b = 0.13, p = 0.01, 95% CI = [0.03, 0.24]; demand: b = 0.001, p = 0.99, 95% CI = [−0.06, 0.07]). After accounting for these three subjective measures, there was still a significant direct effect of age group on the vigilance decrement (b = −0.27, p = 0.009, 95% CI = [−0.47, −0.07]). Thus, the subjective reports could not fully explain why older adults did not show a vigilance decrement.
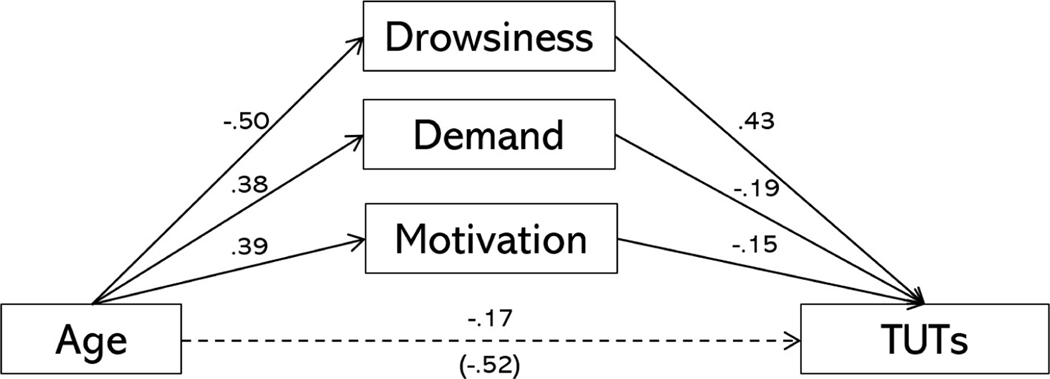
Next, to examine whether these same subjective factors accounted for the age-TUT relation, we specified a model in which age had a direct effect on TUTs and indirect effects through drowsiness, motivation, and demand. The resulting model is depicted in Figure 4. Both demand (indirect effect: b = −0.07, p = 0.02, 95% CI = [−0.14, −0.009]) and drowsiness (indirect effect: b = −0.21, p < .001, 95% CI = [−0.31, −0.12]) significantly mediated the effect of age on TUT, and there was no longer a significant direct path between age and TUT (b = −0.17, p = 0.06, 95% CI = [−0.34, 0.009]). The mediating effect of motivation was not quite significant (indirect effect: b = −0.06, p = 0.06, 95% CI = [−0.12, 0.003]). Therefore, drowsiness, demand, and motivation fully mediated the relation between age and TUT.
Figure 4.
Mediation model in which age was allowed to have a direct and indirect effects on task-unrelated thoughts (TUTs) via motivation, drowsiness, and perceptions of task demand. Solid lines indicate significant paths at p < .05, dashed lines represent non-significant paths. Age is treated as a binary variable (older adults = 1, younger adults = 0) in this model.
Individual differences
Because the present study was designed to examine age-related differences and not individual differences, the sample size was smaller than is typically desired for analyses of individual differences. However, we ran some exploratory analyses examining individual and age-related differences in pupil size and dynamics, sustained attention, and subjective reports. The individual age groups had 60 and 62 participants, respectively. So the correlations within each age group should be interpreted with a degree of caution. Table 3 lists the correlations among dependent variables in the older and younger adults separately.
Table 3.
Correlations among dependent variables in older and younger adult samples
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1. Mean RT | - | .63* | −.25 | .15 | .43* | −.10 | .13 | −.45* | .13 |
| 2. Vigilance decrement | .40* | - | −.08 | .29 | .32 | −.16 | .21 | −.17 | .20 |
| 3. Motivation | .17 | .02 | - | −.32 | −.58* | .07 | −.16 | .32 | .01 |
| 4. Drowsiness | .25 | .19 | .05 | - | .40* | .07 | .28 | −.34 | −.08 |
| 5. Task-unrelated thoughts | .25 | .25* | .05 | .41* | - | −.08 | .19 | −.48* | −.07 |
| 6. Pretrial pupil mean | −.04 | .17 | −.31* | .01 | .15 | - | .49* | −.10 | .00 |
| 7. Pretrial pupil SD | .20 | .24 | −.09 | .25 | .20 | .49* | - | −.25 | .09 |
| 8. TEPR magnitude | −.29* | −.20 | .01 | −.31* | −.07 | −.03 | −.38* | - | .38 |
| 9. TEPR latency | .67* | .31* | .10 | .15 | .19 | −.02 | .26 | −.06 | - |
Note. Correlations among the older adults (N = 62) are listed below the diagonal, and correlations among the younger adults (N = 60) are listed above the diagonal. SD = standard deviation, TEPR = task-evoked pupillary response, asterisks indicate significant correlations at p < .05.
Discussion
The present study indicated that healthy older adults had intact, if not superior, sustained attention compared to younger adults. This finding was present when examining RTs across time, the dynamics of TEPRs across time, and self-reports of TUTs across time.
The processing speed account of cognitive aging predicts that older adults should be slower than younger adults in the PVT. However, older adults were only about 48 ms slower than younger adults on average. When looking at segments of the RT distributions, differences between younger and older adults were observed primarily in slower trials. So although older adults were only slightly slower than younger adults overall, when older adults responded slowly, they tended to respond particularly slowly. These results are consistent with prior work that measures of intraindividual variability and distributional analyses are often a better differentiator of younger and older adults, and older adults with and without Alzheimer’s and dementia, than mean or median RTs (Balota & Yap, 2011; Duchek et al., 2009; Hultsch, MacDonald, & Dixon, 2002; Hultsch, MacDonald, Hunter, Levy-Bencheton, & Strauss, 2000; McAuley, Yap, Christ, & White, 2006; Spieler, Balota, & Faust, 1996; Tse, Balota, Yap, Duchek, & McCabe, 2010; West, Murphy, Armilio, Craik, & Stuss, 2002).
