Abstract
Background
Evidence for the prognostic implications of hyperglycaemia in older adults is inconsistent.
Objective
To evaluate disability-free survival (DFS) in older individuals by glycaemic status.
Methods
This analysis used data from a randomised trial recruiting 19,114 community-based participants aged ≥70 years, who had no prior cardiovascular events, dementia and physical disability. Participants with sufficient information to ascertain their baseline diabetes status were categorised as having normoglycaemia (fasting plasma glucose [FPG] < 5.6 mmol/l, 64%), prediabetes (FPG 5.6 to <7.0 mmol/l, 26%) and diabetes (self-report or FPG ≥ 7.0 mmol/l or use of glucose-lowering agents, 11%). The primary outcome was loss of disability-free survival (DFS), a composite of all-cause mortality, persistent physical disability or dementia. Other outcomes included the three individual components of the DFS loss, as well as cognitive impairment-no dementia (CIND), major adverse cardiovascular events (MACE) and any cardiovascular event. Cox models were used for outcome analyses, with covariate adjustment using inverse-probability weighting.
Results
We included 18,816 participants (median follow-up: 6.9 years). Compared to normoglycaemia, participants with diabetes had greater risks of DFS loss (weighted HR: 1.39, 95% CI 1.21–1.60), all-cause mortality (1.45, 1.23–1.72), persistent physical disability (1.73, 1.35–2.22), CIND (1.22, 1.08–1.38), MACE (1.30, 1.04–1.63) and cardiovascular events (1.25, 1.02–1.54) but not dementia (1.13, 0.87–1.47). The prediabetes group did not have an excess risk for DFS loss (1.02, 0.93–1.12) or other outcomes.
Conclusions
Among older people, diabetes was associated with reduced DFS, and higher risk of CIND and cardiovascular outcomes, whereas prediabetes was not. The impact of preventing or treating diabetes in this age group deserves closer attention.
Keywords: diabetes, disability-free survival, disability, older, cardiovascular, older people
Key Points
Compared with normoglycaemia, diabetes was associated with an increased risk of disability-free survival loss in older people.
Diabetes was also associated with increased risks of all-cause mortality, physical disability, cognitive impairment and CVD.
Prediabetes was not associated with disability-free survival loss and other age-related health outcomes in older people.
Introduction
Diabetes is a major public health challenge that affects older people (>65 years of age), with approximately one in four having diabetes, and half having prediabetes [1]. In addition to major macro- and microvascular complications, diabetes has also been associated with greater risks of cognitive decline, depression, urinary incontinence, injurious falls and persistent pain in older people [2]. The associated high disease burden may lead to impaired physical function and decreased quality of life, increased risk of permanent residential care and premature death in this age group [3].
Prediabetes is a well-accepted indicator of future risk of diabetes and provides an opportune stage at which intervention may prevent progression to diabetes. However, there is an ongoing debate about the utility of identifying prediabetes and whether it should be treated or is medicalising an issue, which does not alter prognosis among older people [4]. Prediabetes was suggested as being less informative as a clinical diagnosis in older people, due to the small proportion of prediabetes cases that progress to diabetes, and the lack of association between prediabetes and cardiovascular complications and mortality risk among older populations [5–7]. In clinical practice, there is a shift away from prolonging life expectancy towards maintaining a healthy and independent life in older age [4]; therefore, robust evidence from large-scale population-based studies with high-quality data is warranted to inform a broader impact of prediabetes in this age group.
We, therefore, conducted an observational analysis using data from a primary prevention clinical trial, ASPirin in Reducing Events in the Elderly (ASPREE) and its post-trial observation study ASPREE-eXTension (ASPREE-XT) [8–11]. Our primary aim was to compare survival free of dementia and persistent physical disability (‘disability-free survival’-DFS) among community-dwelling older people who had normoglycaemia, prediabetes or diabetes at enrolment.
Research design and methods
Data source and study population
ASPREE was a double-blind, randomised, placebo-controlled and trial of low-dose aspirin [8–11]. ASPREE recruited 19,114 community-dwelling participants (87% from Australia and 13% from US) aged ≥70 years (≥65 years for US minorities), who had no prior cardiovascular events, and were free of dementia and physical disability at enrolment [8–11]. After the trial end, 16,317 participants agreed to be followed for a further 5 years post-trial (ASPREE-XT). The study protocol of ASPREE-XT can be found at the ASPREE official website (https://aspree.org).
The present analysis included ASPREE participants with sufficient information to ascertain their diabetes status at baseline, and available follow-up time for each participant to the earliest occurrence of either the second ASPREE-XT post-trial annual visit (last visit completed in August 2019), the event of interest or loss to follow-up/withdrawal. The median follow-up was 6.9 (IQR 5.7, 8.0) years. Glycaemic status was categorised at trial enrolment as diabetes (self-report or fasting plasma glucose [FPG] ≥7.0 mmol/l [≥126 mg/dl] or use of glucose-lowering agents), prediabetes (FPG 5.6 to <7.0 mmol/l [100 to <126 mg/dl]) and normoglycaemia (FPG < 5.6 mmol/l [<100 mg/dl]). Blood collection for FPG occurred after an 8 h fast at a clinic or local pathology centre or the study trial centre (for US participants only).
Study endpoints
The main outcome was loss of DFS, a composite outcome of all-cause mortality, incident dementia or persistent physical disability. Other outcomes included the three individual components of the DFS loss (all-cause mortality, dementia, persistent physical disability) as well as CIND, major adverse cardiovascular events (MACE, including coronary heart disease death, nonfatal myocardial infarction, stroke) and any cardiovascular event (any cardiovascular death, nonfatal myocardial infarction and stroke, hospitalisation for heart failure). Dementia, mortality and CVD outcomes were adjudicated by committees blinded to ASPREE treatment allocation using pre-specified decision rules. CIND was derived from participant scores on the cognitive battery. Details on the outcomes can be found in the Supplemental Methods available in Age and Ageing online [8–11].
