Abstract
Background
The contribution of the virus to the pathogenesis of severe COVID-19 is still unclear. We aimed to evaluate associations between viral RNA load in plasma and host response, complications, and deaths in critically ill patients with COVID-19.
Methods
We did a prospective cohort study across 23 hospitals in Spain. We included patients aged 18 years or older with laboratory-confirmed SARS-CoV-2 infection who were admitted to an intensive care unit between March 16, 2020, and Feb 27, 2021. RNA of the SARS-CoV-2 nucleocapsid region 1 (N1) was quantified in plasma samples collected from patients in the first 48 h following admission, using digital PCR. Patients were grouped on the basis of N1 quantity: VIR-N1-Zero (<1 N1 copies per mL), VIR-N1-Low (1–2747 N1 copies per mL), and VIR-N1-Storm (>2747 N1 copies per mL). The primary outcome was all-cause death within 90 days after admission. We evaluated odds ratios (ORs) for the primary outcome between groups using a logistic regression analysis.
Findings
1068 patients met the inclusion criteria, of whom 117 had insufficient plasma samples and 115 had key information missing. 836 patients were included in the analysis, of whom 403 (48%) were in the VIR-N1-Low group, 283 (34%) were in the VIR-N1-Storm group, and 150 (18%) were in the VIR-N1-Zero group. Overall, patients in the VIR-N1-Storm group had the most severe disease: 266 (94%) of 283 patients received invasive mechanical ventilation (IMV), 116 (41%) developed acute kidney injury, 180 (65%) had secondary infections, and 148 (52%) died within 90 days. Patients in the VIR-N1-Zero group had the least severe disease: 81 (54%) of 150 received IMV, 34 (23%) developed acute kidney injury, 47 (32%) had secondary infections, and 26 (17%) died within 90 days (OR for death 0·30, 95% CI 0·16–0·55; p<0·0001, compared with the VIR-N1-Storm group). 106 (26%) of 403 patients in the VIR-N1-Low group died within 90 days (OR for death 0·39, 95% CI 0·26–0·57; p<0·0001, compared with the VIR-N1-Storm group).
Interpretation
The presence of a so-called viral storm is associated with increased all-cause death in patients admitted to the intensive care unit with severe COVID-19. Preventing this viral storm could help to reduce poor outcomes. Viral storm could be an enrichment marker for treatment with antivirals or purification devices to remove viral components from the blood.
Funding
Instituto de Salud Carlos III, Canadian Institutes of Health Research, Li Ka-Shing Foundation, Research Nova Scotia, and European Society of Clinical Microbiology and Infectious Diseases.
Translation
For the Spanish translation of the abstract see Supplementary Materials section.
Introduction
The pathophysiological processes of severe COVID-19 are currently unclear. The predominant hypothesis during the pandemic has been that severe respiratory failure caused by SARS-CoV-2 infection can be explained by an exacerbated inflammatory response following the resolution of viral replication.1 This model was aligned with the so-called cytokine storm theory, which proposes that severe COVID-19 is caused by a massive release of cytokines to the blood, leading to the induction of a life-threatening systemic inflammatory syndrome.2 Although patients who are critically ill with COVID-19 do show elevated concentrations of cytokines and inflammatory mediators in the blood, these concentrations are nonetheless substantially lower than those observed in patients with bacterial sepsis, chimeric antigen receptor T-cell-induced cytokine release syndrome, or acute respiratory distress syndrome unrelated to COVID-19.3, 4 As proposed by Leisman and colleagues,3 alternative mechanisms other than a cytokine storm should be considered to explain the organ dysfunction induced by SARS-CoV-2 infection.
Research in context.
Evidence before this study
The pathophysiological process of severe COVID-19 is currently unclear. While the cytokine storm theory has been extensively discussed in the literature, it has been challenged by evidence from previous studies. The role of SARS-CoV-2 in the pathogenesis of the organ failure caused by COVID-19 has not been sufficiently studied. A potential reason is that obtaining samples from the lower respiratory tract for investigation in virological studies is challenging. Evidence suggests that profiling viral RNA in plasma is a surrogate of the degree of viral replication in the lower respiratory tract.
We searched PubMed on Aug 10, 2022, for relevant articles published in English from March 11, 2020, to Aug 10, 2022, using the terms “SARS-CoV-2”, “viremia”, “RNAemia”, “viral load”, “plasma”, “serum”, “blood”, “critical”, and “ICU”. We found only six studies quantifying SARS-CoV-2 RNA in plasma from critically ill patients with COVID-19. These studies had small cohorts (usually fewer than 100 patients) and used standard quantitative PCR (qPCR), a technology that is less precise in providing absolute quantification than digital PCR (dPCR). Only two pilot studies used dPCR, a next-generation PCR technology that outperforms qPCR to detect and quantify SARS-CoV-2 RNA.
Added value of this study
To our knowledge, this is the largest study to date to evaluate in parallel viral RNA load in plasma and host responses in critically ill patients with COVID-19. The study included 836 unvaccinated patients across 23 hospitals in Spain. Using dPCR, we identified a group of patients who were admitted to the intensive care unit with a very high viral RNA load in plasma (>2747 SARS-CoV-2 nucleocapsid region [N1] copies per mL [VIR-N1-Storm]), who had a high rate of death (about half of these patients died in the first 90 days following admission). This group represented about one-third of the patients in our cohort, and had more frequent pulmonary and extrapulmonary complications than patients with lower viral load. Most of the patients in the VIR-N1-Storm group also had N-antigenaemia, and they had low concentrations of SARS-CoV-2 anti-S antibodies and biomarker signatures indicating a deep dysregulation of the host response to the infection, involving activation of inflammation, chemotaxis, neutrophil degranulation, antiviral response, immunosuppression, endothelial dysfunction, and tissue damage.
Implications of all the available evidence
This study shows that a noteworthy proportion of patients with severe COVID-19 develop a so-called viral storm, characterised by a massive release of viral components to the blood, which is associated with biological responses similar to those observed in sepsis, the development of frequent complications, and high mortality. Patients who showed no signs of this viral storm at intensive care unit admission had a significantly lower risk of death than patients in the VIR-N1-Storm group, which supports that the early control of viral replication is a key factor to increase survival in COVID-19. Our results encourage the use of strategies aimed to prevent uncontrolled viral replication in patients at high risk of severe COVID-19 (eg, up-to-date vaccination, prophylaxis with anti-S monoclonal antibodies with neutralising activity, and early administration of antivirals during COVID-19). Our findings suggest that evaluating the presence of a viral storm at intensive care unit admission could serve as a predictive enrichment tool for trials testing antiviral therapies or blood purification devices to remove viral components in critically ill patients with COVID-19.