Despite this finding, older adults showed superior sustained attention compared to younger adults. Specifically, older adults did not show a vigilance decrement in RT, a pattern that has been repeatedly observed in younger adults in the PVT (Massar et al., 2016, 2019b; Robison et al., 2021; Unsworth & Robison, 2016, 2020). The younger adults did indeed show a large vigilance decrement, as is typical. The data were also consistent with prior work showing similar patterns in both traditional vigilance tasks (Tomporowski & Tinsley, 1996), and go/no-go tasks like the SART (Brache et al., 2010; Staub et al., 2014a, 2014b, 2015; Vallesi et al., 2021). The present results cannot resolve the discrepancies observed between go/no-go tasks and other cognitive control tasks. That is, older adults tend to show more Stroop interference and reduced proactive control in continuous performance tasks, (e.g, AX-CPT; Braver, Satpute, Rush, Racine, & Barch, 2005; Bugg, 2014; Paxton, Barch, Storandt, & Braver, 2006; Vallesi et al., 2021), but they show fewer no-go errors in the SART (Vallesi et al., 2021). We used the PVT specifically because it does not involve resolving conflict or inhibiting a prepotent response. Thus we cannot fully explain the discrepancies noted above. However, the ability to sustain attention to a particular task for a long period of time appears to be one aspect of cognition that remains intact in healthy aging.
Regarding the age/mind-wandering paradox, the present results were largely consistent a subjective and dispositional account (Jackson & Balota, 2012; Krawietz et al., 2012; Moran et al., 2021; Nicosia & Balota, 2021; Seli et al., 2021). Older adults reported fewer TUTs than younger adults. Older adults also reported being significantly more motivated and alert than younger adults. However, they also reported finding the task significantly more demanding. Mediation analyses showed that accounting for these subjective differences significantly mediated the relation between age and TUT. Specifically, the older adults reported fewer TUTs because they found the task more demanding and because they felt more alert.
The pupillary results also showed several interesting differences between younger and older adults. First, older adults had significantly smaller pupil diameters than younger adults, a finding consistent with prior research (Bak et al., 2017; Birren et al., 1950; Winn et al., 1994). Across blocks, older adults showed a rather typical pattern of a decrease in pretrial pupil diameter (Massar et al., 2016, 2019b; Unsworth & Robison, 2016), but younger adults did not. Based on the behavioral data, we would have expected younger adults to also show a decrease in arousal/pretrial pupil diameter across time, but they did not. This was one finding that was particularly perplexing, and it deserves follow-up investigation. Older adults also showed less variability in pretrial pupil size than younger adults. Previously, intraindividual variability in pretrial/tonic pupil size has been used as a measure of arousal regulation, and the degree to which arousal fluctuates within an individual often correlates with their behavioral performance and how often they report TUTs (Aminihajibashi, Hagen, Andreassen, Laeng, & Espeseth, 2020; M. K. Robison & Brewer, 2020; Robison & Unsworth, 2019; Unsworth & Robison, 2015, 2017a, 2017b). The present results indicate that older adults had more regulated arousal than younger adults, which is consistent with the fact that they also reported fewer TUTs.
The discrepancy between intraindividual variability in RTs, which was larger among older adults, and intraindividual variability in pretrial pupil diameter, which was smaller among older adults, is interesting, as it presents a bit of a paradox. In younger adult samples, intraindividual variability in arousal typically correlates with worse sustained and controlled attention performance. If intraindividual variability in RT is indicative of shifting attentional state (Unsworth & Robison, 2017b, 2017a), older adults should show more variability in arousal than younger adults. The discrepancy here might be that intraindividual variability in RTs measures micro-level shifts in attentional state, lapses that occur over very brief timescales but nonetheless cause slow responding, and macro-level shifts, longer-duration shifts of attention away from the task and to totally unrelated thought streams (i.e., TUT). The fact that older adults showed more intraindividual variability in RT and slower responding in the slow tail of the distribution, but fewer TUTs, would be consistent with this account. However, it is also possible that the significantly smaller pupil diameters in older adults necessarily restricts the range of possible values, limiting the degree to which intraindividual variability can even be observed. To account for large mean differences, we also compared the intraindividual coefficient of variation across groups, and this yielded the same result as the intraindividual standard deviation. Still, this method does not fully account for the range restriction issue. Further research is necessary to disentangle these possibilities.
There were also several key age differences in TEPR dynamics. First, older adults showed larger TEPRs than younger adults. TEPRs have been used as measure of cognitive effort, and can be used to track the degree of effort exerted on a task, or the degree of cognitive demand required by a task (Alnæs et al., 2014; Beatty, 1982; Beatty & Lucero-Wagoner, 2000; Kahneman & Beatty, 1966; Kahneman & Peavler, 1969; Unsworth & Robison, 2015). The fact that older adults showed larger TEPRs is consistent with the fact that they reported greater motivation, greater alertness, and perceived the PVT as more demanding than younger adults. Second, whereas younger adults showed a shallowing of TEPRs across time, consistent with prior work using this task (Unsworth & Robison, 2016), older adults did not. The shallowing of TEPRs across time among younger adults is consistent with the fact that they also exhibited a slowing of RTs across time (i.e., a vigilance decrement). The fact that older adults did not show a shallowing of TEPRs across blocks is consistent with the fact that older adults also did not show a vigilance decrement in their RTs. It is worth noting that TEPRs mimicked changes in task performance across age groups, but pretrial pupil dynamics did not. Previously, changes in both TEPR and pretrial pupil diameter have been used as indices of sustained attention as it wanes across time (Hopstaken et al., 2015a, 2016, 2015b; Massar et al., 2019a, 2016; Unsworth & Robison, 2016). But in the present data, changes in pretrial pupil diameter did not mimic changes in performance. This is a finding that also begs replication in future work. Finally, both younger and older adults showed longer RTs and shallower TEPRs preceding reports of being off-task compared to being on-task. However, these effects did not differ across age groups.
We performed several novel analyses on the TEPRs. Although other studies have shown important TEPR latency differences in other tasks (e.g., Diede & Bugg, 2017; Paivio & Simpson, 1968; Richer & Beatty, 1987), and prior work has noted within- and between-subject differences in TEPR magnitude (Massar et al., 2016, 2019b; Unsworth & Robison, 2016, 2017a, 2017b), to our knowledge no study has given careful consideration to TEPR latency in the PVT. Indeed, the present findings revealed several informative patterns regarding latency. First, older adults’ TEPRs peaked much later than younger adults’, by about 130 ms on average. Thus, it appears TEPRs can be used to characterize between-subject differences in the speed of cognitive, and perhaps neural, processes. This is consistent with a processing speed account of cognitive aging. However, it is worth noting the 130-ms discrepancy in the TEPR peaks was actually larger than the ~48-ms average RT discrepancy. Second, both younger and older adults’ TEPRs were significantly later peaking on trials with slower RTs. Thus, it appears that TEPRs can be used to measure within-person processing speed differences on a trial-by-trial basis. Finally, TEPR latency was a significant correlate of processing speed differences in both younger and older adults (see below). Collectively, these findings highlight a novel and potentially important use of the TEPR - measuring and comparing the speed of cognitive and neural processes.