Statistical analysis
Inverse probability weighting (IPW)-based [12] Cox proportional-hazards regression models with adjustment for ASPREE treatment assignment (aspirin/placebo) were used to estimate weighted hazard ratios (HRs) and the 95% confidence intervals (CIs) for each outcome for the diabetes and prediabetes groups, with the normoglycaemia group as the reference (see Supplementary Methods available in Age and Ageing online). The proportional-hazards assumption was assessed with Schoenfeld residuals, and no violation was found. Weighted cumulative incidence curves were plotted for all outcomes. As sex and age have been suggested to be potential modifiers of the effects of prediabetes and diabetes on multiple clinical outcomes [13–16], subgroup analyses were conducted by sex and age (<75 and ≥75 years) to investigate their modifying effects.
Restricted cubic splines were plotted to visualise the relationship between baseline FPG and each outcome in the entire population and in participants with diabetes, with additional adjustment made for use of glucose-lowering agents including insulin.
Sensitivity analyses examined (i) re-classifying participants with diabetes based on need for therapy (a surrogate for disease severity) into ‘less severe diabetes’ (those who did not use glucose-lowering agents or used metformin alone) and ‘more severe diabetes’ (those using metformin and any other glucose-lowering agents including insulin) and comparing these groups to those with normoglycaemia; (ii) re-classifying participants with diabetes into the prediabetes group if they did not self-report diabetes nor use of glucose-lowering agents but did have an FPG ≥7.0 mmol/l at baseline; (iii) re-defining prediabetes according to the WHO diagnostic criterion as those with FPG 6.0–6.9 mmol/l (110–126 mg/dl) [17] and (iv) using a conventional adjustment approach instead of IPW. Finally, in the model for propensity score generation, we further adjusted for physical activity (self-reported physical activity level in a typical week at present, never/rare; light; moderate and vigorous), for which the data were collected in 12,468 Australian participants through a questionnaire based substudy of the main trial (ALSOP, A Longitudinal Study of Older Persons). The analyses were repeated for all outcomes using newly generated weights in those with available physical activity data.
All statistical tests were two-sided, and a P-value < 0.05 was considered statistically significant. Analyses were conducted using STATA/SE 17.0 for windows.
Results
Among 19,114 ASPREE participants, 298 whose baseline diabetes status could not be ascertained were excluded from this study, leaving 18,816 participants, of which 63% (n = 11,920) had normoglycaemia, 26% (n = 4,851) had prediabetes and 11% (n = 2,045) had diabetes at baseline. (Figure S1 available in Age and Ageing online) Among those with diabetes, 880 (43.0%) reported not taking any glucose-lowering agents, 1,008 (49.3%) reported taking 1 or more oral glucose-lowering agent(s), and 157 (7.7%) reported taking 1 or more oral glucose-lowering agents and/or insulin. Figure 1 illustrates the mode of categorisation of the diabetes diagnosis and Table S1, available in Age and Ageing online, presents the type of glucose-lowering agents used by those with diabetes. Table 1 presents the participant baseline characteristics by their baseline glycaemic status, before weighting. The mean (SD) FPG level in the original cohort of participants with normoglycaemia, prediabetes and diabetes were 5.0 (0.4), 6.0 (0.3) and 7.3 (2.0) mmol/l, respectively. Those classified as having more severe diabetes were more likely to be males (55% versus 49%), non-whites (26% versus 15%) and with a higher prevalence of obesity (53% versus 47%) and chronic kidney disease (45% versus 34%), than those classified as having less severe diabetes. The distribution of all covariates was balanced across the groups after weighting (all ASDs <0.10).
Figure 1.
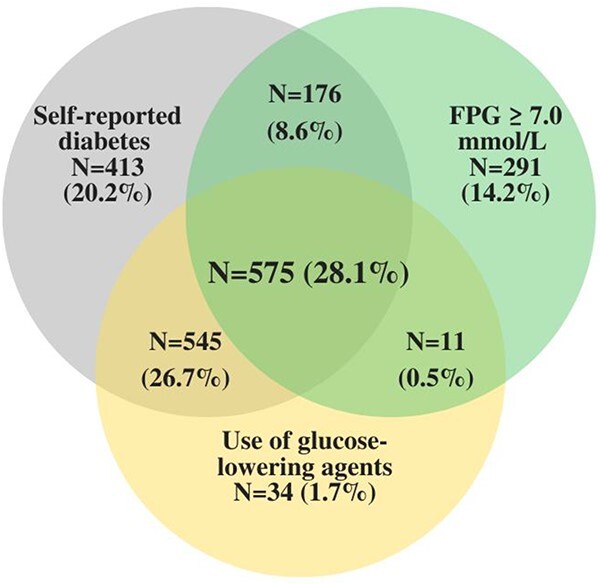
Diabetes diagnosis mode of categorisation (n = 2,045). Legend: The Venn diagram illustrates the mode of classification of the diabetes diagnosis. There were 575 (28.1%) participants with fasting plasma glucose (FPG) ≥7.0 mmol/l who also reported diabetes history and use of glucose-lowering medications; 545 (26.7%) reported diabetes history and medication use but had an FPG < 7.0 mmol/l; 176 (8.6%) with FPG ≥ 7.0 mmol/l reported diabetes history but not medication use; and 11 (0.5%) with FPG ≥ 7.0 mmol/l reported medication use but not diabetes history; 738 (36.1%) met only one criterion for diabetes diagnosis. (self-report diabetes: n = 413; FPG ≥ 7.0 mmol/l; n = 291; use of glucose-lowering medication: n = 34).