The involvement of the virus in the pathophysiology of severe COVID-19 has remained unclear during the pandemic,5 potentially because it is difficult to study. Obtaining samples from the lower respiratory tract for investigation in virological studies is challenging. Such samples can only be collected from patients receiving invasive mechanical ventilation with a clinical indication.6 As a consequence, studies using samples from the lower respiratory tract tend to involve a small number of patients. Plasma can represent an alternative to investigate the role of the virus in critical COVID-19 illness. The presence of SARS-CoV-2 RNA in plasma (RNAemia) is thought to be a consequence of the leakage of viral RNA or virions from the lung to the blood through a damaged alveolar–vascular barrier.7, 8 Evidence from previous studies suggests that the concentration of SARS-CoV-2 RNA in plasma or serum is a surrogate of the degree of viral replication in the lower respiratory tract.9, 10 By contrast with samples from the lower respiratory tract, plasma constitutes a matrix with a more homogeneous composition that can be easily collected from patients with or without invasive mechanical ventilation, contributing to building a more comprehensive picture of the participation of the virus in the critical illness induced by COVID-19. Moreover, viral RNA load profiled in plasma seems to be a better predictor of outcome than that profiled in samples from the lower respiratory tract.11
There are only a few studies quantifying viral RNA in plasma or serum from critically ill patients with COVID-19. These studies have involved small numbers of patients and mostly used standard real-time quantitative PCR (qPCR).9, 11, 12, 13, 14 qPCR quantification provides a semiquantitative evaluation of the viral RNA load in plasma based on the cycle threshold of amplification curves, requiring a standard curve to provide absolute quantification.15 This process affects reproducibility and makes it difficult to compare the results obtained from different studies. Furthermore, performance of qPCR is poor in samples with low concentrations of viral RNA.16 Digital PCR (dPCR) is a disruptive technology due to its enhanced sensitivity17 and its ability to provide absolute quantification.15 Several reports have shown that this technology outperforms qPCR to detect and quantify SARS-CoV-2 RNA.18, 19, 20
To better understand the contribution of the virus to the pathogenesis of severe COVID-19, we aimed to evaluate associations between viral RNA load in plasma and host response, complications, and deaths in critically ill patients with COVID-19.
Methods
Study design and participants
We did a prospective cohort study (a sub-study of CIBERESUCICOVID; NCT04457505) across 23 hospitals in Spain (appendix 2 p 3). We included critically ill patients aged 18 years or older with laboratory-confirmed SARS-CoV-2 who were admitted to an intensive care unit (ICU) between March 16, 2020, and Feb 27, 2021, and had a plasma EDTA (edetic acid) sample collected in the first 48 h following admission. Patients were recruited during the first three epidemic periods of the COVID-19 pandemic as defined by the Spanish National Centre of Epidemiology.21
Exclusion criteria were unconfirmed SARS-CoV-2 infection, missing data at baseline or hospital discharge, insufficient volume in the plasma samples, and ICU admission due to other reasons. None of the patients had received any dose of an anti-SARS-CoV-2 vaccine at recruitment. The study received approval by the Institution's Internal Review Board (Comité Ètic d'Investigació Clínica, HCB/2020/0370). Individual hospitals obtained the respective local ethics committee approval. The study was performed in full compliance with the Declaration of Helsinki and national and international laws on data protection. Written informed consent was obtained from each participant or their legal representative.
Procedures
We recorded data on demographics, comorbidities, and previous treatment. The variable of sex corresponded to the sex assigned at birth. Standard laboratory and clinical data were collected at ICU admission. The pharmacological treatments administered and interventions performed during the hospital admission were also collected. Main complications during hospital stay were reported, including pulmonary complications, hyperglycaemia (plasma glucose concentrations >126 mg/dL), secondary infections, gastrointestinal bleeding, acute kidney injury (an increase in serum creatinine by ≥0·3 mg/dL within 48 h, an increase in serum creatinine to ≥1·5 times baseline, or a urine volume of <0·5 mL/kg per h for 6 h, or a combination thereof), acute hepatic failure (clinical jaundice, hyperbilirubinaemia [ie, blood total bilirubin concentration of twice the upper limit of the normal range (>2·4 mg/dL)], or an increase in alanine aminotransferase or aspartate aminotransferase to twice the upper limit of the normal range [>80 U/L], or a combination thereof), and anaemia (haemoglobin concentrations <13 g/dL in male patients or <12 g/dL in female patients). All data were pseudonymised and stored in a REDCap database hosted in the Centro de Investigación Biomédica en Red (CIBER), Madrid, Spain. Blood donors recruited from the Centro de Hemoterapia y Hemodonación de Castilla y León (Valladolid, Spain) were included as healthy controls. Further details on the study methodology have been published elsewhere.22
Blood samples were collected in EDTA tubes and immediately centrifuged to separate plasma. Plasma was stored at –80°C at the participant sites. Frozen plasma samples were sent to the Biosepsis laboratory in Valladolid, Spain, for viral load quantification and biomarker profiling. A sample was sent to the National Center of Microbiology (Majadahonda, Spain) for antibody quantification.
SARS-CoV-2 RNA was extracted from 140 μL of plasma using the QIAamp Viral RNA Mini Kit (Qiagen, Venlo, Netherlands), according to manufacturer instructions. Quantification of the SARS-COV-2 nucleocapsid region 1 (N1) and RNAse P RNA was done with 5 μL of the eluted solution using the SARS-CoV-2 droplet dPCR kit (Bio-Rad, Hercules, CA, USA) according to manufacturer's specifications on a QX-200 droplet dPCR platform from the same provider. RNAse P is a ubiquitous human intracellular marker that is detectable in circulation when tissues and cells are damaged.23
N-antigenaemia was defined as a positive result for the presence of N-antigen of SARS-CoV-2 in plasma using the Panbio COVID-19 Ag Rapid Test (Abbott, Chicago, IL, USA), as previously described.24
A specific immunoassay was developed to quantify anti-SARS-CoV-2 S IgG and IgM antibodies in plasma, as well as the activity of plasma samples to inhibit the binding between the S protein and the angiotensin-converting enzyme 2 (ACE2) receptor (appendix 2 pp 5–6).
We quantified 40 biomarkers in plasma using the Ella-SimplePlex system (Bio-Techne, Minneapolis, MN, USA) as per manufacturer instructions: lipocalin-2, myeloperoxidase, pentraxin 3 (PTX-3), triggering receptor expressed on myeloid cells (TREM-1), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF; granulocyte biology), intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), E-selectin, endothelin-1, angiopoietin 2 (endothelial dysfunction), D-dimer, u-Plasminogen activator (UPA; coagulation), interleukin (IL)-1β, IL-6, IL-17A, IL-12p70, IL-2, IL-15, tumour necrosis factor alpha (TNF α; inflammation), C-X-C motif chemokine ligand 10 (CXCL10), C-C motif chemokine ligand 2 (CCL2), IL-8, regulated on activation, normal T cell expressed and secreted protein (RANTES; chemotaxis), interferon (IFN)-α, IFN-β, IFN-γ (interferon response), IL-10, IL-1ra, programmed death-ligand 1 (B7-H1 or PD-L1), cytotoxic T-lymphocyte antigen 4 (CTLA-4; immunosuppression), IL-4 (T helper 2 response), IL-7, granzyme A, granzyme B, cluster differentiation 27 molecule (CD27; T-cell biology), FAS (apoptosis), surfactant protein D, epithelial growth factor (EGF), and ferritin (acute phase reactant).
Outcomes
The primary outcome was all-cause death within 90 days after admission to the ICU. The primary outcome was known for all the patients included in the study. Main predictors evaluated were viral N1 load in plasma (as a continuous or categorical variable) and the presence of N-antigenaemia. We also compared the complications (invasive mechanical ventilation, hyperglycaemia, secondary infections, anaemia, acute kidney injury, acute liver failure, renal replacement therapy, pulmonary thromboembolism, hypoglycaemia, and bleeding), time outside ICU, time outside hospital, biomarkers, and antibody concentrations in plasma between the groups.