Our final set of analyses examined individual differences in task performance, subjective reports, and pupillary measures. The results of these analyses demonstrated several potentially important relations. In the younger adult sample, both TEPR magnitude and TEPR latency were significant correlates of RTs. Specifically, participants who exhibited larger and earlier-peaking TEPRs tended to have shorter RTs. In the older adult sample, these same two factors were again significant correlates of RTs. However, the correlation between of TEPR latency was much stronger in the older adults, sharing for about 40% of its variance with mean RTs. While the within-group sample sizes are small for examining these types of relations, this is a large correlation that deserves replication and extension. If it is indeed the case that the speed of cognitive processes can be strongly accounted for by the speed of a physiological response, this could provide a mechanistic explanation for processing speed differences with age.
The present results might also provide a glimpse into relative functioning of the LC-NE neuromodulatory system. If indeed we can measure LC functioning with pupil diameter, then we can potentially leverage pupillary measures to gain better insight into cognitive age- and AD-related cognitive deficits. The present sample of older adults were quite healthy, and reported their health as “excellent” (19%%), “good” (55%%), or “O.K.” (18%%), and most reported that their health limits their daily activities either “Not at all” (55%%) or only “a little” (31%%). Only 5%% of the older adults reported their health as “Fair” and none reported their health as “Poor.” None of the participants had any diagnosed neurological impairments. Regardless, there was a strong correlation between TEPR latency and processing speed. This opens doors for future research using pupillometry, which is low-cost, portable, non-invasive, and easy to implement, to uncover the neural mechanisms underlying processing speed changes with age.
Pupil diameter provides an indirect index of locus coeruleus-norepinephrine system (LC-NE) functioning (Joshi, Li, Kalwani, & Gold, 2016; Rajkowski, 1993; Varazzani, San-Galli, Gilardeau, & Bouret, 2015). Recently, it has been theorized that the relative integrity of the LC-NE system may be a crucial factor underlying age-related changes in cognition (Dahl et al., 2022; Mather & Harley, 2016; Robertson, 2013). Evidence for such a connection comes from both in vivo and postmortem measurements of LC integrity in association with cognitive aging and risk for Alzheimer’s disease (AD). For example, Clewett et al. (2016) used a neuromelanin-sensitive magnetic resonance imaging (MRI) contrast to assess LC neuron density in younger and older adults. Among older adults, there was a positive association between LC density and the composite cognitive reserve score. In postmortem examinations of 165 brains from participants in the Rush Memory and Aging Project, Wilson et al. (2013) measured neuronal density in the substantia nigra, ventral tegmental area, dorsal raphe nucleus, and the LC. Over the course of their participation in the project (about six years, on average), participants’ cognitive functioning was measured annually with 19 tests of episodic memory, working memory, semantic memory, perceptual speed, and visuospatial ability. LC neuron density had a significant protective effect on cognitive decline, even after accounting for neuronal density in the other brain regions of interest. Finally, Dahl et al. (2022) recently showed a relation between in vivo and post mortem LC integrity and cognitive decline in older adults with and without AD. Collectively, these data suggest that LC integrity may be a protective factor in cognitive aging, and may prevent Alzheimer’s-related symptoms.
While obviously very indirect, it is possible that age- and individual differences in TEPR latency measure an important neural mechanism, like the speed of NE delivery to from LC to cortex. However, the LC-NE system is not the only neuromodulatory system that affects pupil diameter, as it also coheres with cholinergic system activity over longer timescales (Reimer et al., 2016). It is also possible that the latency of pupillary responses may indicate differences in neural myelination, which has been hypothesized as a mechanism underlying age-related changes in processing speed (Bartzokis, 2004, 2011; Lu et al., 2011, 2013). If we can use the latency of the TEPR to measure the speed of neural processing, then we can potentially use this measure as an indicator of cognitive decline. Future research should replicate the present design in samples of healthy older adults, older adults with MCIs, and older adults with AD diagnoses. Further, it could be used in combination with more direct measures like MRI to examine whether it does indeed measure an important neural difference, or something different like degeneration of the pupil dilator muscle.
A final implication for this study is one that addresses a more general phenomenon under debate in cognitive psychology - what is the cause of vigilance decrements? Some theories argue that vigilance depletes a limited pool of mental resources, and when this pool of resources declines, performance decrements occur (Smit, Eling, & Coenen, 2004; Warm, Parasuraman, & Matthews, 2008). Other theories argue that a tradeoff occurs between the resources devoted to a task and the resources devoted to other cognitive operations, like mind-wandering, based on a perception of the costs of task completion (effort) and the rewards associated with continuing the task (Kurzban, Duckworth, Kable, & Myers, 2013; Thomson, Besner, & Smilek, 2015). In the present study, the fact that older adults did not show a vigilance decrement and did not show a shallowing of TEPRs across time indicates that, when people are sufficiently motivated and engaged with a task, the vigilance decrement is not an obligatory phenomenon. In fact, the older adults reported the task being even more demanding than younger adults. According to resource theories, more demanding tasks should deplete resources more quickly, causing steeper vigilance decrements. The opposite was true here, as older adults did not show a vigilance decrement despite finding the task more demanding. Thus, the present results also have important implications for how we interpret the vigilance decrement and what causes it. Rather than having a physiological derivation that is a result of repeated use of a limited pool of resources, the present results indicate that the vigilance decrement is caused by other factors. However, those factors are still a bit unclear, given we were unable to fully mediate the association between age and the vigilance decrement with measures of motivation, alertness, and task demands.
Limitations
The present study had several limitations which deserve mention. First, the differential sampling methodology does produce a confounding effect of intrinsic motivation. The younger adults participated in the study to fulfill a course requirement, whereas the older adults participated in exchange for money. The older adults thus elected to participate in the study, whereas the younger adults were required to participate either in this study, another study, or write summaries of research articles to fulfill their requirement. Some studies have shown that differential recruitment methods can lead to different patterns of performance in vigilance and memory tasks (e.g., Tomporowski, Simpson, & Hager, 1993). However, at least one study has shown that this does not fully account for age differences in sustained attention (Tomporowski & Tinsley, 1996). Further, the self-selection into the study by the older adult sample may have driven the high ratings for motivation. The fact that older adults reported greater motivation may not indicate that older adults feel more intrinsically motivated to sustain attention, in general, but the participants who self-selected into this study felt motivated to do well. Additionally, we only inquired about motivation after the task, which may be biased by self-perceptions of performance, and does not account for dynamic shifts of in motivation across time (e.g., Robison et al., 2021; Hopstaken et al., 2015a). Another limitation of the study is that we only had one measure of sustained attention. Part of this decision was intentional, as we wanted to have a longer task (~30 min), which produced a robust vigilance decrement and produced stereotypical pupillary responses in younger adults. However, this prevented us from generalizing to other typical sustained attention tasks (e.g., SART), and estimating age differences at a construct level.