Table 1.
Participant baseline characteristics by baseline glycaemic status
| Normoglycaemia (n = 11,920) | Prediabetes (n = 4,851) | Diabetes (n = 2,045) | ASD | ||
|---|---|---|---|---|---|
| Before weighting | After weighting | ||||
| Age (Median, IQR) | 74.0 (71.6–77.8) | 73.9 (71.6–77.4) | 74.1 (71.5–77.7) | 0.04 | <0.10 |
| Female, n (%) | 7,314 (61.4%) | 2,288 (47.2%) | 999 (48.9%) | 0.14 | <0.10 |
| Race/country, n (%) | |||||
| White Australian | 10,192 (85.5%) | 4,294 (88.5%) | 1,590 (77.8%) | 0.11 | <0.10 |
| White American | 796 (6.7%) | 215 (4.4%) | 74 (3.6%) | 0.03 | <0.10 |
| Black | 515 (4.3%) | 166 (3.4%) | 219 (10.7%) | 0.07 | <0.10 |
| Hispanic/Latino | 278 (2.3%) | 96 (2.0%) | 112 (5.5%) | 0.04 | <0.10 |
| Others | 139 (1.2%) | 80 (1.7%) | 50 (2.4%) | 0.01 | <0.10 |
| BMI categories, n (%) | |||||
| <25 kg/m2 | 3,697 (31.0%) | 931 (19.2%) | 267 (13.1%) | 0.18 | <0.10 |
| ≥25, <30 kg/m2 | 5,367 (45.0%) | 2,223 (45.8%) | 786 (38.4%) | 0.07 | <0.10 |
| ≥30 kg/m2 | 2,854 (24.0%) | 1,697 (35.0%) | 992 (48.5%) | 0.25 | <0.10 |
| Waist circumference, n (%) | |||||
| Normal (women: <80 cm; men:<94 cm) | 2,411 (20.2%) | 608 (12.5%) | 171 (8.4%) | 0.12 | <0.10 |
| High (women: 80–<88 cm; men: 94–<102 cm) | 3,175 (26.6%) | 1,108 (22.8%) | 328 (16.0%) | 0.11 | <0.10 |
| Very high (women: ≥88 cm; men: ≥102 cm) | 6,334 (53.1%) | 3,135 (64.6%) | 1,546 (75.6%) | 0.22 | <0.10 |
| SBP, Mean (SD) | 138.4 (16.6) | 140.6 (16.2) | 140.3 (16.1) | 0.14 | <0.10 |
| DBP, Mean (SD) | 77.2 (10.0) | 77.7 (9.9) | 76.4 (9.8) | 0.14 | <0.10 |
| TC/HDL-C, Mean (SD) | 3.47 (1.01) | 3.68 (1.36) | 3.51 (1.09) | 0.18 | <0.10 |
| CKD, n (%) | 2,775 (24.9%) | 1,207 (26.5%) | 713 (37.2%) | 0.12 | <0.10 |
| Family history of MI, n (%) | 5,182 (43.5%) | 2,012 (41.5%) | 853 (41.7%) | 0.02 | <0.10 |
| Family history of dementia, n (%) | 3,107 (26.1%) | 1,168 (24.1%) | 443 (22.7%) | 0.04 | <0.10 |
| Cancer history, n (%) | 2,227 (18.7%) | 986 (20.3%) | 386 (18.9%) | 0.02 | <0.10 |
| Alcohol use, n (%) | |||||
| Never | 2,167 (18.2%) | 643 (13.3%) | 477 (23.3%) | 0.10 | <0.10 |
| Previous | 676 (5.7%) | 258 (5.3%) | 188 (9.2%) | 0.04 | <0.10 |
| Current | 9,075 (76.2%) | 3,948 (81.4%) | 1,380 (67.5%) | 0.14 | <0.10 |
| Smoking, n (%) | |||||
| Never | 6,891 (57.8%) | 2,480 (51.4%) | 1,041 (50.9%) | 0.07 | <0.10 |
| Previous | 4,601 (38.6%) | 2,167 (44.7%) | 906 (44.3%) | 0.06 | <0.10 |
| Current | 426 (3.6%) | 202 (4.2%) | 98 (4.8%) | 0.01 | <0.10 |
| Education (>12 years), n (%) | 6,634 (55.7%) | 2,647 (54.6%) | 1,034 (50.6%) | 0.05 | <0.10 |
| Statin, n (%) | 3,162 (26.5%) | 1,527 (31.5%) | 1,201 (58.7%) | 0.32 | <0.10 |
| ACE inhibitor/ARB, n (%) | 4,359 (36.6%) | 2,159 (44.5%) | 1,335 (65.3%) | 0.29 | <0.10 |
| Calcium channel blocker, n (%) | 1,742 (14.6%) | 939 (19.4%) | 571 (27.9%) | 0.13 | <0.10 |
| Diuretics, n (%) | 1,875 (15.7%) | 1,065 (22.0%) | 568 (27.8%) | 0.12 | <0.10 |
| β blocker, n (%) | 780 (6.5%) | 477 (9.8%) | 269 (13.2%) | 0.07 | <0.10 |
| Fasting plasma glucose, mean (SD) mmol/l | 5.0 (0.4) | 6.0 (0.3) | 7.3 (2.0) | – | – |
| Self-reported physical activity (n = 12,468) | |||||
| Never/rare | 111 (1.4%) | 43 (1.3%) | 29 (2.4%) | 0.002 | <0.10 |
| Light | 2,478 (31.3%) | 1,083 (32.4%) | 480 (39.7%) | 0.03 | <0.10 |
| Moderate | 4,025 (50.8%) | 1,690 (50.6%) | 563 (46.6%) | 0.01 | <0.10 |
| Vigorous | 1,307 (16.5%) | 523 (15.7%) | 136 (11.3%) | 0.02 | <0.10 |
Missing values from 77 participants [TC (n = 4), HDL-C (n = 19), BMI (n = 38), waist circumference (n = 29), education (n = 1)] were replaced with sex-specific mean values for each variable. BMI was calculated as weight in kilograms divided by height in metres squared. Chronic kidney disease is defined as an estimated glomerular filtration rate <60 ml/min/1.73 m2 or urinary albumin to creatinine ratio ≥3 mg/mmol. Self-reported physical activity was only adjusted in the regression model for propensity score generation in the sensitivity analysis in a subcohort of participants with available physical activity data. Abbreviations: ACE, angiotensin converting enzyme; ARB; angiotensin receptor blockers; ASD, Absolute standardised difference (before and after weighting); BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; IQR: Interquartile range; MI, myocardial infarction; SBP, systolic blood pressure; TC, total cholesterol.
