Introduction and importance:
Neurologic manifestations in primary Sjögren syndrome (SS) range in prevalence from 8 to 49%, and most of the studies suggest a prevalence of 20%. The incidence of SS patients developing movement disorders is about 2%.
Case presentation:
The authors herein report a case of a 40-year-old lady with MRI of the brain mimicking autoimmune encephalitis in SS who presented with chorea. Her MRI findings revealed T2 and FLAIR (fluid-attenuated inversion recovery) high signal intensity areas in bilateral middle cerebellar peduncles, dorsal pons, dorsal midbrain, hypothalami, and medial temporal lobes.
Clinical discussion:
There is still no evidence to support the definite use of MRI in characterizing the central nervous system involvement in primary SS, especially due to overlapping findings with age and cerebrovascular disease. Multiple areas of increased signal intensity in periventricular and subcortical white matter in FLAIR and T2-weighted image is commonly seen in primary SS patients.
Conclusion:
It is crucial to consider autoimmune diseases like SS as a cause of chorea in adults, even in those whose imaging findings are suggestive of autoimmune encephalitis.
Keywords: autoimmune encephalitis, case report, chorea, neurologic manifestation, Sjögren syndrome
Introduction and importance
HIGHLIGHTS
The incidence of movement disorder in a patient with Sjögren syndrome (SS) is about 2%.
MRI findings in SS can mimic changes due to autoimmune encephalitis, age, and cerebrovascular disease.
Multiple areas of T2/FLAIR (fluid-attenuated inversion recovery) high signal intensity can be seen in a patient with SS.
Sjögren’s syndrome (SS) is a chronic autoimmune disease characterized by lymphocytic infiltration of the exocrine glands, most commonly affecting the salivary and lacrimal glands1. It has a wide spectrum of presentations, where ∼80% of the overall patient present with sicca syndrome (dry eyes followed by dry mouth and other sicca symptoms)2. Neurologic manifestations in primary SS range in prevalence from 8 to 49%, and most of the studies suggest a prevalence of 20%3,4. Central nervous system (CNS) manifestations include central focal lesions, conditions that mimic encephalitis, aseptic meningitis, multiple sclerosis, cerebellar syndromes causing ataxia, movement disorders affecting the basal ganglia producing chorea, neuromyelitis optica, and problems with memory and cognition5. The case report is in line with CARE (CAse REport) 2013 criteria6.
We herein report a case of a 40-year-old lady with MRI of the brain mimicking autoimmune encephalitis in SS who presented with chorea.
Case presentation
A 40-year-old right-handed lady presented to our center with progressive irregular, jerky movements of all limbs for the last 1 year that led to difficulty doing the fine movement with her right hand. There were no cognitive impairments, headaches, seizures, visual symptoms, joint pains, rashes, sensory symptoms, weakness of limbs, or bowel–bladder dysfunction. She had no history of pregnancy loss, did not smoke or drink alcohol, and had no family history of similar illnesses. The patient is not allergic to any drugs or foods. On examination, she was conscious and cooperative. Her Mini-Mental State Examination Score was 29. She had generalized, irregular proximal as well as distal jerky movements of her trunk and limbs. She could not sustain persistent handgrip and sticking out of the tongue, suggesting motor impersistence. She did not have any other focal neurological deficits. With the provisional diagnosis of progressive generalized chorea, she was investigated further. Her complete blood counts, serum glucose, renal function tests, liver function tests, thyroid function test, and vitamin B12 levels were within normal range. Similarly, Venereal Disease Research Laboratory, Treponema pallidum haemagglutination, HIV-1 and HIV-2, C-reactive protein, and peripheral smear for acanthocytes were negative. Ophthalmic evaluation of her eyes did not reveal Kayser–Fleischer rings. She had enzyme-linked immunosorbent assay positive for antinuclear antibodies. MRI of her brain was ordered, and it revealed T2 and FLAIR (fluid-attenuated inversion recovery) high signal intensity areas in bilateral middle cerebellar peduncles, dorsal pons, dorsal midbrain, hypothalami, and medial temporal lobes (Figs. 1–3). These lesions neither enhanced with contrast nor had any restricted diffusion. With the provisional diagnosis of autoimmune encephalitis, an injection of methylprednisolone was started. She significantly improved the next day. The results of the extractable nuclear antigen antibodies profile by immunoblot revealed highly positive titers of anti-Ro and anti-La antibodies and negative titers of other markers such as aquaporin-4 and myelin oligodendrocyte glycoprotein protein were also absent. Chorea almost disappeared by day 3 of methylprednisolone. Although the Schirmer test was normal and lip biopsy for mucosal lymphocytic infiltrates revealed a focus score of less than 1, the diagnosis of primary SS with central nervous involvement was made. Lumbar puncture was thus deferred, and serum autoimmune encephalitis profiles were also not done. The patient was discharged on steroids, and later azathioprine was added as a steroid-sparing agent. The patient is doing well in follow-ups in regular outpatient department visits.
