Abstract
The purpose of this investigation was to determine the effect of different relative pressures of blood flow restriction (BFR) on muscle oxygen saturation (SmO2) while walking at 3.0 mph (4.83 kph). Fifteen physically active healthy adults performed seven 5-minute stages of walking at 3.0 mph with a blood flow restriction cuff applied to the proximal portion of the left or right leg while bilateral SmO2 changes were measured using near infra-red spectroscopy (NIRS) on the medial head of the gastrocnemius (GM) and vastus lateralis (VL) muscles. Other measurements including heart rate (HR), blood pressure (BP), rating of perceived exertion (RPE), and ground contact time balance (GCTB) were also collected. SmO2 measurements were analyzed using two-way repeated measures (RM) ANOVA while other measurements were analyzed using one-way RM ANOVA. We observed a significant main effect of LOP% (limb occlusion pressure) on the difference in total area of desaturation that occurred during each occlusion stage (ADS), p < 0.0001 η2 = .336, early ΔSmO2, p < 0.0001 in both the GAS η2 = .132 and VL η2 = .335. The results suggest that there are significant differences in SmO2 desaturation between 40%, 80%, and 100% LOP. Our findings suggest that incremental increases in LOP will bring about greater SmO2 desaturation during walking and may therefore induce a larger adaptive response on the muscles. However, increased LOP% may intensify perception of effort.
Keywords: Blood flow restriction, oxygen saturation, limb occlusion pressure
INTRODUCTION
Blood flow restriction (BFR) is a clinical practice of applying pressurized tourniquet cuffs to the proximal portion of the upper or lower limbs during various exercise modes. Pneumatic cuffs are pressurized to partially restrict arterial inflow and fully restrict venous outflow in working musculature (27). The utilization of BFR combined with both resistance (22, 23, 33) and cardiovascular (32) training can help stimulate favorable adaptations such as muscle hypertrophy and strength gains (1, 11, 28) and improved glucose uptake (5). The major benefit of BFR is its use in low load exercise especially when traditional or high load exercise is not recommended, not practical, or contraindicated (12). By creating an ischemic environment during exercise, BFR can augment the low load exercise by increasing the metabolic demand of the muscle (34).
The use of BFR has been a supplemental training tool for clinicians and performance professionals to provide favorable muscle stress on individuals in a rehabilitative setting. The development of an optimal training program with BFR should provide an adequate stimulus while also minimizing both risk and perceived discomfort. Despite a large body of research on BFR, insight on associated mechanisms and their relative contributions in mediating both acute and potentially chronic physiological effects is still needed. One proposed mechanism of BFR includes acute ischemia leading to increased metabolic stress in skeletal muscles as a result of the external compression of vasculature (21, 34, 39). For example, by restricting venous outflow a previous investigation observed significant increases in the inorganic phosphate (Pi)-to-phosphocreatine (PCr) ratio during low-load BFR plantar flexion exercise compared to a control group performing the exercise condition without BFR (39). Another investigation observed increased lactate and carbon dioxide production (VCO2) in a group performing low-intensity interval cycling at 80% LOP compared to a control group (36). Ischemia of skeletal muscle and surrounding tissues may be one of several mechanisms mediating the positive vascular and muscle adaptations observed from prior BFR research. Measurements of muscle oxygen desaturation invoked by BFR exercise have been used as an indicator of intramuscular metabolic stress (10, 18). Near-infrared spectroscopy is a non-invasive method to measure relative muscle oxygenation in the capillaries and tissue of targeted skeletal muscle (2). Additionally, the use of NIRS to measure muscle deoxygenation has great agreement with measurement of intracellular energetics by P-31 MRS and provides useful information regarding the energy demands of the muscle (31).
Cuff pressure used for BFR is a main determinant that is commonly determined by assessing an individual’s limb occlusion pressure (LOP) at rest in order to limit risk and increase objectivity (20). Limb occlusion pressure is a measurement of the minimum pressure required for 100% occlusion of arterial blood flow in a limb at rest (37) and can be measured through several techniques. An exercise prescription model for continuous or interval exercise recommends cuff pressures at 40%–80% LOP (27). One investigation reported cardiovascular exercise with BFR at moderate intensities to cause increases in heart rate (HR), blood pressure (BP), and ratings of perceived exertion (RPE) compared to without BFR (32). Higher cuff pressures have also shown to cause a greater perception of discomfort compared to that of lower pressures (23). It may be optimal to select external cuff pressures for BFR walking that maximize muscle desaturation in a desired limb without inducing unpleasant cardiovascular or perceptual responses.
Inducing acute skeletal muscle ischemia from BFR has been suggested to be an important driver for metabolic stress in a targeted limb (3, 17, 28, 35). Studies have utilized near-infrared spectroscopy (NIRS) to provide data on the concentration or oxygenation of light-absorbing molecules such as hemoglobin (Hb) and myoglobin (Mb) in skeletal muscle while performing BFR exercise in order to provide indirect insight on localized tissue perfusion (3, 4, 6, 21, 24). Relative pressures between 60%–80% of limb occlusion pressure (LOP) have been used to maintain a hypoxic environment as shown in prior research (9, 13, 14). Cuff pressures between 60%–80% LOP while performing low-load BFR handgrip (16) and knee extension (30) exercises have been shown to produce comparable decline in tissue oxygenation. However, the relationship between cuff pressure and skeletal muscle deoxygenation from BFR walking at recommended occlusion pressures for cardiovascular exercise (27) has not been investigated. Therefore, the purpose of this investigation was to compare the effects different LOP might have on skeletal muscle oxygen saturation, HR, BP, and RPE while walking on a treadmill at 3.0 mph.
METHODS
Participants
This study collected data on 19 physically active healthy adult participants (7 males, 12 females), 19–46 years of age. Inclusion criteria for participants included: being physically active (≥ 3 days/week of at least 30 minutes of moderate-intensity physical activity) and between the ages of 18–64 years old. Four participants were excluded from the final analysis. Three participants were excluded due to a body fat % > 35% and one was excluded due to multiple signal dropouts in two of the NIRS monitors. An a priori power analysis was done using G*Power 3.1.9.7 (Düsseldorf, Germany) for within group main effects of repeated measures ANOVA using and effect size of 0.4 to achieve 85% power. The resulting analysis indicated a sample size of 14 was needed to achieve power.
Table 1 displays descriptive characteristics for participants included in the final analysis.
Table 1.