For DFS loss, 2,829 events were recorded during a median follow-up of 6.9 years. Figure S2 available in Age and Ageing online shows the weighted cumulative incidence curve for each group. The crude incidence rate of DFS loss in participants with normoglycaemia, prediabetes and diabetes were 22.5, 23.5 and 32.5, respectively, per 1,000 person-years. Compared to those with normoglycaemia, the risk of DFS loss was significantly higher in participants with diabetes (weighted HR: 1.39, 95% CI: 1.21–1.60) but not different in those with prediabetes (1.02; 95% CI: 0.93–1.12) (Table 2).
Table 2.
Hazard ratios of study outcomes between participants with baseline normoglycaemia, prediabetes and diabetes
| Loss of disability-free survival | ||||||
| Events (rate) a | Crude HR (95% CI) | Weighted HR (95% CI) b | ||||
| Normoglycaemia | 1,710 (22.5) | 1 (Ref) | 1 (Ref) | |||
| Prediabetes | 724 (23.5) | 1.05 (0.96–1.14) | 1.02 (0.93–1.12) | |||
| Diabetes | 395 (32.5) | 1.48 (1.33–1.65) *** | 1.39 (1.21–1.60) *** | |||
| Secondary outcomes | ||||||
| All-cause mortality | Persistent physical disability c | |||||
| Events (rate) a | Crude HR (95% CI) | Weighted HR (95% CI) b | Events (rate) a | Crude HR (95% CI) | Weighted HR (95% CI) b | |
| Normoglycaemia | 1,099 (13.7) | 1 (Ref) | 1 (Ref) | 441 (6.1) | 1 (Ref) | 1 (Ref) |
| Prediabetes | 491 (15.1) | 1.11 (0.99–1.23) | 1.06 (0.95–1.18) | 168 (5.8) | 0.95 (0.79–1.13) | 0.85 (0.71–1.03) |
| Diabetes | 276 (21.2) | 1.60 (1.40–1.83) *** | 1.45 (1.23–1.72) *** | 117 (10.3) | 1.75 (1.43–2.15) *** | 1.73 (1.35–2.22) *** |
| Incident dementia | Cognitive impairment no dementia | |||||
| Events (rate) a | Crude HR (95% CI) | Weighted HR (95% CI) b | Events (rate) a | Crude HR (95% CI) | Weighted HR (95% CI) b | |
| Normoglycaemia | 561 (7.4) | 1 (Ref) | 1 (Ref) | 2,785 (44.4) | 1 (Ref) | 1 (Ref) |
| Prediabetes | 198 (6.4) | 0.87 (0.74–1.03) | 0.97 (0.82–1.15) | 1,098 (43.5) | 0.99 (0.93–1.07) | 1.00 (0.93–1.08) |
| Diabetes | 102 (8.4) | 1.17 (0.95–1.44) | 1.13 (0.87–1.47) | 511 (52.7) | 1.34 (1.22–1.47) *** | 1.22 (1.08–1.38) ** |
| Major adverse cardiovascular events | Any cardiovascular event | |||||
| Events (rate) a | Crude HR (95% CI) | Weighted HR (95% CI) b | Events (rate) a | Crude HR (95% CI) | Weighted HR (95% CI) b | |
| Normoglycaemia | 648 (8.7) | 1 (Ref) | 1 (Ref) | 797 (10.7) | 1 (Ref) | 1 (Ref) |
| Prediabetes | 322 (10.7) | 1.23 (1.08–1.41) ** | 1.08 (0.94–1.25) | 376 (12.5) | 1.17 (1.04–1.33) * | 1.05 (0.92–1.19) |
| Diabetes | 147 (12.3) | 1.44 (1.20–1.72) *** | 1.30 (1.04–1.63) * | 175 (14.7) | 1.40 (1.19–1.65) *** | 1.25 (1.02–1.54) * |
For the primary outcome, only first event of death, dementia, or persistent physical disability was counted. Abbreviations: CI, confidence interval; HR, hazard ratio.
aRate indicates crude incidence rate per 1,000 person-years.
bWeighted HR was also adjusted for ASPREE randomised treatment (aspirin/placebo).
c366, 148 and 98 participants in the normoglycemia, prediabetes and diabetes group had persistent ADL disability; 75, 20 and 19 participants in the normoglycemia, prediabetes and diabetes group were admitted to care for assistance with activities of daily living.
* P < 0.05.
** P < 0.01.
*** P < 0.001.
Bold values denote statistical significance at the p < 0.05 level.
