ABSTRACT
This interim analysis of an ongoing phase 1 randomized clinical trial evaluated the safety, reactogenicity, and immunogenicity of mRNA-1283, a next-generation SARS-CoV-2 messenger RNA (mRNA)-based vaccine encoding two segments of the spike protein (i.e. receptor binding and N-terminal domains). Healthy adults aged 18–55 years (n = 104) were randomized (1:1:1:1:1) to receive two doses of mRNA-1283 (10, 30, or 100 µg) or mRNA-1273 (100 µg) administered 28 days apart, or a single dose of mRNA-1283 (100 µg). Safety was assessed and immunogenicity was measured by serum neutralizing antibody (nAb) or binding antibody (bAb) responses. At the interim analysis, no safety concerns were identified and no serious adverse events, adverse events of special interest, or deaths were reported. Solicited systemic adverse reactions were more frequent with higher dose levels of mRNA-1283 than with mRNA-1273. At day 57, all dose levels of the 2-dose mRNA-1283 regimen (including the lowest dose level [10 µg]) induced robust nAb and bAb responses that were comparable to those of mRNA-1273 (100 µg). mRNA-1283 was generally safe in adults, with all dose levels of the 2-dose regimen (10, 30, and 100 µg) eliciting similar immunogenicity as the 2-dose mRNA-1273 regimen (100 µg).
Clinical Trials Registration: Clinicaltrials.gov, NCT04813796.
KEYWORDS: Severe acute respiratory syndrome coronavirus-2, COVID-19, vaccination, clinical study, mRNA vaccines
Introduction
An unprecedented global response to the coronavirus disease 2019 (COVID-19) pandemic led to the rapid development of vaccines against the causative pathogen, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The messenger RNA (mRNA)-based vaccine mRNA-1273 (SPIKEVAX; Moderna, Inc., Cambridge, MA, USA)1 is authorized and approved for use in many populations worldwide, available for individuals as young as ≥ 6 months.2 In the phase 3 clinical trial in adults, mRNA-1273 had an acceptable safety profile and was efficacious against symptomatic infection and severe disease.3 Subsequent postauthorization real-world studies provided evidence of mRNA-1273 effectiveness against symptomatic infection and hospitalization4–6 but demonstrated reduced effectiveness against infection caused by several SARS-CoV-2 variants of concern, including B.1.351 (beta), AY lineages (delta), and BA lineages (omicron).7–9 A booster dose is recommended in individuals aged ≥ 12 years to maintain effectiveness.10
The response to the COVID-19 pandemic demonstrated that the mRNA-lipid nanoparticle (LNP) platform affords several advantages, including expeditious development of safe and efficacious vaccines;11 however, the refrigerated shelf life of some of these vaccines can limit vaccine distribution in certain markets.12–15 Next-generation mRNA-based SARS-CoV-2 vaccines that offer longer refrigerated shelf life and/or are immunogenic at a lower dose than mRNA-1273, or broaden protection by targeting multiple SARS-CoV-2 strains or other viruses (ie, multivalent or combination vaccines), may potentially further prevent disease-associated morbidity and mortality.
While mRNA-1273 encodes the full-length SARS-CoV-2 S protein to elicit robust immunogenicity, the investigational mRNA-1283 vaccine candidate aims to elicit immune responses by encoding only two segments of the SARS-CoV-2 S protein that contain key SARS-CoV-2 neutralization epitopes, the N-terminal domain (NTD) and the receptor binding domain (RBD),16,17 connected by a short G3SG3 flexible linker and also anchored into the membrane with a C-terminal influenza hemagglutinin transmembrane segment. Including only the major antigenic domains of SARS-CoV-2 has the potential to focus the neutralizing antibody (nAb) responses to these target regions, while the shorter mRNA length of mRNA-1283 facilitates higher levels of protein expression at similar or lower doses compared to mRNA-1273 as well as longer storage under refrigeration. Inclusion of the influenza hemagglutinin transmembrane segment allows for the presentation of the tandem NTD-RBD domains on the cell surface for specific B-cell recognition.18 A preclinical evaluation of mRNA-1283 in mice demonstrated the vaccine had comparable protection and immunogenicity characteristics as mRNA-1273 with potential dose-sparing properties, while indicating that mRNA-1283 showed improved storage stability under refrigeration than mRNA-1273.18
Here, we report preliminary safety, reactogenicity, and immunogenicity results for mRNA-1283 from a phase 1, randomized, observer-blind, dose-ranging study in healthy adults aged 18–55 years.
Methods
Trial design and participants
This is an ongoing phase 1, randomized, observer-blind study being conducted at 5 sites in the United States to evaluate the safety, reactogenicity, and immunogenicity of mRNA-1283 (NCT04813796). Eligible participants were healthy adults (aged 18–55 years) with negative SARS-CoV-2 serology, no known history of SARS-CoV-2 infection or exposure to SARS-CoV-2 or COVID-19 in the previous 30 days, and were not previously administered any coronavirus vaccine. A full list of the study inclusion/exclusion criteria is in the Supplement.
Three dose levels of mRNA-1283 (10 µg, 30 µg, and 100 µg) and one dose level of mRNA-1273 (100 µg) were administered on a 2-dose schedule with 28 days between doses; the mRNA-1283 100-µg dose level was also assessed as a single-dose regimen. Participants were randomly assigned in parallel (1:1:1:1:1) using interactive response technology (Figure S1; see the Supplement for details). Participants were followed for safety, reactogenicity, and immunogenicity, with a planned interim analysis to evaluate data on ≥ 80 participants through day 57. After the interim analysis, participants who received the single mRNA-1283 100-µg dose were offered an additional injection of mRNA-1273 (100 µg).
