Introduction and importance:
Chronic-encapsulated intracerebral hematomas are a rare type of hematoma. They tend to be mistaken for abscesses or tumors. The etiology of these hematomas is not yet clear, although they have mainly been linked to arteriovenous malformations, cavernomas, and head trauma. Surgical evacuation is effective in improving neurological symptoms with a good prognosis. However, the lesion may be difficult to diagnose.
Case presentation:
Here, the authors report a case of a chronic-encapsulated and calcified intracerebral hematoma following recurrent mild head injuries mimicking a supratentorial hemangioblastoma in a healthy 26-year-old female patient presented with progressive raised intracranial pressure and left body heaviness with good outcomes after en bloc surgical resection.
Clinical discussion:
The chronic-encapsulated intracerebral hematoma was first described by Hirsh et al. in 1981. Their etiology is not yet clear, although they have mainly been linked to arteriovenous malformations, cavernomas, and head trauma. Pathologically, they are characterized by the presence of a fibrous capsule composed of an outer collagen layer and an inner granulated layer. Radiologically, they appear as cystic lesions with a homogeneous high signal on T1-weighted and T2-weighted images associated with a lower signal ring sign and ring enhancement after gadolinium administration that may suggest hemangioblastoma.
Conclusion:
Although chronic parenchymal hematomas remain a rare phenomenon, it has become increasingly logical to consider this entity in differential diagnoses with other lesions. In cases with recurrent head trauma, a detailed investigation will aid in making the diagnosis of such a rare pathology.
Keywords: chronic-encapsulated hematoma, hemangioblastoma, MRI, surgery
Introduction and importance
Highlights
Chronic-encapsulated intracerebral hematomas tend to be mistaken for abscesses or tumors.
Pathologically, they are characterized by the presence of a fibrous capsule composed of an outer collagen layer and an inner granulated layer.
Radiologically, they appear as cystic lesions with a homogeneous high signal on T1-weighted and T2-weighted images associated with a lower signal ring sign and ring enhancement that may suggest hemangioblastoma.
Since these hematomas rarely disappear spontaneously, their surgical management would be indicated in symptomatic cases.
Intracerebral hematomas usually resolve spontaneously. However, after a variable period of time, some may slowly expand and behave as space-occupying lesions, leading to neurological deficits and damage1. Since chronic-encapsulated intracerebral hematomas are a rare type of hematoma, they tend to be mistaken for tumors or abscesses, being correctly diagnosed only preoperatively around 20%. The etiology of these hematomas is not yet clear, although they have mainly been linked to arteriovenous malformations, cavernomas, and head trauma1. Surgical evacuation is effective in improving neurological symptoms with a good prognosis. However, the lesion may be difficult to diagnose.
Here, the authors report a case of a chronic-encapsulated and calcified intracerebral hematoma following recurrent mild head injuries mimicking a supratentorial hemangioblastoma in a healthy 26-year-old female patient presented with progressive raised intracranial pressure and left body heaviness with good outcomes after en bloc surgical resection.
This case report has been reported in line with the SCARE (Surgical CAse REport) Criteria2.
Case presentation
A previously healthy 26-year-old married housewife patient was admitted to our department of neurosurgery for the progressive onset of raised intracranial pressure syndrome made of holocranial headache, several episodes of vomiting, and bilateral blurred vision with the sensation of left body heaviness, clumsiness in both hands, and dizziness without response to common analgesics from 15 days beforehand. There was no associated episode of seizures or fever. Upon neurological examination, she was awake and well oriented to time and space with good coordination and normal reflexes. There was no cranial nerve impairment. Motor ability examination revealed a 4/5 left hemiparesis, mainly affecting the upper limb without facial involvement. Dilated fundus examination showed a bilateral grade III papilledema.