Summary of Participants (n = 15) Descriptive Outcomes
| Characteristics | Descriptive Outcomes (Mean ± SD) |
|---|---|
| Age | 27.7 ± 7.3 |
| Gender (Males/Females) | 7 Males/8 Females |
| Height (cm) | 168.6 ± 9.1 |
| Weight (kg) | 69.2 ± 13.0 |
| Body Fat % | 26.8 ± 7.5 |
| Occluded Limb (Right/Left) | 6 Right/9 Left |
| Resting Heart Rate (bpm) | 70.9 ± 9.2 |
| Resting Systolic Blood Pressure (mmHg) | 113.9 ± 6.0 |
| Resting Diastolic Blood Pressure (mmHg) | 69.7 ± 9.2 |
Note. SD: Standard Deviation; cm: Centimeter; kg: Kilogram; bpm: Beats Per Minute; mmHg; millimeter of mercury = unit of pressure
Informed consent was obtained from all participants in accordance with the Sam Houston State University Institutional Review Board (IRB) which approved the study IRB-2020-256. Additionally, this research was carried out fully in accordance with the ethical standards of the International Journal of Exercise Science (26). A health history questionnaire (HHQ) was administered to participants after signing the informed consent. This information determined participant eligibility based on both inclusion and exclusion criteria.
Protocol
A Delfi Personalized Tourniquet System for BFR unit (Delfi Medical Innovations Inc., Vancouver, BC) was used to measure participant’s LOP, manipulate cuff pressure based on LOP measurement, and to perform lower-limb bilateral calibration. Four Moxy monitors (Fortiori Design LLC, Hutchinson, MN) measured local muscle oxygen saturation (SmO2) and total heme (tHb) bilaterally on the medial head of the gastrocnemius and vastus lateralis muscles at the positions recommended by (29). A Garmin Running Dynamics Pod (Garmin Ltd., Olathe, KS) was clipped onto the participant’s waistband (on backside) prior to exercise testing. This device allowed assessors to view and analyze walking/running metrics real-time. Data regarding ground contact balance (GCTB) was collected from the Garmin Pod. Participants wrapped a Garmin chest heart rate (HR) strap (Garmin Ltd., Olathe, KS) around their chest to record HR. A Welch Allyn trigger aneroid sphygmomanometer (Welch Allyn Inc., Skaneateles Falls, NY) and 3M Littmann Classic II S.E. stethoscope (3M Littman, Saint Paul, MN) was used to manually assess blood pressure (BP). Participant’s height, weight, and body fat percentage were measured using a stadiometer and SECA mBCA 514 bioelectrical impedance scale (SECA, Hamburg, Germany). A Cosmed T170 Treadmill (Cosmed, Rome, Italy) was used for walking during exercise testing. A poster of a 10-point RPE scale was posted in front of the treadmill for participants to see.
Data from the Moxy and Garmin devices was wirelessly collected to Perfpro software (Hartware Technologies, Rockford MI) using a laptop computer during the exercise protocol. This software connects to wireless ANT+ enabled devices. This allowed the assessors to view SmO2, tHb, HR, GCTB metrics, and other data in real-time from one source.
This study involved participants performing a walking protocol with a BFR cuff applied to the proximal portion of the left or right leg while changes in muscle oxygen saturation were measured using near infra-red spectroscopy (NIRS) in gastrocnemius and vastus lateralis muscles. Blood flow restriction cuff placement on the participant’s right or left upper leg as well as the order of occlusion pressure was randomized. Other measurements including HR, BP, rated perceived exertion (RPE), and GCTB were also measured during testing. This study also used a self-reported health history questionnaire as an intake for health history, physical activity levels, and medication use. Participants completed testing in one visit that lasted approximately 1.5–1.75 hours in length.
Participants who arrived for testing had their forehead temperature taken and completed a COVID-19 symptom-related questionnaire. A seated resting BP was taken manually on the side determined to be occluded during the walking protocol.
While lying in a prone position on a cushioned table, LOP was determined automatically using the Delfi BFR unit. Once LOP was determined, the base of the BFR cuff was wrapped with microspore tape and flex wrap to prevent the cuff from falling while not inflated during portions of the exercise protocol. Moxy monitor placement on the vastus lateralis muscles were positioned on a reference line 9.4 cm from the superior lateral side of the patella to the anterior superior iliac spine (29). Monitor placement on the gastrocnemius muscles were placed approximately 50%–60% of the distance along a reference line that began from the Achilles tendon insertion to the medial side of the popliteus cavity. Sites were traced with a non-permanent marker before securing the monitors with micropore tape and flex wrap. Participants warmed-up by walking on a treadmill for 5–10 minutes at 2.0 mph. The exercise protocol immediately followed the warm-up. Exercise testing involved walking continuously on a treadmill for seven 5-minute stages at 3.0 mph while the BFR cuff pressure during each stage was manipulated. Cuff pressures for stages 1, 3, 5, and 7 were at 0% LOP. Cuff pressure order for stages 2, 4, and 6 were randomized between 40%, 80%, and 100% LOP. Data from the Moxy monitors, HR monitor, and Dynamic Pod were continuously monitored. During exercise testing, the same author took all BP readings to limit interobserver variability. A second assessor monitored data on PerfPro, asked for RPE, and recorded data at each stage. Verbal feedback regarding GCTB was given if and when the L/R GCTB % deviated below 45% or above 55%. RPE, BP, and HR were recorded at 3 and 5 minutes of each stage.
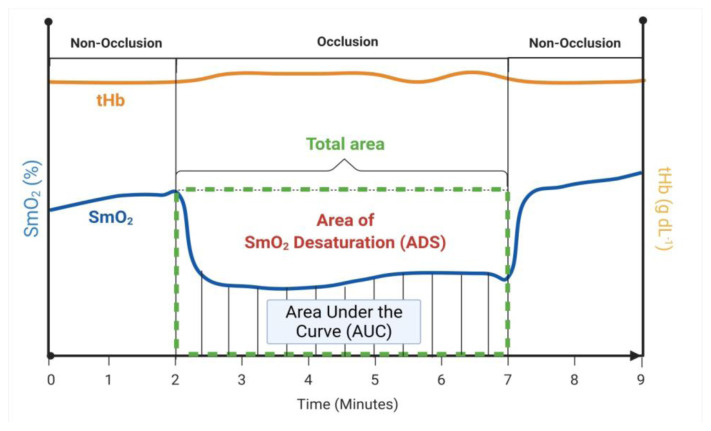
Near infrared spectroscopy data was analyzed three separate ways: (a) area of desaturation (ADS), (b) initial change (Δ) in SmO2 and tHb, and (c) final change (Δ) in SmO2 and tHb. These analyses were performed on both BFR and control (CON) limbs. The ADS analyses were used to approximate the total area of SmO2 desaturation during each occlusion stage and illustrated in Figure 1. The purpose of the ADS calculation was to quantify the strain placed on the muscles during each occlusion stage. The ADS was calculated through the following steps in both the BFR and CON limbs:
Figure 1.