The protocol was approved by the central institutional review board (Advarra, Inc; Columbia, MD) and the study was conducted in accordance with the protocol, applicable laws and regulatory requirements, the International Council for Harmonization Good Clinical Practice guidelines, and the ethical principles derived from the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethical Guidelines. All participants provided written informed consent before any study procedures were conducted.
Vaccines
mRNA-1283 is an mRNA-LNP vaccine encoding for a chimeric protein (NTD-RBD-HATM) comprising the SARS-CoV-2 S protein NTD and RBD, linked together by a flexible peptide linker and anchored to a 23-amino acid transmembrane domain from influenza hemagglutinin (HATM). mRNA-1273 is an mRNA-LNP vaccine encoding for the full-length, 2-proline prefusion stabilized SARS-CoV-2 S protein (S2P). Placebo was a 0.9% sodium chloride (normal saline) injection.
Vaccines were provided as a sterile liquid for injection at 0.2 mg/mL (mRNA-1273) or 0.5 mg/mL (mRNA-1283; diluted with 0.9% saline to the appropriate dose) concentrations; all vaccines were administered at 0.5-mL dose volume into the deltoid muscle. For the 2-dose regimen, each dose level of mRNA-1283 or mRNA-1273 was administered 28 days apart (day 1 and day 29). The 1-dose regimen of mRNA-1283 had placebo administered on day 1 and mRNA-1283 (100 µg) administered on day 29.
Objectives and endpoints
The primary objective was to evaluate the safety and reactogenicity of mRNA-1283 (10 µg, 30 µg, and 100 µg) and mRNA-1273 (100 µg), each administered on a 2-dose schedule (28 days apart), and mRNA-1283 (100 µg) administered as a single dose. Safety endpoints included solicited local (i.e., pain, erythema, or hardness at the injection site; or axillary swelling or tenderness) and systemic (i.e., headache, fatigue, myalgia, arthralgia, nausea, chills, and fever) adverse reactions (ARs) ≤ 7 days of each dose, as recorded by participants using an electronic diary; safety laboratory abnormalities ≤ 7 days of each dose (see the Supplement); unsolicited adverse events (AEs) ≤ 28 days of each dose; and any serious AEs (SAEs), medically attended AEs (MAAEs), AEs leading to discontinuation, and AEs of special interest (AESIs) from day 1 to end of study. Safety data were monitored by an unblinded, independent data and safety monitoring board, a blinded internal safety team, and a blinded safety oversight team. This report summarizes interim safety assessments after a median of 127 days (> 4 months) after dose 2.
Secondary objectives were to evaluate the immunogenicity of mRNA-1283 administered as a 2-dose schedule (10 µg, 30 µg, and 100 µg) or a single-dose schedule (100 µg) and mRNA-1273 administered as a 2-dose schedule (100 µg) as measured by nAb titer or level of binding antibody (bAb). Serum nAb titers were measured by a validated lentivirus assay19,20 (PsVN assay; see the Supplement) against Wuhan-Hu-1 (with D614G mutation) SARS-CoV-2 or the B.1.351 (beta) variant with a 50% inhibitory dilution; titers against Wuhan-Hu-1 (without D614G mutation) were also measured by a validated live virus 50% microneutralization assay21 (see the Supplement). Levels of serum bAbs against Wuhan-Hu-1 SARS-CoV-2 were measured by a SARS-CoV-2 4-PLEX ligand binding assay (Meso Scale Discovery [MSD] modified 3-PLEX assay;22 see the Supplement). For this interim analysis, only assessments on samples collected on day 1 (baseline) and days 29, 36, and 57 were summarized.
Statistical analyses
Sample size determination is described in the Supplement. Safety was assessed in the safety and solicited safety populations (defined in the Supplement). All safety analyses were summarized according to the actual vaccine received. Safety was descriptively analyzed as counts and percentages.
Immunogenicity was assessed in the per-protocol population (defined in the Supplement). For each vaccine group, the geometric mean titer (GMT) or levels of SARS-CoV-2 nAb and bAb were computed at each sampling time point, as well as the geometric mean fold rise (GMFR) of titers or levels from baseline to each post-baseline time point; values were summarized alongside corresponding 95% confidence intervals (CIs) calculated based on the t distribution of the log-transformed values, then back-transformed to the original scale for presentation. The geometric mean ratio (GMR) calculated as the mean of the difference in nAb titers and bAb levels between mRNA-1283 and mRNA-1273 and the corresponding 2-sided 95% CI were provided. Percentages of participants with vaccine seroresponse were also calculated by time point and vaccine group alongside 2-sided 95% CIs using the Clopper-Pearson method. Seroresponse was defined as an increase in titer or level from less than the lower limit of quantification [LLOQ] at baseline to ≥ LLOQ, or a ≥ 4-fold increase over baseline in participants with preexisting baseline titers or levels that were ≥ LLOQ. All analyses were performed using SAS software, version 9.4 (SAS Institute).
Results
Participants
Between March 11, 2021, and July 28, 2021, a total of 104 participants were randomly assigned to receive two doses of mRNA-1283 (10 µg, n = 21; 30 µg, n = 22; 100 µg, n = 21), one dose of mRNA-1283 (100 µg; n = 18), or two doses of mRNA-1273 100 µg (n = 22). All randomly assigned participants received dose 1; 75 (91.5%) and 20 participants (90.9%) received dose 2 in the mRNA-1283 and mRNA-1273 groups, respectively (Figure 1). Overall, 68 participants (82.9%) in the mRNA-1283 groups and 18 participants (81.8%) in the mRNA-1273 group were included in the per-protocol immunogenicity population for this interim analysis. Participant baseline demographics are summarized in Table 1.