Conclusions
The present study indicated that older adults showed superior sustained attention compared to younger adults. Specifically, older adults did not show a vigilance decrement in a 30-minute task that produces a robust decrement among younger adults. The RT data also indicated that processing speed differences were largely due to the slowest RTs. When older adults reacted slowly, they tended to react particularly slowly. Older adults also reported fewer task-unrelated thoughts (mind-wandering and mind-blanking), and exhibited both larger and more stable task-evoked pupillary responses across time, despite slower-peaking pupillary responses. The pupillary data also suggested that the latency of task-evoked pupillary responses was a strong correlate of individual differences in processing speed, particularly among older adults. Finally, the fact that older adults did not show a vigilance decrement, nor a shallowing of task-evoked pupillary responses across time, casts doubt on resource theories of vigilance, and suggests the vigilance decrement is driven by other factors. In sum, the ability to sustain attention to a task over a long period of time is preserved in healthy cognitive aging.
Figure 5.
Mediation model in which age was allowed to have a direct and indirect effects on the vigilance decrement via motivation, drowsiness, and perceptions of task demand. Standardized parameter estimates are shown. Solid lines indicate significant paths at p < .05, dashed lines represent non-significant paths. Age is treated as a binary variable (older adults = 1, younger adults = 0) in this model.
Public significance:
Although many cognitive abilities tend to decline with advanced age, one ability that remains intact is sustained attention, our ability to maintain focus on a specific task for an extended period of time. Using a combination of reaction time measures and measurement of pupil diameter, the present study demonstrated that older adults can sustain their attention even better than younger adults, despite delays in processing speed. Older adults also expressed feeling more motivated to perform well on the task, finding the task more cognitively demanding, and they did not mind-wander as much as younger adults. However, these differences did not totally account for why older adults sustained their attention longer than younger adults.
Acknowledgments
We would like to thank Jeff Shi and Dillon Seals for their assistance with data collection. This research was supported by National Institute of Aging Grant T32AG000030-40, awarded to Julie M. Bugg / Washington University in St. Louis. This work was also supported by funds provided by the McDonnell Center for Systems Neuroscience at Washington University in St. Louis. Portions of these data were presented in a poster atthe Dallas Aging and Cognition Conference (February, 2022).
All data and analysis code are publicly available on the Open Science Framework (https://osf.io/63wcj/).
Footnotes
This pattern of results is nearly identical if using coefficient of variation rather than standard deviation as a measure of intraindividual variability.
References
- Alnæs D, Sneve MH, Espeseth T, Endestad T, Pavert SHP van de, & Laeng B. (2014). Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. Journal of Vision, 14, 1–1. 10.1167/14.4.1 [DOI] [PubMed] [Google Scholar]
- Aminihajibashi S, Hagen T, Andreassen OA, Laeng B, & Espeseth T. (2020). The effects of cognitive abilities and task demands on tonic and phasic pupil sizes. Biological Psychology, 156, 107945. 10.1016/j.biopsycho.2020.107945 [DOI] [PubMed] [Google Scholar]
- Aust F, & Barth M. (2018). papaja: Create APA manuscripts with R Markdown. Retrieved from https://github.com/crsh/papaja
- Bak E, Yoo YJ, Yang HK, & Hwang JM (2017). Quantitative pupillometry of the pupillary light reflex in koreans. Journal of the Korean Ophthalmological Society, 58 (6), 712–717. 10.3341/jkos.2017.58.6.712 [DOI] [Google Scholar]
- Balota DA, & Yap MJ (2011). Moving beyond the mean in studies of mental chronometry: The power of response time distributional analyses. Current Directions in Psychological Science, 20 (3), 160–166. 10.1177/963721411408885 [DOI] [Google Scholar]
- Bartzokis G. (2004). Age-related myelin breakdown: A developmental model of cognitive decline and alzheimer’s disease. Neurobiology of Aging, 25 (1), 5–18. 10.1016/j.neurobiolaging.2003.03.001 [DOI] [PubMed] [Google Scholar]
- Bartzokis G. (2011). Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiology of Aging, 32 (8), 1341–1371. 10.1016/j.neurobiolaging.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty J. (1982). Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin, 91 (2), 276–292. 10.1037/0033-2909.91.2.276 [DOI] [PubMed] [Google Scholar]
- Beatty J, & Lucero-Wagoner B. (2000). The pupillary system. In Cacioppo JT, Tassinary LG, & Bernston GG (Eds.), Handbook of psychophysiology (Vol. 2, pp. 142–162). Cambridge University Press. [Google Scholar]
- Birren JE, Casperson RC, & Botwinick J. (1950). Age changes in pupil size. Journal of Gerontology, 5 (3), 216–221. 10.1093/geronj/5.3.216 [DOI] [PubMed] [Google Scholar]
- Brache K, Scialfa C, & Hudson C. (2010). Aging and vigilance: Who has the inhibition deficit? Experimental Aging Research, 36 (2), 140–152. 10.1080/03610731003613425 [DOI] [PubMed] [Google Scholar]
- Braver TS, Satpute AB, Rush BK, Racine CA, & Barch DM (2005). Context processing and context maintenance in healthy aging and early stage dementia of the alzheimer’s type. Psychology and Aging, 20 (1), 33–46. 10.1037/0882-7974.20.1.33 [DOI] [PubMed] [Google Scholar]
- Bugg JM (2014). Conflict-triggered top-down control: Default mode, last resort, or no such thing? Journal of Experimental Psychology: Learning, Memory, and Cognition, 40 (2), 567. 10.1037/a0035032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett DV, Lee T-H, Greening S, Ponzio A, Margalit E, & Mather M. (2016). Neuromelanin marks the spot: Identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiology of Aging, 37, 117–126. 10.1016/j.neurobiolaging.2015.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, & Byrd M. (1982). Aging and cognitive deficits. In Craik FI & Trehub S. (Eds.), Aging and cognitive processes (pp. 191–211). Springer. 10.1007/978-1-4684-4178-9 [DOI] [Google Scholar]
