Summary
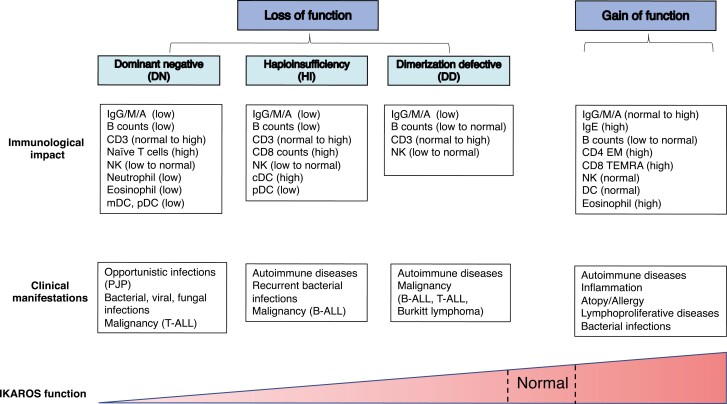
IKAROS/IKZF1 plays a pivotal role in lymphocyte differentiation and development. Germline mutations in IKZF1, which have been shown to be associated with primary immunodeficiency, can be classified through four different mechanisms of action depending on the protein expression and its functional defects: haploinsufficiency, dimerization defective, dominant negative, and gain of function. These different mechanisms are associated with variable degrees of susceptibility to infectious diseases, autoimmune disorders, allergic diseases, and malignancies. To date, more than 30 heterozygous IKZF1 germline variants have been reported in patients with primary immunodeficiency. Here we review recent discoveries and clinical/immunological characterization of IKAROS-associated diseases that are linked to different mechanisms of action in IKAROS function.
Keywords: IKAROS, IKZF1, infections, primary immunodeficiency, inborn errors of immunity, hypogammaglobulinemia, infections, autoimmune diseases, malignancies, B-ALL, T-ALL, Burkitt lymphoma, atopy, allergy
IKAROS/IKZF1 plays a pivotal role in lymphocyte differentiation and development. Germline mutations in IKZF1 have been associated with primary immunodeficiency. Here we review recent discoveries and clinical/immunological characterization of IKAROS-associated disorders that are linked to different mechanisms of action in IKAROS function.
Graphical Abstract
Graphical Abstract.
Introduction
IKAROS, encoded by IKZF1, is one of the IKAROS zinc finger transcription factor family members [1]. The IKAROS family, consisting of total five proteins: IKAROS (IKZF1), HELIOS (IKZF2), AIOLOS (IKZF3), EOS (IKZF4), and PEGASUS (IKZF5), have a high degree of homology to each other [2–5]. Structurally, IKAROS family members contain two major functional domains, the DNA-binding domain (N-terminal zinc fingers) and the dimerization domain (C-terminal zinc fingers) [6]. IKAROS, AIOLOS, and HELIOS are highly expressed in lymphocytes and have been shown to play an essential role in lymphocyte development [7]. The normal expression and function of IKAROS have been shown to be critical for hematopoietic development. Somatic and germline IKAROS deletions and point mutations in humans have been described in lymphocyte malignances, particularly B-cell acute lymphoblastic leukemia (B-ALL) [8–10]. In recent years, our group and others have shown that germline IKAROS point mutations and deletions were associated with patients with primary immunodeficiency (PID), immune dysregulation as well as malignancies [11–24]. The majority of these variants are either located in or affect the main DNA binding or dimerization functional domains, leading to different clinical manifestations through four different mechanisms of action: 1) IKAROS haploinsufficiency (HI) mutations more frequently present with a higher risk of bacterial infections, autoimmune diseases, and malignancies [13, 15], 2) dimerization defective (DD) mutations mainly manifest with hematologic diseases (autoimmune diseases and malignances) [24], 3) dominant negative (DN) mutations increase susceptibility to severe/opportunistic infections, and T-ALL [12], and 4) gain-of-function (GOF) mutations are commonly associated with autoimmunity, allergy, and lymphoproliferative disorders [23]. Different mechanisms of action in the same gene can disrupt proper transcriptional regulation affecting lymphocyte and/or myeloid cell development, differentiation, and also impaired immune functions. While the immune and clinical phenotypes associated with IKAROS mutations are diverse, certain overlapping features between the variants with different mechanisms of action are also seen. Understanding of the molecular mechanisms related to IKAROS mutations will hopefully help us find targeted therapeutic options to treat these rare diseases. In this review, we will discuss the current understanding of IKAROS-associated diseases, which include both loss-of-function (LOF; acting by HI, DD, or DN) and GOF mutations underlying PID/IEI, and describe the genetic, cellular and molecular effects, immunological and clinical manifestations, as well as therapeutic options for patients carrying IKAROS mutations.