Area of SmO2 Desaturation (ADS).
Illustration of calculations for the total area of SmO2, area under the SmO2 curve (AUC), and area of SmO2 desaturation (ADC) during each occlusion stage.
Starting SmO2 for each occlusion stage was determined by averaging SmO2 five seconds prior to the start of each occlusion stage.
The starting SmO2 was then multiplied by the time difference of the first- and final-time interval of each occlusion stage to approximate the total area.
The trapezoidal rule was used to approximate the area underneath the SmO2 curve (AUC).
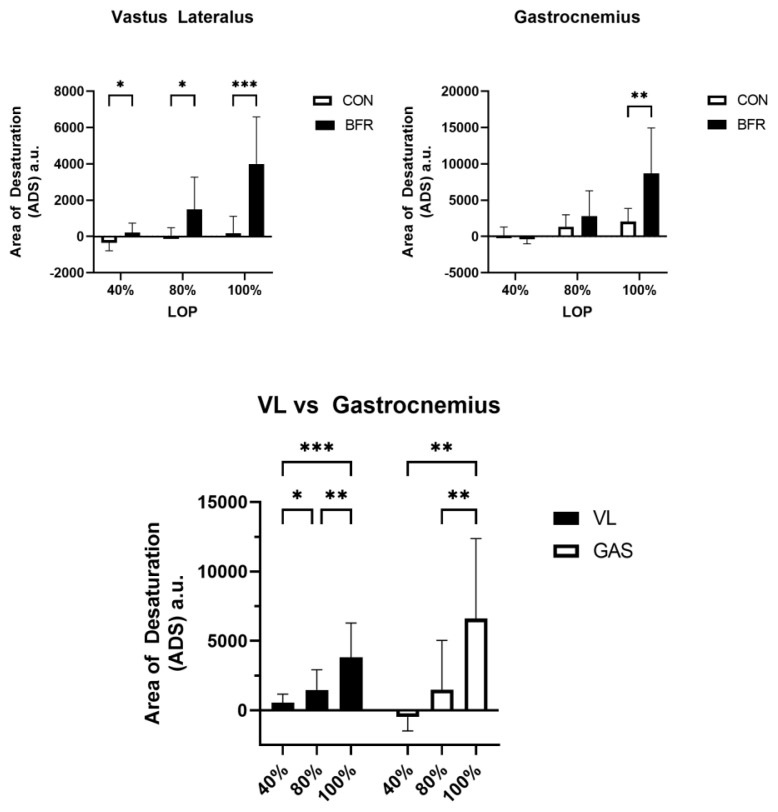
ADS for each muscle was calculated by subtracting the AUC from the total area and assigned an arbitrary unit (a.u.). These values were calculated and reported for both BFR and CON limbs in figure 3 a & b.
Finally, in order to make comparisons between the VL and GAS, the differences in BFR and CON limbs were calculated by subtracting CON data from BFR values. The resulting ADS values are reported in figure 3c.
Figure 3.
Area of SmO2 Desaturation (ADS) Between LOP% and Muscles
Comparison of the area of SmO2 desaturation (ADS) for each LOP% stage and muscles. Data shown as means ± standard deviation. Significant main effect of LOP% on ADS p < 0.0001. Asterisks indicate significant pairwise comparisons * p < 0.05, ** p < 0.01, *** p < 0.001
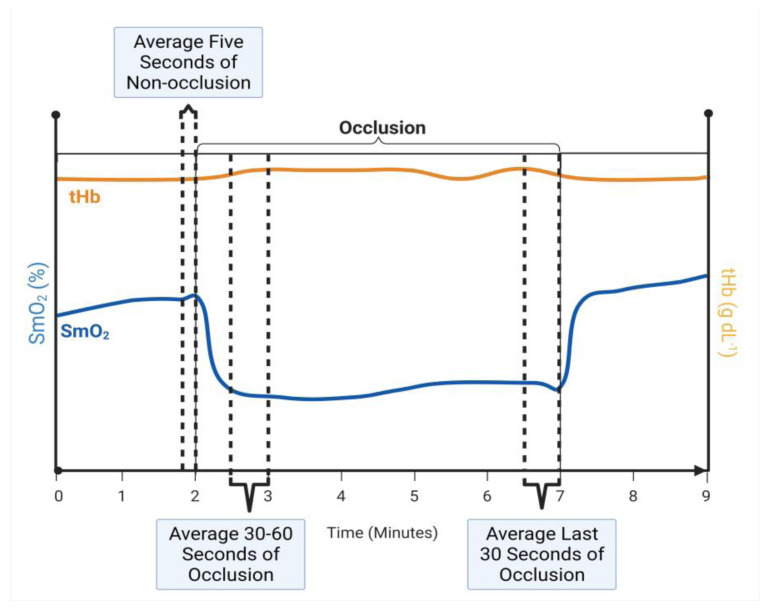
To capture some of the change over time during the occlusion period, we calculated both the early and late ΔSmO2 and ΔtHb for each occlusion stage. The purpose of these calculations was to quantify changes in muscle oxygenation after the first 30 seconds of occlusion and during the final 30 seconds of occlusion. We calculated ΔSmO2 and ΔtHb through the following calculations and is illustrated in figure 2.
Figure 2.
Initial and Final Change in SmO2 and tHb
Illustration of calculations for the averages for the last 5 seconds of the non-occlusion stage, 30–60 seconds of each occlusion stage, and last 30 seconds of the occlusion stage. These averages were calculated for both SmO2 and tHb. The average SmO2 and tHb during both the early or late 30 second interval of each occlusion stage was subtracted from the average SmO2 and tHb 5-seconds prior to each occlusion stage (Stages 1, 3, and 5).
The average SmO2 and tHb 5 seconds prior to each occlusion stage was determined in both the BFR and CON limbs.
The average SmO2 and tHb during seconds 30–60 and final 30 seconds of each occlusion stage was determined in both the BFR and CON limbs.
Early and late ΔSmO2 and ΔtHb were calculated by subtracting the values obtained in step 2 from the respective values obtained in step 1.
Finally, to control for background noise the CON values were subtracted from the BFR values.
Ground contact balance deviation values represent the mean % deviation from the occluded limb. For example, a 52% left-ground contact balance with right leg occlusion would represent a 4% deviation (52% left – 48% right) in favor of the non-occluded leg while walking. Negative SmO2 and tHb values in the analysis represent a positive increase from baseline comparisons. Negative GCTB values represent the % deviation in favor of the occluded limb.