- Dahl MJ, Mather M, Werkle-Bergner M, Kennedy BL, Guzman S, Hurth K, Miller CA, Qiao Y, Shi Y, Chui HC, & Ringman JM (2022). Locus coeruleus integrity is related to tau burden and memory loss in autosomal-dominant Alzheimer’s disease. Neurobiology of Aging, 112, 39–54. 10.1016/j.neurobiolaging.2021.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton JE, & Parasuraman R. (1993). Sensory and cognitive vigilance: Effects of age on performance and subjective workload. Human Performance, 6 (1), 71–97. 10.1207/s15327043hup0601_4 [DOI] [Google Scholar]
- Diede NT, & Bugg JM (2017). Cognitive effort is modulated outside of the explicit awareness of conflict frequency: Evidence from pupillometry. Journal of Experimental Psychology: Learning, Memory, and Cognition, 43 (5), 824–835. 10.1037/xlm0000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diede NT, Gyurkovics M, Nicosia J, Diede A, & Bugg JM (2022). The effect of context on mind-wandering in younger and older adults. Consciousness and Cognition, 97, 103256. 10.1016/j.concog.2021.103256 [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Tse C-S, Holtzman DM, Fagan AM, & Goate AM (2009). The utility of intraindividual variability in selective attention tasks as an early marker for alzheimer’s disease. Neuropsychology, 23 (6), 746. 10.1037/a0016583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein GO, & Mcdaniel MA (1997). Aging and mind wandering: Reduced inhibition in older adults? Experimental Aging Research, 23 (4), 343–354. 10.1080/03610739708254035 [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, Dermen D, & Harman HH (1976). Manual for kit of factor-referenced cognitive tests (Vol. 102). Educational Testing Service, Princeton, NJ. [Google Scholar]
- Frank DJ, Nara B, Zavagnin M, Touron DR, & Kane MJ (2015). Validating older adults’ reports of less mind-wandering: An examination of eye movements and dispositional influences. Psychology and Aging, 30 (2), 266–278. 10.1037/pag0000031 [DOI] [PubMed] [Google Scholar]
- Giambra LM (1973). Daydreaming in males from seventeen to seventy-seven: A preliminary report. In Proceedings of the Annual Convention of the American Psychological Association (pp. 769–770). American Psychological Association. [Google Scholar]
- Giambra LM (1989). Task-unrelated thought frequency as a function of age: A laboratory study. Psychology and Aging, 4 (2), 136–143. [DOI] [PubMed] [Google Scholar]
- Giambra LM (2000). Daydreaming characteristics across the life-span: Age differences and seven to twenty year longitudinal changes. In Kunzedorf RG & Wallace B. (Eds.), Individual Differences in Conscious Experience (pp. 147–206). John Benjamins Amsterdam. 10.1075/aicr.20 [DOI] [Google Scholar]
- Hambrick DZ, Salthouse TA, & Meinz EJ (1999). Predictors of crossword puzzle proficiency and moderators of age–cognition relations. Journal of Experimental Psychology: General, 128 (2), 131–164. 10.1037/0096-3445.128.2.131 [DOI] [PubMed] [Google Scholar]
- Hart SG, & Staveland LE (1988). Development of nasa-tlx (task load index): Results of empirical and theoretical research. In Hancock PA & Meshkati N. (Eds.), Advances in Psychology; (Vol. 52, pp. 139–183). Elsevier. [Google Scholar]
- Hasher L, Lustig C, & Zacks R. (2007). Inhibitory mechanisms and the control of attention. In Conway ARA, Jarrold C, Kane MJ, Miyake A, & Towse JN (Eds.) Variation in working memory (pp. 227–249). Oxford University Press. 10.1093/acprof:oso/9780195168648.003.0009 [DOI] [Google Scholar]
- Hasher L, & Zacks RT (1988). Working memory, comprehension, and aging: A review and a new view. In Bower G. (Ed.), Psychology of Learning and Motivation (Vol. 22,pp. 193–225). Elsevier. 10.1016/S0079-7421(08)60041-9 [DOI] [Google Scholar]
- Hess EH, & Polt JM (1964). Pupil size in relation to mental activity during simple problem-solving. Science, 143 (3611), 1190–1192. 10.1126/science.143.3611.1190 [DOI] [PubMed] [Google Scholar]
- Hopstaken JF, Linden D. van der, Bakker AB, & Kompier MA (2015a). The window of my eyes: Task disengagement and mental fatigue covary with pupil dynamics. Biological Psychology, 110, 100–106. 10.1016/j.biopsycho.2015.06.013 [DOI] [PubMed] [Google Scholar]
- Hopstaken JF, Linden D. van der, Bakker AB, Kompier MA, & Leung YK(2016). Shifts in attention during mental fatigue: Evidence from subjective, behavioral, physiological, and eye-tracking data. Journal of Experimental Psychology: Human Perception and Performance, 42 (6), 878–889. 10.1037/xhp0000189 [DOI] [PubMed] [Google Scholar]
- Hopstaken JF, Van Der Linden D, Bakker AB, & Kompier MA (2015b). A multifaceted investigation of the link between mental fatigue and task disengagement. Psychophysiology, 52 (3), 305–315. 10.1111/psyp.12339 [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, & Dixon RA (2002). Variability in reaction time performance of younger and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57 (2), P101–P115. 10.1093/geronb/57.2.P101 [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, Hunter MA, Levy-Bencheton J, & Strauss E. (2000). Intraindividual variability in cognitive performance in older adults: Comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology, 14 (4), 588–598. 10.1037/0894-4105.14.4.588 [DOI] [PubMed] [Google Scholar]
- Jackson JD, & Balota DA (2012). Mind-wandering in younger and older adults: Converging evidence from the sustained attention to response task and reading for comprehension. Psychology and Aging, 27 (1), 106–119. 10.1037/a0023933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JD, Weinstein Y, & Balota DA (2013). Can mind-wandering be timeless? Atemporal focus and aging in mind-wandering paradigms. Frontiers in Psychology, 4, 742. 10.3389/fpsyg.2013.00742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerison HJ, & Pickett RM (1964). Vigilance: The importance of the elicited observing rate. Science, 143 (3609), 970–971. 10.1126/science.143.3609.970 [DOI] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani RM, & Gold JI (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron, 89, 221–234. 10.1016/j.neuron.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, & Beatty J. (1966). Pupil diameter and load on memory. Science, 154, 1583–1585. 10.1126/science.154.3756.1583 [DOI] [PubMed] [Google Scholar]