A head computed tomography (CT) scan (Fig. 1) was performed, demonstrating a right frontoparietal lesion with a triple fleshy, cystic, and calcium component. The cystic component was predominant, exerting a mass effect on the right lateral ventricle and the midline. A further examination with brain MRI (Fig. 2) showed the right frontoparietal lesion measuring 72×53 mm in diameter, exerting a midline shift estimated at 10 mm. This lesion had three different components. The cystic component had a hyposignal on T1-weighted sequences and hypersignal on T2-weighted sequences without restricted diffusion on diffusion-weighted sequences. The solid component had a heterogeneous signal on T1-weighted sequences and a frank hyposignal on T2-weighted sequences with major enhancement after chelates of gadolinium injection and significant hyperperfusion on perfusion-weighted imaging. The third component was a calcium deposit which appeared as a signal void (flow void) with a hyposignal on the gradient echo sequence. The lesion was associated with dilatation of the ventricles with transependymal resorption of cerebrospinal fluid at the level of the occipital horns on T2 fluid-attenuated inversion recovery sequences. All these findings were compatible with the diagnosis of supratentorial hemangioblastoma. A chest radiography, complete blood count, serum electrolytes test, and hemostasis assessment were performed and revealed no abnormalities. A full-body CT scan (Fig. 3) was performed in search of other tumor locations, and to rule out the possibility of von Hippel–Lindau disease did not show any abnormalities. Bilateral kidneys also showed up normal.
Figure 1.
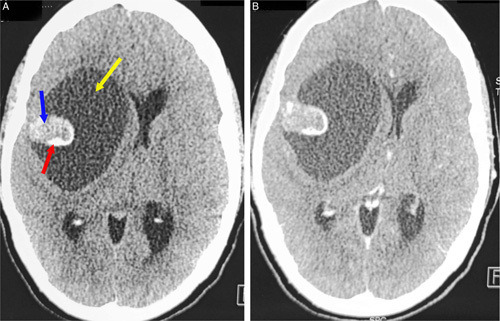
Axial brain computed tomography scan in parenchymal bone before (A) and after (B) enhancement showing a right frontoparietal lesion with a triple fleshy (blue arrow), cystic (white arrow), and calcium component (red arrow). The cystic component was predominant, exerting a mass effect on the right lateral ventricle and the midline.
Figure 2.
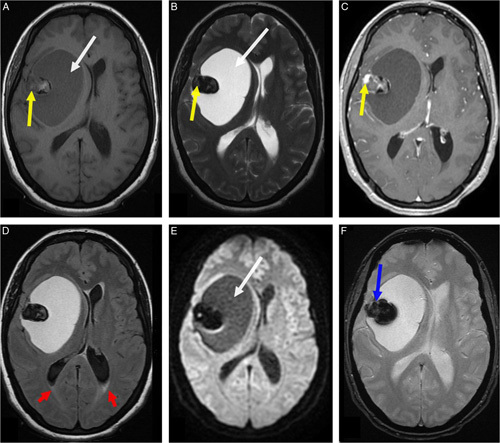
Axial brain MRI showing the right frontoparietal lesion measuring 72×53 mm in diameter exerting a midline shift estimated at 10 mm. This lesion had three different components. The cystic component had a hyposignal on T1-weighted sequences (A, white arrow) and hypersignal on T2-weighted sequences (B, white arrow) without restricted diffusion on diffusion-weighted sequences (E, white arrow). The solid component (mural nodule) had a heterogeneous signal on T1-weighted sequences (A, yellow arrow) and a frank hyposignal on T2-weighted sequences (B, yellow arrow) with major enhancement after chelates of gadolinium injection (C, yellow arrow). Note the third component, which was a calcium deposit having a signal void with a hyposignal on the gradient echo sequence (F, blue arrow). There was no peritumoral edema except a minor transependymal cerebrospinal fluid resorption at the level of the occipital horns on T2 fluid-attenuated inversion recovery sequences (D, red arrows).
Figure 3.
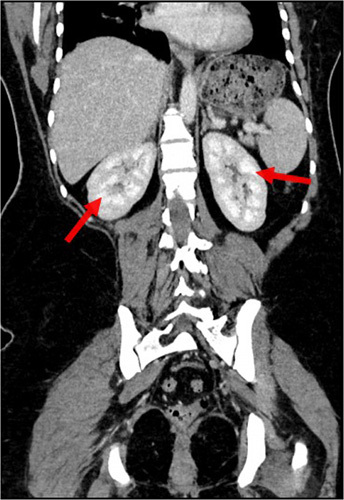
A full-body enhanced computed tomography scan (portal venous phase) in the coronal plane showing no other tumor locations as well as normal bilateral kidneys (red arrows).
After a well-written consent, the patient underwent an en bloc resection and total cyst soft aspiration through a c-shaped skin incision and frontoparietal bone flap centered on the tumor in a supine position with head turned to the left side and put in a classic horseshow headrest (Fig. 4). Preoperatively, the cystic component was viscous and yellowish on aspiration and the solid component was firm and reddish in appearance, given its high vascularity (Figs. 5 and 6). The postoperative course was uneventful, and the postoperative CT scan confirmed the gross total tumor resection.