Figure S2 available in Age and Ageing online shows the weighted cumulative incidence curves for the secondary outcomes for each group. When the components of the DFS loss were considered separately, the risks of all-cause mortality (weighted HR: 1.45, 95% CI: 1.23–1.72) and persistent physical disability (1.73, 95% CI: 1.35–2.22) were significantly higher in those with diabetes compared with the normoglycaemia group but not for dementia (1.13, 95% CI: 0.87–1.47). Participants with diabetes also had a higher risk of CIND (1.22, 95% CI: 1.08–1.38), MACE (1.30, 95% CI: 1.04–1.63), and any cardiovascular event (1.25, 95% CI: 1.02–1.54). Compared with the normoglycaemia group, those with prediabetes did not have excess risks for the other secondary outcomes (all P-values >0.05) (Table 2).
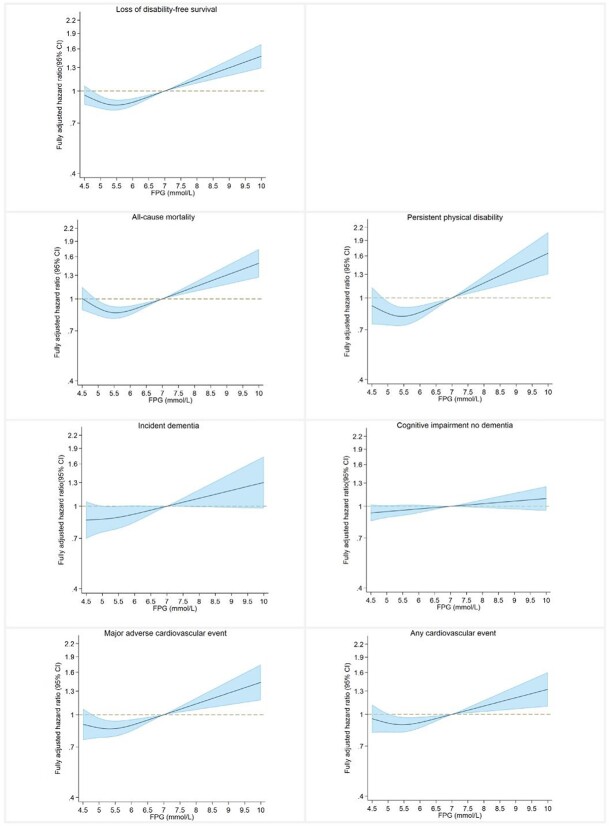
Figure 2 shows the restricted cubic splines for each outcome by baseline FPG levels in the entire study population. There was a non-linear relationship between FPG levels and DFS loss (nadir 5.6 mmol/l). The HR were roughly static for DFS loss until the FPG level approached 7.0 mmol/l at which point a positive linear association emerged. A similar trend was also seen for the relationship for persistent physical disability, all-cause mortality, MACE and any cardiovascular event. The relationship for incident dementia and CIND was more linear, with the risk increasing slowly with higher FPG levels. Among participants with diabetes, each 1 mmol/l increase in FPG was associated with equally 6% increased risks of the DFS loss (95% CI: 1.01–1.12) and all-cause mortality (95% CI:1.00–1.13), 9% increased risk of persistent physical disability (95% CI: 0.99–1.20), 13% increased risk of MACE (95% CI: 1.05–1.22) and 12% increased risk of any cardiovascular event (95% CI: 1.04–1.19). The positive associations between baseline FPG and risk of dementia (HR: 1.05, 0.94–1.17) and CIND (HR: 1.01, 0.96–1.06) were nonsignificant (Table S2 available in Age and Ageing online).
Figure 2.
Restricted cubic spline plots for each study outcome by baseline fasting plasma glucose levels. Legend: Restricted cubic spline was applied on baseline FPG with three knots, with the reference of FPG 7.0 mmol/l. Conventional adjustment was made for all covariates listed in Table 1 and for oral hypoglycaemic agents and insulin use. Spline curves were truncated at FPG of 4.5 and 10 mmol/l as less than 2% participants with FPG levels out of this range. The solid lines indicate the hazard ratios, and the shades denote 95% CIs.
Table S3 available in Age and Ageing online shows the baseline characteristics of participants with prediabetes and diabetes in males and females. The mean FPG level was similar in males and females with prediabetes (6.0 mmol/l) and was lower in females with diabetes compared to males with diabetes (7.1 versus 7.5 mmol/l). Among participants with diabetes who reported use of glucose-lowering medication, females were more likely to record an FPG <7 mmol/l than males.
Sex modified the association between diabetes and persistent physical disability, when compared with normoglycaemia (P-value for interaction <0.01); females with diabetes were significantly more likely to experience this outcome than males (Table 3). Sex also modified the associations between prediabetes, DFS loss and all-cause mortality, when compared with normoglycaemia (P for interaction <0.05); females with prediabetes were more likely to experience these outcomes than males and appear to be at an increased risk (HR [95% CI] for DFS loss was 1.15 (1.01–1.32) and for all-cause mortality was 1.29 [1.09–1.52]). No significant interaction was found between age and baseline glycaemic status for any outcome (Table S4 available in Age and Ageing online).
Table 3.