Statistical analysis
A multivariable logistic regression analysis model was built to evaluate the association of viral N1 load with 90-day mortality, adjusted by the most relevant clinical variables. Because this initial analysis supported that viral N1 load was an important factor influencing mortality, we next divided the cohort into three groups on the basis of viral N1 load to compare their biological and clinical characteristics. We avoided using arbitrary thresholds of viral RNA load to build the three groups. Accordingly, we used two objective thresholds: absence of viral N1 RNA in plasma for the lower threshold, and the best value of viral RNA load to predict mortality as the upper threshold. Therefore, patients with less than 1 viral N1 RNA copy per mL of plasma were included in the VIR-N1-Zero group. The remaining patients were distributed into one of two further groups based on the threshold of viral N1 RNA predicting 90-day mortality more accurately in the receiver operating characteristic curve, as indicated by the Youden index (2747 N1 copies per mL; appendix 2 p 7). Patients with 1–2747 N1 copies per mL were included in the VIR-N1-Low group, and patients with more than 2747 N1 copies per mL were included in the VIR-N1-Storm group. The resulting categorical variable was tested by the multivariable analysis for its association with mortality, as was N-antigenaemia. Missing values were not imputed, because only 2·3% of patients were excluded from the multivariable analysis due to missing values. Differences between groups were assessed using the χ2 test for categorical variables and the Kruskal–Wallis test for continuous variables. The level of significance was set at 0·05. Statistical analysis was done using IBM SPSS Statistics (version 25.0).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
1068 patients met the inclusion criteria, of whom 117 had insufficient plasma samples and 115 had key information missing. 836 patients were included in the analysis, of whom 403 (48%) were in the VIR-N1-Low group, 283 (34%) were in the VIR-N1-Storm group, and 150 (18%) were in the VIR-N1-Zero group (table 1 ). Most patients were male. Patients in the VIR-N1-Storm group were on average older than patients in the other groups. Hypertension, diabetes of any type, obesity, chronic cardiac disease, and chronic pulmonary disease were the most common comorbidities, and prevalence of diabetes was highest in the VIR-N1-Storm group. Patients in the VIR-N1-Storm group had the worst respiratory function on admission to the ICU, as assessed by the ratio of arterial oxygen partial pressure to fractional inspired oxygen. Patients in the VIR-N1-Zero group were on average admitted to the ICU later after the onset of symptoms than patients in the other groups, and were less frequently treated with hydroxychloroquine, lopinavir or ritonavir, or interferon beta. By contrast, patients in the VIR-N1-Storm group received remdesivir less frequently than patients in the VIR-N1-Low group, but no other differences were found regarding remdesivir treatment between the three groups of patients. Most patients in our cohort received corticosteroids (n=807 [97%]) and one-fifth received tocilizumab (n=187 [22%]), with a similar proportion of patients receiving these drugs in each of the three groups. Neither the viral N1 load nor the prevalence of N-antigenaemia were different in patients who received remdesivir, lopinavir or ritonavir, tocilizumab, hydroxychloroquine, or interferon beta compared with those who did not receive these drugs (all p values >0·05, data not shown).
Table 1.
Participant characteristics and outcomes
| VIR-N1-Zero group (n=150) | VIR-N1-Low group (n=403) | VIR-N1-Storm group (n=283) | p value for VIR-N1-Zero vs VIR-N1-Low | p value for VIR-N1-Zero vs VIR-N1-Storm | p value for VIR-N1-Low vs VIR-N1-Storm | ||
|---|---|---|---|---|---|---|---|
| Characteristics | |||||||
| Age, years | 60 (51–71) | 64 (56–71) | 68 (60–75) | 0·22 | <0·0001 | <0·0001 | |
| Sex | |||||||
| Female | 53 (35%) | 121 (30%) | 86 (30%) | .. | .. | .. | |
| Male | 97 (65%) | 282 (70%) | 197 (70%) | 0·23 | 0·29 | 0·92 | |
| Time from symptoms onset to ICU admission, days | 11 (8–15) | 10 (7–12) | 9 (7–12) | 0·0074 | 0·0001 | 0·31 | |
| SOFA score | 4 (3–6) | 5 (4–7) | 5 (4–7) | 0·015 | <0·0001 | 0·014 | |
| PaO2:FiO2 ratio | 118·84 (91·22–160·42) | 109·00 (80·00–143·06) | 97·71 (70·00–128·89) | 0·041 | <0·0001 | 0·015 | |
| Mean arterial pressure, mm Hg | 87·73 (74·08–100·00) | 88·80 (76·67–100·00) | 88·80 (76·67–97·67) | .. | .. | .. | |
| Haemoglobin concentration, g/dL | 13·40 (11·78–14·53) | 13·40 (12·23–14·50) | 13·20 (11·90–14·60) | .. | .. | .. | |
| Comorbidities | |||||||
| Hypertension | 70 (47%) | 209 (52%) | 162 (57%) | 0·28 | 0·036 | 0·16 | |
| Obesity | 62 (41%) | 145 (36%) | 92 (33%) | 0·25 | 0·068 | 0·35 | |
| Diabetes | 32 (21%) | 98 (24%) | 89 (31%) | 0·46 | 0·026 | 0·039 | |
| Chronic pulmonary disease | 11 (7%) | 48 (12%) | 46 (16%) | 0·12 | 0·0090 | 0·10 | |
| Chronic cardiac disease | 13 (9%) | 51 (13%) | 38 (13%) | 0·19 | 0·14 | 0·77 | |
| Immunosuppression | 12 (8%) | 35 (9%) | 34 (12%) | 0·80 | 0·19 | 0·15 | |
| Gastrointestinal or pancreatic comorbidity | 12 (8%) | 34 (8%) | 24 (9%) | 0·87 | 0·86 | 0·98 | |
| Chronic neurological disease | 9 (6%) | 24 (6%) | 27 (10%) | 0·98 | 0·20 | 0·078 | |