IKAROS LOF mutations causing IKAROS haploinsufficiency
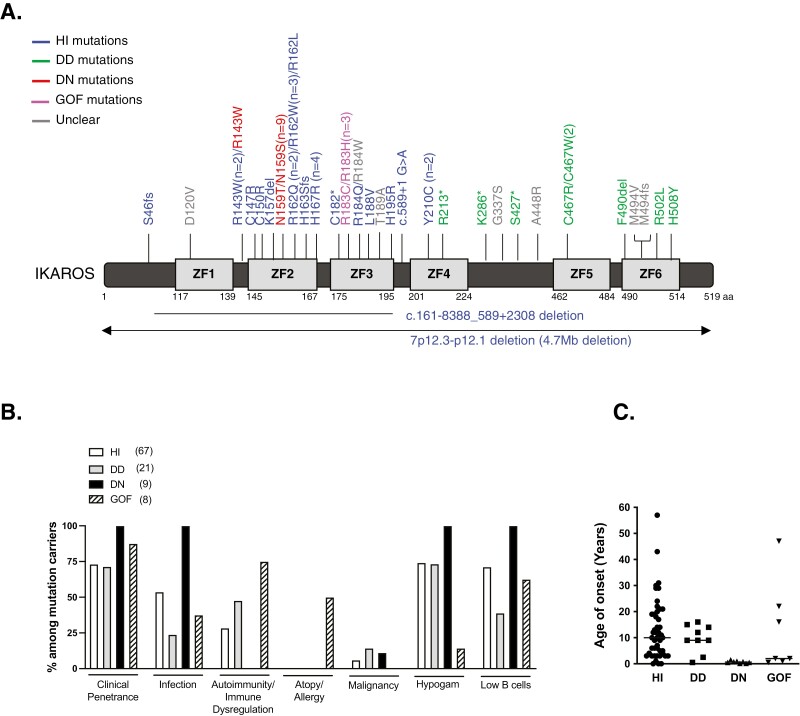
The first IKAROS mutation (Y210C) associated with a PID/IEI was reported in 2012 in a single patient case report of an infant with pancytopenia, immunodeficiency, absent B cells, very low NK cells, and bone marrow hypoplasia; limited information about the mechanism of disease was known at that time [25]. Later, when our and other groups evaluated Y210C biology, a partial DNA-binding haploinsufficiency defect was detected [13, 15]. In 2016, we and a large group of collaborators reported six different IKAROS mutations in 29 patients from six unrelated families presenting with a CVID phenotype [13]. These mutations rendered the protein non-functional as determined through the absence of pericentromeric targeting and DNA binding, yet the mutations did not affect WT IKAROS function, suggesting haploinsufficiency (HI) as the pathophysiological mechanism in these individuals. IKAROS HI can be caused by either missense mutations or deletions involving the IKZF1 gene, primarily clustered in the DNA binding domain. Since that report, more variants acting by haploinsufficiency have been identified in over 60 patients with PID (Fig. 1A) [14-22, 26]. The age of onset of clinical manifestations varies between less than a year to more than 50 years, with the median age of presentation at 10 years (Fig. 1C). About 25% of mutation carriers are asymptomatic, suggesting a clinical penetrance (or delayed onset of disease at the time of report) at around ~75% (reviewed in [11, 27, 28]).
Figure 1:
Pathogenic germline IKAROS variants reported in PID patients. (A) Schematic of the IKAROS isoform 1 (NM_006060) with germline variants reported in patients with PID/IEI. ZF indicates zinc finger. Loss-of-function mutations acting by haploinsufficiency (HI) are indicated in blue, mutations causing the dimerization defect (DD) of the IKAROS family are indicated in green, mutations presenting a dominant-negative (DN) effect over WT IKAROS protein are depicted in red, those germline mutations reported in IKAROS gain-of-function (GOF) are indicated in pink, mutations found in PID patients with an unclear/untested mechanism are indicated in gray. Numbers next to the mutations indicate the number of different families reported to carry the same mutation. The R143W mutation was reported by three different groups, two as an HI mutation and one as a partial DN mutation. Our group tested and confirmed that the mutant C467W impaired dimerization with the IKAROS WT protein (unpublished data). (B) The incident rates of clinical manifestations in individuals carrying IKAROS mutations. The number next to the mutations indicates the total number of individuals reported in PID cases carrying IKAROS mutations. The graph for the “low B-cell number” was based on the absolute B-cell counts, with the exception of the GOF group (only a percentage of B-cell data from lymphocytes was available for the GOF group). “Hypogammaglobulinemia (Hypogam)” and “low B cells” data were calculated from mutation carriers whose laboratory data were available. (C) Age of onset of clinical manifestations. The horizontal bars indicate the median values.
Among IKZF1 HI mutations carriers (n = 67 published; reviewed in [11, 27, 28]), bacterial infections were the most common complication and present in >50% of them. Four cases of viral (HSV, HPV) and one case of mycobacterial and parasitic infections were also described (reviewed in [11, 27, 28]). The second most common clinical manifestations were autoimmune diseases/immune dysregulation (~30% among HI mutation carriers) which included idiopathic thrombocytopenic purpura (ITP), arthritis, systemic lupus erythematosus (SLE), and antiphospholipid syndrome (reviewed in [28]). Four cases of malignances were reported: three pediatric B-ALL cases (presenting between the ages of 3-7 years), and one case with pancreatic tumor at 22 years old (Fig. 1B) [13, 17, 20]. As the IKAROS-family of transcription factors are exclusively expressed in hematopoietic cells, the relationship between IKAROS variants (or in other family members), and non-hematopoietic tumors is unlikely to be direct; however, an indirect link (e.g., due to poor immune surveillance or anti-tumor effect) could not be formally excluded.
A progressive decline of B-cell numbers and serum immunoglobulin levels was detected in patients with HI mutations, as about 60-70% had both low B-cell counts and low IgG, IgM, and IgA levels. Bone marrow sample studies have found a very early although incomplete B-cell arrest (upstream of pre-pro B cells), with normal numbers of plasma cells detected in the cases analyzed [13]. Most patients had poor antibody responses to protein and polysaccharide vaccines, suggesting that abnormal B-cell development as well as function are both associated with IKAROS HI mutations. T-cell counts were normal to high with a trend for increased CD8 T cells. This was more prevalent in patients carrying heterozygous loss-of-function (LOF) missense variants (opposed to gene deletions) and lead to inverted CD4/CD8 ratios. Low NK-cell numbers were observed in ~20% of these patients, also more frequently seen in those with missense mutations. An expansion of cDC1 and decreased pDC was reported in patients with HI mutations [29].