Limitations for this study include population demographics and data collection methods. The population demographics were limited to physically active healthy adults to ensure participants were prepared to meet the physical demands of exercise testing and to also minimize risk. This limits how results can be generalized to clinical populations. While NIRS is both non-invasive and cost effective for the purpose of investigating local muscle oxygenation, adipose tissue thickness at the sites of measurement is another limitation that may affect measurements according one review (15). Intensities of cardiovascular exercise with BFR are generally low in nature (24) but are not standardized. A walking speed of 3.0 mph is realistic to a physically active healthy adult and is within the light-moderate intensity for walking.
Statistical Analysis
Data collected in PerfPro was exported along with data collected by the assessors to Microsoft Excel for processing prior to being analyzed on GraphPad Prism (version 9.2.0) (GraphPad Software, San Diego, CA).
For comparison between CON and BFR limbs, a repeated measures two-way analysis of variance (ANOVA) was performed to determine the between group main effects of occlusion and within group main effects of LOP%. Sphericity was not assumed, and the Geisser-Greenhouse correction was used. A Sidak post-hoc comparison was performed to compare the effects of occlusion at each level of pressure.
For comparison of the differences in ADS between the VL and GAS two-way repeated measures ANOVA test was performed using the differences in ADS data to determine the between group main effects of muscle and within group main effects of LOP%. Sphericity was not assumed, and the Geisser-Greenhouse correction was used. A Tukey post hoc test was performed to compare ADS of both muscles.
Two-way repeated measures ANOVA tests were performed to compare early vs late ΔSmO2 and ΔtHb and determine the within group main effects. A Sidak’s post hoc test was used for multiple comparisons if the within group main effect was also significant.
One-way repeated measures ANOVA tests were conducted to measure within group main effects of 5-minute HR, SBP, DBP, RPE, and GCTB data of each occlusion stage. Comparisons for HR, SBP, and DBP for each occlusion stage were compared using Tukey’s test. Significance for all statistical comparisons was determined at an alpha level of 0.05.
RESULTS
Our main hypothesis of this study was that there would be no difference in ADS between different occlusion pressures during walking. Based on our findings we reject this hypothesis as we observed a significant between group main effect of occlusion on ADS in both the VL p < 0.0001; η2 = .237; 95% CI [1214, 2666] and GAS p = 0.0066; η2 = .089; 95% CI [761, 4316] muscles (Figure 3 a & b). We also observed significant within group main effects of LOP% on ADS in both the VL p < 0.0001; η2 = .199 and GAS p < 0.0001; η2 = .280 muscles indicating that when occlusion pressure increases, so does ADS. However, when comparing the VL and the GAS muscles we did not observe a significant difference in ADS between the two muscles p = 0.587; η2 = .006 95% CI [−2241, 1045] (Figure 3c).
In both the VL and GAS, increasing LOP% (40%, 80%, 100%) resulted in an increase in ADS of 38 ± 972, 1475 ± 2668, 4865 ± 4162 a.u. respectively (grand means). Tukey’s multiple comparison test of ADS revealed (Figure 3c) a significant difference for VL 40% vs. 80% LOP (p = 0.0182; Hedge’s g = −0.354; 95% CI [−1666, −154]), 40% vs 100% LOP (p = 0.0005; Hedge’s g = −1.234; 95% CI [−4940, 1559]), and 80% vs. 100% LOP (p = 0.0098, Hedge’s g = −0.910, 95% CI [−4098, −581]). On the other hand, ADS comparisons for the GAS 40% vs. 100% LOP (p = 0.0010; Hedge’s g = − 2.755; 95% CI [−11079, −3090]) and 80% vs.100% LOP (p = 0.0012; Hedge’s g = −1.992; 95% CI [−8076, −2168]), but not 40% vs 80% LOP (p = 0.0821; Hedge’s g = −0.763; 95% CI [−4202, 278]) showed a significant difference. There was a statistically significant interaction between the effects of LOP% and Muscle on SmO2 desaturation p = 0.0160; η2 = 0.046.
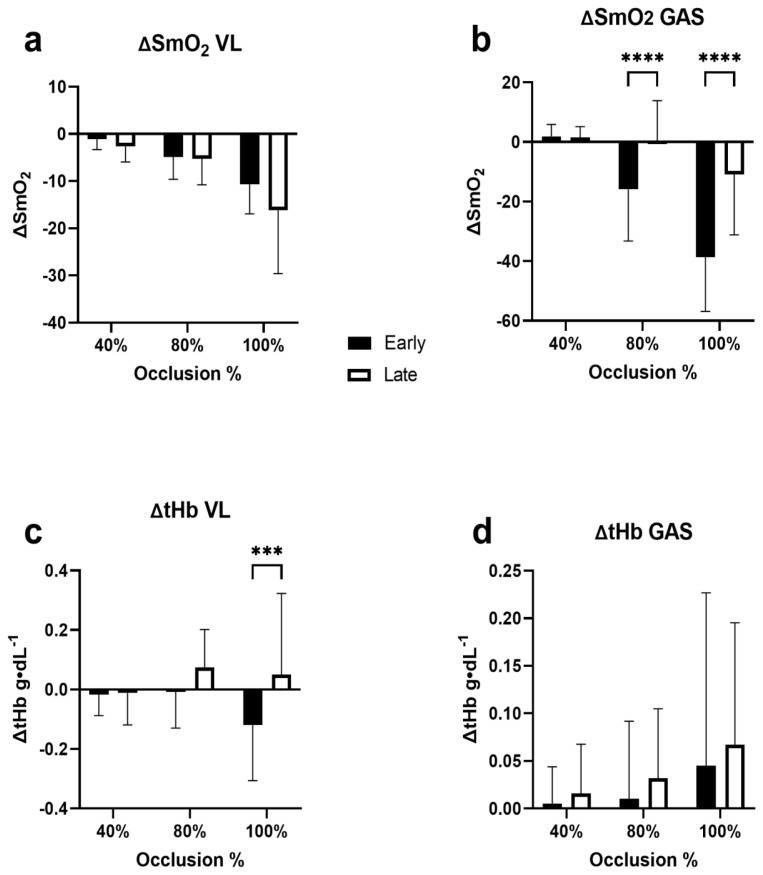
The analysis of the early ΔSmO2 and ΔtHb showed that there were no significant differences in how SmO2 changed in the early period of occlusion compared with the late period of occlusion in the VL (p = 0.081; η2 = .021; 95% CI [−0.35, 5.93]) (Figure 4a). But there was a significant main effect of pressure on the magnitude of change (p < 0.0001; η2 = .335). In the GAS there was a significant main effect difference between the early and late periods of occlusion (p < 0.0001; η2 = .132; 95% CI [−19.59, −9.56]) (Figure 4b). Post-hoc analysis showed significant difference between early and late occlusion at 80% (p < 0.0001; Hedge’s g = −2.00; 95% CI [−23.94, −8.77]) and 100% LOP (p < 0.0001; Hedge’s g = −3.40; 95% CI [−35.40, −20.23]) but not at 40% LOP (p = 0.999; Hedge’s g = .054; 95% CI [−7.15, 8.03]). Additionally, there was a main effect of pressure on the magnitude of change in the GAS (p < 0.0001; η2 = .029).