Risk of study outcomes between participants with normoglycaemia, prediabetes and diabetes, by sex
| Males (n = 8,215) | Females (n = 10,601) | P for interaction | |||
|---|---|---|---|---|---|
| Events (rate)a | HR (95% CI)b | Events (rate)a | HR (95% CI)b | ||
| Loss of disability-free survival | |||||
| Normoglycaemia | 783 (27.0) | 1 (Ref) | 927 (19.7) | 1 (Ref) | |
| Prediabetes | 398 (24.4) | 0.89 (0.79–1.01) | 326 (22.4) | 1.15 (1.01–1.32) | 0.005 |
| Diabetes | 217 (34.9) | 1.24 (1.03–1.51) | 178 (30.0) | 1.54 (1.26–1.87) | 0.13 |
| All-cause mortality | |||||
| Normoglycaemia | 557 (18.2) | 1 (Ref) | 542 (10.9) | 1 (Ref) | |
| Prediabetes | 276 (16.2) | 0.88 (0.76–1.02) | 215 (13.9) | 1.29 (1.09–1.52) | 0.001 |
| Diabetes | 174 (26.5) | 1.49 (1.20–1.84) | 102 (15.8) | 1.36 (1.05–1.78) | 0.62 |
| Incident dementia | |||||
| Normoglycaemia | 242 (8.4) | 1 (Ref) | 319 (6.8) | 1 (Ref) | |
| Prediabetes | 106 (6.5) | 0.83 (0.65–1.05) | 92 (6.3) | 1.10 (0.86–1.40) | 0.10 |
| Diabetes | 55 (8.8) | 1.07 (0.75–1.55) | 47 (7.9) | 1.17 (0.81–1.71) | 0.74 |
| Persistent physical disability | |||||
| Normoglycaemia | 173 (6.3) | 1 (Ref) | 268 (6.0) | 1 (Ref) | |
| Prediabetes | 81 (5.3) | 0.76 (0.58–1.00) | 87 (6.4) | 0.93 (0.72–1.20) | 0.30 |
| Diabetes | 43 (7.4) | 1.08 (0.72–1.63) | 74 (13.5) | 2.32 (1.70–3.16) | 0.004 |
| CIND | |||||
| Normoglycaemia | 1,118 (45.9) | 1 (Ref) | 1,667 (43.5) | 1 (Ref) | |
| Prediabetes | 590 (43.7) | 0.96 (0.86–1.06) | 508 (43.3) | 1.03 (0.93–1.15) | 0.31 |
| Diabetes | 249 (49.6) | 1.14 (0.95–1.36) | 262 (56.0) | 1.30 (1.10–1.53) | 0.29 |
| MACE | |||||
| Normoglycaemia | 334 (11.8) | 1 (Ref) | 314 (6.8) | 1 (Ref) | |
| Prediabetes | 206 (13.0) | 1.01 (0.85–1.21) | 116 (8.1) | 1.17 (0.93–1.46) | 0.33 |
| Diabetes | 95 (15.8) | 1.28 (0.96–1.71) | 52 (8.8) | 1.30 (0.91–1.86) | 0.97 |
| Any cardiovascular event | |||||
| Normoglycaemia | 393 (13.9) | 1 (Ref) | 404 (8.7) | 1 (Ref) | |
| Prediabetes | 231 (14.7) | 0.96 (0.81–1.14) | 145 (10.2) | 1.14 (0.94–1.39) | 0.20 |
| Diabetes | 108 (18.0) | 1.21 (0.93–1.59) | 67 (11.4) | 1.27 (0.93–1.75) | 0.84 |
Abbreviations: CI, confidence interval; CIND, cognitive impairment no dementia; HR, hazard ratio; MACE, major adverse cardiovascular events.
aRate indicates crude incidence rate per 1,000 person-years.
bHR was weighted HR and was adjusted for ASPREE randomised treatment (aspirin/placebo).
Bold values denote statistical significance at the p < 0.05 level.
Table S5 available in Age and Ageing online shows that participants with more severe diabetes (those reporting use of metformin as well as other glucose-lowering agents including insulin) had the greatest risk of DFS loss (weighted HR, 1.51; 95% CI: 1.18–1.95), all-cause mortality (1.75, 1.31–2.34), CVD (1.87, 1.36–2.58) and MACE (1.85, 1.30–2.63), followed by those with less severe diabetes (those who did not use glucose-lowering agents or used metformin alone), compared to those with normoglycaemia.
After re-classifying 291 participants with diabetes into the prediabetes group (diagnosed on FPG without self-report of diabetes or use of glucose-lowering agents), the results were consistent with the main findings (Table S6 available in Age and Ageing online) as they were when using the WHO criteria for prediabetes (Table S7 available in Age and Ageing online).
Adjusting for all covariates in Cox models instead of using IPW (Table S8 available in Age and Ageing online) did not materially change the findings. Using newly generated weights with further adjustment for participants’ physical activity levels yielded similar results to the main findings, except for an attenuated association between diabetes and CIND and wider Cis, as expected with the smaller sample size (Table S9 available in Age and Ageing online).
Discussion
In this observational analysis, compared with the normoglycaemia group, participants with diabetes had greater risks of DFS loss, all-cause mortality, persistent physical disability, CIND, MACE and any cardiovascular event. The prediabetes group did not have an overall excess risk of DFS loss nor other outcomes. Sex modified the association between diabetes and persistent physical disability, where a higher risk was observed for females. Sex also modified the associations between prediabetes, DFS loss and all-cause mortality, with higher risks seen in females with prediabetes compared with their male counterparts. The overall findings regarding diabetes reflect the substantial and broad adverse impact of diabetes imposed on ageing communities, highlighting the necessity of targeting prevention of diabetes in this age group. Our subgroup analysis results emphasised that an increased awareness of the sex differences in terms of the clinical implications of hyperglycaemia in older populations is warranted.