| Chronic kidney disease | 7 (5%) | 27 (7%) | 25 (9%) | 0·38 | 0·12 | 0·30 | |
| Current smoker | 11 (7%) | 30 (7%) | 18 (6%) | 0·97 | 0·70 | 0·58 | |
| Asthma | 8 (5%) | 24 (6%) | 16 (6%) | 0·78 | 0·89 | 0·87 | |
| Genitourinary disease | 4 (3%) | 18 (5%) | 22 (8%) | 0·34 | 0·033 | 0·069 | |
| Alcoholism* | 10 (7%) | 19 (5%) | 9 (3%) | 0·36 | 0·092 | 0·32 | |
| Mild chronic liver disease | 5 (3%) | 13 (3%) | 15 (5%) | 0·95 | 0·35 | 0·18 | |
| Treatment during hospital admission | |||||||
| Corticosteroids | 143 (97%) | 389 (97%) | 275 (98%) | 0·66 | 0·70 | 0·31 | |
| Tocilizumab | 29 (20%) | 88 (22%) | 70 (25%) | 0·56 | 0·22 | 0·37 | |
| Remdesivir | 29 (20%) | 87 (22%) | 39 (14%) | 0·60 | 0·12 | 0·0095 | |
| Lopinavir or ritonavir | 4 (3%) | 53 (13%) | 29 (10%) | 0·0003 | 0·0050 | 0·25 | |
| Hydroxychloroquine | 1 (1%) | 45 (11%) | 24 (9%) | <0·0001 | 0·0010 | 0·25 | |
| Interferon beta | 0 | 24 (6%) | 15 (5%) | 0·0024 | 0·0043 | 0·72 | |
| Complications | |||||||
| Invasive mechanical ventilation | 81 (54%) | 318 (79%) | 266 (94%) | <0·0001 | <0·0001 | <0·0001 | |
| Hyperglycaemia | 86 (59%) | 282 (70%) | 205 (73%) | 0·011 | 0·0023 | 0·40 | |
| Secondary infection | 47 (32%) | 208 (52%) | 180 (65%) | <0·0001 | <0·0001 | 0·0009 | |
| Anaemia | 57 (39%) | 199 (49%) | 182 (65%) | 0·027 | <0·0001 | <0·0001 | |
| Acute kidney injury | 34 (23%) | 126 (31%) | 116 (41%) | 0·063 | 0·0002 | 0·0078 | |
| Acute liver failure | 33 (22%) | 85 (21%) | 78 (28%) | 0·73 | 0·23 | 0·044 | |
| Renal replacement therapy | 6 (4%) | 32 (8%) | 37 (13%) | 0·11 | 0·0030 | 0·029 | |
| Pulmonary thromboembolism | 14 (10%) | 20 (5%) | 21 (8%) | 0·062 | 0·51 | 0·18 | |
| Hypoglycaemia | 6 (4%) | 27 (7%) | 23 (8%) | 0·25 | 0·11 | 0·47 | |
| Bleeding | 7 (5%) | 25 (6%) | 18 (6%) | 0·52 | 0·50 | 0·92 | |
| Outcomes by 90 days after ICU admission | |||||||
| Deaths | 26 (17%) | 106 (26%) | 148 (52%) | 0·028 | <0·0001 | <0·0001 | |
| Time outside ICU among survivors, days | 82 (77–85) | 77 (64–82) | 64 (43–76) | <0·0001 | <0·0001 | <0·0001 | |
| Time outside hospital among survivors, days | 72 (60–78) | 65 (47–73) | 48 (19–64) | <0·0001 | <0·0001 | <0·0001 | |
Data are median (IQR) or n (%) unless otherwise stated. p values were calculated using the Kruskal–Wallis test with Bonferroni post-hoc test adjustment for continuous variables, and χ2 test for categorical variables. Data were missing for haemoglobin (n=36), hydroxychloroquine (n=4), lopinavir or ritonavir (n=4), remdesivir (n=4), corticosteroids (n=5), tocilizumab (n=4), interferon beta (n=4), invasive mechanical ventilation (n=1), renal replacement therapy (n=3), pulmonary thromboembolism (n=35), acute liver failure (n=5), acute kidney injury (n=4), secondary infection (n=14), hyperglycaemia (n=5), hypoglycaemia (n=4), bleeding (n=4), and anaemia (n=4). N1=SARS-CoV-2 nucleocapsid region. ICU=intensive care unit. SOFA=Sequential Organ Failure Assessment. PaO2=arterial oxygen partial pressure. FiO2=fractional inspired oxygen.
Alcoholism was defined as a mantained excessive alcohol consumption (>4 drinks per day for women or >5 drinks per day for men) in the 6 months before COVID-19 symptoms.
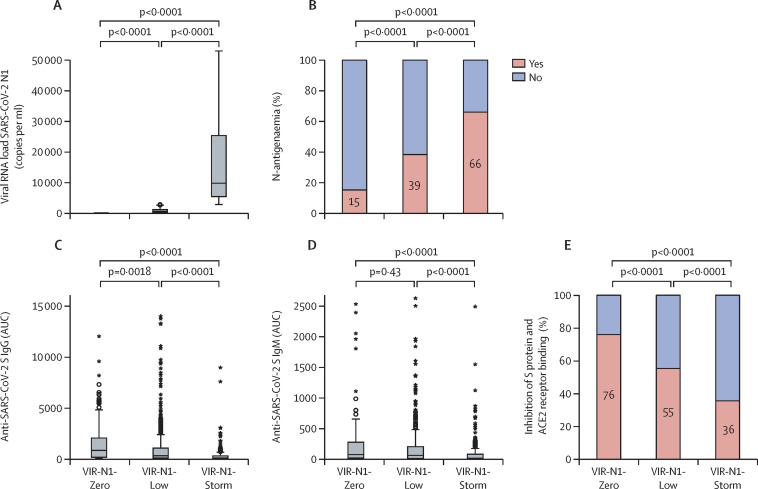
Before admission to the ICU, lopinavir or ritonavir was initiated in two patients (<1%), remdesivir was initiated in 16 (2%), interferon beta was initiated in four (<1%), hydroxychloroquine was initiated in two (<1%), tocilizumab was initiated in 20 (2%), and corticosteroids were initiated in 62 (7%), with no significant differences between the three groups. Viral RNA load in plasma for the three groups is shown in figure 1 . A progressive increase in the prevalence of N-antigenaemia was observed in the groups, from lowest in the VIR-N1-Zero group (23 [15%] of 150 patients) to highest in the VIR-N1-Storm group (187 [66%] of 283; figure 1). Viral N1 load in plasma yielded an area under the curve of 0·74 to detect N-antigenaemia (appendix 2 p 8).
Figure 1.
Virological and antibody profiles
(A) Viral RNA load in plasma (copies of N1 mRNA per mL of plasma). (B) Prevalence of N-antigenaemia in each group. (C) Anti-SARS-CoV-2 S IgG concentrations in plasma. (D) Anti-SARS-CoV-2 S IgM concentrations in plasma. (E) Percentage of plasma binding inhibition of SARS-CoV-2 S protein to ACE2 receptor. Patients were distributed in two categories in each group (those with an inhibitory activity of 50% or greater and those with an inhibitory activity of less than 50%). N1=SARS-CoV-2 nucleocapsid region. AUC=area under the curve. ACE2=angiotensin-converting enzyme 2.
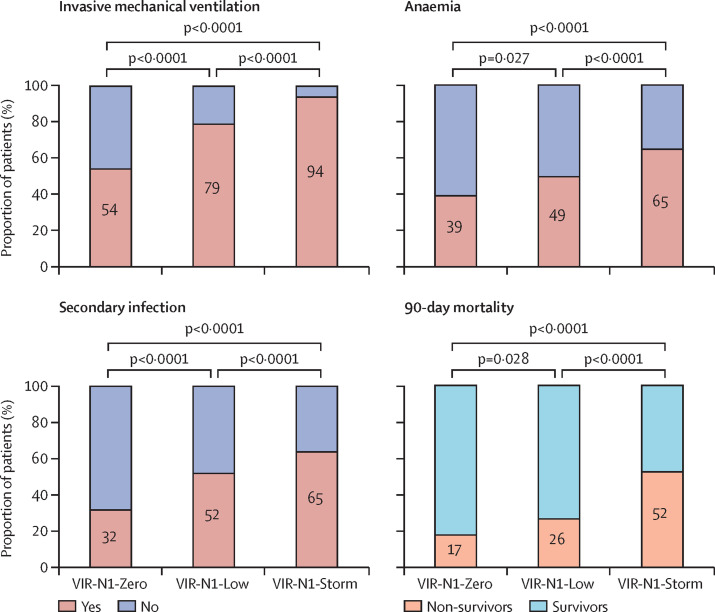
Patients in the VIR-N1-Zero group less frequently received mechanical ventilation during their ICU admission than patients in the other groups (figure 2 ). By contrast, patients in the VIR-N1-Storm group received mechanical ventilation more frequently than patients in the other groups. Patients in the VIR-N1-Storm group also more frequently had acute kidney injury and secondary infections during the ICU admission than patients in the other groups (secondary infections are detailed in appendix 2 [p 9]). Although haemoglobin concentrations at ICU admission did not differ significantly between groups with different viral load, anaemia as a complication was more frequent in the VIR-N1-Storm group than in the other groups (figure 2). Patients in the VIR-N1-Zero group less frequently had hyperglycaemia than those in the other groups.