Prophylactic antibiotics and IgG replacement therapy (IgGRT) were used for the patients with a history of recurrent infections. Patients with ITP were treated with corticosteroids, high dose intravenous IgG therapy, anti-IgD therapy, and rituximab. Patients with antiphospholipid syndrome were managed with steroids and anticoagulation. Immunosuppressant therapies were prescribed for those who developed SLE. Two cases of hematopoietic stem cell transplant (HSCT) were reported in the patients with HI mutations; one patient died with multiorgan failure a week after a second stem cell infusion because of delayed engraftment and infection; the other patient was transplanted because of relapsed B-ALL with his HLA-matched IKAROS-mutated brother (IKAROS deficiency and the donor status were unknown at the time of transplantation). Four years after the transplant, the patient was doing well and off IgG replacement therapy [13, 25].
IKAROS LOF mutations causing a dimerization defect
In 2020, we and our collaborators published a novel group of IKAROS allelic variants that primarily disrupt IKAROS homo- and heterodimerization with IKAROS family members. These variants extended the phenotypical spectrum of IKAROS-associated diseases and provided new insights into the underlying mechanistic consequences of IKAROS mutations [24]. These mutations were either located in the dimerization domain (ZF5 and ZF6) or create premature stop codons upstream of the dimerization domain thereby impairing homo- and heterodimerization. In transfection experiments, expression of the mutant alleles alone failed to target PC-HC but did not affect WT IKAROS function, suggesting the mutations were likely acting by a dimerization haploinsufficiency mechanism. Unlike the HI mutants, which are unable to bind to target sequences, dimerization defective (DD) mutations can bind its target DNA but mostly as monomers. Moreover, the mutations affect protein stability, post-translational modification (e.g. SUMOylation), and recruitment of the nucleosome remodeling and deacetylase (NuRD) complex, all of which are not affected by HI mutations, thereby determining different underlying pathophysiologic mechanisms between HI and DD mutations.
The clinical phenotypes of patients with DD mutations are largely associated with non-malignant and malignant hematologic disorders which include multiple hematopoietic cytopenia presenting as Evans syndrome, autoimmune neutropenia, ITP, autoimmune hemolytic anemia, as well as lymphoproliferative disorders such as T-ALL, and Burkitt lymphoma [16, 24, 30-33]. The age of onset of clinical symptoms is mostly within the first decade of life but could also present later (Fig. 1C). The overall type of clinical manifestations is similar to those in HI patients, but the incidence of autoimmune diseases/immune dysregulation and malignancies are higher than in the latter (Fig. 1B). On the other hand, bacterial infections are less prevalent and less severe in DD patients (~25% of DD mutation carriers) when compared to the HI patients (>50% of mutation carriers). Germline and somatic IKAROS DD mutations have been shown to be associated with lymphoid and myeloid malignancies, highlighting the necessary role of the dimerization process in IKAROS tumor suppression [8, 34, 35]. One-third of the patients carrying deleterious DD variants are clinically asymptomatic suggesting incomplete penetrance or very late onset (Fig. 1B).
T- and B-cell phenotypes are less impacted by DD mutations when compared with other allelic variants. Patients with DD mutations also showed progressive B-cell lymphopenia (~40% of mutation carriers) and elevated CD8 T cells (~20%) but these numbers are less prevalent than those seen in patients carrying HI mutations (60-70% and ~40%, respectively). Despite the lesser impact on B-cell numbers, more than 70% of patients with DD mutations had mild to moderate hypogammaglobulinemia (Fig. 1B), indicating that B-cell dysregulation is associated with IKAROS mutations, regardless of the mechanism of action. Slightly low NK-cell numbers were observed in one-third of patients with DD mutations.
Most DD patients presenting with cytopenia were treated with steroids and high dose intravenous IgG therapy. Rituximab, mycophenolate mofetil, and sirolimus were used to control these complications in some patients. The two patients with hematologic malignancies (T-ALL or Burkitt lymphoma) received specific chemotherapy regimens and achieved complete remission [24].
IKAROS LOF variants with a dominant negative effect
A recent study identified mostly de novo germline heterozygous IKAROS N159S/T mutations in 7 different patients with early-onset combined immunodeficiency and complete clinical penetrance [12]. Molecular studies revealed that when WT and mutant(s) were co-expressed, the N159S/T mutations inhibited the WT IKAROS protein’s PC-HC localization, strongly suggesting that these variants exert a dominant negative (DN) effect on IKAROS WT function. Two more patients carrying IKAROS N159S mutation were reported in combined immunodeficiency (CID) and PID cohorts [32, 36]. Most patients with IKAROS DN mutations had a history of Pneumocystis jirovecii pneumonia (PJP; 8/9) first presenting between the ages of 6 months to 2 years, and repeated episodes were not uncommon. Unlike the patients with HI mutations who presented with mainly recurrent bacterial infections, patients with DN variants showed severe and invasive complications including bacterial (7/9), viral (6/9), fungal (8/9), mycobacterial (2/9), and parasitic (1/9) infections [12, 32, 36]. Neither autoimmune diseases/immune dysregulation nor atopy/allergic manifestations have been reported among patients carrying IKAROS DN mutations.
One patient had Cryptosporidium species cholangitis that led to cirrhosis, and one patient developed T-ALL in early teenage years. Interestingly, somatic N159S and N159Y mutations were reported as hot spot mutations in large cohort studies of patients with AML and B-ALL, respectively, indicating that N159 variants, either germline or somatic, are likely associated with a leukemia driver [34, 35].