Figure 4.
Initial and Final ΔSmO2 for Each Occlusion Stage. Comparison of the initial and final ΔSmO2 (%) for each LOP% stage. Data shown as means ± standard deviation. Significant within group main effects found for Initial ΔSmO2 p < 0.001 and Final ΔSmO2 p = 0.0016. Asterisks indicate significant pairwise comparisons *p < 0.05, ** p < 0.01, *** p < 0.001
In the VL there was a significant main effect of occlusion period on the magnitude of change in tHb (p = 0.003; η2 = .0645; 95% CI [−0.136, −0.035]) (Figure 4c). Post-hoc analysis indicated a significant difference only during 100% LOP between the early and late period of occlusion (p = 0.001; Hedge’s g = −1.77; 95% CI [−0.257, −0.080]). In the GAS (n = 14) there were no significant differences in period of occlusion or pressure on the change in tHb (p = 0.366; η2 = .008; 95% CI [−0.06, 0.02] (Figure 4d).
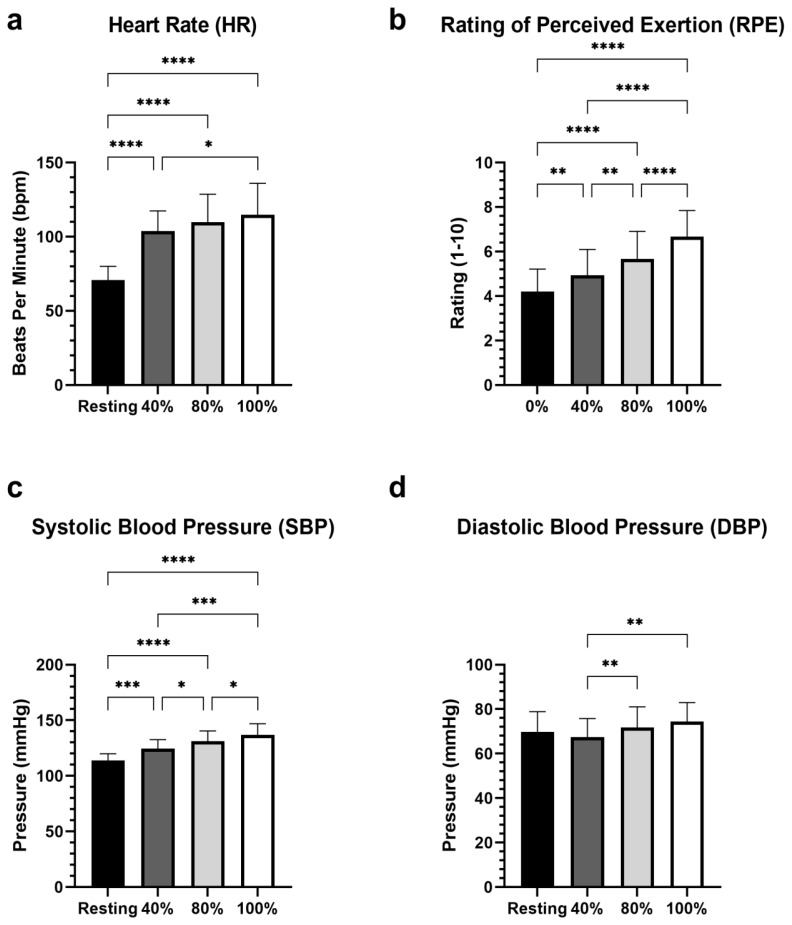
The results of the repeated measures ANOVA determined that there was a statistically significant main effect of LOP% on HR, (p < 0.0001; η2 = .541), with increases in LOP% corresponding to increases in heart rate. The SBP also increased along with LOP% SBP, (p < 0.000; η2 = .515) as did DBP (p = 0.0022; η2 = .082). The pairwise comparisons are shown in figure 5. Similarly, RPE significantly increased along with increases in LOP%, (p < 0.0001; η2 = .403). We observed no statistically significant difference (p = 0.1264; η2 = .034) in GCTB between stages (Figure 6).
Figure 5.
Comparisons of HR, RPE, SBP, and DBP. Stage comparison of HR, RPE, SBP, and DBP. Means ± standard deviation. Significant within group main effects observed for HR, RPE, SBP p < 0.0001, and DBP p = 0.0022. Asterisks indicate significant pairwise comparisons *p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
Figure 6.
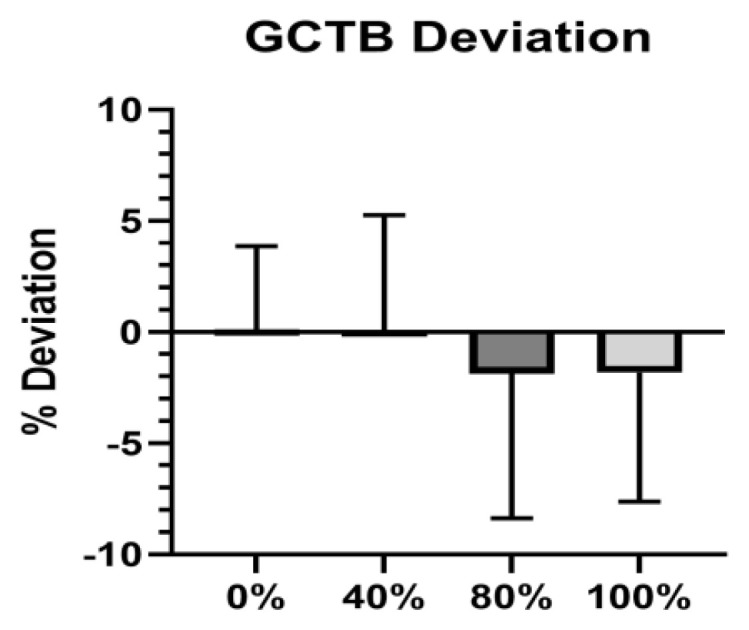
GCTB Deviation. Comparison of the GCTB deviation (%) during stage one and at each occlusion stage. Means ± standard deviation. Positive values indicate favoring of the non-occluded leg.