Figure 2.
Complications and 90-day mortality by viral RNA N1 load group
N1=SARS-CoV-2 nucleocapsid region.
The multivariable analysis showed that viral N1 load (as a continuous variable) was a predictor of 90-day all-cause mortality, independently of age, sex, COVID-19 severity at admission, duration of disease evolution, epidemic period, number of comorbidities, antecedent of immunosuppression, type of treatment, and complications (table 2 ). When viral N1 load was introduced in the model as a categorical variable, this analysis showed that patients in the VIR-N1-Zero group had the lowest odds of death within 90 days after admission as compared with patients in the VIR-N1-Storm group, followed by those in the VIR-N1-Low group (table 2). By 90 days after admission, 26 (17%) of 150 patients in the VIR-N1-Zero group, 106 (26%) of 403 in the VIR-N1-Low group, and 148 (52%) of 283 in the VIR-N1-Storm group had died (table 1, figure 2; appendix 2 p 10). Patients who survived in the VIR-N1-Zero group had a greater number of days out of the ICU and out of hospital in the first 90 days after admission to the ICU than those in the other groups (table 1). The multivariable analysis showed that the presence of N-antigenaemia was associated with increased mortality, using the same adjusting variables (table 2).
Table 2.
Multivariable logistic regression analysis for odds of 90-day mortality
|
Model 1 |
Model 2 |
Model 3 |
|||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | ||
| Viral RNA load in plasma, copies per mL | 1·15 (1·08–1·22) | <0·0001 | .. | .. | .. | .. | |
| Viral RNA load category | |||||||
| VIR-N1-Storm | .. | .. | 1 (ref) | .. | .. | .. | |
| VIR-N1-Zero | .. | .. | 0·30 (0·16–0·55) | <0·0001 | .. | .. | |
| VIR-N1-Low | .. | .. | 0·39 (0·26–0·57) | <0·0001 | .. | .. | |
| N-antigenaemia (yes vs no) | .. | .. | .. | .. | 1·57 (1·09–2·26) | 0·015 | |
| Age, years | 1·07 (1·05–1·09) | <0·0001 | 1·07 (1·05–1·09) | <0·0001 | 1·08 (1·06–1·10) | <0·0001 | |
| Sex (male) | 1·04 (0·71–1·53) | 0·84 | 1·02 (0·69–1·51) | 0·92 | 1·02 (0·70–1·50) | 0·91 | |
| SOFA score | 1·13 (1·04–1·23) | 0·0050 | 1·14 (1·04–1·24) | 0·0040 | 1·13 (1·04–1·23) | 0·0050 | |
| Time since symptoms onset, days | 1·03 (0·99–1·08) | 0·14 | 1·03 (0·99–1·07) | 0·19 | 1·02 (0·98–1·07) | 0·28 | |
| Epidemic period | |||||||
| Period 1 | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | |
| Period 2 | 0·33 (0·17–0·64) | 0·0010 | 0·36 (0·19–0·69) | 0·0020 | 0·35 (0·19–0·67) | 0·0010 | |
| Period 3 | 0·55 (0·28–1·09) | 0·089 | 0·58 (0·29–1·16) | 0·12 | 0·62 (0·32–1·20) | 0·15 | |
| Multimorbidity | 0·96 (0·85–1·09) | 0·53 | 0·96 (0·84–1·09) | 0·48 | 0·96 (0·85–1·09) | 0·54 | |
| Immunosuppression | 2·36 (1·32–4·22) | 0·0040 | 2·32 (1·29–4·18) | 0·0050 | 2·52 (1·41–4·49) | 0·0020 | |
| Remdesivir | 0·92 (0·57–1·48) | 0·72 | 0·97 (0·60–1·57) | 0·90 | 0·88 (0·55–1·41) | 0·60 | |
| Tocilizumab | 0·83 (0·54–1·28) | 0·41 | 0·82 (0·53–1·27) | 0·38 | 0·92 (0·60–1·42) | 0·71 | |
| Invasive mechanical ventilation | 3·29 (1·62–6·67) | 0·0010 | 3·37 (1·66–6·84) | 0·0010 | 4·11 (2·06–8·20) | <0·0001 | |
| Acute kidney injury | 2·17 (1·48–3·20) | <0·0001 | 2·21 (1·50–3·26) | <0·0001 | 2·10 (1·44–3·07) | <0·0001 | |
| Acute liver failure | 1·38 (0·91–2·10) | 0·13 | 1·38 (0·90–2·10) | 0·14 | 1·42 (0·94–2·14) | 0·096 | |
| Secondary infection | 1·01 (0·69–1·50) | 0·95 | 0·99 (0·67–1·47) | 0·97 | 1·02 (0·70–1·50) | 0·91 | |
| Hyperglycaemia | 1·64 (1·06–2·54) | 0·026 | 1·68 (1·08–2·60) | 0·021 | 1·65 (1·07–2·54) | 0·023 | |
| Anaemia | 1·25 (0·84–1·87) | 0·27 | 1·23 (0·82–1·84) | 0·31 | 1·27 (0·85–1·87) | 0·24 | |
Model 1 evaluated viral N1 load as a continuous variable, model 2 evaluated viral N1 load as a categorical variable, and model 3 evaluated N-antigenaemia. Each of the models included 817 patients. Copies per mL of viral RNA were transformed to Napierian log values to reach a normal distribution. Epidemic period 1 was from March 16 to June 21, 2020; epidemic period 2 was from June 22 to Dec 6, 2020; and epidemic period 3 was from Dec 7, 2020, to Feb 27, 2021. Multimorbidity was a composed variable obtained from the summation of hypertension, diabetes, obesity, chronic cardiac disease, chronic neurological disease, chronic kidney disease, chronic pulmonary disease, gastrointestinal or pancreatic comorbidity, asthma, genitourinary disease, and mild chronic liver disease. N1=SARS-CoV-2 nucleocapsid region. OR=odds ratio. SOFA=Sequential Organ Failure Assessment.
Patients in the VIR-N1-Zero group had the lowest concentrations of C-reactive protein in plasma, while patients in the VIR-N1-Storm group had the highest (appendix 2 p 11). By contrast, patients in the VIR-N1-Zero group had the highest monocyte counts. Patients in the VIR-N1-Storm group had the highest plasma glucose concentrations upon admission to the ICU.