Figure 1.
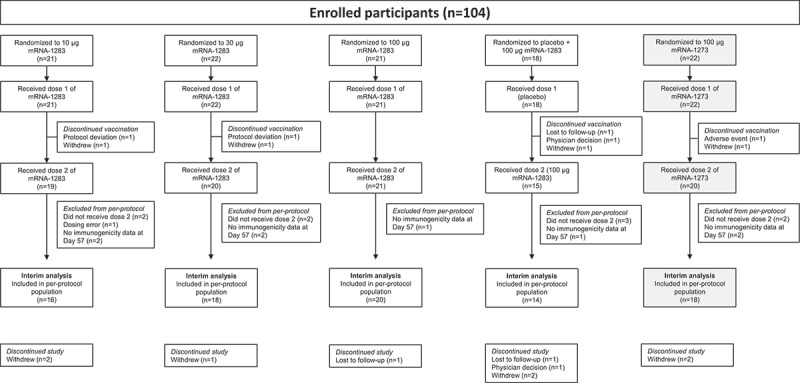
Participant disposition. One participant in the mRNA-1273 100-µg group discontinued vaccination because of an adverse event, which was considered related to study vaccination. All randomly assigned participants who received dose 1 of the study vaccination were included in the safety population. Reasons for exclusion from the per-protocol immunogenicity population are described in the Supplement. Abbreviation: mRNA, messenger RNA.
Table 1.
Participant baseline demographics (safety population).
| mRNA-1283 |
mRNA-1273 |
||||
|---|---|---|---|---|---|
| 10 µg (n = 21) |
30 µg (n = 22) |
100 µg (n = 21) |
Placebo + 100 µg (n = 18) |
100 µg (n = 22) |
|
| Age, y | |||||
| Mean ± SD | 38.1 ± 9.5 | 34.8 ± 10.4 | 34.8 ± 10.8 | 33.7 ± 10.4 | 42.0 ± 10.3 |
| Median (range) | 41.0 (23–51) | 30.5 (21–54) | 36.0 (19–51) | 30.5 (19–49) | 43.0 (22–55) |
| Sex, n (%) | |||||
| Male | 9 (42.9) | 13 (59.1) | 12 (57.1) | 12 (66.7) | 14 (63.6) |
| Female | 12 (57.1) | 9 (40.9) | 9 (42.9) | 6 (33.3) | 8 (36.4) |
| Race, n (%) | |||||
| White | 15 (71.4) | 16 (72.7) | 16 (76.2) | 10 (55.6) | 16 (72.7) |
| Black/African American | 1 (4.8) | 2 (9.1) | 1 (4.8) | 0 | 1 (4.5) |
| Asian | 0 | 1 (4.5) | 2 (9.5) | 1 (5.6) | 1 (4.5) |
| American Indian or Alaska Native | 0 | 0 | 0 | 1 (5.6) | 0 |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 1 (5.6) | 0 |
| Multiracial | 1 (4.8) | 0 | 0 | 2 (11.1) | 0 |
| Other | 0 | 0 | 0 | 0 | 0 |
| Not reported | 4 (19.0) | 3 (13.6) | 2 (9.5) | 3 (16.7) | 4 (18.2) |
| Ethnicity, n (%) | |||||
| Not Hispanic or Latino | 14 (66.7) | 15 (68.2) | 16 (76.2) | 13 (72.2) | 14 (63.6) |
| Hispanic or Latino | 7 (33.3) | 7 (31.8) | 5 (23.8) | 4 (22.2) | 7 (31.8) |
| Not reported | 0 | 0 | 0 | 1 (5.6) | 1 (4.5) |
| Weight, kg | |||||
| Mean ± SD | 81.3 ± 14.4 | 76.9 ± 17.1 | 73.4 ± 15.5 | 82.5 ± 19.9 | 81.5 ± 11.9 |
| Height, cm | |||||
| Mean ± SD | 173.5 ± 11.2 | 171.8 ± 10.0 | 172.1 ± 12.1 | 175.0 ± 9.2 | 172.3 ± 8.4 |
| BMI, kg/m2 | |||||
| Mean ± SD | 27.0 ± 3.8 | 25.9 ± 4.1 | 24.6 ± 3.3 | 26.7 ± 4.5 | 27.5 ± 3.6 |
All randomly assigned participants who received dose 1 of the study vaccination were included in the safety population.
Abbreviations: BMI, body mass index; mRNA, messenger RNA; SD, standard deviation.
Safety
Solicited local and systemic adverse reactions
Across mRNA-1283 groups, solicited local ARs ≤ 7 days of vaccination were reported by 6–18 participants (33.3–85.7%) after dose 1 and 12–19 participants (73.7–90.5%) after dose 2 (Figure 2(a)); 21 (95.5%) and 19 mRNA-1273 recipients (95.0%) reported solicited local ARs after dose 1 and dose 2, respectively. The majority of solicited local ARs were mild to moderate (grades 1 and 2), with no grade 4 events reported. Pain at the injection site was most common, reported by > 70% of participants across vaccine groups following dose 2. The median duration of solicited local ARs was 2–3 days after mRNA-1283 and 3 days after mRNA-1273.
Figure 2.