- Kahneman D, & Peavler WS (1969). Incentive effects and pupillary changes in association learning. Journal of Experimental Psychology, 79 (2p1), 312–318. 10.1037/h0026912 [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, & Kwapil TR (2007). For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science, 18, 614–621. 10.1111/j.1467-9280.2007.01948.x [DOI] [PubMed] [Google Scholar]
- Kane MJ, Meier ME, Smeekens BA, Gross GM, Chun CA, Silvia PJ, & Kwapil TR (2016). Individual differences in the executive control of attention, memory, and thought, and their associations with schizotypy. Journal of Experimental Psychology: General, 145 (8), 1017–1048. 10.1037/xge0000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawietz SA, Tamplin AK, & Radvansky GA (2012). Aging and mind wandering during text comprehension. Psychology and Aging, 27 (4), 951–958. 10.1037/a0028831 [DOI] [PubMed] [Google Scholar]
- Kurzban R, Duckworth A, Kable JW, & Myers J. (2013). An opportunity cost model of subjective effort and task performance. Behavioral and Brain Sciences, 36 (6), 661–679. 10.1017/S0140525X12003196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82 (13), 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Laeng B, Ørbo M, Holmlund T, & Miozzo M. (2011). Pupillary Stroop effects. Cognitive Processing, 12 (1), 13–21. 10.1007/s10339-010-0370-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH, Lee GJ, Raven EP, Tingus K, Khoo T, Thompson PM, & Bartzokis G. (2011). Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. Journal of Clinical and Experimental Neuropsychology, 33 (10), 1059–1068. 10.1080/13803395.2011.595397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH, Lee GJ, Tishler TA, Meghpara M, Thompson PM, & Bartzokis G. (2013). Myelin breakdown mediates age-related slowing in cognitive processing speed in healthy elderly men. Brain and Cognition, 81 (1), 131–138. 10.1016/j.bandc.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Massar SA, Lim J, & Huettel SA (2019a). Sleep deprivation, effort allocation and performance. In Dongen Hans Van WPA , Hinson JM, Honn KA, & Chee MW (Eds.), Progress in brain research (Vol. 246, pp. 1–26). Elsevier. 10.1016/bs.pbr.2019.03.007 [DOI] [PubMed] [Google Scholar]
- Massar SA, Lim J, Sasmita K, & Chee MW (2016). Rewards boost sustained attention through higher effort: A value-based decision making approach. Biological Psychology, 120, 21–27. 10.1016/j.biopsycho.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Massar SA, Lim J, Sasmita K, & Chee MW (2019b). Sleep deprivation increases the costs of attentional effort: Performance, preference and pupil size. Neuropsychologia, 123, 169–177. 10.1016/j.neuropsychologia.2018.03.032 [DOI] [PubMed] [Google Scholar]
- Mather M, & Harley CW (2016). The locus coeruleus: Essential for maintaining cognitive function and the aging brain. Trends in Cognitive Sciences, 20 (3), 214–226. 10.1016/j.tics.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley T, Yap M, Christ SE, & White DA (2006). Revisiting inhibitory control across the life span: Insights from the ex-gaussian distribution. Developmental Neuropsychology, 29 (3), 447–458. 10.1207/s15326942dn2903_4 [DOI] [PubMed] [Google Scholar]
- McVay JC, & Kane MJ (2009). Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35 (1), 196. 10.1037/a0014104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, & Kane MJ (2010). Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008). Psychological Bulletin, 136 (2), 188–197. 10.1037/a0018298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, & Kane MJ (2012a). Drifting from slow to “d’oh!”: Working memory capacity and mind wandering predict extreme reaction times and executive control errors. Journal of Experimental Psychology: Learning, Memory, and Cognition, 38 (3), 525–549. 10.1037/a0025896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, & Kane MJ (2012b). Why does working memory capacity predict variation in reading comprehension? On the influence of mind wandering and executive attention. Journal of Experimental Psychology: General, 141, 302. 10.1037/a0025250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Meier ME, Touron DR, & Kane MJ (2013). Aging ebbs the flow of thought: Adult age differences in mind wandering, executive control, and self-evaluation. Acta Psychologica, 142 (1), 136–147. 10.1016/j.actpsy.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minear M, & Shah P. (2008). Training and transfer effects in task switching. Memory & Cognition, 36 (8), 1470–1483. 10.3758/MC.336.8.1470 [DOI] [PubMed] [Google Scholar]
- Moran CN, McGovern DP, Warren G, Grálaigh RÓ, Kenney JP, Smeaton A, & Dockree PM (2021). Young and restless, old and focused: Age-differences in mind-wandering frequency and phenomenology. Psychology and Aging, 36 (2), 252–267. 10.1037/pag0000526 [DOI] [PubMed] [Google Scholar]
- Mrazek MD, Smallwood J, Franklin MS, Chin JM, Baird B, & Schooler JW (2012). The role of mind-wandering in measurements of general aptitude. Journal of Experimental Psychology: General, 141 (4), 788–798. 10.1037/a0027968 [DOI] [PubMed] [Google Scholar]
- Nicosia J, & Balota D. (2021). Dispositional factors account for age differences in self-reported mind-wandering. Psychology and Aging, 36 (4), 421–432. 10.1037/pag0000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivio A, & Simpson HM (1968). Magnitude and latency of the pupillary response during an imagery task as a function of stimulus abstractness and imagery ability. Psychonomic Science, 12 (2), 45–46. 10.3758/BF03331181 [DOI] [Google Scholar]
- Parasuraman R. (1979). Memory load and event rate control sensitivity decrements in sustained attention. Science, 205 (4409), 924–927. 10.1126/science.472714 [DOI] [PubMed] [Google Scholar]
- Parasuraman R, & Mouloua M. (1987). Interaction of signal discriminability and task type in vigilance decrement. Perception & Psychophysics, 41 (1), 17–22. 10.3758/BF03208208 [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Nestor PG, & Greenwood P. (1989). Sustained-attention capacity in young and older adults. Psychology and Aging, 4 (3), 339. 10.1037/0882-7974.4.3.339 [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Storandt M, & Braver TS (2006). Effects of environmental support and strategy training on older adults’ use of context. Psychology and Aging, 21 (3), 499–509. 10.1037/0882-7974.21.3.499 [DOI] [PubMed] [Google Scholar]