Patients in the VIR-N1-Zero group had the highest concentrations of anti-SARS-CoV-2 S IgG antibodies in plasma at ICU admission (figure 1; appendix 2 p 11). By contrast, patients in the VIR-N1-Storm group had the lowest concentrations of both anti-SARS-CoV-2 S IgG and IgM antibodies. In parallel, plasma from patients in the VIR-N1-Zero group showed the strongest inhibitory activity of the interaction between the SARS-CoV-2 S protein and the ACE2 receptor, and plasma from patients in the VIR-N1-Storm group showed the lowest inhibitory activity (figure 1).
Patients in the VIR-N1-Zero group had the lowest plasma concentrations of RNAse P mRNA (appendix 2 pp 11–12). By contrast, concentrations of this marker were raised markedly in patients in the VIR-N1-Storm group. Concentrations of this marker were highest in patients who received invasive mechanical ventilation and in those who died (median RNAse P mRNA concentration 90 817 copies per mL [IQR 44 257–170 161] in patients who received invasive mechanical ventilation vs 45 278 copies per mL [20 867–81 532] in patients not requiring invasive mechanical ventilation, p<0·0001; median RNAse P mRNA concentration 95 604 copies per mL [IQR 52 148–201 275] in patients who died vs 70 023 copies per mL [32 010–128 565] in patients who remained alive, p<0·0001).
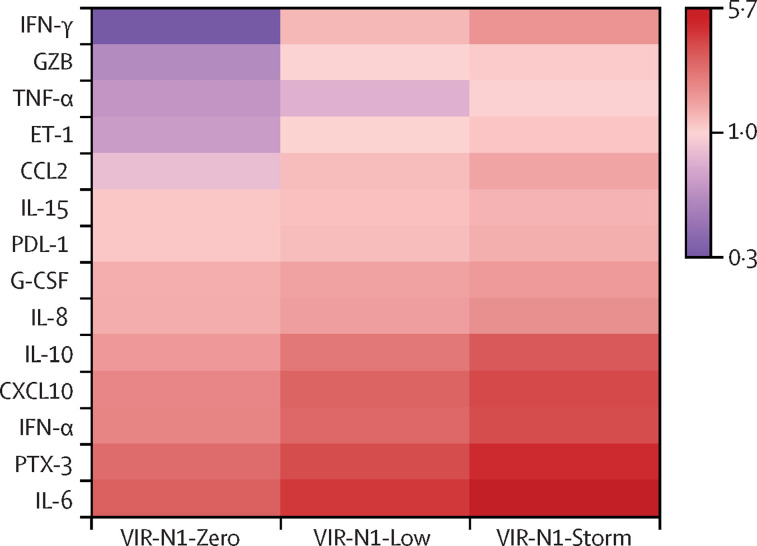
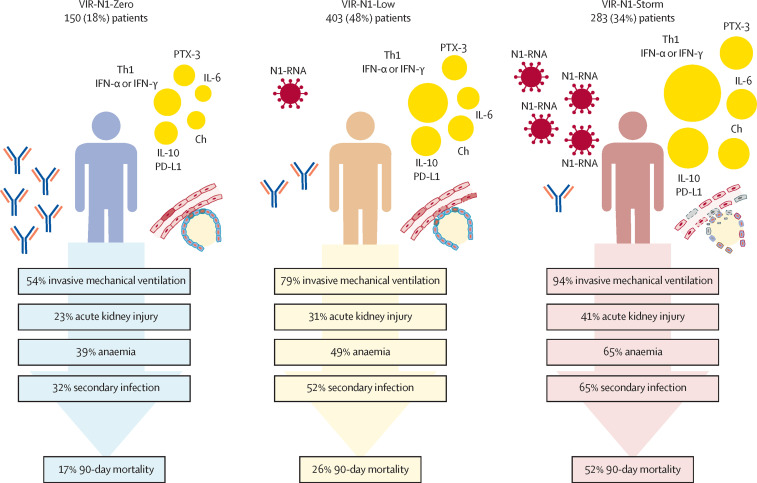
As with RNAse P mRNA, and by contrast to what was observed for antibodies, there was a progressive increase in the concentrations of several biomarkers involved in innate immunity, endothelial dysfunction, and immunosuppression across the three compared groups, with patients in the VIR-N1-Zero group showing the lowest concentrations and patients in the VIR-N1-Storm group showing the highest (figure 3 ; appendix 2 pp 13–14). These biomarkers were T helper 1 or interferon pro-inflammatory mediators (TNF-α, IL-15, IFN-α, IFN-γ), molecules with chemotactic activity for monocytes (CCL2), granulocytes (IL-8 and G-CSF) and T cells (CXCL10 or IP-10), a potent vasoconstrictor peptide produced by vascular endothelial cells (ET-1), an immunomodulatory cytokine (IL-10), a checkpoint inhibitor of T-cell function (PD-L1), and a serin-protease mediating apoptosis of infected cells by T-CD8 cells and natural killer cells (granzyme B). An activator of the complement pathway (PTX-3), together with a T helper 2 or T helper 17 cytokine (IL-6), were among the mediators that showed the greatest increases from the VIR-N1-Zero group to the VIR-N1-Storm group. Patients in the VIR-N1-Storm group showed the highest concentrations of a marker of neutrophil degranulation (MPO), of the immunomodulatory mediator IL-1ra, the inflammatory marker TREM-1, and finally of another marker of endothelial dysfunction, VCAM-1. The three groups of patients did not show induced secretion of IFN-β and IL-1 β, IL-12p70, UPA, IL-17A, and lipocalin-2, had low concentrations of the adaptive immunity stimulator IL-2, and had below average concentrations of granzyme A (a T-cell effector molecule) and IL-4 (a T helper 2 cytokine). Figure 4 summarises the biological responses and clinical outcomes in the three groups.
Figure 3.
Heat map showing biomarker concentrations by viral RNA N1 load group
Results are provided as log 2 (median in each group divided by median in healthy controls). Differences in the concentrations of the biomarkers shown here were significant (p<0·05) for all the comparisons between the three groups (VIR-N1-Zero, VIR-N1-Low, and VIR-N1-Storm). N1=SARS-CoV-2 nucleocapsid region. IFN=interferon. GZB=granzyme B. TNF=tumour necrosis factor. ET=endothelin. CCL=C-C motif chemokine ligand. IL=interleukin. PD-L=programmed death-ligand. G-CSF=granulocyte colony-stimulating factor. CXCL=C-X-C motif chemokine ligand. PTX=pentraxin.
Figure 4.
Biological responses and clinical outcomes in patients in the VIR-N1-Zero, VIR-N1-Low, and VIR-N1-Storm viral load groups
N1=SARS-CoV-2 nucleocapsid region. PTX=pentraxin. Th1=T helper 1 cytokines. IFN=interferon. Ch=chemokines. IL=interleukin. PD-L=programmed death-ligand.