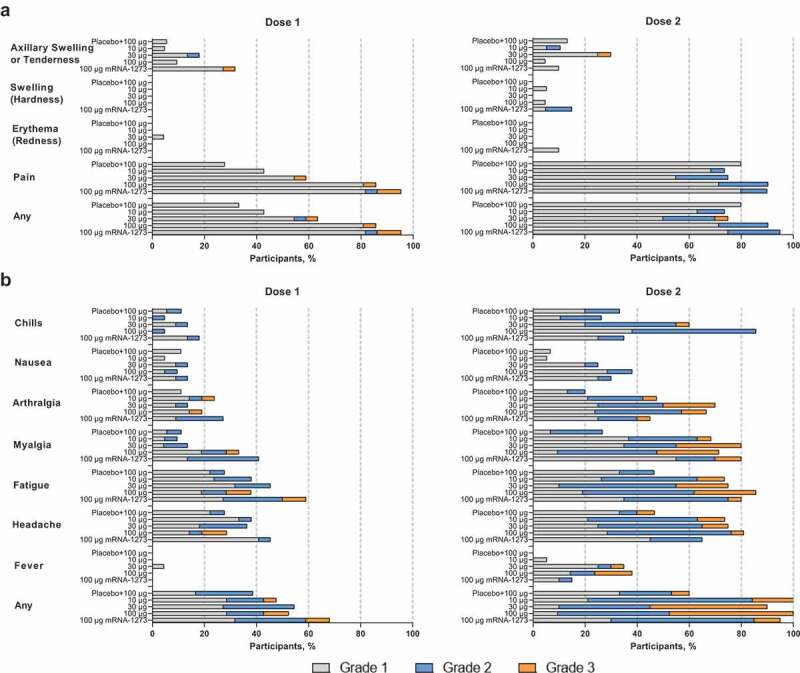
Solicited (a) local and (b) systemic adverse reactions within 7 days of each dose. Percentages are based on participants in the solicited safety population, consisting of all randomly assigned participants receiving ≥1 dose and contributing any solicited adverse reaction data. Number of participants in the dose 1 solicited safety population: mRNA-1283 10 µg, n = 21; mRNA-1283 30 µg, n = 22; mRNA-1283 100 µg, n = 21; placebo + mRNA-1283 100 µg, n = 18; and mRNA-1273 100 µg, n = 22. Number of participants in the dose 2 solicited safety population: mRNA-1283 10 µg, n = 19; mRNA-1283 30 µg, n = 20; mRNA-1283 100 µg, n = 21; placebo + mRNA-1283 100 µg, n = 15; and mRNA-1273 100 µg, n = 20. Abbreviation: mRNA, messenger RNA.
After dose 1, solicited systemic ARs ≤ 7 days of vaccination were reported by 7–12 participants (38.9–54.5%) across mRNA-1283 groups and 15 participants (68.2%) in the mRNA-1273 group (Figure 2(b)). Systemic ARs were more frequent after dose 2, reported by 9–21 participants (60–100%) across mRNA-1283 groups and 19 participants (95.0%) in the mRNA-1273 group. Headache, fatigue, and myalgia were most frequent. Solicited systemic ARs were predominantly mild to moderate after dose 1 of mRNA-1283, but increased in severity after dose 2, with 3 to 12 (20.0–63.2%) and 1 to 10 participants (6.7–47.6%) reporting grade 2 and 3 events, respectively; no grade 4 events were reported. Increased severity was predominantly reported at higher mRNA-1283 dose levels. Fever and chills were largely reported after dose 2 and for participants who received mRNA-1283 at higher dose levels. The median duration of solicited systemic ARs was 2–3 days after mRNA-1283 and 2 days after mRNA-1273.
Unsolicited adverse events
No prespecified study pause rules were met, and no SAEs, AESIs, severe AEs, or fatal AEs were reported ≤ 28 days after any dose of mRNA-1283 or mRNA-1273. Unsolicited treatment-emergent AEs (TEAEs) were reported by 4–9 participants (22.2–42.9%) across mRNA-1283 groups and 7 participants (31.8%) in the mRNA-1273 group (Table 2). Overall, 2–4 (9.5–19.0%) and 4 participants (18.2%) reported unsolicited TEAEs considered related to either mRNA-1283 or mRNA-1273, respectively. Across mRNA-1283 groups, a total of 2–3 participants (9.1–14.3%) reported MAAEs; two participants reported vaccine-related MAAES (n = 1, allergic dermatitis [mRNA-1283 30 µg]; n = 1, headache, fatigue, arthralgia, and myalgia [mRNA-1283 100 µg]). Three mRNA-1273 recipients (13.6%) reported MAAEs, and one MAAE (vomiting, 4.5%) was considered related to vaccination. One participant in the mRNA-1273 group discontinued vaccination because of transient elevation in liver transaminase levels 7 days after dose 1 that subsequently returned to normal, which was considered vaccine-related. Clinical laboratory findings are presented in the Supplement.
Table 2.