Figure 1.
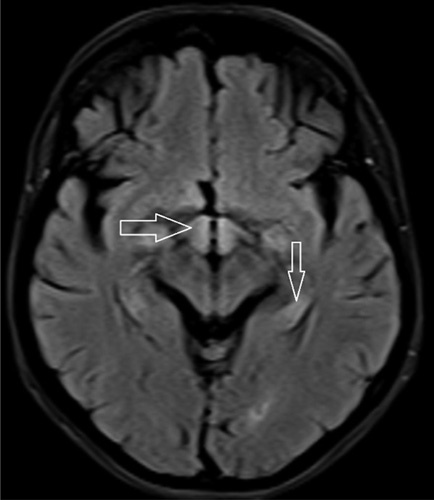
MRI of the brain showing signal intensity in the hypothalamus (horizontal arrow) and medial temporal lobe (vertical arrow).
Figure 3.
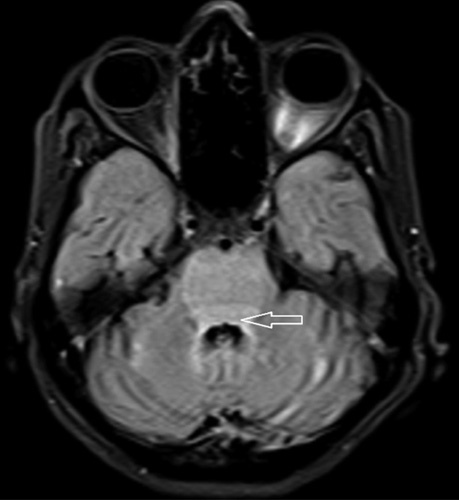
MRI of the brain showing high signal intensity in posterior pons (horizontal arrow).
Figure 2.
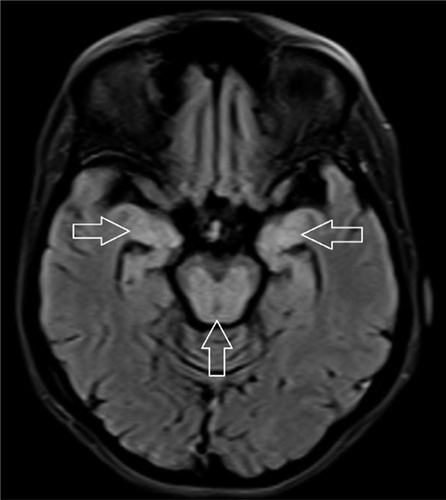
MRI of the brain showing signal intensity in the bilateral medial temporal lobe (horizontal arrows) and midbrain (vertical arrow).
Clinical discussion
Mikulicz first described SS in 1892, and in 1933, the Danish ophthalmologist Sjögren described clinical and histological findings, and the keratoconjunctivitis sicca term was introduced for this syndrome7. B-cell activation and formation of autoantibodies is a consistent immunoregulatory abnormality in Sjögren’s syndrome7. Like other autoimmune diseases, SS has a female predominance with a high female-to-male ratio (9 : 1) with the peak incidence in the 40–55-year age group2. Sjögren’s syndrome presents with various sicca features (such as dry eyes and dry mouth), and besides these features, 70% of patient complains of easy fatiguability causing functional disability8.