Discussion
By using dPCR, a robust next-generation PCR method, we found that the viral RNA load in plasma measured in the first 48 h following ICU admission was directly associated with 90-day all-cause mortality in a large cohort of critically ill patients with COVID-19. We identified a specific group of patients (the VIR-N1-Storm group), who were admitted to the ICU with a high concentration of viral RNA in plasma (>2747 copies per mL of N1), who had a particularly high mortality (about half of these patients died in the first 90 days after ICU admission, a mortality rate similar to that of septic shock or acute respiratory distress syndrome). Patients in this group represented about one-third of the cohort included in this study, and more frequently received invasive mechanical ventilation and had disease complications (acute kidney injury requiring renal replacement therapy, secondary infections, and anaemia) compared with patients in the other groups. Most of the patients (187 [66%] of 283) in the VIR-N1-Storm group had presence of the viral protein N in plasma (N-antigenaemia), which was also an independent predictor of mortality in our study. Patients in the VIR-N1-Storm group had low concentrations of SARS-CoV-2 S antibodies, and their profile of biomarkers denoted the existence of a wide dysregulation of the host response to the infection, involving activation of inflammation, chemotaxis, neutrophil degranulation and antiviral response, immunosuppression, endothelial dysfunction, and tissue damage. These findings are in line with previous results that have shown links between viral RNAemia and hypercytokinaemia in critically ill patients.25 Patients in the VIR-N1-Storm group were older and more frequently had diabetes, which was likely to contribute to mounting impaired or less effective immunological responses against SARS-CoV-2.26, 27 Alternatively, anti-S antibodies could be being removed from circulation due to their consumption during the antiviral response (eg, via Fc receptor clearance).
dPCR helped us to identify a subgroup of patients with undetectable concentrations of viral N1 RNA in plasma (150 [18%] of 836 patients in the cohort), and this group had the lowest frequency of complications and the lowest mortality rate (four in every five patients in this group were still alive 3 months after ICU admission). Only a minority of patients in this group had N-antigenaemia (n=23 [15%]). At the time of ICU admission, these patients had already developed a robust response of SARS-CoV-2 S antibodies, and showed milder alterations in their host response than patients in the other groups. The remaining patients in our cohort, those with detectable but low concentrations of viral RNA in plasma, showed intermediate severity, frequency of complications, magnitude of the dysregulated response, and 90-day mortality (n=106 [26%]) compared with patients in the other groups.
Although the existence of a potential cytokine storm as a major causal event in the pathogenesis of severe COVID-19 has been extensively discussed in the literature, the available evidence seems to challenge this theory.3, 4 By contrast, our findings show that a substantial proportion of patients admitted to the ICU with COVID-19 show a viral storm, characterised by the massive release of viral material to the blood from the lungs or other tissues, which probably reflects the inability of these patients to control viral replication. This viral storm is accompanied by biological alterations similar to those observed in sepsis,28 which suggests that it is probably the initial driver of organ failure in these patients. Our results suggest that ICU admission without signs of this viral storm translates into a milder impact of the disease and a lower risk of mortality.
This study has some limitations. First, we could not elucidate why some patients with undetectable concentrations of viral N1 RNA in plasma showed N-antigenaemia. Because the N protein is likely to be less prone to degradation than RNA, it could persist in plasma even in the absence of ongoing viral replication.29 Second, we did not culture SARS-CoV-2 in plasma or blood, so we could not confirm or exclude the potential contribution of live virus to the viral storm. Nonetheless, the presence of SARS-CoV-2 RNA or proteins in plasma could induce direct harmful effects through activation of innate immune pathways.30 Third, we did not profile cellular immunity. Finally, our study was developed during the first year of the COVID-19 pandemic, so the effect of the omicron (B.1.1.529) variant and vaccination on our results is unknown.
Despite these limitations, this study has a major strength. Identifying biological differences between subgroups of critically ill patients with severe COVID-19 requires a sufficient sample size, otherwise these differences could be missed.9 To our knowledge, this is the largest study to date to profile in parallel viral and immunological signatures in critically ill patients with COVID-19, providing important clues to understand the pathophysiology of the most severe forms of the disease caused by SARS-CoV-2 infection. Our results encourage strategies to prevent uncontrolled viral replication in patients at high risk of severe disease, to decrease morbidity and mortality (eg, up-to-date vaccination, prophylaxis with anti-S monoclonal antibodies with neutralising activity in uninfected patients, or early administration of antivirals in patients with COVID-19). Our findings also suggest that the presence of a viral storm could serve as a predictive enrichment tool for trials evaluating antivirals or blood purification devices to remove viral components from the blood in critically ill patients with COVID-19.
Data sharing
The datasets used or analysed in this study are available from the corresponding author on reasonable request.
Declaration of interests
JFB-M, AT, FB, RA, JME, and APT have a patent application on SARS-CoV-2 antigenaemia as a predictor of mortality in COVID-19. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by grants from the Instituto de Salud Carlos III (FONDO-COVID19, COV20/00110, CIBERES, 06/06/0028; AT), Proyectos de Investigación en Salud (PI19/00590; JFB-M), Miguel Servet (CP20/00041; DdG-C), Sara Borrell (CD018/0123; APT), and Predoctorales de Formación en Investigación en Salud (FI20/00278; AdF). We also received funds from Programa de Donaciones Estar Preparados, UNESPA (Madrid, Spain), and from the Canadian Institutes of Health Research (CIHR OV2–170357; DJK and JFB-M), Research Nova Scotia, Li-Ka Shing Foundation (DJK), and finally by a Research Grant 2020 from ESCMID (APT). COV20/00110, PI19/00590, CP20/00041, CD018/0123, FI20/00278 were co-funded by European Regional Development Fund and European Social Fund (A way to make Europe, and Investing in your future). We thank the IRB-Lleida Biobank 119 (B.0000682) and Plataforma Biobancos PT17/0015/0027 in Lleida, the Hospital Clinic Barcelona (HCB)-IDIBAPS biobank in Barcelona, and the National DNA Bank and the Hospital Universitario de Salamanca biobank (both in Salamanca) for their logistical support with sample processing and storage. We are indebted to the Fundació Glòria Soler for its contribution and support to the COVIDBANK of HCB-IDIBAPS Biobank. This work was not supported by any pharmaceutical company or other agency.
CIBERES-UCI-COVID Group
Contributors
JFB-M, DJK, FB, and AT participated in protocol development, study design, and study management. JFB-M and SR developed the statistical analysis plan. JFB-M wrote the first draft of the manuscript. NG-M participated in the coordination of the clinical study, analysed the data, and drafted the figures. AM participated in the conception of the work and in the coordination of the study. LT, PRM, EB-M, EGC, AÚ-I, MdCdlT, ÁE, SC-F, IMV, FP-G, LS, JLM, PV-C, VSM, MG-R, NC, MCMD, LJV, CM-L, RNJG, EM, AL-V, WT, JAB, RH-M, JB, PE, AMdlG, CR, GA, GR, JBM, RCA, MSV, EBP, EG, FJCM, MRN, JMSC, YPM, MTGU, MTBV, AM-R, LPB, LdR-C, NAM, JMG, MLB, JC, CB, JG, MTN, JN-dO, EP-S, LGG, JC-R and JME recruited the patients and collected the clinical data. SR, IM, MM-V, MJM-G, VM, MV, and OC did the antibody assays. LSR performed the extraction of viral RNA. AO developed the dPCR works. APT and AdF analysed the viral load data. AO, TP, and NJ profiled the biomarkers. DdG-C participated in the study design. AC, LFB, and RA participated in the study design and coordination. All authors critically revised the manuscript for important intellectual content and approved the final version. All authors agree to be accountable in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors had full access to all the data in the study, verified the underlying data reported, and accept responsibility for the decision to submit for publication.