DISCUSSION
Our main hypothesis that there would be no significant differences in SmO2 between 40%, 80%, and 100% LOP while walking at 3.0 mph (4.83 kph) was rejected. Our analysis of SmO2 ADS data revealed a statistically significant main effect for LOP% on SmO2 desaturation. This finding suggests that incremental increases in LOP will bring about greater SmO2 desaturation and may therefore impose a greater metabolic demand burden on the muscles for the same workload. For example, Lauber et al found that low load BFR increased post exercise lactate (19). The non-significant main effect for muscle on ADS suggests that both the vastus lateralis and gastrocnemius muscles responded similarly to occlusion during our study protocol.
During our investigation we observed changes in SmO2 from start to finish of occlusion stages. For example, an initial drop in SmO2 was sometimes followed by a slow rise in SmO2 despite continued occlusion. This is likely due to the action of the muscle pump during exercise (7). Because of this observation, we thought it was fitting to measure both the early and late change in SmO2 in both the VL and GAS separately. Our analyses of early and late ΔSmO2 were used to represent the change in SmO2 during the early portion as well as the final thirty seconds of the occlusion stages respectively. In each analysis we observed higher LOP were associated with greater decreases in SmO2 in both the VL and the GAS. However, in the VL there was no difference in the early or late phase of occlusion suggesting that the desaturation remained relatively constant throughout the occlusion period. In the GAS, there was a significant difference between the early and late phase of occlusion with the late phase having less desaturation. This suggests that over the period of occlusion in the GAS, some blood flow is restored despite continued occlusion. This observation would be supported by the changes that we observed in tHb (Figure 5). For example, in the VL we observed a higher tHb value in the late phase than the early phase, which suggests greater blood pooling in the VL. However, we did not see any significant difference in tHb between the early and late phases in the GAS.
The differences we observed between the GAS and VL may be explained in part by the difference in blood supply. The VL is supplied primarily by the lateral femoral circumflex artery which is a branch of the deep femoral artery. The GAS is primarily supplied by the popliteal artery which is a continuation of the superficial femoral artery.
We found an increase in perceptual and physiological variables of physical exertion alongside increased relative BFR pressures. Our results of RPE appear to be consistent with prior research (38) that measured perceived exertion at different relative pressures. Prior to testing, participants were instructed to report their RPE based on perception of effort rather than pain and/or discomfort. While there was no increase in exercise intensity, participants in our study reported greater perception of effort with increased levels of occlusion. Increases in HR, SBP, and DBP were congruent with findings reported in one review (32) which covered acute responses of cardiovascular exercise with BFR. Displayed in Figure 5, these measurements, with the exception of DBP also increased from resting/control values. Caution should be taken when interpreting these blood pressure responses to a clinical population because we used physically active healthy adult participants in our investigation.
We observed non-significant changes in walking symmetry to different LOP% based on average GCTB deviation, represented in Figure 6. These results indicate that GCTB did not significantly differ with increased LOP%. Our findings are supported by Faras et. al. who found only minor changes in gait parameters in the non-occluded leg during walking (8). The non-significant changes in walking symmetry indicate we were effective in preventing participants from significantly favoring one limb over the other during occlusion stages.
We sought to quantify and compare SmO2 at several different LOP% while walking on a treadmill. To our knowledge, no previous investigation had explored changes in SmO2 during BFR-walking at recommended occlusion pressures for cardiovascular exercise (25). Several studies have investigated the impact different relative pressures of BFR had on microvascular oxygenation using NIRS technology (14, 16, 30, 38). These investigations are important because relative muscle oxygen desaturation has provided indirect insight on localized tissue perfusion and has been used as an indicator of intramuscular metabolic stress (10, 18, 28). Given that BFR exercise commonly uses low exercise intensities and loads, optimizing metabolic stress in order to promote positive muscle adaptations should be a priority. The results of our study suggest that different LOP% can generate varying magnitudes of SmO2 desaturation. Increased SmO2 desaturation as a result of higher LOP% may necessitate lower training workloads. However, our results also suggest that higher LOP% may intensify perception of effort and physiological variables. Despite the potentially heightened stimulus of using higher LOP%, practitioners and exercise professionals should program such levels of LOP% with caution.
Comparison of our results with other studies is difficult due to differences in study methodology. One investigation (38) analyzed relative tissue oxygenation on participants while they performed seven 5-minute continuous stages of cycling. Five of the stages were with BFR at 40%, 50%, 60%, and 80% of an estimated LOP. They analyzed the changes in tissue oxygen saturation by subtracting the average thirty seconds at the end of the occlusion stage from the minute prior to their exercise protocol. Their comparisons did not find significant differences in the reduction of relative tissue oxygenation between 40%–80% LOP of the VL. We observed a non-significant reduction in SmO2 for the VL between 40% and 80% LOP. Our study design similarly used a cardiovascular exercise modality in contrast to investigations utilizing either knee extension (30) or hand grip exercises (14, 16). Despite these non-significant changes in measurements of tissue oxygen saturation, our study demonstrated that changes in SmO2 were not consistent during a 5-minute occlusion period. We therefore believe associating change in tissue muscle oxygen saturation during a brief portion of BFR exercise with total muscle stress to be misleading and a matter of conjecture.
Another study (30) compared total area of deoxygenated heme in the VL during unilateral knee extensions at 40%, 60%, and 80% LOP. In parallel to their findings, we observed an increase in accumulated oxygen deoxygenation with increased relative pressures of BFR. Their results also revealed that there were significant differences in accumulated oxygen extraction between 40% and 80% LOP. Our findings go along with there being significant differences in total SmO2 desaturation between 40% and 80% LOP. We did not analyze differences in lower-limb SmO2 desaturation between 0% and 40%. The study by Reis and colleagues (30) found that 40% LOP had significantly greater total desaturation than no BFR. Our findings showed there was minimal lower-limb SmO2 desaturation at 40% while walking at 3.0 mph and may suggest the futility of conducting BFR walking at 40% LOP if lower-limb SmO2 desaturation is the goal. Reverting to recommendations for AE with BFR (25), we found 80% LOP to be more meaningful than 40%. We acknowledge that we did not investigate LOP between 40%–80% and the effect these LOP had on other physiological variables or adaptations. Our findings suggest using LOP < 80% for BFR with cardiovascular exercise may not be optimal to enhance muscle adaptations. One major finding of their study was that there were non-significant differences in oxygen extraction above 60% LOP during low-intensity knee extension exercise. Reis and colleagues did not investigate the effects full arterial occlusion may have had on deoxygenated heme. Our findings suggest 100% LOP will give rise to significantly greater total SmO2 desaturation than 80% LOP.