Figure 4.
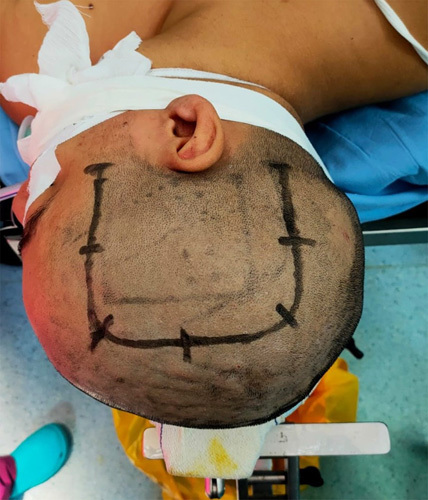
Preoperative photograph showing the c-shaped frontoparietal skin incision centered on the tumor in a supine position with head turned to the left side and put in classic horseshow headrest.
Figure 5.
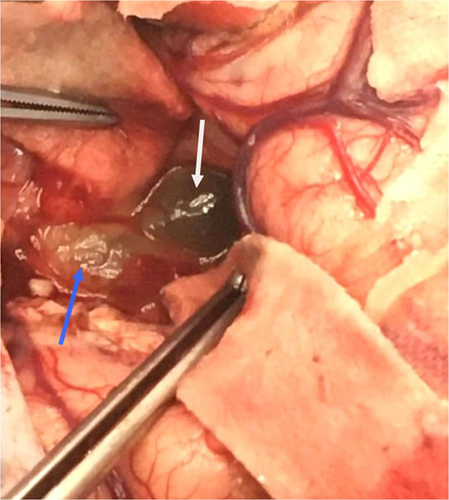
Peroperative view after bone removal, durotomy, and corticotomy showing the cystic (white arrow) and solid component (blue arrow) of the tumor.
Figure 6.
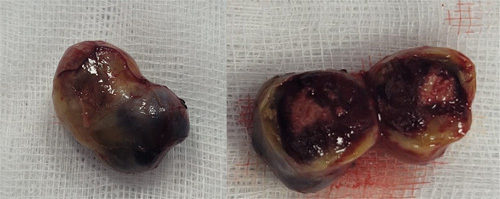
Immediate postoperative photograph of the tumor’s solid component. Note its yellowish and reddish appearance, given its high vascularity.
Histological examination (Fig. 7) of the specimen showed that the nodule described corresponds to a hematoma well limited by a thick fibrous wall focally calcified and which is the site of a localized mononuclear inflammatory infiltrate rich in siderophages. This wall is sometimes covered by one or more layers of regular endothelial cells. The lumen is filled with fibrino-cruoric material. In immunohistochemistry, immunostaining was negative for anti-inhibin and CD34 antibodies.
Figure 7.
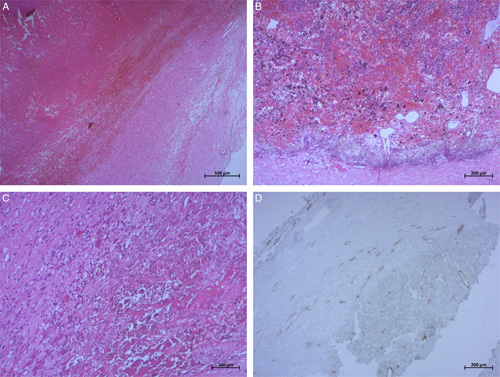
Photomicrographs of surgical specimen hematoxylin and eosin (H&E) stain: (A) (H&E, ×25), (B) (H&E, ×50), and (C) (H&E, ×100) showing that pseudocystic cavity contains fibrinous hemorrhagic material with an underlying fibrous capsule containing inflammatory cells and siderophages. An immunohistochemistry study (D) showed that the capsule is lined by endothelial cells negative for CD34 (×50).
The patient was discharged from our department 5 days postoperatively with persistent left hemiparesis and put under levetiracetam at a dose of 2000 mg per day and degressive systemic corticosteroids with an appointment at the outpatient clinic in 1 month. At that date, the patient was awake and in good clinical condition, with total disappearance of his left body deficit.