All nine patients with IKAROS DN mutations showed severe B-cell lymphopenia and hypo/agammaglobulinemia, indicating complete immunological and clinical penetrance (Figure 1B). A very early B-cell developmental arrest similar to that described in HI patients (severe reduction in pre-pro B cells, pro-B cells as well as mature B cells) was identified in bone marrow studies [12]. However, unlike HI patients, plasma cells were absent in DN patients, indicating a more severe B-cell maturation defect in DN patients.
T-cell absolute numbers vary among DN patients but markedly increased naïve phenotypes and low memory T cells were consistently detected. While most of the patients’ T cells were positive for recent thymic emigrant makers (CD45RA+ CD31+), Th1, Th2, Th17, and Treg-committed populations were almost absent due to low memory T cells [12]. In addition to the abnormal T-cell phenotypes, impaired T-cell functions were reported. T-cell receptor (TCR)-induced T-cell activation and proliferation were impaired at low-dose TCR stimulation, and this defect was not observed when the cells were stimulated with higher concentration of α-CD3 stimuli or bead-conjugated TCR stimulation. IL-2-induced phosphorylation of STAT5 was markedly diminished in the patients’ T cells and RNAseq data from CD4 T cells showed abnormal transcriptome patterns, suggesting that T-cell maturation and differentiation were also affected in these patients [12].
Other than the abnormal T- and B-cell phenotypes, myeloid abnormalities were also observed in DN patients. Progressive eosinopenia and neutropenia were detected, and monocyte dysfunction and abnormal transcriptional regulation was also reported. DC phenotypes were tested in two DN patients and showed a mild reduction in pDCs, and one patient showed a severe reduction in myeloid DC (mDC). NK cell counts were normal in about 70% of the patients while decreased in the rest.
DN patients were treated with broad and aggressive antimicrobial regimes and IgGRT due to severe and invasive infections. Five out of nine patients underwent HSCT because of their early and severe clinical and immunological phenotypes; of note, four patients were transplanted before their genetic diagnosis was identified. HSCT was well tolerated in four reported patients without chronic GVHD complications and patients were off antimicrobial prophylaxis and IgGRT during follow-up. Unfortunately, one patient who had Cryptosporidium sclerosing cholangitis before HSCT progressed to liver failure and died a year after the procedure [36]. Despite the limited accumulated experience with IKZF1 DN patients (n = 9), HSCT should be considered as an early curative option in these patients based on their severe phenotypes and life-threatening complications.
IKAROS gain-of-function variants
Very recently, Hoshino et al. reported germline heterozygous IKAROS gain-of-function (GOF) mutations in eight individuals from four unrelated families [23]. The mutations were located at residue R183 (R183H and R183C) in the highly conserved ZF3. When compared with WT IKAROS protein, these variants functionally increased IKAROS binding capacity to their target DNA sequences. Unlike the previously reported IKAROS allelic variants, which mostly associated with low B-cell numbers and hypogammaglobulinemia leading to recurrent infections, GOF patients had mostly normal B-cell numbers with normal to elevated serum immunoglobulin levels, including IgE. GOF patients also had bacterial infections but were generally not severe (4/8; sinopulmonary infections and otitis media). Interestingly, GOF patients presented with atopic and allergic diseases (4/8; asthma, rhinitis, dermatitis, food allergy, and eosinophilic esophagitis among others), and plasma cell (PC) hyper-proliferation (4/8), which have not been reported in other IKAROS allelic variants. Histopathological analysis showed that three GOF patients had IgG4-related diseases as increased infiltration of the IgG4-positive PCs was observed in lymph nodes, intestine, and/or bile duct. Polyclonal PC proliferation was observed in one patient’s bone marrow sample. The age of onset varied from less than 1 year to over 40 years (Figure 1C). Autoimmunity/immune dysregulation was profound and a hallmark in the GOF patients (75%, 6/8) compared with the IKAROS HI (~30% of mutation carriers) and DD patients (~50% of mutation carriers) (Figure 1B). While autoimmune diseases reported in HI and DD variants were mostly limited to the hematologic compartment or a single disease [24], GOF patients developed multiple autoimmune diseases, including type 1 diabetes mellitus, enteritis, autoimmune hepatitis, Hashimoto thyroiditis, leukocytoclastic vasculitis, vitiligo, alopecia, and cytopenia. Malignancy has not been reported in GOF patients so far, but we are aware of patients carrying the germline R183H variant who were diagnosed with different hematologic malignancies as multiple myeloma and lymphomas (HSK and SDR, personal communication). Similar to the HI and DD variants, among whom the presence of clinically asymptomatic individuals is not uncommon, one patient with a GOF mutation did not present with any clinical manifestations but did show immunological penetrance [23].
