Abstract
The incidence, presentation, and predisposing factors of post-acute sequelae of COVID-19 (PASC) are currently poorly understood. Lung explants may provide a rare insight into terminal SARS-CoV-2-associated lung damage and its pathophysiology. A 62-year-old man presented with progressively worsening respiratory symptoms after recovering from mild COVID-19 3 months earlier. No underlying pulmonary comorbidities were reported. A chest CT revealed bilateral extensive ground-glass and reticular opacities, suspicious of pulmonary fibrosis. Despite initial high-dose glucocorticoid therapy, the interstitial lung disease progressed, and after exhausting all viable therapeutic options, bilateral lung transplantation was successfully conducted. Histological analysis revealed extensive end-stage interstitial fibrosis with diffuse dendriform ossification and bronchiolar and transitional cell metaplasia. Signs of interstitial remodeling such as an increased interstitial collagen deposition, a pathological accumulation of CD163+/CD206+ M2-polarized macrophages with an increased expression of phosphorylated ERK, and an increased density of CD105+ newly formed capillaries were observed. qRT-PCR and immunohistochemistry for SARS-CoV-2 N-protein in the endothelium of medium-sized vessels confirmed a persistence of SARS-CoV-2. Our findings highlight a highly unusual presentation of SARS-CoV-2-associated lung fibrosis, implying that incomplete viral clearance in the vascular compartment may play a vital pathophysiological role in the development of PASC.
Keywords: COVID-19, SARS-COV-2, Post-COVID-19, Dendriform pulmonary ossification, Pulmonary fibrosis, Lung transplant
Established Facts
A subset of COVID-19 patients develops post-acute sequelae of COVID-19, encompassing a broad range of mostly unspecific symptoms currently poorly characterized.
Observational studies report that more than half of hospitalized COVID-19 patients suffer from abnormal lung function 90 days after the initial symptom onset, typically in the form of decreased diffusion capacity for carbon monoxide. This is due to varying degrees of residual pulmonary pathology on imaging.
In some cases of severe chronic COVID-19-associated respiratory disease, lung transplantation offers the only viable curative treatment option and has been successfully conducted in several expert centers worldwide, with initially promising results.
Novel Insights
We present a case study of a 62-year-old male with terminal COVID-19-associated lung fibrosis and persistence of SARS-CoV-2 despite experiencing mild COVID-19 3 months earlier, indicating that chronic disease may develop irrespective of initial disease severity in rare instances.
Diffuse dendriform ossification, which developed alongside diffuse interstitial fibrosis, may be an additional histological feature of COVID-19-associated lung fibrosis.
Incomplete viral clearance of the pulmonary vasculature, as in the present case, may play an important role in triggering the development of specific post-acute sequelae of COVID-19.
Introduction
While the clinical characteristics of acute COVID-19 are well known, the incidence, severity, and risk factors of its long-term effects remain poorly characterized. Most patients return to their baseline state of health after recovering from acute severe acute respiratory infection coronavirus 2 (SARS-CoV-2) infection; however, a subset presents with a persistent, mostly unspecific range of symptoms such as fatigue, dyspnea, arthralgia and chest pain weeks to months after recovery [1, 2]. A WHO-led Delphi process defined the “post-COVID-19 condition” also called “long COVID-19” or “post-acute sequelae of COVID-19” (PASC) as symptoms present 3 months after acute disease that last for at least 2 months [3]. Importantly, PASC develops irrespective of initial disease severity and may affect a multitude of organ systems, although a particularly high prevalence is noted among cases with initially severe disease [4, 5]. The incidence of chronic pulmonary disease remains unclear, although observational studies have found that more than half of hospitalized COVID-19 patients present with abnormal lung function 90 days after the symptom onset [6, 7]. Chest CT abnormalities are a frequent finding up to 1 year post-COVID-19, although the incidence varies between datasets [8, 9, 10]. One radiological study reported a 56% incidence of radiological changes 3 months after infection, with 12% of cases demonstrating fibrosis [9]. Prospective follow-up on SARS-CoV-1 patients has shown 2–6% of cases with persistent radiological changes after 15 years, although it is unclear whether this applies to SARS-CoV-2 [11].
