Abstract
Objectives:
The present study aimed to investigate the clinical relevance of lateral pelvic lymph node dissection (LPLND) in low rectal cancer without preoperative treatment, with a focus on the presence of LPLN enlargement in preoperative imaging.
Methods:
Consecutive patients with cT3 to T4 low rectal cancer who underwent mesorectal excision and LPLND without preoperative treatment between 2007 and 2018 at a single dedicated cancer center were included. LPLN short-axis diameter (SAD) measured using preoperative multi-detector row computed tomography (MDCT) was evaluated retrospectively.
Results:
A total of 195 consecutive patients were analyzed. Overall, 101 (51.8%) and 94 (48.2%) patients had visible and no visible LPLNs in preoperative imaging, including 56 (28.7%), 28 (14.4%), and 17 (8.7%) patients had SADs of <5 mm, 5-7 mm, and ≥7 mm, respectively. Incidence of pathologically confirmed LPLN metastasis were 18.1%, 21.4%, 28.6%, and 52.9%, respectively. Overall, thirteen (6.7%) patients developed local recurrence (LR), including one patient who developed lateral recurrence, yielding a 5-year cumulative risk for LR of 7.4%. Five-year RFS and OS for all patients were 69.7% and 85.7%, respectively. No differences were observed in the cumulative risk for LR and OS between any pairs of groups.
Conclusions:
No significant difference was observed in the cumulative risk for LR and OS regardless of LPLN SAD, implying the good impact of LPLND on the prevention of lateral recurrence, as well as the difficulty of predicting LPLN metastasis using only LPLN SAD in preoperative imaging.
Keywords: lateral pelvic lymph node dissection, local recurrence, preoperative chemoradiation, rectal cancer, total mesenteric excision
Introduction
Colorectal cancer is the third most common cancer in both sexes worldwide, with approximately 1.8 million new cases reported in 2018[1]. Although rectal cancer and colon cancer share many underlying biological features, the former occurs in a specific anatomical setting (i.e., in the narrow pelvis near other organs) and thus requires a unique therapeutic approach not required for treating the latter.
There are two major concerns with treating rectal cancer: local pelvic recurrence and distant organ metastasis after surgical resection. Local pelvic recurrence is further categorized into two types: lateral recurrence due to lateral pelvic lymph node (LPLN) metastasis and central recurrence due to microscopic residual disease in the circumferential resection margin (CRM). Many colorectal surgeons have emphasized preoperative pelvic radiotherapy to manage extra-mesorectal disease in locally advanced rectal cancer since the Dutch trial[2]. Treatment outcomes for locally advanced rectal cancer have drastically improved with the combination of (chemo)radiation and perioperative systemic chemotherapy in the last two decades[3,4]. Meanwhile, an insufficient therapeutic effect was recently reported for neoadjuvant chemoradiation alone with total mesenteric excision (TME) for enlarged LPLNs[5]. Therefore, there has been an increasing interest in the surgical management of enlarged LPLNs.
In Japan, colorectal surgeons have taken on the challenge of addressing lateral recurrence with LPLN dissection (LPLND) without preoperative treatment[6]. The autonomic nerve-preserving procedure was established in the 1990s and significantly contributed to the spread of LPLND in Japan[7]. A Japanese nationwide multi-institutional study revealed a high incidence of LPLN metastasis (approximately 15-20%) for T3 to T4 rectal cancers below the peritoneal reflection[8]. The results of that study provide support for current guidelines of the Japanese Society for Cancer of the Colon and Rectum (JSCCR), which recommend adding LPLND to mesorectal excision (ME) for clinical stage II or III locally advanced low rectal cancer without any preoperative treatment[9]. However, the Japan Clinical Oncology Group (JCOG) 0212 trial demonstrated that prophylactic LPLND had a minor impact on survival, although the trial did not confirm noninferiority of ME alone compared to ME with LPLND[10]. Several meta-analyses have also found that adding LPLND to TME had a minimal impact when performed for all rectal cancers[11,12], implying the need to reconsider the indications for LPLND.
Although both Western and Eastern treatment strategies have provided satisfactory control for local recurrence and survival outcomes, there is still room for improvement. We have a common goal to implement tailored strategies based on risk stratification for each recurrent pattern of lateral recurrence, central recurrence, and distant organ recurrence. Hence, in the present study, we reviewed the preoperative clinical characteristics and oncologic outcomes of patients with low rectal cancer, with a focus on the presence of LPLN enlargement in preoperative imaging, in order to better understand the clinical relevance of LPLND in low rectal cancer without preoperative treatment in Eastern countries.
Patients and Methods
Patient identification
Consecutive patients with American Joint Committee on Cancer (AJCC)[13] cT3 to T4 low rectal adenocarcinoma who underwent proctectomy with curative intent between January 2007 and December 2018 were identified from a prospectively collected database of the Aichi Cancer Center Hospital (ACCH). In the present study, patients with tumors for which the lower edge was located below the peritoneal reflection were considered to have low rectal cancer according to the JSCCR guidelines[9].