Clinical discussion
The chronic-encapsulated intracerebral hematoma was first described by Hirsh et al. in 19813. To our knowledge, there are currently around 68 published cases of this entity in the literature. These hematomas are characterized by a slow and progressive expansion due to repeated internal microbleeding from the capsule that surrounds them.
The etiology of these hematomas is not yet clear, although they have mainly been linked to arteriovenous malformations, cavernomas, and head trauma1. In our patient and by pushing the interrogation, it turned out that the latter was a victim of domestic violence past last year, where she suffered head injuries at each conflict with her husband. Cases without associated vascular malformation or other known causes have also been reported. Other possible causes described are coagulopathies, cerebral arteriosclerosis, small cerebral aneurysms, and chronic inflammation, and recent cases after stereotactic radiosurgery for arteriovenous malformations have been found4.
Pathologically, these hematomas are characterized by the presence of a fibrous capsule composed of an outer collagen layer and an inner granulated layer3. Its histology is similar to that of the capsule of chronic subdural hematomas, and it is thought to develop by gradual growth due to repeated bleeding of new blood vessels into the capsule5. The presence of this capsule is essential to be able to define this entity since there are published cases of chronic fluid intracerebral hematomas instead of encapsulated hematomas, which should not be confused5. Wetzel et al.6 postulated that in the subacute phase of slow bleeding, fibroblasts penetrate the brain parenchyma and begin to form a capsule around the hematoma. As the capsule forms, it begins to vascularize through chemical signaling that includes vascular endothelial growth factor. This crumbly capsule may bleed again, increasing the size of the hematoma.
From a radiological point of view, Cai et al.7 described the behavior of five patients who were diagnosed with a chronic hematoma during surgery. On CT scan, the hematoma appeared as an almost circular or elliptical lesion, hypodense and with clear borders and representative surrounding edema. The five cases presented ring contrast enhancement, three of them moderately. In our case, the lesion contained fleshy, cystic, and calcium components. On administration of contrast, richly vascularized granulation tissue may be seen as a ring-like uptake pattern. On magnetic resonance, these hematomas present as cystic lesions with a homogeneous high signal on T1-weighted and T2-weighted images associated with a lower signal ring sign on susceptibility-weighted imaging and ring enhancement after gadolinium administration7. In our case, the hematoma had three different components. The cystic component had a hyposignal on T1-weighted sequences and hypersignal on T2-weighted sequences without restricted diffusion. The solid component had a heterogeneous signal on T1-weighted sequences and a frank hyposignal on T2-weighted sequences with major enhancement after chelates of gadolinium injection and significant hyperperfusion. The third component was a calcium deposit which appeared as a signal void with a hyposignal on the gradient echo sequence.
Regarding location, most of the chronic hematomas described previously in the literature, including ours, were intraparenchymal, although six intraventricular locations have been described, two of them in the pediatric population6.
Regarding treatment, in asymptomatic patients, it is reasonable to carry out conservative management with a close clinical and radiological follow-up. However, since these hematomas rarely disappear spontaneously, their surgical management would be indicated in symptomatic cases5. Our patient underwent surgery as she presented with progressive raised intracranial pressure and left body heaviness, and the first diagnosis made on imaging was a supratentorial hemangioblastoma.
Most of the cases described in the literature have been operated on by craniotomy, including our patient. All but two with good results. Pozzati and Kosnik8 in their series of 10 cases described two deaths due to postoperative rebleeding. These good results are partly due to the fact that the presence of the capsule makes it easy to separate the hematoma from the brain parenchyma, as it was made in our case. In the case of endoscopic surgery, surgical time and brain exposure are shorter, so there is less risk of bleeding. Although, if there is, it is more difficult to control by endoscopy. Therefore, careful manipulation of the hematoma should be performed, and preparations for a craniotomy in the case of massive bleeding should be performed5. Another possible minimally invasive option to manage this kind of hematoma is the CT-guided stereotactic aspiration, described in 2014 by Nishiara et al.9. However, this could leave more of the hematoma, which would lead to a recurrence, and, in addition, controlling the bleeding would be more difficult.
On the other hand, the need to remove the capsule or not is controversial. Although excision of the capsule has been recommended to prevent the recurrence of the hematoma, Yashon et al.10 demonstrated that only evacuating the hematoma without the capsule may lead to a better prognosis. However, Pozzati and Kosnik8, as well as other authors, consider simple aspiration of the hematoma insufficient. In our patient, we removed the entire hematoma with its capsule, as there was a good cleavage plane between the hematoma and the brain, and tried to reduce the intracranial pressure as much as possible, especially after the cystic soft aspiration.