Lung transplantation has been performed on a small subset of COVID-19 patients suffering from end-stage disease with promising posttransplantation outcomes [12, 13, 14, 15, 16, 17]. This has led to the introduction of selection criteria by a consortium of transplant centers worldwide in an effort to systematize indications, timing and postoperative care [18]. Nevertheless, further interdisciplinary research needs to be conducted to characterize the presentation and development of chronic COVID-19-associated lung disease. Herein, we report an unusual presentation of pulmonary PASC in need of lung transplantation with unusual histomorphological features and persistent SARS-CoV-2 viral load in the explant.
Case Report
Clinical History and Radiological Findings
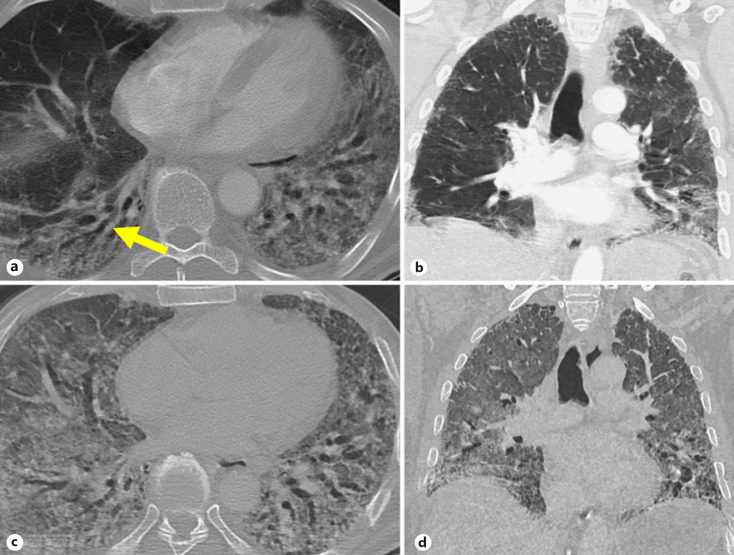
A 62-year-old man presented with acute dyspnea at rest, requiring high-flow oxygen therapy with a fraction of inspired oxygen of 80% in February 2021. A nasopharyngeal SARS-CoV-2 PCR was negative; however, the patient reported contracting a mild case of COVID-19 in December 2020, which was treated in an outpatient setting. Known comorbidities included hypertension, hyperlipidemia, and diabetes mellitus type II with a negative history of smoking and lung disease. A chest CT scan performed at admission revealed bilateral, diffuse ground-glass opacities, bronchiectasis and subpleural reticular changes consistent with pulmonary fibrosis predominantly in the middle and lower lobes (Fig. 1a, b).
Fig. 1.
CT images at admission and before transplantation at admission: axial (a) and coronal (b) chest CT shows ground-glass and reticular opacities in the dependent lung with bronchiectasis (arrow). Before transplantation: axial (c) and coronal (d) chest CT shows extensive bilateral ground-glass opacities, reticulation, and bronchiectasis consistent with ARDS pattern. ARDS, acute respiratory distress syndrome.
Treatment with high-dose glucocorticoids was initiated, starting with 10 mg/day dexamethasone for 21 days with a switch to oral 25 mg/day prednisolone and 1,500 mg/day mycophenolate mofetil. However, therapy remained ineffective, and lung function progressively worsened (forced expiratory volume of 1.23 L [42%], total lung capacity of 3.1 L [51%] 2 weeks after admission). Imaging demonstrated a rapid decline of his condition with an acute respiratory distress syndrome pattern, upon which the patient was intubated (Fig. 1c, d). The indication of lung transplantation was established in line with previously suggested selection criteria [18]. As a bridge to transplantation, a venovenous extracorporeal membrane oxygenation was implanted a month after admission. A week later, bilateral lung transplantation was successfully conducted.
No major postoperative complications were reported. After undergoing an intensive rehabilitative program, the patient was discharged 2 months later with steadily improving forced expiratory volume values (2.9 L [92%] in June 2021).