Neurologic manifestations in primary SS range in prevalence from 8 to 49%, and most studies suggest a prevalence of 20%3,4. The neurologic manifestations of primary SS can be divided anatomically into two categories: peripheral neuropathies and CNS vasculopathy9. The prevalence of CNS vasculopathy is 3–20%, and they can have antibodies against Sjögren’s-syndrome-related antigen A (SSA) (Ro/SSA)8. It is mainly thought to be due to small vessel vasculitis, and cerebral angiographic findings have correlated with anti-Ro antibody positivity10,11. Various brain involvements ranging from motor and sensory deficit, brain stem syndrome, cerebellar syndrome, seizures, cognitive dysfunction, movement disorder (Parkinson, dystonia, and chorea), and psychiatric disorders can be seen in SS patients12.
CNS involvement generally precedes the diagnosis of primary SS and occurs as recurrent, episodic, which is separated by long disease-free intervals resulting in insidious and progressive neurologic deficits3. The incidence of SS patients developing movement disorder is about 2%13. Our patient presented with chorea without a family history, and no other etiology of the chorea could be determined. MRI finding of signal intensity was seen in bilateral middle cerebellar peduncles, dorsal pons, dorsal midbrain, hypothalami, and medial temporal lobes (Figs. 1–3). Patients with normal MRI findings have an antineuronal antibody in their pathogenesis; our patient has abnormal MRI findings which are mostly seen in cases of vasculitis pathogenesis of SS14. MRI findings in a case of autoimmune encephalitis include T2/FLAIR signal intensity commonly in the mesial temporal lobe, and bilaterality is seen in 60% of cases15. There is still no evidence to support the definite use of MRI in characterizing CNS involvement in primary SS, especially due to overlapping findings with age and cerebrovascular disease5. Multiple areas of increased signal intensity in periventricular and subcortical white matter in FLAIR and T2-weighted image is commonly seen in primary SS patient16.
Lymphocytic infiltrate around salivary glands is the hallmark of SS, and based on this, the focus score is calculated. A focus score of 1 or more is indicative of SS (sensitivity 63–81.8%)8. There are various classification criteria developed for the research purpose, but some can be used to guide the clinical diagnosis of SS. The diagnosis of primary SS was made in our patient based on the American College of Rheumatology/European League Against Rheumatism classification criteria17. In the absence of a large randomized clinical trial, the recommendation for treatment of clinically significant neurological manifestations of primary SS varies based on the underlying pathology and commonly used drugs include aggressive therapy with glucocorticoids and immunosuppressive agents5. Although SS patients have compromised quality of life due to their wide range of symptoms, their life expectancy is comparable to the general population18.
Conclusion
It is crucial to consider autoimmune diseases like SS as a cause of chorea in adults, even in those whose imaging findings are suggestive of autoimmune encephalitis.
Ethical approval
This is a case report; therefore, it did not require ethical approval from our ethics committee.
Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
The study did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
S.G., B.P.G., N.P., and S.K.: collected all the required case information, images, and reports; reviewed the literature and contributed to both writing and editing the manuscript; R.K., R.R., and R.O: reviewed and edited the manuscript; B.G., S.J., A.S., and N.G.: were involved in the counseling and treatment of the patient.
Conflicts of interest disclosure
The authors report no conflicts of interest.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Subarna Giri accepts full responsibility for the work and the conduct of the study, has access to the data, and controlled the decision to publish.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Author agreement statement
We, the undersigned, declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We understand that the Corresponding Author is the sole contact for the Editorial process. He/she is responsible for communicating with the other authors about progress, submissions of revisions, and final approval of proofs.
Acknowledgments
None.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online ■ ■
Contributor Information
Bikram P. Gajurel, Email: Bikram_gajurel@iom.edu.np.
Subarna Giri, Email: giri.subarna2054@iom.edu.np.
Navin Poudel, Email: navinpoudel77@iom.edu.np.