Maintaining function, independence and quality of life has become a clear goal for diabetes management of older populations. [4] Previous studies have suggested that diabetes in older adults is associated with a higher burden of functional decline [18], physical disability [19, 20], all-cause mortality [6] and a reduced physical DFS [21, 22], when defined as a composite outcome of physical disability and all-cause mortality. DFS in our study was defined as survival free from both physical and cognitive disability that is more reflective of a contemporary healthcare goal in older populations. We found that ASPREE participants with diabetes at baseline carried a greater risk of DFS loss compared with normoglycaemic participants. This was driven by increased persistent physical disability and death. DFS loss in the older population with diabetes is likely to be an aggregated result of diabetes-induced pathophysiological damage and complications, and its associated comorbidities that are potentially disabling and debilitating. [23, 24] We also found a stronger association between diabetes and persistent physical disability in females compared to males (weighted HR: 2.32 versus 1.08). This means that careful consideration of the optimal management of diabetes in older women who are otherwise relatively healthy is particularly needed. Congruent with our findings, a longitudinal cohort study using a nationally representative sample of 5,632 older Italians also found a significant association between diabetes and disability based on ADL limitations in women but not in men. [25]
Although no significant association between diabetes and incident dementia was observed in ASPREE participants in either sex, the inconsistency with previous studies [26, 27] may be explained by the fact that ASPREE required participants to be cognitively healthy at trial enrolment. Thus, recruited individuals may have initially been less susceptible to the development of dementia over the short term. However, diabetes was associated with a 22% increased risk of CIND, a precursor of dementia. The potential deleterious effect of diabetes on cognition may arise from its impact on the brain vasculature, blood flow, delivery of substrates essential to cognition and loss of brain volume. [28] We also found significant associations between diabetes and CVD outcomes that are consistent with the findings of other studies. [1] Results from the sensitivity analyses using proxies of more/less severe diabetes based on need for therapy and from the analyses assessing the prognostic implication of FPG in diabetes participants suggest that the high risk of DFS loss and CVD presented in those with more severe diabetes may indicate the need for more focus on optimal glucose control to maintain health among ageing populations.
Prediabetes was not associated with DFS loss nor other outcomes in the entire cohort. Previous studies have found that older adults with prediabetes had a similar risk of all-cause mortality and CVD events when compared with their normoglycaemic counterparts. [6, 7] An analysis by Rooney et al. [5] found that only 9% of older people with prediabetes progressed to diabetes, while 13% returned to normal glycaemic status over a median 5-year follow-up. These results suggest that prediabetes may not be as strong a risk factor in older adults. Furthermore, results from our subgroup analysis revealed a female preponderance in the mortality risks associated with prediabetes. Previous literature suggests that the excess risk of prediabetes and diabetes in older women has multiple explanations, with contributions from biological (steroidal and sex hormones), socioeconomic and psychological factors. [29]
The major study strength was its use of a large-scale, contemporary, well-characterised, community-based cohort of older individuals who were comprehensively followed for a median of 7 years with formal adjudication of major events, and serial assessment of cognition and physical disability. It is the first study investigating the clinical impact of prediabetes and diabetes on DFS loss in older populations, a single composite outcome that encapsulates major adverse outcomes in older adults. Given that the natural history of prediabetes and its clinical relevance in older adults remains incompletely characterised [5], our study findings of no association between prediabetes and DFS and other outcomes suggest that management of prediabetes per se may not be necessary for preserving cognitive and physical function in older populations.
This analysis has several limitations. First, it is an associative analysis and a causal relationship between diabetes and study outcomes cannot be established. Second, we lacked information on the duration of participants’ diabetes history. We also lack information on participants’ baseline HbA1c value and oral glucose tolerance test (OGTT) data, and thus were unable to distinguish prediabetes phenotypes in terms of impaired glucose tolerance, impaired fasting glucose, or the combination of the two. These phenotypes may have different prognostic significance [30]. However, it is less common for older adults to undertake OGTT with FPG ≤7.0 mmol/l and our results on the impact of impaired fasting glucose are consistent across different outcomes and robust. Third, it is possible that participants with prediabetes were prescribed with metformin for prevention of diabetes. Given that only 26 participants with FPG ≤7.0 mmol/l and without self-reported diabetes took metformin monotherapy at baseline, any potential misclassification bias, if it existed, would have little impact. Fourth, as most ASPREE participants were white, our study findings may not be generalised to African American, Hispanic and other ethnic groups. Finally, the sensitivity analysis adjusting for physical activity was conducted in a sub-cohort of those who participated in the questionnaire-based substudy (two-thirds of main trial participants). The results were generally consistent with the main results, suggesting the robustness of the main findings.
Conclusions
Diabetes in community-dwelling older adults was associated with an adverse impact on healthy ageing, including loss of disability-free survival, increased risks of persistent physical disability, all-cause mortality, cognitive decline with no dementia and cardiovascular disease. Prediabetes was not associated with any of these outcomes in the entire cohort but appeared to be linked to a higher risk of DFS loss and all-cause mortality in older females. The sex difference in prognostic implications of diabetes and prediabetes needs further investigation. The findings highlight the importance of diabetes prevention in older people and glucose control in individuals with diabetes, particularly older females.
Supplementary Material
Contributor Information
Zhen Zhou, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia; Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia.
Andrea J Curtis, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
Alice Owen, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
Rory Wolfe, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
Anne M Murray, Division of Geriatrics, Department of Medicine Hennepin HealthCare, Berman Centre for Outcomes and Clinical Research, Hennepin Healthcare Research Institute, Minneapolis, MN, USA; University of Minnesota, Minneapolis, MN, USA.
Andrew M Tonkin, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
Michael E Ernst, Department of Pharmacy Practice and Science, College of Pharmacy, The University of Iowa, Iowa, IA, USA; Department of Family Medicine, Carver College of Medicine, The University of Iowa, Iowa, IA, USA.
Suzanne G Orchard, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
Chao Zhu, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Prudence R Carr, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
Christopher M Reid, School of Public Health, Curtin University, Perth, WA, Australia.
Sara E Espinoza, Sam and Ann Barshop Institute, UT Health San Antonio, San Antonio, TX, USA; Geriatrics Research, South Texas Veterans Health Care System, San Antonio, TX, USA.
Raj C Shah, Department of Family and Preventive Medicine, The Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, IL, USA.