Histopathological Findings
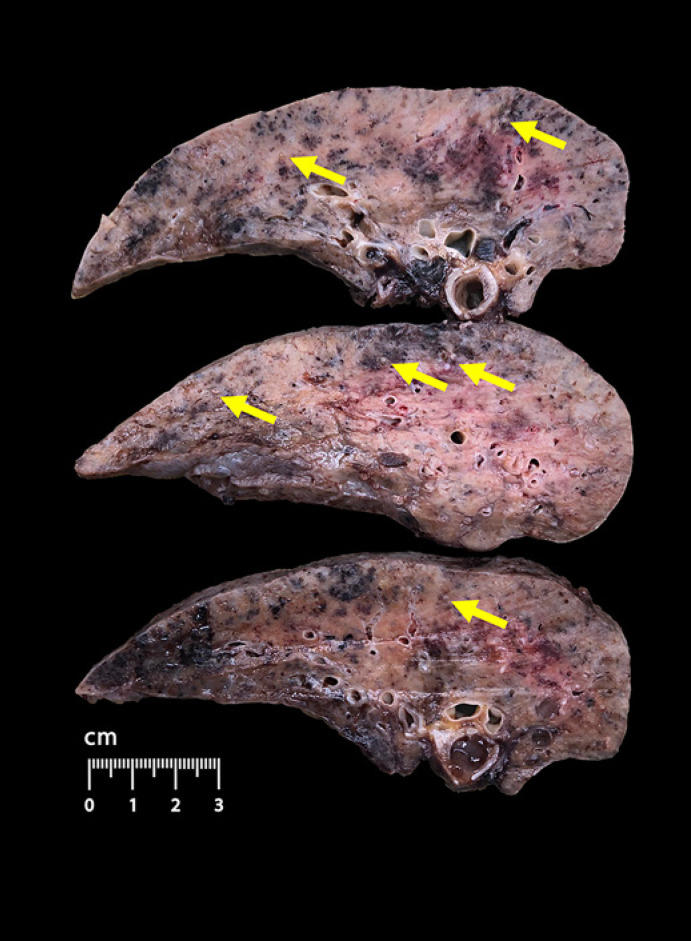
The lung explant was examined on grossing and sent for further histological and molecular analysis. The lungs were heavy, firm, with brownish yellowish carnification of the parenchyma, and showed signs of diffuse calcification (Fig. 2), as evidenced by a video demonstration of the macroscopic examination (see online suppl. Materials; see www.karger.com/doi/10.1159/000525457 for all online suppl. material).
Fig. 2.
Gross findings of lung explant. Dense parenchyma with areas of brownish yellowish carnification and diffuse calcification (yellow arrows).
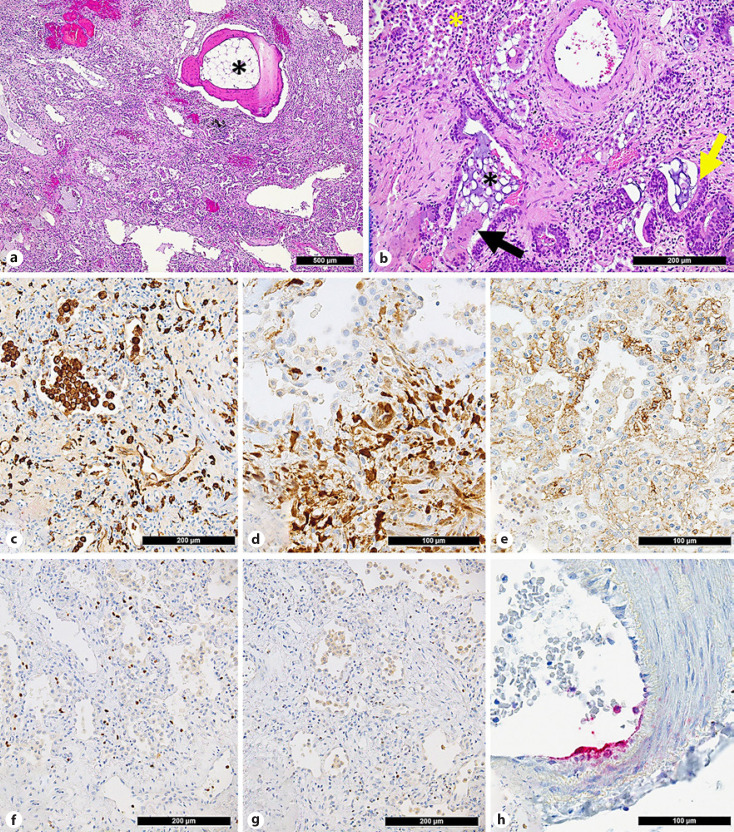
As a correlate to the calcification, histology revealed extensive, diffuse interstitial dendriform ossification, comprising of lamellar bone with fatty marrow with focal hematopoiesis (Fig. 3a). Diffuse interstitial fibrosis with bronchiolar, mucinous and transitional cell metaplasia of the alveolar epithelium (Fig. 3b); traction bronchiectasis; bronchiolar mucostasis; fibrinous pleuritic; and multifocal alveolar hemorrhages were evident throughout the parenchyma. Hemosiderin-laden macrophages were a frequent finding (online suppl. Fig. 1). A proliferation of interstitial and intra-alveolar bronchiogenic giant cells exhibiting partial positivity for thyroid transcription factor 1 was observed (not shown). There were various signs of pulmonary remodeling, as evidenced by an increase of interstitial collagen and a disarray of elastic fibers, proliferative diffuse alveolar damage (DAD) and an interstitial accumulation of M2-polarized CD163+ and CD206+ macrophages (Fig. 3c) with an increased expression of phospho-ERK (Fig. 3d) and increased neoangiogenesis (CD105+ vessels) (Fig. 3e). Fibrin staining revealed sparse intra-alveolar fibrinous exudates, corresponding to residual exudative DAD without microthrombi.