The causes of the decrease in muscle oxygenation is presumably due to impaired muscle blood flow. Interestingly, the impact of occlusion pressure on blood flow has often been reported to be non-linear, such that moderate pressures often elicit the same reduction in blood flow as higher pressures (7, 25). For example, Crossley observed no difference in the reduction in blood flow through the superficial femoral artery (i.e., predominantly flow to lower leg) when a cuff was inflated around the lower thigh to 40% and 80% LOP at rest (7). This is partially consistent with our data indicating no significant difference in the ADS and ΔSMO2 of the GAS at 40% and 80% LOP. Nevertheless, the differing magnitude of ADS in the VL at 40% and 80% LOP in our study is inconsistent with the findings of Crossley et al. Thus, measurements made at rest in one muscle may not be representative of what occurs during exercise in another muscle. More research is needed to determine the impact of %LOP on muscle blood flow during exercise.
In application, higher relative pressures for BFR walking may be beneficial to produce greater skeletal muscle deoxygenation and aid in causing stress to targeted musculature. However, these higher pressures may be accompanied by greater perceived exertion and hemodynamic variables that could potentially make BFR walking at such pressures uncomfortable for individuals. Based on our results, programming BFR walking at 3.0 mph using 40% LOP may not be an adequate stimulus for SmO2 desaturation in the lower limb. The calculation of personalized tourniquet pressures using the Delfi BFR device appeared to be practical and a tool for objective measurements which could be easily taken prior to each BFR session. Overall walking with BFR may be a valuable exercise modality in a rehabilitative setting due to its advantage of being easy to perform and familiarity.
As represented by standard deviation for SmO2, we detected a spread in data points at each stage of occlusion and as LOP% increased. We suspect such variability at greater LOP% transpired due to individual differences within our sample that we did not account for. We speculate thickness of adipose tissue was one potential reason, considering this is a known limitation of NIRS technology. While we excluded participants who met a body fat % > 35%, we did not have exclusion for adiposity at measurement sites.
We did not observe earlier occlusion stages having an impact on SmO2 for later stages. We are unsure if our study design had any impact on the accuracy of SmO2 measurements in later stages. While our study incorporated randomized LOP% order and a non-occluded stage between each occlusion stage, future research may benefit from measuring muscle oxygen saturation changes during isolated stages of occlusion. Our study could have benefited from using relative exercise intensities from maximal exercise testing rather than a set speed of 3.0 mph. Additional research is needed to investigate the effect different relative pressures outside of 40%, 80%, and 100% have on SmO2 while walking. Future investigations of SmO2 using NIRS technology should apply more strict criteria of adiposity in participants, especially at the measurement sites where muscle oxygen sensors are used.
The findings of our study revealed there are some significant differences of BFR walking at 40%, 80%, and 100% LOP on SmO2 desaturation. We sought to determine if lower relative occlusion pressures would induce comparable SmO2 desaturation as higher occlusion pressures. Additionally, if perceived exertion and hemodynamic variables at lower occlusion levels were significantly less than higher pressures it may not be necessary to use higher pressures in practice. Heart rate was also found to be non-significant between these stages. While we saw significant changes in SmO2 during the initial and final portion of occlusion stages, we don’t speculate that these measurements provided an extensive outlook of the total demand placed on the VL and GM within each occlusion stage. Some of our results for ΔSmO2 appear to be consistent with prior research that has investigated changes in oxygen saturation at different relative pressures. However, comparisons with results of other investigations are difficult due to discrepancy of methods used. Further research is needed to investigate the differences in SmO2 at additional LOP beyond what was included in our study.
ACKNOWLEDGEMENTS
This project was made possible in part by the help of Ben Weatherford, PT, DPT at Owens Recovery Science who loaned us the Delfi BFR unit.
REFERENCES
- 1.Abe T, Kearns CF, Sato Y, Kearns CF, Muscle YS. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100(5):1460–6. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- 2.Barstow TJ. Understanding near infrared spectroscopy and its application to skeletal muscle research. J Appl Physiol. 2019;126(5):1360–76. doi: 10.1152/japplphysiol.00166.2018. [DOI] [PubMed] [Google Scholar]
- 3.Biazon TMPC, Ugrinowitsch C, Soligon SD, Oliveira RM, Bergamasco JG, Borghi-Silva A, et al. The association between muscle deoxygenation and muscle hypertrophy to blood flow restricted training performed at high and low loads. Front Physiol. 2019;10:446. doi: 10.3389/fphys.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cayot TE, Lauver JD, Silette CR, Scheuermann BW. Effects of blood flow restriction duration on muscle activation and microvascular oxygenation during low-volume isometric exercise. Clin Physiol Funct Imaging. 2016;36(4):298–305. doi: 10.1111/cpf.12228. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen D, Eibye KH, Hostrup M, Bangsbo J. Blood flow-restricted training enhances thigh glucose uptake during exercise and muscle antioxidant function in humans. Metabolism. 2019;98:1–15. doi: 10.1016/j.metabol.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Corvino RB, Rossiter HB, Loch T, Martins JC, Caputo F. Physiological responses to interval endurance exercise at different levels of blood flow restriction. Eur J Appl Physiol. 2017;117(1):39–52. doi: 10.1007/s00421-016-3497-5. [DOI] [PubMed] [Google Scholar]
- 7.Crossley KW, Porter DA, Ellsworth J, Caldwell T, Feland JB, Mitchell U, et al. Effect of cuff pressure on blood flow during blood flow-restricted rest and exercise. Med Sci Sports Exerc. 2020;52(3):746–53. doi: 10.1249/MSS.0000000000002156. [DOI] [PubMed] [Google Scholar]
- 8.Faras TJ, Laporte MD, Sandoval R, Najjar F, Ade V, Stubbs P. The effect of unilateral blood flow restriction on temporal and spatial gait parameters. Heliyon. 2019;5(1):1–16. doi: 10.1016/j.heliyon.2019.e01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson RA, Hunt JEA, Lewis MP, Martin NRW, Player DJ, Stangier C, et al. The acute angiogenic signalling response to low-load resistance exercise with blood flow restriction. Eur J Sport Sci. 2018;18(3):397–406. doi: 10.1080/17461391.2017.1422281. [DOI] [PubMed] [Google Scholar]