- Porter G, Tales A, Troscianko T, Wilcock G, Haworth J, & Leonards U. (2010). New insights into feature and conjunction search: I. Evidence from pupil size, eye movements and ageing. Cortex, 46 (5), 621–636. [DOI] [PubMed] [Google Scholar]
- Rajkowski J. (1993). Correlations between locus coeruleus (LC) neural activity, pupil diameter and behavior in monkey support a role of lc in attention. Soc. Neurosc., Abstract, Washington, DC, 1993. [Google Scholar]
- Raven JC, Raven JC, & Court JH (1962). Advanced progressive matrices. HK Lewis; London. [Google Scholar]
- Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, McCormick DA, & Tolias AS (2016). Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nature Communications, 7 (1), 1–7. 10.1038/ncomms13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W. (2018). Psych: Procedures for psychological, psychometric, and personality research. Evanston, Illinois: Northwestern University. Retrieved from https://CRAN.R-project.org/package=psych [Google Scholar]
- Richer F, & Beatty J. (1987). Contrasting effects of response uncertainty on the task-evoked pupillary response and reaction time. Psychophysiology, 24 (3), 258–262. 10.1111/j.1469-8986.1987.tb00291.x [DOI] [PubMed] [Google Scholar]
- Robertson IH (2013). A noradrenergic theory of cognitive reserve: Implications for Alzheimer’s disease. Neurobiology of Aging, 34 (1), 298–308. 10.1016/j.neurobiolaging.2012.05.019 [DOI] [PubMed] [Google Scholar]
- Robison MK (2018). Regulating mind-wandering and sustained attention with goal-setting, feedback, and incentives on sustained attention. Doctoral Dissertation. [Google Scholar]
- Robison MK, & Brewer GA (2020). Individual differences in working memory capacity and the regulation of arousal. Attention, Perception, & Psychophysics, 82, 3273–3290. 10.3758/s13414-020-02077-0 [DOI] [PubMed] [Google Scholar]
- Robison MK, Gath KI, & Unsworth N. (2017). The neurotic wandering mind: An individual differences investigation of neuroticism, mind-wandering, and executive control. The Quarterly Journal of Experimental Psychology, 70 (4), 649–663. 10.1080/17470218.2016.1145706 [DOI] [PubMed] [Google Scholar]
- Robison MK, Miller AL, & Unsworth N. (2020). A multi-faceted approach to understanding individual differences in mind-wandering. Cognition, 198, 104078. 10.1016/j.cognition.2019.104078 [DOI] [PubMed] [Google Scholar]
- Robison MK, & Unsworth N. (2015). Working memory capacity offers resistance to mind-wandering and external distraction in a context-specific manner. Applied Cognitive Psychology, 29 (5), 680–690. 10.1002/acp.3150 [DOI] [Google Scholar]
- Robison MK, & Unsworth N. (2019). Pupillometry tracks fluctuations in working memory performance. Attention, Perception, & Psychophysics, 81, 407–419. 10.3758/s13414-018-1618-4 [DOI] [PubMed] [Google Scholar]
- Robison MK, Unsworth N, & Brewer GA (2021). Examining the effects of goal-setting, feedback, and incentives on sustained attention. Journal of Experimental Psychology: Human Perception and Performance, 47 (6), 869–891. 10.1037/xhp0000926 [DOI] [PubMed] [Google Scholar]
- Rosseel Y. (2012). lavaan: An R package for structural equation modeling. Journal of Statistical Software, 48, 1–36. Retrieved from http://www.jstatsoft.org/v48/i02/ [Google Scholar]
- Rummel J, & Boywitt CD (2014). Controlling the stream of thought: Working memory capacity predicts adjustment of mind-wandering to situational demands. Psychonomic Bulletin & Review, 21, 1309–1315. 10.3758/s13423-013-0580-3 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1993). Attentional blocks are not responsible for age-related slowing. Journal of Gerontology, 48 (6), 263–270. 10.1093/geronj/48.6.P263 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103 (3), 403–428. 10.1037/0033-295X.103.3.403 [DOI] [PubMed] [Google Scholar]
- Seli P, O’Neill K, Carriere JS, Smilek D, Beaty RE, & Schacter DL (2021). Mind-wandering across the age gap: Age-related differences in mind-wandering are partially attributable to age-related differences in motivation. The Journals of Gerontology: Series B, 76 (7), 1264–1271. 10.1093/geronb/gbaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shake MC, Shulley LJ, & Soto-Freita AM (2016). Effects of individual differences and situational features on age differences in mindless reading. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 71 (5), 808–820. 10.1093/geronb/gbv012 [DOI] [PubMed] [Google Scholar]
- Simon JR (1990). The effects of an irrelevant directional cue on human information processing. In Proctor RW & Reeve TG (Eds.), Advances in psychology (Vol. 65, pp. 31–86). Elsevier. [Google Scholar]
- Smallwood J, & Schooler JW (2006). The restless mind. Psychological Bulletin, 132 (6), 946–958. 10.1037/0033-2909.132.6.946 [DOI] [PubMed] [Google Scholar]
- Smit AS, Eling PA, & Coenen AM (2004). Mental effort causes vigilance decrease due to resource depletion. Acta Psychologica, 115 (1), 35–42. 10.1016/j.actpsy.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, & Faust ME (1996). Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. Journal of Experimental Psychology: Human Perception and Performance, 22 (2), 461–479. 10.1037/0096-1523.22.2.461 [DOI] [PubMed] [Google Scholar]
- Staub B, Doignon-Camus N, Bacon E, & Bonnefond A. (2014a). Investigating sustained attention ability in the elderly by using two different approaches: Inhibiting ongoing behavior versus responding on rare occasions. Acta Psychologica, 146, 51–57. 10.1016/j.actpsy.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Staub B, Doignon-Camus N, Bacon É, & Bonnefond A. (2014b). The effects of aging on sustained attention ability: An ERP study. Psychology and Aging, 29 (3), 684–695. 10.1037/a0037067 [DOI] [PubMed] [Google Scholar]
- Staub B, Doignon-Camus N, Després O, & Bonnefond A. (2013). Sustained attention in the elderly: What do we know and what does it tell us about cognitive aging? Ageing Research Reviews, 12 (2), 459–468. 10.1016/j.arr.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Staub B, Doignon-Camus N, Marques-Carneiro JE, Bacon E, & Bonnefond A. (2015). Age-related differences in the use of automatic and controlled processes in a situation of sustained attention. Neuropsychologia, 75, 607–616. 10.1016/j.neuropsychologia.2015.07.021 [DOI] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18 (6), 643–662. 10.1037/h0054651 [DOI] [Google Scholar]