Fig. 3.
Major histological findings of the lung explant. a Extensive interstitial pulmonary fibrosis with dendriform ossification with the formation of lamellar bone and fatty bone marrow (asterisk). b Higher magnification reveals bronchiolocentric, diffuse interstitial fibrosis with bronchiolar/mucinous and transitional cell metaplasia (yellow arrow) and mucostasis (black asterisk) as well as intra-alveolar and intrabronchiolar desquamation of macrophages (yellow asterisk) and occasional multinucleated giant cells (black arrow). c Accumulation of CD206+ (M2-polarized) macrophages in alveolar spaces and the interstitium. d Overexpression of phosphorylated ERK in macrophages but not in alveolar pneumocytes (upper half of the figure). e Increased density of CD105+ vessels indicative of neoangiogenesis. Lymphocyte characterization shows a predominance of Tbet+ TH1 cells (f) over GATA3+ TH2 cells (g). h Positivity for N-protein of SARS-CoV-2 in the endothelial lining of an arteriole.
Interstitial lymphocytes were predominantly T cells (ratio of CD3+ T cells to CD20+ B cells is 2:1) with an exhausted phenotype (weak PD1 positivity). Cytotoxic T cells (CD8+) and T-helper 1 subsets (Tbet+ TH1) were more frequent than T-helper 2 subsets (GATA3+ TH2) (Fig. 3f, g). T-regulatory cells (FoxP3+ Treg) and gammadelta T cells (γδ+) comprised 2–3% of lymphocytes. Plasma cells were polytypic; there was no plasmablast excess (no MUM1+ cells). Mast cells (as evidenced by mast cell tryptase stain) were increased.
Quantitative reverse transcriptase polymerase chain reaction for SARS-CoV-2 revealed low residual viral load in the explanted lung tissue (N-Gene: Ct [cycle threshold] value 36.5) (PCR protocol as previously described [19]). This result was confirmed with the orthogonal method − droplet digital PCR (N1 gene: 0 copies/μL; N2 gene 0.0307 copies/μL). Immunohistochemistry for SARS-CoV-2 nucleocapsid (N)-antigen showed focal endothelial deposition in arterioles (Fig. 3h). Links of the scanned H&E sections as well as all antibody protocols can be found in online supplementary Materials.
Discussion/Conclusion
This is an unusual presentation of COVID-19 with initially mild symptoms rapidly progressing to end-stage interstitial pulmonary fibrosis after 3 months. In this case, histological analysis of the explant enabled a correlation between clinical and radiological features, providing a rare glimpse into the complex pathophysiology of pulmonary PASC. Previous histopathological findings of lung explants were heterogeneous, ranging from varying degrees of proliferative DAD to interstitial remodeling, peribronchiolar metaplasia, microvascular thrombosis and focal microscopic honeycomb change [13, 20]. These changes are likely due to a combination of factors, both direct viral effects and/or immune-mediated, which collectively contribute to fibrosis.