- 10.Ganesan G, Cotter JA, Reuland W, Cerussi AE, Tromberg BJ, Galassetti P. Effect of blood flow restriction on tissue oxygenation during knee extension. Med Sci Sports Exerc. 2015;47(1):185–93. doi: 10.1249/MSS.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavanda S, Isenmann E, Schloder Y, Roth R, Freiwald J, Schiffer T, et al. Low-intensity blood flow restriction calf muscle training leads to similar functional and structural adaptations than conventional lowload strength training: a randomized controlled trial. PLoS One. 2020;15(6):1–13. doi: 10.1371/journal.pone.0235377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes L, Paton B, Rosenblatt B, Gissane C, Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2017;51(13):1003–11. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- 13.Hunt JEA, Stodart C, Ferguson RA. The influence of participant characteristics on the relationship between cuff pressure and level of blood flow restriction. Eur J Appl Physiol. 2016;116(7):1421–32. doi: 10.1007/s00421-016-3399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilett MJ, Rantalainen T, Keske MA, May AK, Warmington SA. The effects of restriction pressures on the acute responses to blood flow restriction exercise. Front Physiol. 2019;10:1018. doi: 10.3389/fphys.2019.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones S, Chiesa ST, Chaturvedi N, Hughes AD. Recent developments in near-infrared spectroscopy (NIRS) for the assessment of local skeletal muscle microvascular function and capacity to utilise oxygen. Artery Res. 2016;16:25–33. doi: 10.1016/j.artres.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilgas MA, McDaniel J, Stavres J, Pollock BS, Singer TJ, Elmer SJ. Limb blood flow and tissue perfusion during exercise with blood flow restriction. Eur J Appl Physiol. 2019;119(2):377–87. doi: 10.1007/s00421-018-4029-2. [DOI] [PubMed] [Google Scholar]
- 17.Kon M, Ikeda T, Homma T, Suzuki Y. Effects of low-intensity resistance exercise under acute systemic hypoxia on hormonal responses. J strength Cond Res. 2012;26(3):611–7. doi: 10.1519/JSC.0b013e3182281c69. [DOI] [PubMed] [Google Scholar]
- 18.Larkin KA, Macneil RG, Dirain M, Sandesara B, Manini TM, Buford TW. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med Sci Sports Exerc. 2012;44(11):2077–83. doi: 10.1249/MSS.0b013e3182625928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauber B, König D, Gollhofer A, Centner C. Isometric blood flow restriction exercise: acute physiological and neuromuscular responses. BMC Sports Sci Med Rehabil. 2021;13(12) doi: 10.1186/s13102-021-00239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurentino G, Ugrinowitsch C, Aihara AY, Fernandes AR, Parcell AC, Ricard M, et al. Effects of strength training and vascular occlusion. Int J Sports Med. 2008;29(8):664–7. doi: 10.1055/s-2007-989405. [DOI] [PubMed] [Google Scholar]
- 21.Lauver JD, Cayot TE, Rotarius T, Scheuermann BW. The effect of eccentric exercise with blood flow restriction on neuromuscular activation, microvascular oxygenation, and the repeated bout effect. Eur J Appl Physiol. 2017;117(5):1005–15. doi: 10.1007/s00421-017-3589-x. [DOI] [PubMed] [Google Scholar]
- 22.Lixandrão ME, Ugrinowitsch C, Berton R, Vechin FC, Conceição MS, Damas F, et al. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med. 2018;48(2):361–78. doi: 10.1007/s40279-017-0795-y. [DOI] [PubMed] [Google Scholar]
- 23.Loenneke JP, Wilson JM, Marín PJ, Zourdos MC, Bemben MG. Low intensity blood flow restriction training: a meta-analysis. Eur J Appl Physiol. 2012;112(5):1849–59. doi: 10.1007/s00421-011-2167-x. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney SJ, Dicks ND, Lyman KJ, Christensen BK, Hackney KJ. Acute cardiovascular, metabolic, and muscular responses to blood flow restricted rowing exercise. Aerosp Med Hum Perform. 2019;90(5):440–6. doi: 10.3357/AMHP.5258.2019. [DOI] [PubMed] [Google Scholar]
- 25.Mouser JG, Ade CJ, Black CD, Bemben DA, Bemben MG. Brachial blood flow under relative levels of blood flow restriction is decreased in a nonlinear fashion. Clin Physiol Funct Imaging. 2018;38(3):425–30. doi: 10.1111/cpf.12432. [DOI] [PubMed] [Google Scholar]
- 26.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson SD, Hughes L, Warmington S, Burr J, Scott BR, Owens J, et al. Blood flow restriction exercise: considerations of methodology, application, and safety. Front Physiol. 2019;10:533. doi: 10.3389/fphys.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45(2):187–200. doi: 10.1007/s40279-014-0264-9. [DOI] [PubMed] [Google Scholar]
- 29.Rainoldi A, Melchiorri G, Caruso I. A method for positioning electrodes during surface EMG recordings in lower limb muscles. J Neurosci Methods. 2004;134(1):37–43. doi: 10.1016/j.jneumeth.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Reis JF, Fatela P, Mendonca GV, Vaz JR, Valamatos MJ, Infante J, et al. Tissue oxygenation in response to different relative levels of blood-flow restricted exercise. Front Physiol. 2019;10:407. doi: 10.3389/fphys.2019.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol. 2013;115(12):1757–66. doi: 10.1152/japplphysiol.00835.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva JCG, Domingos-Gomes JR, Freitas EDS, Neto GR, Aniceto RR, Bemben MG, et al. Physiological and perceptual responses to aerobic exercise with and without blood flow restriction. J strength Cond Res. 2021;35(9):2479–85. doi: 10.1519/JSC.0000000000003178. [DOI] [PubMed] [Google Scholar]
- 33.Slysz J, Stultz J, Burr JF. The efficacy of blood flow restricted exercise: a systematic review & meta-analysis. J Sci Med Sport. 2016;19(8):669–75. doi: 10.1016/j.jsams.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M, et al. Intramuscular metabolism during low-intensity resistance exercise with blood flow restriction. J Appl Physiol. 2009;106(4):1119–24. doi: 10.1152/japplphysiol.90368.2008. [DOI] [PubMed] [Google Scholar]
- 35.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88(6):2097–106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 36.Thomas HJ, Scott BR, Peiffer JJ. Acute physiological responses to low-intensity blood flow restriction cycling. J Sci Med Sport. 2018;21(9):969–74. doi: 10.1016/j.jsams.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Weatherholt AM, Vanwye WR, Lohmann J, Owens JG. The effect of cuff width for determining limb occlusion pressure: a comparison of blood flow restriction devices. Int J Exerc Sci. 2019;12(3):136–43. doi: 10.70252/RWVU7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei J, Nassis GP, Gu Z, Zou Y, Wang X, Li Y. Acute physiological and perceptual responses to moderate intensity cycling with different levels of blood flow restriction. Biol Sport. 2021;38(3):437–43. doi: 10.5114/biolsport.2021.100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagisawa O, Sanomura M. Effects of low-load resistance exercise with blood flow restriction on high-energy phosphate metabolism and oxygenation level in skeletal muscle. Interv Med Appl Sci. 2017;9(2):67–75. doi: 10.1556/1646.9.2017.2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]