Summary of unsolicited TEAEs through 28 days following any vaccination (safety population).
| mRNA-1283 |
mRNA-1273 | ||||
|---|---|---|---|---|---|
| n (%) | 10 µg (n = 21) |
30 µg (n = 22) |
100 µg (n = 21) |
Placebo + 100 µg (n = 18) |
100 µg (n = 22) |
| All unsolicited TEAEs | |||||
| All AEs | 8 (38.1) | 9 (40.9) | 9 (42.9) | 4 (22.2) | 7 (31.8) |
| SAEs | 0 | 0 | 0 | 0 | 0 |
| Fatal AEs | 0 | 0 | 0 | 0 | 0 |
| MAAEs | 3 (14.3) | 2 (9.1) | 2 (9.5) | 2 (11.1) | 3 (13.6) |
| AEs leading to vaccination discontinuation | 0 | 0 | 0 | 0 | 1 (4.5) |
| AEs leading to study discontinuation | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| AESIs | 0 | 0 | 0 | 0 | 0 |
| Related unsolicited TEAEs | |||||
| All AEs | 2 (9.5) | 4 (18.2) | 4 (19.0) | 2 (11.1) | 4 (18.2) |
| SAEs | 0 | 0 | 0 | 0 | 0 |
| Fatal AEs | 0 | 0 | 0 | 0 | 0 |
| MAAEs | 0 | 1 (4.5) | 1 (4.8) | 0 | 1 (4.5) |
| AEs leading to vaccination discontinuation | 0 | 0 | 0 | 0 | 1 (4.5) |
| AEs leading to study discontinuation | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 |
| AESIs | 0 | 0 | 0 | 0 | 0 |
A TEAE is any event not present before exposure to study vaccination or any event already present that worsened in intensity or frequency after exposure.
Abbreviations: AE, adverse event; AESI, adverse event of special interest; MAAE, medically attended adverse event; mRNA, messenger RNA; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Immunogenicity
Serum neutralizing antibodies against SARS-CoV-2
For the 2-dose regimen, pseudovirus nAb GMTs to D614G SARS-CoV-2 increased from baseline after dose 1 (day 29) of mRNA-1273 (100 µg) and all dose levels of mRNA-1283 (10 µg, 30 µg, and 100 µg; Figure 3a). At 28 days following dose 2 (day 57), pseudovirus nAb GMTs (95% CI) for all dose levels of mRNA-1283 administered were comparable to those of mRNA-1273 (mRNA-1283, 10 µg: 1,370 [888–2,112], 30 µg: 1091 [645–1,846], and 100 µg: 1679 [1,133–2,487]; mRNA-1273: 1,190 [826–1,713]; Figure 3(a); Table S1); generally similar findings were observed against Wuhan-Hu-1 (without D614G mutation) with the live-virus microneutralization assay (Figure S2; Table S1). As measured by pseudovirus nAb titers against D614G, seroresponse at day 57 occurred in 100% of participants in all groups who received two doses of mRNA vaccines. Geometric mean ratios (95% CI; Table S1) of GMTs for 2-dose regimens of mRNA-1283 versus mRNA-1273 at day 57 were 1.15 (0.67–1.98), 0.92 (0.50–1.70), and 1.41 (0.84–2.38) for the mRNA-1283 10-µg, 30-µg, and 100-µg dose levels, respectively. For the 1-dose mRNA-1283 regimen (placebo + mRNA-1283 100 µg), a slight increase from baseline was observed 28 days after mRNA-1283 administration (day 57) but remained lower than 2-dose regimens; the GMR (95% CI) at day 57 relative to two doses of mRNA-1273 was 0.05 (0.01–0.18) and seroresponse occurred in 28.6% of participants.
Figure 3.
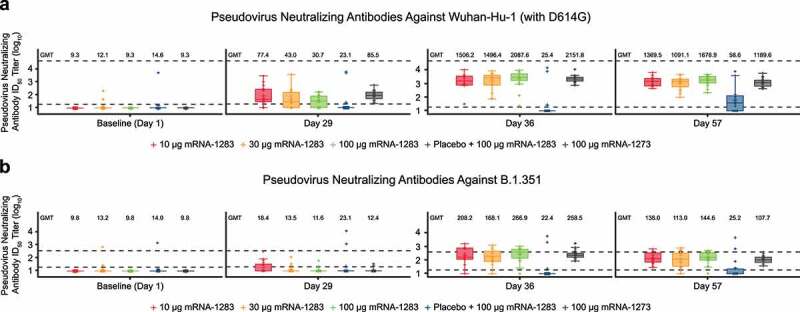
Pseudovirus neutralizing antibody ID50 titers against (a) Wuhan-Hu-1 (with D614G mutation) SARS-CoV-2 or (b) B.1.351 variant through day 57. Neutralizing antibody titers on the pseudovirus neutralization assay at ID50 (log scale) are shown for those participants in the per-protocol immunogenicity population. In both panels, boxplots are based on log-transformed values; boxes and horizontal bars denote IQR range and ID50, respectively, with whisker end points equal to the values below or above the median at 1.5 times the IQR. Dots represent individual results and the dotted lines represent either the LLOQ or ULOQ. Antibody values reported as below the LLOQ were replaced by 0.5 × LLOQ, whereas values greater than the ULOQ were converted to the ULOQ if the actual values were unavailable. In panel a, the LLOQ was 18.5 (log10[LLOQ] = 1.267) and the ULOQ was 45,118 (log10[ULOQ] = 4.654). In panel b, the LLOQ was 19.5 (log10[LLOQ] = 1.290) and the ULOQ was 385.7 (log10[ULOQ] = 2.586).
Abbreviations: GMT, geometric mean titer; ID50, 50% inhibitory dose; IQR, interquartile range; LLOQ, lower limit of quantification; mRNA, messenger RNA; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; ULOQ, upper limit of quantification.