Contributor Information
CIBERES-UCI-COVID Group:
Alicia Ortega, Amanda de la Fuente, Raquel Almansa, Tamara Postigo, Noelia Jorge, Ana P Tedim, Laura González-González, Lara Sánchez Recio, Wysali Trapiello, José Ángel Berezo, Rubén Herrán-Monge, Jesús Blanco, Pedro Enríquez, Isidoro Martínez, María Martín-Vicente, María José Muñoz-Gómez, Vicente Más, Mónica Vázquez, Olga Cano, Amalia Martínez de la Gándara, Covadonga Rodríguez, Gloria Andrade, Gloria Renedo, Juan Bustamante-Munguira, Ramón Cicuendez Ávila, María Salgado-Villén, Enrique Berruguilla-Pérez, Estel Güell, Fernando Javier Casadiego Monachello, María Recuerda Núñez, Juan Manuel Sánchez Calvo, Yhivian Peñasco-Martín, María Teresa García Unzueta, Adrián Ceccato, Laia Fernández-Barat, María Teresa Bouza Vieiro, Ana Moreno-Romero, Leire Pérez Bastida, Lorena del Río-Carbajo, Noelia Albalá Martínez, José Manuel Gómez, María Luisa Blasco, Jesús Caballero, Carme Barberà, Jessica González, María Teresa Nieto, Jorge Nieto-del Olmo, Estefanía Prol-Silva, Joan Canseco-Ribas, and Jose María Eiros
Supplementary Materials
References
- 1.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA. 2020 doi: 10.1001/jama.2020.17052. published online Sept 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermejo-Martin JF, Almansa R, Tedim AP, et al. Mounting evidence of impaired viral control in severe COVID-19. Lancet Microbe. 2021;2:e228–e229. doi: 10.1016/S2666-5247(21)00084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulaiman I, Chung M, Angel L, et al. Microbial signatures in the lower airways of mechanically ventilated COVID-19 patients associated with poor clinical outcome. Nat Microbiol. 2021;6:1245–1258. doi: 10.1038/s41564-021-00961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D, Kearney MF, O'Regan A, et al. Therapeutic implications of ongoing alveolar viral replication in COVID-19. Lancet Rheumatol. 2022;4:e135–e144. doi: 10.1016/S2665-9913(21)00322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs JL, Bain W, Naqvi A, et al. Severe acute respiratory syndrome coronavirus 2 viremia is associated with coronavirus disease 2019 severity and predicts clinical outcomes. Clin Infect Dis. 2022;74:1525–1533. doi: 10.1093/cid/ciab686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs JL, Naqvi A, Shah FA, et al. Plasma SARS-CoV-2 RNA levels as a biomarker of lower respiratory tract SARS-CoV-2 infection in critically ill patients with COVID-19. J Infect Dis. 2022;226:2089–2094. doi: 10.1093/infdis/jiac157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ynga-Durand M, Maaß H, Milošević M, et al. SARS-CoV-2 viral load in the pulmonary compartment of critically ill COVID-19 patients correlates with viral serum load and fatal outcomes. Viruses. 2022;14 doi: 10.3390/v14061292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinrich F, Nentwich MF, Bibiza-Freiwald E, et al. SARS-CoV-2 blood RNA load predicts outcome in critically ill COVID-19 patients. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bermejo-Martin JF, González-Rivera M, Almansa R, et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit Care. 2020;24:691. doi: 10.1186/s13054-020-03398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutmann C, Takov K, Burnap SA, et al. SARS-CoV-2 RNAemia and proteomic trajectories inform prognostication in COVID-19 patients admitted to intensive care. Nat Commun. 2021;12 doi: 10.1038/s41467-021-23494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Järhult JD, Hultström M, Bergqvist A, Frithiof R, Lipcsey M. The impact of viremia on organ failure, biomarkers and mortality in a Swedish cohort of critically ill COVID-19 patients. Sci Rep. 2021;11 doi: 10.1038/s41598-021-86500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merino I, de la Fuente A, Domínguez-Gil M, Eiros JM, Tedim AP, Bermejo-Martín JF. Digital PCR applications for the diagnosis and management of infection in critical care medicine. Crit Care. 2022;26:63. doi: 10.1186/s13054-022-03948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suo T, Liu X, Feng J, et al. ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg Microbes Infect. 2020;9:1259–1268. doi: 10.1080/22221751.2020.1772678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veyer D, Kernéis S, Poulet G, et al. Highly sensitive quantification of plasma SARS-CoV-2 RNA sheds light on its potential clinical value. Clin Infect Dis. 2021 doi: 10.1093/cid/ciaa1196. published online Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Feng J, Zhang Q, et al. Analytical comparisons of SARS-COV-2 detection by qRT-PCR and ddPCR with multiple primer/probe sets. Emerg Microbes Infect. 2020;9:1175–1179. doi: 10.1080/22221751.2020.1772679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793–798. doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ram-Mohan N, Kim D, Zudock EJ, et al. SARS-CoV-2 RNAemia predicts clinical deterioration and extrapulmonary complications from COVID-19. Clin Infect Dis. 2022;74:218–226. doi: 10.1093/cid/ciab394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Red Nacional de Vigilancia Epidemiológica (RENAVE) Informe no 87: situación de COVID-19 en España. 2021. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/GRIPE/Informes%20semanales/Temporada_2020-21/Informe%20n%C2%BA%2087%20Situaci%C3%B3n%20de%20COVID-19%20en%20Espa%C3%B1a%20a%2014%20de%20julio%20de%202021.pdf
- 22.Torres A, Motos A, Ceccato A, et al. Methodology of a large multicenter observational study of patients with COVID-19 in Spanish intensive care units. Arch Bronconeumol. 2022;58(suppl 1):22–31. doi: 10.1016/j.arbres.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruneau T, Wack M, Poulet G, et al. Circulating ubiquitous RNA, a highly predictive and prognostic biomarker in hospitalized coronavirus disease 2019 (COVID-19) patients. Clin Infect Dis. 2022;75:e410–e417. doi: 10.1093/cid/ciab997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almansa R, Eiros JM, de Gonzalo-Calvo D, et al. N-antigenemia detection by a rapid lateral flow test predicts 90-day mortality in COVID-19: a prospective cohort study. Clin Microbiol Infect. 2022;28 doi: 10.1016/j.cmi.2022.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa449. published online July 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oishi K, Horiuchi S, Frere J, Schwartz RE, tenOever BR. A diminished immune response underlies age-related SARS-CoV-2 pathologies. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.111002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Z-H, Yu W-L, Sun Y. Multiple immune function impairments in diabetic patients and their effects on COVID-19. World J Clin Cases. 2021;9:6969–6978. doi: 10.12998/wjcc.v9.i24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubio I, Osuchowski MF, Shankar-Hari M, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis. 2019;19:e422–e436. doi: 10.1016/S1473-3099(19)30567-5. [DOI] [PubMed] [Google Scholar]
- 29.Olea B, Albert E, Torres I, et al. SARS-CoV-2 N-antigenemia in critically ill adult COVID-19 patients: frequency and association with inflammatory and tissue-damage biomarkers. J Med Virol. 2022;94:222–228. doi: 10.1002/jmv.27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin DE. Why does viral RNA sometimes persist after recovery from acute infections? PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used or analysed in this study are available from the corresponding author on reasonable request.