Sanjeev Kharel, Email: kharel_sanjeev@iom.edu.np.
Saket Jha, Email: sakjhaa@gmail.com.
Ragesh Karn, Email: rageshkarn16@gmail.com.
Reema Rajbhandari, Email: reemarajbhandari@hotmail.com.
Rajeev Ojha, Email: rajeevnet@hotmail.com.
Niraj Gautam, Email: gautamniraj123@gmail.com.
Aashish Shrestha, Email: aacss420@gmail.com.
References
- 1. Jonsson R, Brokstad KA, Jonsson MV, et al. Current concepts on Sjögren’s syndrome – classification criteria and biomarkers. Eur J Oral Sci 2018;126(suppl 1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vivino FB. Sjögren’s syndrome: clinical aspects. Clin Immunol 2017;182:48–54. [DOI] [PubMed] [Google Scholar]
- 3. Delalande S, de Seze J, Fauchais AL, et al. Neurologic manifestations in primary Sjögren syndrome: a study of 82 patients. Medicine 2004;83:280–91. [DOI] [PubMed] [Google Scholar]
- 4. Mori K, Iijima M, Koike H, et al. The wide spectrum of clinical manifestations in Sjögren’s syndrome-associated neuropathy. Brain 2005;128:2518–2534. [DOI] [PubMed] [Google Scholar]
- 5. Margaretten M. Neurologic manifestations of primary Sjögren syndrome. Rheum Dis Clin North Am 2017;43:519–29. [DOI] [PubMed] [Google Scholar]
- 6. Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218–235. [DOI] [PubMed] [Google Scholar]
- 7. Fox RI. Sjögren’s syndrome. Lancet 2005;366:321–331. [DOI] [PubMed] [Google Scholar]
- 8. Mavragani CP, Moutsopoulos HM. Sjögren syndrome. CMAJ 2014;186:E579–E586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mellgren SI, Göransson LG, Omdal R. Primary Sjögren’s syndrome associated neuropathy. Can J Neurol Sci 2007;34:280–287. [DOI] [PubMed] [Google Scholar]
- 10. Alexander EL. Neurologic disease in Sjögren’s syndrome: mononuclear inflammatory vasculopathy affecting central/peripheral nervous system and muscle. A clinical review and update of immunopathogenesis. Rheum Dis Clin North Am 1993;19:869–908. [PubMed] [Google Scholar]
- 11. Soliotis FC, Mavragani CP, Moutsopoulos HM. Central nervous system involvement in Sjögren’s syndrome. Ann Rheum Dis 2004;63:616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Massara A, Bonazza S, Castellino G, et al. Central nervous system involvement in Sjögren’s syndrome: unusual, but not unremarkable – clinical, serological characteristics and outcomes in a large cohort of Italian patients. Rheumatology 2010;49:1540–1549. [DOI] [PubMed] [Google Scholar]
- 13. Alvarado-Franco NL, Gonzalez-Marques C, Olguín-Ramírez LA, et al. Athetoid movements as initial manifestation of primary Sjögren syndrome. Tremor Other Hyperkinet Mov 2018;8:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fanchke PV, Venegas Fanchke P, Sinning M, et al. Primary Sjögren’s syndrome presenting as a generalized chorea. Parkinsonism Relat Disord 2005;11:193–194. [DOI] [PubMed] [Google Scholar]
- 15. Moore, C, The Radswiki. Autoimmune encephalitis. Radiopaedia.org; 2010. http://radiopaedia.org/articles/12686
- 16. Tzarouchi LC, Tsifetaki N, Konitsiotis S, et al. CNS involvement in primary Sjögren syndrome: assessment of gray and white matter changes with MRI and voxel-based morphometry. AJR Am J Roentgenol 2011;197:1207–1212. [DOI] [PubMed] [Google Scholar]
- 17. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome. Ann Rheum Diss 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 18. Stefanski AL, Tomiak C, Pleyer U, et al. The diagnosis and treatment of Sjögren’s syndrome. Dtsch Arztebl Int 2017;114:354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