After dose 2, all 2-dose mRNA-1283 groups (10 µg, 30 µg, and 100 µg) had comparable pseudovirus nAb GMTs to B.1.351 compared with mRNA-1273 (Figure 3(b); Table S1). The 1-dose mRNA-1283 100 µg regimen did not induce a substantial nAb response to B.1.351 at day 57 (Figure 3(b)). At day 57, seroresponse to B.1.351 occurred in 61.1–85.0% and 66.7% of participants in the 2-dose mRNA-1283 groups and mRNA-1273 group, respectively (Table S1); 14.3% of participants in the 1-dose mRNA-1283 regimen had a seroresponse to B.1.351.
Serum binding antibodies against SARS-CoV-2
For the 2-dose regimens, bAb immunoglobulin G (IgG) responses to Wuhan-Hu-1 full-length S protein were low or undetectable at baseline for all vaccine groups and increased after dose 1 of mRNA-1283 (10 µg, 30 µg, and 100 µg) and mRNA-1273 (100 µg; Figure 4(a)). At 28 days after dose 2 (day 57), bAb responses were generally comparable between mRNA-1283 and mRNA-1273 regardless of dose level (Figure 4(a); Table S2), with geometric mean (GM) levels (95% CI) reported as 322,671 (232,477–447,858), 280,454 (196,077–401,139), 416,620 (312,082–556,175), and 303,004 (242,750–378,212) for the mRNA-1283 10-µg, 30-µg, and 100-µg and mRNA-1273 100-µg dose levels, respectively. Seroresponse at day 57 occurred in 100% of mRNA-1283 (all dose levels) or mRNA-1273 100 µg recipients. Similar trends were observed for bAb IgG responses to Wuhan-Hu-1 S protein RBD and NTD (Figure 4(b,c); Table S2), with all dose levels of mRNA-1283 eliciting similar responses to either domain at day 57 as compared with mRNA-1273; seroresponses were observed in 100% of 2-dose vaccine recipients at day 57.
Figure 4.
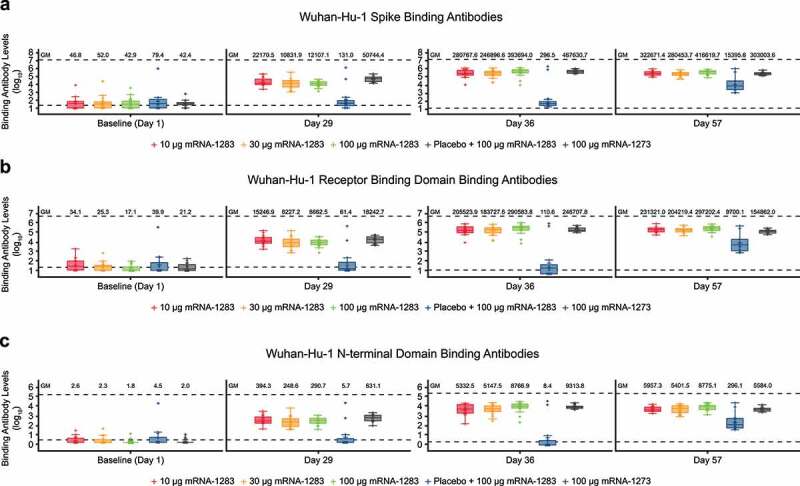
Binding antibody levels through day 57 specific to Wuhan-Hu-1 SARS-CoV-2 (a) S protein, (b) RBD, or (C) NTD Binding IgG antibody levels by MSD 4-PLEX assay are shown for those participants in the per-protocol immunogenicity population. Boxplots are based on log-transformed values; boxes and horizontal bars denote IQR range and GM, respectively, with whisker end points equal to the values below or above the median at 1.5 times the IQR. Individual results are represented as dots and the dotted lines represent either the LLOQ or ULOQ. Antibody values reported as below the LLOQ were replaced by 0.5 × LLOQ, whereas values greater than the ULOQ were converted to the ULOQ if actual values were unavailable. In panel a, the LLOQ was 23 (log10[LLOQ] = 1.362) and the ULOQ was 14,000,000 (log10[ULOQ] = 7.146). In panel b, the LLOQ was 19 (log10[LLOQ] = 1.279) and the ULOQ was 6,000,000 (log10[ULOQ] = 6.778). In panel c, the LLOQ was 3 (log10[LLOQ] = 0.477) and the ULOQ was 200000 (log10[ULOQ] = 5.301).
Abbreviations: GM, geometric mean; ID50, 50% inhibitory dose; IgG, immunoglobulin G; IQR, interquartile range; LLOQ, lower limit of quantification; mRNA, messenger RNA; MSD, Meso Scale Discovery; NTD, N-terminal domain; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; ULOQ, upper limit of quantification.
The 1-dose mRNA-1283 regimen (placebo + mRNA-1283 100 µg) showed an increase from baseline in bAb responses to Wuhan-Hu-1 S protein, RBD, and NTD at 28 days after mRNA-1283 was administered (day 57). GM levels (95% CI) were 15,396 (4,855–48,820) for S protein, 9,700 (3,110–30,252) for RBD, and 296 (95–919) for NTD; seroresponse against S protein, RBD, and NTD was 92.9%.
Discussion
Here, we present interim safety and immunogenicity findings of a phase 1 clinical study in healthy adults of a next-generation SARS-CoV-2 vaccine candidate (mRNA-1283), which encodes the SARS-CoV-2 S protein RBD and NTD segments versus full-length S protein encoded by mRNA-1273. Preliminary findings indicated that all mRNA-1283 dose levels (10 µg, 30 µg, and 100 µg) administered on a 2-dose schedule (28 days apart) were generally safe and elicited robust immune responses that were comparable to mRNA-1273 (100 µg). The lowest investigated mRNA-1283 dose level (10 µg) had the most favorable tolerability profile and induced robust nAb and bAb responses to SARS-CoV-2 after dose 2 that were similar to those of mRNA-1273. Overall, these findings suggest that a 2-dose regimen of mRNA-1283 at a lower dose level may achieve similar vaccine efficacy to mRNA-1273.