T-cell phenotypes were skewed to CD4 effector memory cells (CD45RA−/CCR7−) and terminally differentiated memory CD8 T phenotypes (CD57+ CD8 T cells and CD8+ TEMRA). Despite increased TEMRA and senescent T-cell populations, CD3-induced T-cell proliferation was comparable to the healthy controls. While the GOF patients’ CD4 T-cell blasts exhibited a dramatic reduction in IL-2 production and Th1 differentiation, increased Th2 differentiation was detected. These abnormal T-cell phenotypes were confirmed by in vitro studies using naïve CD4 T cells transduced with each IKAROS allelic variants. Similar to the results from the patients’ T-cell blasts, T cells expressing GOF mutations showed increased IL-4 (Th2) production, and decreased IL-2 and IFNγ production (Th1). This contrasts with IKAROS HI mutations, where increased IL-2 and IFNγ, and reduced IL-4 production are observed in vitro. The IKAROS DN mutation showed an overall blunted Th differentiation [12, 23]. Of note, GOF patients showed an absence of effector Treg and increased Tfh population, suggesting T-cell differentiation is compromised by abnormal IL-2 production. Abnormal IL-2 production and effector Treg population in these patients may contribute the autoimmune manifestations as in other IEI patients with impaired Treg numbers and/or function (e.g., IPEX, CD25 deficiency and CTLA4 patients, among others) [37]. Interestingly, in vitro use of lenalidomide, known to degrade IKAROS and AIOLOS protein [38], normalized these abnormal T phenotypes to a similar level of WT IKAROS (IL-2, IFNγ, and IL-4 levels) [23]. Lenalidomide should be considered as a possible treatment option for the IKAROS GOF patients as this drug is widely used for the treatment of multiple myeloma patients through inhibition of PC proliferation; further studies are warranted to prove clinical efficacy of such treatment.
Other than T- and B-cell phenotypes, NK and DC numbers were largely within normal ranges, while eosinophil numbers and hemoglobin levels were elevated [23].
Treatments for these patients were based on their specific clinical conditions and include standard therapies for infections, autoimmune disorders, allergies or immune dysregulatory conditions. Rituximab, sirolimus, and steroids were used to treat some of the latter diseases. Two patients received IgGRT due to low B-cell counts, hypogammaglobulinemia, and/or recurrent (but not severe) bacterial infections. We have unreported data of another R183H patient who received an HSCT due to severe clinical manifestations (i.e., diffuse large B-cell lymphoma, cutaneous T-cell lymphoma, allergies/anaphylaxis, poor response to lenalidomide) and the disease seems to be cured after HSCT (HSK and SDR, unpublished).
Conclusions
The important role of IKAROS in lymphoid and myeloid cells have been demonstrated in both mouse models and human diseases [39]. The characterization of genotype and phenotype correlation of human IKAROS-associated diseases has built on recent studies of IEI. Proper IKAROS expression levels and functions are crucial for normal hematologic development, differentiation and function, as both IKAROS LOF (including HI, DD, and DN variants) and GOF lead to severe outcomes of immunodeficiency and immunodysregulation in humans. The genetic and functional dissections of various IKAROS mutations have led to four different allelic variants of inborn errors of human IKAROS diseases, including 1) haploinsufficiency, 2) dimerization defective, 3) dominant-negative, and 4) gain-of-function mutations. Too little IKAROS function, such as in DN mutations, is associated with a predisposition to opportunistic infections, severe and invasive bacterial, viral, and fungal infections as well as the compromised lymphocyte differentiation. Interestingly, no autoimmune, allergic or immune dysregulation features have been described in patients with IKAROS DN mutations. Conversely, too much IKAROS function, such as in GOF mutations, is associated with allergy, autoimmunity, immunodysregulation, and lymphoproliferative disorders. Achieving one half to one-quarter of IKAROS expression or function are not an exception either. Recurrent bacterial infections, progressive loss of B cells, and immunoglobulin levels are profound in patients with HI mutations. Interestingly, DD mutations, which fall under the same category of haploinsufficiency, are mostly associated with hematologic diseases with reduced impact on infectious disease susceptibility and T-cell lymphocyte differentiation. These differences are probably due to different underlying mechanisms. While the IKAROS HI mutants alone have completely lost DNA binding without any effect on dimerization, IKAROS DD mutants have not only lost their function but also have impaired dimerization with IKAROS family members, likely influencing IKAROS family’s functions too. Moreover, reduced protein stability and abnormal post-translational modifications and NuRD complex recruitment by DD mutations likely contribute to IKAROS expression/function and its downstream signaling. Of the IKAROS mutations reported in PID/IEI patients, 6 mutations were categorized as unclear mechanisms (Fig. 1A). The IKAROS D120V mutation was reported in a cohort of SLE patients. This mutation is the only one reported in the ZF1 domain, and the mutant alone was defective in DNA binding and pericentromeric targeting. Despite the functional defect, there is no heterozygous condition data available, so we categorized this mutant under the unclear mechanism. Moreover, this patient also carries a likely deleterious heterozygous LYN mutation, so it is challenging to identify the IKAROS mutation as the main cause of the disease [40]. The remaining five mutations’ mechanism was unclear because there was either no data available to prove their loss of function, or their function in the heterozygous condition was uncertain [16, 32].
Not surprisingly, other IKAROS family members (AIOLOS and HELIOS) variants have been shown to associate with PID/IEI. Two germline AIOLOS (IKZF3) variants were reported in PID patients; one (G159R) has a dominant negative effect over both AIOLOS and IKAROS WT protein, and the other (N160S) has a dominant negative effect only on AIOLOS WT protein [41, 42]. Despite only one amino acid being different between these two variants, the patients presented with very different phenotypes. G159R is associated with B-cell deficiency, recurrent sinopulmonary infections, EBV infection, and a higher risk of B-cell lymphoma, whereas the N160S mutation has shown to associate with abnormal T- and B-cell differentiation and function, increased susceptibility to Pneumocystis jirovecii pneumonia, and chronic lymphocytic leukemia. Of note, AIOLOS N160S is a homologous variant to IKAROS N159S, with which it resembles several clinical and immunological features.
Very recently, two groups published HELIOS (IKZF2) variants in PID patients. Hetemaki et al. identified a non-sense heterozygous mutation in HELIOS ZF4 (Y200*) [43]. The mutant failed to dimerize with IKAROS family members and prevented Mi-2/NuRD chromatin remodeling complex activity. Patients presented with a CID phenotype with chronic T-cell activation and increased proinflammatory cytokines in effector and regulatory T cells. Affected patients had recurrent upper respiratory infections, thrush and mucosal ulcers, and chronic lymphadenopathy with dysregulated germinal centers and aberrations in antibody production.