The pathogenesis of lung fibrosis has been studied in other viral infections. The TGFβ pathway, one of the most critical cascades underlying pulmonary fibrosis, has been shown to be activated by αvβ6 integrin and toll-like receptor 3 in influenza [21]. TGFβ-mediated immune responses and an upregulation of interferon-stimulated genes were also found in SARS-CoV-2 [22, 23]. Additionally, a SARS-CoV-2-mediated depletion of angiotensin-converting enzyme 2 has been postulated to stimulate pro-fibrotic signal transduction in the pulmonary microenvironment [24, 25, 26]. These findings collectively imply a virally mediated dysregulation of cytokine response which, exacerbated by chronic oxidative stress, increases the susceptibility of fibrotic change.
A particularly remarkable histological finding in this case is extensive dendriform ossification throughout the lung parenchyma. Metaplastic ossification is a characteristic finding of cicatricial organizing pneumonia, which is classified into a nodular and a dendriform subtype (DPO) [27, 28]. DPO is a notably uncommon histological finding, with a prevalence of 0.5% in retrospective autopsy studies [27]; it may be idiopathic or a result of a dysregulated calcium metabolism, previous lung injury or underlying usual interstitial pneumonia. A high pH in the pulmonary microenvironment has been postulated to stimulate scar formation and a precipitation of calcium salts. These effects are further exacerbated by interstitial iron deposition which was also observed in this case (online suppl. Fig. 1). Interestingly, the gene for bone morphogenic protein shares sequence homology with TGFβ, thus playing a potential role in osteoblastogenesis and bone formation at the site of inflammation [29]. In this case, other etiologies of pulmonary calcification/ossification, such as chronic venous lung congestion or secondary hypercalcemia in end-stage renal disease, were clinically excluded, so that its pathophysiology is likely linked to COVID-19. Only one other case in the literature reported histologically confirmed SARS-CoV-2-associated DPO [30], making its presentation in COVID-19 patients extraordinarily rare.
Histological signs of aberrant macrophage activation, as evidenced by an accumulation of CD163+/CD206+ macrophages, were detected in this case (Fig. 3c). Macrophage activation syndrome leading to dysregulated antigen response may be linked to the cytokine storm in severe COVID-19 [31, 32, 33, 34]. CD163+ macrophages have been shown to be associated with pulmonary fibrosis [35], increased extracellular matrix deposition and fibroblast activation [36]. CD163, considered to be an effective biomarker for alternatively activated (M2-polarized) macrophages, is involved in the uptake of free hemoglobin released after hemolysis and is accordingly increased in a broad range of chronic inflammatory diseases [37]. An increased expression of phospho-ERK in this subgroup of macrophages (Fig. 3d) implies activation of MAPK/MEK/ERK signaling. MEK has previously been shown to induce M2-type polarization by activating peroxisome proliferator-activated receptor gamma [38], thus implying a potential pharmacological target in COVID-19-associated lung fibrosis [39].
Lastly, the detection of residual SARS-CoV-2 viral load by means of quantitative reverse transcriptase polymerase chain reaction, as well as positivity along the endothelial lining in greater vessels (Fig. 3h) shows an unusual failure of viral clearing in this patient 3 months after the symptom onset and emphasizes the angiocentricity of COVID-19. Long-term SARS-CoV-2 RNA detection is a frequent finding within weeks to months after symptom resolution [40, 41, 42, 43] and may even occur in mild to asymptomatic cases [41, 42, 44, 45]. It has been hypothesized to be caused by negative immunological feedback loops due to viral superantigens, although its exact mechanisms remain to be investigated [46]. Potential risk factors facilitating SARS-CoV-2 persistence include immunosuppression, increased IL-6, mechanical ventilation [43], older age (>60 years) [41], and decreased SARS-CoV-2 IgM titers [47]. Importantly, our case demonstrated a potential link between incomplete viral clearing and the development of chronic pulmonary disease. In line with our findings, one prospective study found a correlation between chronic SARS-CoV-2-associated pulmonary disease and high viral loads, weak antibody response and high levels of matrix metalloproteinase-9 [48]. Thus, further investigations are required to establish the pathophysiological link between viral persistence and PASC.
In conclusion, this case presented a highly unusual development of symptoms and histological findings of PASC with persistence of SARS-CoV-2 months after initial infection. There is an urgent need to characterize the incidence, clinical presentation and significance of SARS-CoV-2-associated long-term effects in order to allow optimal follow-up and risk stratification in postinfection management.