No safety concerns were identified and no deaths, SAEs, or AESIs were reported. Both local and systemic ARs after dose 1 of mRNA-1283 were generally mild to moderate, characterized by pain at the injection site, headache, fatigue, and myalgia. After dose 2, solicited systemic ARs generally increased in frequency and severity. Overall, compared with mRNA-1273, solicited systemic ARs occurred more frequently with higher dose levels of mRNA-1283.
Following a 2-dose schedule, all mRNA-1283 dose levels (including the lowest dose [10 µg]) induced nAb responses after dose 2 to prototype SARS-CoV-2 that were comparable to mRNA-1273 (100 µg). Notably, the pseudovirus nAb responses after dose 2 of mRNA-1273 in this study align with those among adults in the pivotal phase 3 trial,23 wherein two doses of mRNA-1273 (100 µg) administered 28 days apart were efficacious against symptomatic COVID-19 infection and severe COVID-19.3,24 Additionally, levels of bAb responses to full-length S protein, NTD, and RBD were similar between mRNA-1273 and all mRNA-1283 dose levels at 28 days following dose 2. Our interim phase 1 findings raise the possibility that a lower mRNA-1283 dose administered via a 2-dose regimen might achieve similar efficacy and effectiveness as mRNA-1273 among adults. Notably, although the 1-dose regimen of mRNA-1283 (100 µg) induced bAb responses at 28 days, levels remained lower than those for 2-dose mRNA-1283 and mRNA-1273 regimens; further, neutralizing responses to D614G SARS-CoV-2 remained low after one dose, together supporting a 2-dose, primary series schedule of mRNA-1283. The 2-dose schedule of mRNA-1283 (all dose levels) and mRNA-1273 also elicited comparable nAb responses to the SARS-CoV-2 B.1.351 variant after dose 2; further studies evaluating variant-specific modifications of mRNA-1283 as a booster dose are currently underway (NCT05137236).25
These data suggest that higher mRNA-1283 dose levels administered in a 2-dose regimen do not result in higher nAb responses; this is in contrast to a previous phase 1 study of mRNA-1273 in adults, which showed a dose-dependent increase in neutralizing responses.26 Preclinical findings also observed that two doses of mRNA-1283 administered at lower dose levels than mRNA-1273 elicited higher nAb titers and bAb responses in mice, which may be due to greater antigen expression with mRNA-1283 at lower dose levels.18 In addition, antibodies to the RBD and NTD regions are the primary contributors to the neutralization response observed in pseudovirus assays,27,28 and the design of mRNA-1283 to focus the immune response to these 2 regions may shift the dose-dependent response of the vaccine to dose levels lower than 10 µg. Whether these findings extend to T-cell responses in humans is currently being assessed. Because the severity of solicited systemic ARs was increased at the mRNA-1283 100-µg dose level, it remains possible that increased systemic inflammation at higher dose levels, potentially through complex interactions of type 1 interferons or other innate immune effector molecules, may suppress B- and/or T-cell responses and warrants further study.29–31
This study was strengthened by its randomized design, which allowed for descriptive comparisons to mRNA-1273, a widely administered SARS-CoV-2 vaccine with known efficacy in preventing COVID-19. A limitation was the small number of participants enrolled. This interim report presents findings through day 57 of an ongoing phase 1 trial that will continue to evaluate mRNA-1283 safety and immunogenicity, including long-term antibody responses to inform on immune response durability, which remains important amid reports of waning immunity within 6 months of mRNA-1273 primary vaccination.20,32
In conclusion, these preliminary findings support the continued evaluation of mRNA-1283, a next-generation SARS-CoV-2 vaccine. All dose levels of mRNA-1283 administered via a 2-dose regimen, 28 days apart, were generally safe in healthy adults aged 18–55 years, with the lowest dose level (10 µg) inducing comparable immunogenicity to the approved mRNA-1273 100 µg regimen. Clinical evaluation of mRNA-1283 is ongoing to assess the applicability of booster doses at lower dose levels and immune responses against other SARS-CoV-2 variants.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Murray Kimmel (Optimal Research, Melbourne, Florida, USA), Dr. Daniel Brune (Optimal Research, Peoria, Illinois, USA), and Dr. Randall Severance (AES/Optimal Research, Chandler, Arizona, USA) for their contributions to the study. Medical writing and editorial assistance were provided by Emily Stackpole, PhD, of MEDiSTRAVA in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna, Inc., and under the direction of the authors.
Funding Statement
This work was supported by Moderna, Inc.
Disclosure statement
PY and MH have no conflicts to declare. YDP, LS, AA, US, and RP are employees and shareholders in Moderna, Inc.
Data sharing statement
Upon request, and subject to review, Moderna, Inc. will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Moderna, Inc. may also provide access to the related individual anonymized participant data.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2190690.
Contributions
YDP, US, and RP were involved in the study concept and design. Data collection was performed by PY, MH, and LS. Analysis and interpretation of the data were undertaken by YDP, US, AA, and RP. All authors contributed to the drafting and critical review of this manuscript and approved the final draft.
References
- 1.FDA . Package Insert - SPIKEVAX. 2022. [accessed 2022 Mar 15]. https://www.fda.gov/media/155675/download.