Shahin et al. reported a biallelic missense mutation in Exon 8 (I325V), between the DNA binding and the dimerization domain, demonstrating that this variant did not affect any of these crucial functions, but impacts on epigenetic regulation of HELIOS remodeling partners, resulting in decreased IL-2 locus accessibility and IL-2 production and T-cell function [44]. This patient presented with recurrent infections, leukopenia (low B, CD4, and NK cell numbers), hypogammaglobulinemia, and poor antibody responses as well as other features such as failure to thrive, hypothyroidism, and osteopenia. Following this report, the same group also reported five more HELIOS variants in PID patients (R291*, R106W, N220S, V347M, Y359C) [45]. These patients presented with hypogammaglobulinemia, SLE, immune thrombocytopenia or EBV-associated hemophagocytic lymphohistiocytosis. These data suggested that LOF of HELIOS is associated with immunodeficiency.
IKAROS and IKAROS family’s mutations are associated with a board spectrum of immunological and clinical phenotypes. The diversity of the phenotypes likely relies on gene dosage effects due to deletions and loss of expression, loss-of-function, dominant negative, and gain-of-function effects. This diversity underlying the different mechanisms of action makes it hard to predict the potential deleterious effect of variants of unknown significance (VUS) without formal testing. The current patient care should be structured based on the identification of specific IKAROS mutations (e.g. IKAROS N159S/T, from PJP prophylaxis to HSCT recommendation), and prospective reporting of new variants and their functional impact will help to advance our understanding of IKAROS-associated diseases and their management. Future studies with a larger number of patients with IKAROS mutations will certainly help to improve delineation of the genotype and phenotype correlations, pathophysiology, penetrance and expressivity, and better targeted clinical management for IKAROS-associated diseases.
Acknowledgments
This work was supported by the Intramural Research Program, National Institutes of Health Clinical Center. The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Glossary
Abbreviations
- AIHA
autoimmune hemolytic anemia
- ALL
acute lymphoblastic leukemia
- cDC
conventional dendritic cells
- CID
combined immunodeficiency
- CLL
chronic lymphocytic leukemia
- DD
dimerization defective
- DN
dominant negative
- EBV
Epstein-Barr virus
- GOF
gain of function
- GVHD
graft versus host disease
- HI
haploinsufficiency
- HPV
human papillomavirus
- HSV
Herpes Simplex Virus
- HSCT
hematopoietic stem cell transplantation
- IEI
inborn errors of immunity
- IgGRT
IgG replacement therapy
- ITP
idiopathic thrombocytopenic purpura
- mDC
myeloid DC; NuRD: the nucleosome remodeling and deacetylase
- PC-HC
pericentromeric heterochromatin
- PID
primary immunodeficiency
- PJP
Pneumocystis jirovecii pneumonia
- PC
plasma cell
- pDC
plasmacytoid dendritic cells
- SLE
systemic lupus erythematosus
- TEMRA
terminally differentiated effector memory
Contributor Information
Hye Sun Kuehn, Immunology Service, Department of Laboratory Medicine, National Institutes of Health Clinical Center, Bethesda, MD, USA.
Brigette Boast, Immunology Service, Department of Laboratory Medicine, National Institutes of Health Clinical Center, Bethesda, MD, USA.
Sergio D Rosenzweig, Immunology Service, Department of Laboratory Medicine, National Institutes of Health Clinical Center, Bethesda, MD, USA.
Ethical statement
Approval was received by the NIH Institutional Review Board (IRB) for this study, and an informed consent of all participating subjects studied at NIH was obtained.
Conflict of interests
None declared.
Funding
This work was supported by the Intramural Research Program, National Institutes of Health (NIH) Clinical Center and National Institute of Allergy and Infectious Diseases.
Data availability
Not applicable.
Author contribution
All authors contributed to the manuscript writing and approved the submitted version.