Through our patient, we were able to add to the literature another rare case of chronic-encapsulated cerebral hematoma following recurrent mild head trauma mimicking radiologically a solitary calcified supratentorial hemangioblastoma which remains in itself a rare localization. An association between these two pathologies was not found in the literature. We insist on the importance of imaging as well as histological and immunohistochemical study to make the diagnosis and choose the best surgical management of these hematomas.
Conclusion
Although chronic parenchymal hematomas remain a rare phenomenon, it has become increasingly logical to consider this entity in differential diagnoses with other lesions to choose the best management. In cases with recurrent head trauma, a detailed patient history, radiographic study, and a comprehensive list of differential diagnoses will aid in making the diagnosis of such a rare pathology.
Ethical approval
The study is exempt from ethical approval in our institution.
Patient consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Patient perspective
During hospitalization and at discharge, the patient was given the opportunity to share her perspective on the intervention she received, and she was satisfied with the care.
Sources of funding
There are no sources of funding for this research.
Author contribution
M.B. and S.A.: study concept, data interpretation, and writing the paper; M.B., S.A., and A.A.: writing the paper; M.B., S.A., A.A., M.B., and M.Z.B.: study concept.
Conflicts of interest disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of the article.
Research registration unique identifying number (UIN)
None.
Guarantor
Mehdi Borni, Department of Neurosurgery, UHC Habib Bourguiba, Sfax, Tunisia. E-mail: borni.mehdi13@gmail.com
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgments
All authors would like to kindly thank all the medical staff in the Department of Neurosurgery and all doctors involved in this work. Special thanks to the radiologists and medical assistants who were also involved in managing our patients.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online ■ ■
Contributor Information
Mehdi Borni, Email: borni.mehdi13@gmail.com.
Souhir Abdelmouleh, Email: abdelmoulehsouhir@gmail.com.
Anis Abdelhedi, Email: anisabdelhedi86@gmail.com.
Ameur Affes, Email: affesameur@hotmail.com.
Mohamed Z. Boudawara, Email: zaher.boudawara@rns.tn.
References
- 1. Sakaida H, Sakakura M, Tochio H, et al. Chronic encapsulated intracerebral hematoma associated with angiographically occult arteriovenous malformation – case report. Neurol Med Chir (Tokyo) 1993;33:638–642. [DOI] [PubMed] [Google Scholar]
- 2. Agha RA, Franchi T, Sohrab C, et al. The SCARE 2020 guideline: updating consensus Surgical Case Report (SCARE) guidelines. Int J Surg 2020;84:226–230. [DOI] [PubMed] [Google Scholar]
- 3. Hirsh LF, Spector HB, Bogdanoff B. Chronic encapsulated intracerebral hematoma. Neurosurgery 1981;9:169–172. [DOI] [PubMed] [Google Scholar]
- 4. Takeuchi S, Takasato Y, Masaoka H, et al. Development of chronic encapsulated intracerebral hematoma after radiosurgery for a cerebral arteriovenous malformation. Acta Neurochir 2009;151:1513–1515. [DOI] [PubMed] [Google Scholar]
- 5. Uriel Lavín R, Hernández Valido AV, Diana Martín R, et al. Hematoma intracerebral crónico encapsulado: un reto diagnóstico. Ocronos 2022;5:21. [Google Scholar]
- 6. Wetzel J, Bray D, Wrubel D. Chronic encapsulated intraventricular hematoma in a pediatric patient: case report. J Neurosurg Pediatr 2018;22:68–73. [DOI] [PubMed] [Google Scholar]
- 7. Cai S, Zhou B, Liao H, et al. Imaging diagnosis of chronic encapsulated intracerebral hematoma, a comparison of computed tomography (CT) and magnetic resonance imaging (MRI) characteristics. Pol J Radiol 2017;82:578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pozzati E, Giuliani G, Gaist G, et al. Chronic expanding intracerebral hematoma. J Neurosurg 1986;65:611–614. [DOI] [PubMed] [Google Scholar]
- 9. Nishiyama A, Toi H, Takai H, et al. Chronic encapsulated intracerebral hematoma: three case reports and a literature review. Surg Neurol Int 2014;5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yashon D, Kosnik E. Protect intracerebral hematoma. Neurosurgery 1978;2:103–106. [DOI] [PubMed] [Google Scholar]