Statement of Ethics
This study has received approval by the Ethics Committee of Northwestern and Central Switzerland (ID 2020-00629). Written informed consent was obtained from the patient for publication of the details of their medical case and accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare. Alexandar Tzankov steps back in all functions as a co-editor in consideration of this manuscript.
Funding Sources
This study was funded by the Botnar Research Centre for Child Health (BRCCH), Grant No. FTC-2020-10.
Author Contributions
The manuscript was written by Jasmin Dionne Haslbauer and Alexandar Tzankov. Clinical information was provided by Judith Löffler-Ragg, Katharina Cima, Anna-Katharina Luger, Florian Augustin, Christoph Krapf, Daniel Hoefer, and Ivan Tancevski. Preparation of radiological images and analysis were performed by Anna-Katharina Luger. Preparation of gross and histological images and analysis were performed by Katja Schmitz, Alexandar Tzankov, and Jasmin Dionne Haslbauer. RT-PCR and ddPCR were performed by Ivana Bratic-Hench. Critical appraisal and approval of the final version of the manuscript were performed by Jasmin Dionne Haslbauer, Ivana Bratic-Hench, Katharina Cima, Anna-Katherina Luger, Katja Schmitz, Florian Augustin, Christoph Krapf, Daniel Hoefer, Ivan Tancevski, Alexandar Tzankov, and Judith Löffler-Ragg.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. A scanned H&E-stained slide was provided in an online link. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplemental Video
Acknowledgments
The authors would like to thank the patient, his relatives, Michèle Baumann, Susi Grieshaber, Petra Hirschmann and Chantal Tresch for providing immunohistochemical stains, as well as Martin Portmann and Petra Huber for scanning slides and making them available online for the purpose of this case study.
Funding Statement
This study was funded by the Botnar Research Centre for Child Health (BRCCH), Grant No. FTC-2020-10.
References
- 1.Ladds E, Rushforth A, Wieringa S, Taylor S, Rayner C, Husain L, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020 Dec;20((1)):1144. doi: 10.1186/s12913-020-06001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020 Aug;324((6)):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2021 Dec;22((4)):e102–7. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021 Aug;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirschtick JL, Titus AR, Slocum E, Power LE, Hirschtick RE, Elliott MR, et al. Population-based estimates of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) prevalence and characteristics. Clin Infect Dis. 2021 Dec;73((11)):2055–2064. doi: 10.1093/cid/ciab408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Shen C, Wang L, Majumder S, Zhang D, Deen MJ, et al. Pulmonary fibrosis and its related factors in discharged patients with new corona virus pneumonia: a cohort study. Respir Res. 2021;22((1)):203. doi: 10.1186/s12931-021-01798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2021 Jul–Aug;27((4)):328–337. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luger AK, Sonnweber T, Gruber L, Schwabl C, Cima K, Tymoszuk P, et al. Chest CT of lung injury 1 year after COVID-19 pneumonia: the CovILD study. Radiology. 2022 Mar;:211670. doi: 10.1148/radiol.211670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vijayakumar B, Tonkin J, Devaraj A, Philip KEJ, Orton CM, Desai SR, et al. CT lung abnormalities after COVID-19 at 3 months and 1 year after hospital discharge. Radiology. 2022;303((2)):444–444. doi: 10.1148/radiol.2021211746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han X, Fan Y, Alwalid O, Zhang X, Jia X, Zheng Y, et al. Fibrotic interstitial lung abnormalities at 1-year follow-up CT after severe COVID-19. Radiology. 2021 Dec;301((3)):E438–40. doi: 10.1148/radiol.2021210972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Li J, Liu H, Han N, Ju J, Kou Y, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020 Feb;8((1)):8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharat A, Querrey M, Markov NS, Kim S, Kurihara C, Garza-Castillon R, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020 Dec;12((574)):eabe4282. doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flaifel A, Kwok B, Ko J, Chang S, Smith D, Zhou F, et al. Pulmonary pathology of end-stage COVID-19 disease in explanted lungs and outcomes after lung transplantation. Am J Clin Pathol. 