References
- 1. Georgopoulos K, Moore DD, Derfler B.. IKAROS, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science 1992, 258, 808–12. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 2. Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, et al. Aiolos, a lymphoid restricted transcription factor that interacts with IKAROS to regulate lymphocyte differentiation. EMBO J 1997, 16, 2004–13. doi: 10.1093/emboj/16.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hahm K, Cobb BS, McCarty AS, Brown KE, Klug CA, Lee R, et al. Helios, a T cell-restricted IKAROS family member that quantitatively associates with IKAROS at centromeric heterochromatin. Genes Dev 1998, 12, 782–96. doi: 10.1101/gad.12.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kelley CM, Ikeda T, Koipally J, Avitahl N, Wu L, Georgopoulos K, et al. Helios, a novel dimerization partner of IKAROS expressed in the earliest hematopoietic progenitors. Curr Biol 1998, 8, 508–15. doi: 10.1016/s0960-9822(98)70202-7. [DOI] [PubMed] [Google Scholar]
- 5. Perdomo J, Holmes M, Chong B, Crossley M.. Eos and pegasus, two members of the IKAROS family of proteins with distinct DNA binding activities. J Biol Chem 2000, 275, 38347–54. doi: 10.1074/jbc.M005457200. [DOI] [PubMed] [Google Scholar]
- 6. Molnar A, Georgopoulos K.. The IKAROS gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol Cell Biol 1994, 14, 8292–303. doi: 10.1128/mcb.14.12.8292-8303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Powell MD, Read KA, Sreekumar BK, Oestreich KJ.. IKAROS zinc finger transcription factors: regulators of cytokine signaling pathways and CD4(+) T helper cell differentiation. Front Immunol 2019, 10, 1299. doi: 10.3389/fimmu.2019.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Churchman ML, Qian M, Te Kronnie G, Zhang R, Yang W, Zhang H, et al. Germline genetic IKZF1 variation and predisposition to childhood acute lymphoblastic leukemia. Cancer Cell 2018, 33, 937–948.e8. doi: 10.1016/j.ccell.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med 2009, 360, 470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of IKAROS. Nature 2008, 453, 110–4. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 11. Kuehn HS, Nunes-Santos CJ, Rosenzweig SD.. Germline IKZF1 mutations and their impact on immunity: IKAROS-associated diseases and pathophysiology. Expert Rev Clin Immunol 2021, 17, 407–16. doi: 10.1080/1744666X.2021.1901582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boutboul D, Kuehn HS, Van de Wyngaert Z, Niemela JE, Callebaut I, Stoddard J, et al. Dominant-negative IKZF1 mutations cause a T, B, and myeloid cell combined immunodeficiency. J Clin Invest 2018, 128, 3071–87. doi: 10.1172/JCI98164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuehn HS, Boisson B, Cunningham-Rundles C, Reichenbach J, Stray-Pedersen A, Gelfand EW, et al. Loss of B cells in patients with heterozygous mutations in IKAROS. N Engl J Med 2016, 374, 1032–43. doi: 10.1056/NEJMoa1512234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bogaert DJ, Kuehn HS, Bonroy C, Calvo KR, Dehoorne J, Vanlander AV, et al. A novel IKAROS haploinsufficiency kindred with unexpectedly late and variable B-cell maturation defects. J Allergy Clin Immunol 2018, 141, 432–435.e7. doi: 10.1016/j.jaci.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoshino A, Okada S, Yoshida K, Nishida N, Okuno Y, Ueno H, et al. Abnormal hematopoiesis and autoimmunity in human subjects with germline IKZF1 mutations. J Allergy Clin Immunol 2017, 140, 223–31. doi: 10.1016/j.jaci.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 16. Eskandarian Z, Fliegauf M, Bulashevska A, Proietti M, Hague R, Smulski CR, et al. Assessing the functional relevance of variants in the IKAROS family zinc finger protein 1 (IKZF1) in a cohort of patients with primary immunodeficiency. Front Immunol 2019, 10, 568. doi: 10.3389/fimmu.2019.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Groth DJ, Lakkaraja MM, Ferreira JO, Feuille EJ, Bassetti JA, Kaicker SM.. Management of chronic immune thrombocytopenia and presumed autoimmune hepatitis in a child with IKAROS haploinsufficiency. J Clin Immunol 2020, 40, 653–7. doi: 10.1007/s10875-020-00781-y. [DOI] [PubMed] [Google Scholar]
- 18. Banday AZ, Jindal AK, Kaur A, Saka R, Parwaiz A, Sachdeva MUS, et al. Cutaneous IgA vasculitis-presenting manifestation of a novel mutation in the IKZF1 gene. Rheumatology (Oxford) 2021, 60, e101–e3. [DOI] [PubMed] [Google Scholar]
- 19. Dieudonne Y, Guffroy A, Vollmer O, Carapito R, Korganow AS.. IKZF1 loss-of-function variant causes autoimmunity and severe familial antiphospholipid syndrome. J Clin Immunol 2019, 39, 353–7. doi: 10.1007/s10875-019-00643-2. [DOI] [PubMed] [Google Scholar]
- 20. Chen QY, Wang XC, Wang WJ, Zhou QH, Liu DR, Wang Y.. B-cell deficiency: a de novo IKZF1 patient and review of the literature. J Investig Allergol Clin Immunol 2018, 28, 53–6. doi: 10.18176/jiaci.0207. [DOI] [PubMed] [Google Scholar]
- 21. Van Nieuwenhove E, Garcia-Perez JE, Helsen C, Rodriguez PD, van Schouwenburg PA, Dooley J, et al. A kindred with mutant IKAROS and autoimmunity. J Allergy Clin Immunol 2018, 142, 699–702.e12. doi: 10.1016/j.jaci.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sriaroon P, Chang Y, Ujhazi B, Csomos K, Joshi HR, Zhou Q, et al. Familial immune thrombocytopenia associated with a novel variant in IKZF1. Front Pediatr 2019, 7, 139. doi: 10.3389/fped.2019.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoshino A, Boutboul D, Zhang Y, Kuehn HS, Hadjadj J, Ozdemir N, et al. Gain-of-function IKZF1 variants in humans cause immune dysregulation associated with abnormal T/B cell late differentiation. Sci Immunol 2022, 7, eabi7160. doi: 10.1126/sciimmunol.abi7160. [DOI] [PubMed] [Google Scholar]