- Thomson DR, Besner D, & Smilek D. (2015). A resource-control account of sustained attention: Evidence from mind-wandering and vigilance paradigms. Perspectives on Psychological Science, 10 (1), 82–96. 10.1177/1745691614556681 [DOI] [PubMed] [Google Scholar]
- Thurstone LL (1938). Primary mental abilities (Vol. 119). University of Chicago Press; Chicago. [Google Scholar]
- Tomporowski PD, Simpson RG, & Hager L. (1993). Method of recruiting subjects and performance on cognitive tests. The American Journal of Psychology, 106 (4), 499–521. 10.2307/1422966 [DOI] [PubMed] [Google Scholar]
- Tomporowski PD, & Tinsley VF (1996). Effects of memory demand and motivation on sustained attention in young and older adults. The American Journal of Psychology, 187–204. 10.2307/1423272 [DOI] [PubMed] [Google Scholar]
- Tse C-S, Balota DA, Yap MJ, Duchek JM, & McCabe DP (2010). Effects of healthy aging and early stage dementia of the Alzheimer’s type on components of response time distributions in three attention tasks. Neuropsychology, 24 (3), 300. 10.1037/a0018274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, & McMillan BD (2013). Mind wandering and reading comprehension: Examining the roles of working memory capacity, interest, motivation, and topic experience. Journal of Experimental Psychology: Learning, Memory, and Cognition, 39 (3), 832–842. 10.1037/a0029669 [DOI] [PubMed] [Google Scholar]
- Unsworth N, & McMillan BD (2014). Similarities and differences between mind-wandering and external distraction: A latent variable analysis of lapses of attention and their relation to cognitive abilities. Acta Psychologica, 150, 14–25. 10.1016/j.actpsy.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Unsworth N, McMillan BD, Brewer GA, & Spillers GJ (2012). Everyday attention failures: An individual differences investigation. Journal of Experimental Psychology: Learning, Memory, and Cognition, 38 (6), 1765–1772. 10.1037/a0028075 [DOI] [PubMed] [Google Scholar]
- Unsworth N, & Robison MK (2015). Individual differences in the allocation of attention to items in working memory: Evidence from pupillometry. Psychonomic Bulletin & Review, 22 (3), 757–765. 10.3758/s13423-014-0747-6 [DOI] [PubMed] [Google Scholar]
- Unsworth N, & Robison MK (2016). Pupillary correlates of lapses of sustained attention. Cognitive, Affective, & Behavioral Neuroscience, 16, 601–615. 10.3758/s13415-016-0417-4 [DOI] [PubMed] [Google Scholar]
- Unsworth N, & Robison MK (2017a). A locus coeruleus-norepinephrine account of individual differences in working memory capacity and attention control. Psychonomic Bulletin & Review, 24, 1282–1311. 10.3758/s13423-016-1220-5 [DOI] [PubMed] [Google Scholar]
- Unsworth N, & Robison MK (2017b). The importance of arousal for variation in working memory capacity and attention control: A latent variable pupillometry study. Journal of Experimental Psychology: Learning, Memory, and Cognition, 43, 1962–1987. 10.1037/xlm0000421 [DOI] [PubMed] [Google Scholar]
- Unsworth N, & Robison MK (2018). Tracking arousal state and mind wandering with pupillometry. Cognitive, Affective, & Behavioral Neuroscience, 18, 638–664. 10.3758/s13415-018-0594-4 [DOI] [PubMed] [Google Scholar]
- Unsworth N, & Robison MK (2020). Working memory capacity and sustained attention: A cognitive-energetic perspective. Journal of Experimental Psychology: Learning, Memory, and Cognition, 46 (1), 77–103. 10.1037/xlm0000712 [DOI] [PubMed] [Google Scholar]
- Unsworth N, Robison MK, & Miller AL (2021). Individual differences in lapses of attention: A latent variable analysis. Journal of Experimental Psychology: General, 150 (7), 1303–1331. 10.1037/xge0000998 [DOI] [PubMed] [Google Scholar]
- Vallesi A, Tronelli V, Lomi F, & Pezzetta R. (2021). Age differences in sustained attention tasks: A meta-analysis. Psychonomic Bulletin & Review, 28, 1755–1775. 10.3758/s13423-021-01908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varazzani C, San-Galli A, Gilardeau S, & Bouret S. (2015). Noradrenaline and dopamine neurons in the reward/effort trade-off: A direct electrophysiological comparison in behaving monkeys. Journal of Neuroscience, 35 (20), 7866–7877. 10.1523/JNEUROSCI.0454-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warm JS, Parasuraman R, & Matthews G. (2008). Vigilance requires hard mental work and is stressful. Human Factors, 50 (3), 433–441. 10.1518/001872008X312152 [DOI] [PubMed] [Google Scholar]
- Wel P. van der, & Steenbergen van H. (2018). Pupil dilation as an index of effort in cognitive control tasks: A review. Psychonomic Bulletin & Review, 25 (6), 2005–2015. 10.3758/s13423-018-1432-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Murphy KJ, Armilio ML, Craik FI, & Stuss DT (2002). Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and Cognition, 49 (3), 402–419. 10.1006/brcg.2001.1507 [DOI] [PubMed] [Google Scholar]
- Wickham H. (2017). Tidyverse: Easily install and load the ‘tidyverse’. Retrieved from https://CRAN.R-project.org/package=tidyverse
- Wilke CO (2020). Cowplot: Streamlined plot theme and plot annotations for ‘ggplot2’. Retrieved from https://CRAN.R-project.org/package=cowplot
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Schneider JA, & Bennett DA (2013). Neural reserve, neuronal density in the locus ceruleus, and cognitivedecline. Neurology, 80 (13), 1202–1208. 10.1212/WNL.0b013e3182897103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn B, Whitaker D, Elliott DB, & Phillips NJ (1994). Factors affecting light-adapted pupil size in normal human subjects. Investigative Ophthalmology & Visual Science, 35 (3), 1132–1137. [PubMed] [Google Scholar]
- Zavagnin M, Borella E, & De Beni R. (2014). When the mind wanders: Age-related differences between young and older adults. Acta Psychologica, 145, 54–64. 10.1016/j.actpsy.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Zekveld AA, Kramer SE, & Festen JM (2011). Cognitive load during speech perception in noise: The influence of age, hearing loss, and cognition on the pupil response. Ear and Hearing, 32 (4), 498–510. 10.1097/AUD.0b013e31820512bb [DOI] [PubMed] [Google Scholar]