2022 Jan;157((6)):908–926. doi: 10.1093/ajcp/aqab208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang C, Jaksch P, Hoda MA, Lang G, Staudinger T, Tschernko E, et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med. 2020 Oct;8((10)):1057–1060. doi: 10.1016/S2213-2600(20)30361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepper PM, Langer F, Wilkens H, Schäfers HJ, Bals R. Lung transplantation for COVID-19-associated ARDS. Lancet Respir Med. 2021 Sep;9((9)):e88. doi: 10.1016/S2213-2600(21)00278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo WR, Yu H, Gou JZ, Li XX, Sun Y, Li JX, et al. Histopathologic findings in the explant lungs of a patient with COVID-19 treated with bilateral orthotopic lung transplant. Transplantation. 2020 Nov;104((11)):e329–31. doi: 10.1097/TP.0000000000003412. [DOI] [PubMed] [Google Scholar]
- 17.Rohr JM, Strah H, Berkheim D, Siddique A, Radio SJ, Swanson BJ. Pulmonary hypertensive changes secondary to COVID-19 pneumonia in a chronically SARS-CoV-2-infected bilateral lung explant. Int J Surg Pathol. 2022 Jun;30((4)):443–447. doi: 10.1177/10668969211064208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharat A, Machuca TN, Querrey M, Kurihara C, Garza-Castillon R, Kim S, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021 May;9((5)):487–497. doi: 10.1016/S2213-2600(21)00077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 Jun;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aesif SW, Bribriesco AC, Yadav R, Nugent SL, Zubkus D, Tan CD, et al. Pulmonary pathology of COVID-19 following 8 weeks to 4 months of severe disease: a report of three cases, including one with bilateral lung transplantation. Am J Clin Pathol. 2021 Mar;155((4)):506–514. doi: 10.1093/ajcp/aqaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolly L, Stavrou A, Vanderstoken G, Meliopoulos VA, Habgood A, Tatler AL, et al. Influenza promotes collagen deposition via αvβ6 integrin-mediated transforming growth factor β activation. J Biol Chem. 2014 Dec;289((51)):35246–63. doi: 10.1074/jbc.M114.582262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira-Gomes M, Kruglov A, Durek P, Heinrich F, Tizian C, Heinz GA, et al. SARS-CoV-2 in severe COVID-19 induces a TGF-β-dominated chronic immune response that does not target itself. Nat Commun. 2021 Mar;12((1)):1961. doi: 10.1038/s41467-021-22210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nienhold R, Ciani Y, Koelzer VH, Tzankov A, Haslbauer JD, Menter T, et al. Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat Commun. 2020 Oct;11((1)):5086. doi: 10.1038/s41467-020-18854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catarata MJ, Ribeiro R, Oliveira MJ, Robalo Cordeiro C, Medeiros R. Renin-angiotensin system in lung tumor and microenvironment interactions. Cancers. 2020 Jun;12((6)):1457. doi: 10.3390/cancers12061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bösmüller H, Matter M, Fend F, Tzankov A. The pulmonary pathology of COVID-19. Virchows Arch. 2021 Jan;478((1)):137–150. doi: 10.1007/s00428-021-03053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haslbauer JD, Stalder A, Zinner C, Bassetti S, Mertz KD, Went P, et al. Immunohistochemical and transcriptional analysis of SARS-CoV-2 entry factors and renin-angiotensin-aldosterone system components in lethal COVID-19. Pathobiology. 2022;89((3)):166–177. doi: 10.1159/000520221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lara JF, Catroppo JF, Kim DU, da Costa D. Dendriform pulmonary ossification, a form of diffuse pulmonary ossification: report of a 26-year autopsy experience. Arch Pathol Lab Med. 2005 Mar;129((3)):348–353. doi: 10.5858/2005-129-348-DPOAFO. [DOI] [PubMed] [Google Scholar]
- 28.Bin Saeedan M, Farver C, Mehta AC, Yadav R. Cicatricial organizing pneumonia with dendriform pulmonary ossification: an unusual cause for a recurrent pneumothorax. Case Rep Pulmonol. 2019 Dec;2019:2379145. doi: 10.1155/2019/2379145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015 Apr;3:15005. doi: 10.1038/boneres.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García Moreno B, Buitrago Weiland G, Sánchez Alegre ML, Vanegas Rodríguez JE. Accelerated pulmonary ossification as a sequela of SARS-CoV-2 pneumonia. Radiol Cardiothorac Imaging. 2021 Apr;3((2)):e200598. doi: 10.1148/ryct.2021200598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020 Jun;19((6)):102537–7. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Wu H, Yao X, Zhang D, Zhou Y, Fu B, et al. Pyroptotic macrophages stimulate the SARS-CoV-2-associated cytokine storm. Cell Mol Immunol. 2021 May;18((5)):1305–1307. doi: 10.1038/s41423-021-00665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roussel M, Ferrant J, Reizine F, Le Gallou S, Dulong J, Carl S, et al. Comparative immune profiling of acute respiratory distress syndrome patients with or without SARS-CoV-2 infection. Cell Rep Med. 2021 Jun;2((6)):100291. doi: 10.1016/j.xcrm.2021.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendisch D, Dietrich O, Mari T, von Stillfried S, Ibarra IL, Mittermaier M, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021 Dec;184((26)):6243–e27. doi: 10.1016/j.cell.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nouno T, Okamoto M, Ohnishi K, Kaieda S, Tominaga M, Zaizen Y, et al. Elevation of pulmonary CD163+ and CD204+ macrophages is associated with the clinical course of idiopathic pulmonary fibrosis patients. J Thorac Dis. 2019 Sep;11((9)):4005–4017. doi: 10.21037/jtd.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, et al. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol. 2004 Dec;204((5)):594–604. doi: 10.1002/path.1667. [DOI] [PubMed] [Google Scholar]
- 37.Etzerodt A, Moestrup SK. CD163 and inflammation: biological, diagnostic, and therapeutic aspects. Antioxid Redox Signal. 2013 Jun;18((17)):2352–2363. doi: 10.1089/ars.2012.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He L, Jhong JH, Chen Q, Huang KY, Strittmatter K, Kreuzer J, et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021 Nov;37((5)):109955. doi: 10.1016/j.celrep.2021.109955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long ME, Eddy WE, Gong KQ, Lovelace-Macon LL, McMahan RS, Charron J, et al. MEK1/2 inhibition promotes macrophage reparative properties. J Immunol. 2017 Jan;198((2)):862–872. doi: 10.4049/jimmunol.1601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh KA, Spillane S, Comber L, Cardwell K, Harrington P, Connell J, et al. The duration of infectiousness of individuals infected with SARS-CoV-2. J Infect. 2020 Dec;81((6)):847–856. doi: 10.1016/j.jinf.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartman WR, Hess AS, Connor JP. Persistent viral RNA shedding after COVID-19 symptom resolution in older convalescent plasma donors. Transfusion. 2020;60((10)):2189–2191. doi: 10.1111/trf.15927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carmo A, Pereira-Vaz J, Mota V, Mendes A, Morais C, da Silva AC, et al. Clearance and persistence of SARS-CoV-2 RNA in patients with COVID-19. J Med Virol. 2020;92((10)):2227–2231. doi: 10.1002/jmv.26103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vena A, Taramasso L, Di Biagio A, Mikulska M, Dentone C, De Maria A, et al. Prevalence and clinical significance of persistent viral shedding in hospitalized adult patients with SARS-CoV-2 infection: a prospective observational study. Infect Dis Ther. 2021 Mar;10((1)):387–398. doi: 10.1007/s40121-020-00381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corsini Campioli C, Cano Cevallos E, Assi M, Patel R, Binnicker MJ, O'Horo JC. Clinical predictors and timing of cessation of viral RNA shedding in patients with COVID-19. J Clin Virol. 2020 Sep;130:104577. doi: 10.1016/j.jcv.2020.104577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis. 2020 Nov;71((16)):2249–2251. doi: 10.1093/cid/ciaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacobs JJL. Persistent SARS-2 infections contribute to long COVID-19. Med Hypotheses. 2021 Apr;149:110538. doi: 10.1016/j.mehy.2021.110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu Y, Li Y, Guo E, He L, Liu J, Yang B, et al. Dynamics and correlation among viral positivity, seroconversion, and disease severity in COVID-19: a retrospective study. Ann Intern Med. 2021 Apr;174((4)):453–461. doi: 10.7326/M20-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lerum TV, Maltzahn NN, Aukrust P, Trøseid M, Henriksen KN, Kåsine T, et al. Persistent pulmonary pathology after COVID-19 is associated with high viral load, weak antibody response, and high levels of matrix metalloproteinase-9. Sci Rep. 2021 Dec;11((1)):23205. doi: 10.1038/s41598-021-02547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplemental Video
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. A scanned H&E-stained slide was provided in an online link. Further inquiries can be directed to the corresponding author.