- 2.World Health Organization . Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19. 2022. [accessed 2023 Mar 13]. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-mRNA-1273-2021.3. [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–9. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, Tian Y, Florea A, Takhar HS, Tubert JE, et al. Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: interim results from a prospective observational cohort study. Lancet Reg Health Am. 2021:100134. doi: 10.2139/ssrn.3916094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, Malek JA, Coyle P, Ayoub HH, Al Kanaani Z, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–21. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 6.Pilishvili T, Gierke R, Fleming-Dutra KE, Farrar JL, Mohr NM, Talan DA, Krishnadasan A, Harland KK, Smithline HA, Hou PC, et al. Effectiveness of mRNA Covid-19 vaccine among U.S. health care personnel. N Engl J Med. 2021;385(25):e90. doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, Tian Y, Florea A, Aragones M, Tubert JE, et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375:e068848. doi: 10.1136/bmj-2021-068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, AlMukdad S, Coyle P, Ayoub HH, Al Kanaani Z, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 delta variant in Qatar. Nat Med. 2021;27(12):2136–43. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 9.Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, Bruxvoort KJ, Tubert JE, Florea A, Ku JH, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med. 2022;28(5):1063–71. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, Lewis N, Natarajan K, Stenehjem E, Grannis SJ, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–63. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhary N, Weissman D, Whitehead KA.. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20(11):817–38. doi: 10.1038/s41573-021-00283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uddin MN, Roni MA.. Challenges of storage and stability of mRNA-Based COVID-19 vaccines. Vaccines. 2021;9(9):1033. doi: 10.3390/vaccines9091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–34. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acharya KP, Ghimire TR, Subramanya SH. Access to and equitable distribution of COVID-19 vaccine in low-income countries. NPJ Vaccines. 2021;6(1):54. doi: 10.1038/s41541-021-00323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crommelin DJA, Anchordoquy TJ, Volkin DB, Jiskoot W, Mastrobattista E. Addressing the cold reality of mRNA vaccine stability. J Pharm Sci. 2021;110(3):997–1001. doi: 10.1016/j.xphs.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouwer PJM, Caniels TG, van der Straten K, Snitselaar JL, Aldon Y, Bangaru S, Torres JL, Okba NMA, Claireaux M, Kerster G, et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369(6504):643–50. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Wang P, Nair MS, Yu J, Rapp M, Wang Q, Luo Y, Chan JFW, Sahi V, Figueroa A, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584(7821):450–56. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 18.Stewart-Jones GBE, Elbashir SM, Wu K, Lee D, Renzi I, Ying B, Koch M, Sein CE, Choi A, Whitener B, et al. Development of SARS-CoV-2 mRNA vaccines encoding spike N-terminal and receptor binding domains. bioRxiv. 2022; 2022.10.07.511319. [DOI] [PubMed]
- 19.Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J, Yoon H, Li D, Haynes BF, Sanders KO, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29(4):529–39.e3. doi: 10.1016/j.chom.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi A, Koch M, Wu K, Chu L, Ma L, Hill A, Nunna N, Huang W, Oestreicher J, Colpitts T, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27(11):2025–31. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu L, McPhee R, Huang W, Bennett H, Pajon R, Nestorova B, Leav B. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine. 2021;39(20):2791–99. doi: 10.1016/j.vaccine.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu L, Vrbicky K, Montefiori D, Huang W, Nestorova B, Chang Y, Carfi A, Edwards DK, Oestreicher J, Legault H, et al. Immune response to SARS-CoV-2 after a booster of mRNA-1273: an open-label phase 2 trial. Nat Med. 2022;28(5):1042–49. doi: 10.1038/s41591-022-01739-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Sahly HM, Baden LR, Essink B, Montefiori D, McDermont A, Rupp R, Lewis M, Swaminathan S, Griffin C, Fragoso V, et al. Humoral immunogenicity of the mRNA-1273 vaccine in the phase 3 COVE trial. J Infect Dis. 2022;226(10):1731–42. doi: 10.1093/infdis/jiac188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, Campbell TB, Clark J, Jackson LA, Fichtenbaum CJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385(19):1774–85. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ClinicalTrials.gov . A study to evaluate the immunogenicity and safety of mRNA-1283 COVID-19 vaccine boosters. 2021.
- 26.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. 2020;383(20):1920–31. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCallum M, De Marco A, Lempp FA, Tortorici MA, Pinto D, Walls AC, Beltramello M, Chen A, Liu Z, Zatta F, et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell. 2021;184(9):SS0092-1 doi: 10.1016/j.cell.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrell AG, Dadonaite B, Greaney AJ, Eguia R, Loes AN, Franko NM, Logue J, Carreño JM, Abbad A, Chu HY, et al. Receptor-binding domain (RBD) antibodies contribute more to SARS-CoV-2 neutralization when target cells express high levels of ACE2. Viruses. 2022;14(9):2061. doi: 10.3390/v14092061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15(2):87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darrah PA, Hegde ST, Patel DT, Lindsay RW, Chen L, Roederer M, Seder RA. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J Exp Med. 2010;207(7):1421–33. doi: 10.1084/jem.20092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arunachalam PS, Scott MKD, Hagan T, Li C, Feng Y, Wimmers F, Grigoryan L, Trisal M, Edara VV, Lai L, et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature. 2021;596(7872):410–16. doi: 10.1038/s41586-021-03791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pegu A, O’Connell SE, Schmidt SD, O’Dell S, Talana CA, Lai L, Albert J, Anderson E, Bennett H, Corbett KS, et al. Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. Science. 2021;373(6561):1372–77. doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