- 24. Kuehn HS, Niemela JE, Stoddard J, Ciullini Mannurita S, Shahin T, Goel S, et al. Germline IKAROS dimerization haploinsufficiency causes hematologic cytopenias and malignancies. Blood 2021, 137, 349–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldman FD, Gurel Z, Al-Zubeidi D, Fried AJ, Icardi M, Song C, et al. Congenital pancytopenia and absence of B lymphocytes in a neonate with a mutation in the IKAROS gene. Pediatr Blood Cancer 2012, 58, 591–7. doi: 10.1002/pbc.23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuehn HS, Gloude NJ, Dimmock D, Tokita M, Wright M, Rosenzweig SD, et al. Abnormal SCID newborn screening and spontaneous recovery associated with a novel haploinsufficiency IKZF1 mutation. J Clin Immunol 2021, 41, 1241–9. doi: 10.1007/s10875-021-01035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuehn HS, Nunes-Santos CJ, Rosenzweig SD.. IKAROS-associated diseases in 2020: genotypes, phenotypes, and outcomes in primary immune deficiency/inborn errors of immunity. J Clin Immunol 2021, 41, 1–10. doi: 10.1007/s10875-020-00936-x. [DOI] [PubMed] [Google Scholar]
- 28. Nunes-Santos CJ, Kuehn HS, Rosenzweig SD.. IKAROS family zinc finger 1-associated diseases in primary immunodeficiency patients. Immunol Allergy Clin North Am 2020, 40, 461–70. doi: 10.1016/j.iac.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cytlak U, Resteu A, Bogaert D, Kuehn HS, Altmann T, Gennery A, et al. IKAROS family zinc finger 1 regulates dendritic cell development and function in humans. Nat Commun 2018, 9, 1239. doi: 10.1038/s41467-018-02977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoshino A, Toyofuku E, Mitsuiki N, Yamashita M, Okamoto K, Yamamoto M, et al. Clinical courses of IKAROS and CTLA4 deficiencies: a systematic literature review and retrospective longitudinal study. Front Immunol 2021, 12, 784901. doi: 10.3389/fimmu.2021.784901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okano T, Imai K, Naruto T, Okada S, Yamashita M, Yeh TW, et al. Whole-exome sequencing-based approach for germline mutations in patients with inborn errors of immunity. J Clin Immunol 2020, 40, 729–40. doi: 10.1007/s10875-020-00798-3. [DOI] [PubMed] [Google Scholar]
- 32. Thaventhiran JED, Lango Allen H, Burren OS, Rae W, Greene D, Staples E, et al.; Primary Immunodeficiency Consortium for the NIHR BioresourcePrimary Immunodeficiency Consortium for the NIHR Bioresource. Publisher Correction: whole-genome sequencing of a sporadic primary immunodeficiency cohort. Nature 2020, 584, E2. doi: 10.1038/s41586-020-2556-6. [DOI] [PubMed] [Google Scholar]
- 33. Kuijpers TW, Tromp SAM, van Leeuwen EMM, de Bree GJ.. Case report: a highly variable clinical and immunological presentation of IKAROS deficiency in a single family. Front Immunol 2022, 13, 865838. doi: 10.3389/fimmu.2022.865838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li JF, Dai YT, Lilljebjorn H, Shen SH, Cui BW, Bai L, et al. Transcriptional landscape of B cell precursor acute lymphoblastic leukemia based on an international study of 1,223 cases. Proc Natl Acad Sci USA 2018, 115, E11711–20. doi: 10.1073/pnas.1814397115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang X, Zhang X, Li X, Lv Y, Zhu Y, Wang J, et al. The specific distribution pattern of IKZF1 mutation in acute myeloid leukemia. J Hematol Oncol 2020, 13, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kellner ES, Krupski C, Kuehn HS, Rosenzweig SD, Yoshida N, Kojima S, et al. Allogeneic hematopoietic stem cell transplant outcomes for patients with dominant-negative IKFZ1/IKAROS mutations. J Allergy Clin Immunol 2019, 144, 339–42. [DOI] [PubMed] [Google Scholar]
- 37. Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the international union of immunological societies expert committee. J Clin Immunol 2020, 40, 24–64. doi: 10.1007/s10875-019-00737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014, 343, 301–5. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boast B, Nunes-Santos CJ, Kuehn HS, Rosenzweig SD.. IKAROS-associated diseases: from mice to humans and back again. Front Pediatr 2021, 9, 705497. doi: 10.3389/fped.2021.705497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Belot A, Rice GI, Omarjee SO.. Contribution of rare and predicted pathogenic gene variants to childhood-onset lupus: a large, genetic panel analysis of British and French cohorts (vol 2, pg e99, 2020). Lancet Rheumatol 2020, 2, E6–64-E. [DOI] [PubMed] [Google Scholar]
- 41. Yamashita M, Kuehn HS, Okuyama K, Okada S, Inoue Y, Mitsuiki N, et al. A variant in human AIOLOS impairs adaptive immunity by interfering with IKAROS. Nat Immunol 2021, 22, 893–903. doi: 10.1038/s41590-021-00951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuehn HS, Chang J, Yamashita M, Niemela JE, Zou C, Okuyama K, et al. T and B cell abnormalities, pneumocystis pneumonia, and chronic lymphocytic leukemia associated with an AIOLOS defect in patients. J Exp Med 2021, 218, e20211118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hetemaki I, Kaustio M, Kinnunen M, Heikkila N, Keskitalo S, Nowlan K, et al. Loss-of-function mutation in IKZF2 leads to immunodeficiency with dysregulated germinal center reactions and reduction of MAIT cells. Sci Immunol 2021, 6, eabe3454. doi: 10.1126/sciimmunol.abe3454. [DOI] [PubMed] [Google Scholar]
- 44. Shahin T, Kuehn HS, Shoeb MR, Gawriyski L, Giuliani S, Repiscak P, et al. Germline biallelic mutation affecting the transcription factor Helios causes pleiotropic defects of immunity. Sci Immunol 2021, 6, eabe3981. doi: 10.1126/sciimmunol.abe3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shahin T, Mayr D, Shoeb MR, Kuehn HS, Hoeger B, Giuliani S, et al. Identification of germline monoallelic mutations in IKZF2 in patients with immune dysregulation. Blood Adv 2022, 6, 2444–51. doi: 10.1182/bloodadvances.2021006367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.