Robyn L Woods, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
Joanne Ryan, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
John J McNeil, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
Mark R Nelson, Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia.
Sophia Zoungas, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia.
Declaration of Conflicts of Interest
A.M.T. reported receiving research support or honoraria from Amgen, Boehringer-Ingelheim, Merck and Pfizer, as well as materials in the ASPREE trial from Bayer. M.R.N. reported receiving honoraria from Sanofi and Amgen as well as Bayer for materials in ASPREE. S.Z. has received payment to the institution (Monash University) from Eli Lilly Australia, Boehringer-Ingelheim, Merck Sharp & Dohme Australia, AstraZeneca, Novo Nordisk, Sanofi and Servier for consultancy work outside the submitted work. Others declare no conflict of interest.
Declaration of Sources of Funding
The ASPREE and ASPREE-XT studies are supported by grants (U01AG029824 and U19AG062682) from the National Institute on Ageing and the National Cancer Institute at the National Institutes of Health in the United States, by grants (334047 and 1127060) from the National Health and Medical Research Council of Australia, and by Monash University and the Victorian Cancer Agency.
Data Availability
Requests for data access will be via the ASPREE Principal Investigators with details for applications provided through https://aspree.org/aus/for-researchers/ or https://aspree.org/usa/for-researchers/.
References
- 1. Halter JB, Musi N, McFarland Horne Fet al. Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes 2014; 63: 2578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roman de Mettelinge T, Cambier D, Calders P, Van Den Noortgate N, Delbaere K. Understanding the relationship between type 2 diabetes mellitus and falls in older adults: a prospective cohort study. PloS One 2013; 8: e67055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown AF, Mangione CM, Saliba D, Sarkisian CA. California healthcare foundation/American Geriatrics Society panel on improving care for elders with diabetes. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc 2003; 51: S265–80. [DOI] [PubMed] [Google Scholar]
- 4. Sinclair A, Dunning T, Rodriguez-Manas L. Diabetes in older people: new insights and remaining challenges. Lancet Diabetes Endocrinol 2015; 3: 275–85. [DOI] [PubMed] [Google Scholar]
- 5. Rooney MR, Rawlings AM, Pankow JSet al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med 2021; 181: 511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang O, Matsushita K, Coresh Jet al. Mortality implications of prediabetes and diabetes in older adults. Diabetes Care 2020; 43: 382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deedwania P, Patel K, Fonarow GCet al. Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population-based cohort study. Int J Cardiol 2013; 168: 3616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McNeil JJ, Nelson MR, Woods RLet al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med 2018; 379: 1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McNeil JJ, Wolfe R, Woods RLet al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018; 379: 1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McNeil JJ, Woods RL, Nelson MRet al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med 2018; 379: 1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. ASPREE Investigator Group . Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials 2013; 36: 555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mansournia MA, Altman DG. Inverse probability weighting. BMJ 2016; 352: i189. [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Wu H, Fan Bet al. The role of age on the risk relationship between prediabetes and major morbidities and mortality: analysis of the Hong Kong diabetes surveillance database of 2 million Chinese adults. Lancet Reg Health West Pac 2022; 22: 100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nanayakkara N, Curtis AJ, Heritier Set al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses. Diabetologia 2021; 64: 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, O'Neil A, Jiao Yet al. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med 2019; 17: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Regensteiner JG, Golden S, Huebschmann AGet al. Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American Heart Association. Circulation 2015; 132: 2424–47. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva, Switzerland: WHO Press; 2011. [PubMed]
- 18. Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev 2010; 6: 134–43. [DOI] [PubMed] [Google Scholar]
- 19. Gregg EW, Beckles GL, Williamson DFet al. Diabetes and physical disability among older U.S. adults. Diabetes Care 2000; 23: 1272–7. [DOI] [PubMed] [Google Scholar]
- 20. Godino JG, Appel LJ, Gross ALet al. Diabetes, hyperglycemia, and the burden of functional disability among older adults in a community-based study. J Diabetes 2017; 9: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huo L, Shaw JE, Wong E, Harding JL, Peeters A, Magliano DJ. Burden of diabetes in Australia: life expectancy and disability-free life expectancy in adults with diabetes. Diabetologia 2016; 59: 1437–45. [DOI] [PubMed] [Google Scholar]
- 22. Andrade FC. Measuring the impact of diabetes on life expectancy and disability-free life expectancy among older adults in Mexico. J Gerontol B Psychol Sci Soc Sci 2010; 65B: 381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong E, Backholer K, Gearon Eet al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2013; 1: 106–14. [DOI] [PubMed] [Google Scholar]
- 24. Forbes A. Reducing the burden of mortality in older people with diabetes: a review of current research. Front Endocrinol (Lausanne) 2020; 11: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maggi S, Noale M, Gallina Pet al. Physical disability among older Italians with diabetes. The ILSA study. Diabetologia 2004; 47: 1957–62. [DOI] [PubMed] [Google Scholar]
- 26. Cholerton B, Baker LD, Montine TJ, Craft S. Type 2 diabetes, cognition, and dementia in older adults: toward a precision health approach. Diabetes Spectr 2016; 29: 210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chatterjee S, Peters SA, Woodward Met al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016; 39: 300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberts RO, Knopman DS, Przybelski SAet al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 2014; 82: 1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kautzky-Willer A, Harreiter J, Abrahamian Het al. Sex and gender-specific aspects in prediabetes and diabetes mellitus-clinical recommendations (update 2019). Wien Klin Wochenschr 2019; 131: 221–8. [DOI] [PubMed] [Google Scholar]
- 30. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 2016; 37: 278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for data access will be via the ASPREE Principal Investigators with details for applications provided through https://aspree.org/aus/for-researchers/ or https://aspree.org/usa/for-researchers/.