Patient characteristics were reviewed and augmented by secondary chart review. Patients were excluded if they did not receive LPLND for any reason, e.g., enrolling in a clinical trial, or having unfit performance status, low cardiopulmonary function, or severe atherosclerosis intolerable to LPLND. Patients were also excluded if they received preoperative chemotherapy primarily for unresectable tumors. Patients with recurrent or metastatic colorectal cancer, primary colon cancer spreading to the rectum, or those who had undergone urgent surgery without preoperative examination were also excluded from the present analysis.
Treatment
All rectal cancer patients received a colonoscopy, barium or gastrografin enema, and computed tomography (CT) scanning of the chest, abdomen, and pelvis for preoperative staging. Multi-detector row CT (MDCT) was used for the detailed evaluation of lymph node involvement and growth into circumferent organs or structures. Endoscopic ultrasound and pelvic magnetic resonance imaging (MRI) were also recommended for preoperative staging, but was not performed for all cases during the study period.
Standard treatment for rectal cancer at ACCH during the study period included upfront TME or tumor-specific ME with upward lymph node dissection toward the root of the inferior mesenteric artery (IMA) without preoperative CRT[14]. Multivisceral resection was included when clinical T4 tumors were detected on preoperative imaging without any preoperative treatment. Bilateral LPLND was performed for all clinical stage II or III tumors for which the lower edge of the tumor was located below the peritoneal reflection. The current JSCCR classification regards all internal iliac, hypogastric, obturator, external iliac, common iliac, lateral sacral, presacral, and sacral promontory nodes as regional LPLNs for low rectal cancer[9]; thus, the extent of LPLND included all of these nodes.
After surgical treatment and pathological evaluation, the benefit of postoperative chemotherapy was discussed in a multidisciplinary team conference for each patient. Meanwhile, some people refused postoperative chemotherapy after providing informed consent from medical oncologists.
Follow-up and survival
After surgical treatment (and postoperative chemotherapy), all patients were recommended to undergo follow-up, which entailed physical examinations and collection of laboratory data including carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels every three months for the first two years and every six months for the subsequent three years, and CT scanning of the chest, abdomen, and pelvis every six months for five years. All follow-up visits, laboratory tests, and radiological examinations were performed at ACCH.
All patients were followed for ten years postoperatively, up to when any event occurred, or up to March 2021, whichever was earlier. Survival time was defined as the time from primary rectal cancer surgery to each event. The cumulative risk for local recurrence (LR) was calculated as the time to LR as a first relapse, relapse-free survival (RFS) was calculated as the time to first recurrence or death from any cause, and overall survival (OS) was calculated as the time to death from any cause.
Statistical analysis
For all patients who met the inclusion criteria, LPLN short-axis diameter (SAD) was retrospectively evaluated using preoperative MDCT at slice thickness of 5 mm or less. Patients were categorized into four groups according to LPLN diameter: No visible LPLN, LPLN SAD less that 5 mm (<5 mm), LPLN SAD 5 mm or more and less than 7 mm (5-7 mm), and LPLN SAD 7 mm or more (≥7 mm). Associations between LPLN diameter and pathologic positivity were assessed. The correlation between LPLN diameter and oncologic outcomes was also evaluated by the cumulative risk for LR, RFS, and OS.
Categorical variables were analyzed using Pearson's χ2 test. Continuous variables were presented as medians with interquartile ranges (IQRs) and analyzed using the Kruskal-Wallis H test. Odds ratios (ORs) for LPLN metastasis were calculated by univariate and multivariate logistic regression analyses. Cumulative risks for LR, RFS, and OS were estimated by Kaplan-Meier survival analysis and compared with the log-rank test. Multivariate models were developed with backward selection using covariates with p values < 0.10 in the univariate analysis. P values < 0.05 were considered statistically significant. SPSS 27.0 (IBM Corporation, Armonk, NY, US) was used for all statistical analyses.
Ethical approval
The present experimental protocols were approved by the institutional review committee at Aichi Cancer Center Hospital (2020-1-297).
Results
Patient, surgical, and pathological characteristics
A total of 620 consecutive patients with early-stage rectal cancer during the study period were identified from a prospectively collected database, and 206 patients met the inclusion criteria of the present study. Eleven patients lacked preoperative imaging data and thus were excluded. Accordingly, 195 patients were included and analyzed in the present study.
Patient, surgical, and pathological characteristics are shown in Table 1. Overall, 20 (10.3%) patients had cT4 tumors, and 74 (37.9%) had clinically positive nodes in the mesorectum. One hundred and twenty-four (63.6%) and 71 (36.4%) patients underwent sphincter-preserving surgery and non-sphincter-preserving surgery, respectively. Twenty-four (12.3%) patients underwent multivisceral resection, of which the most frequent organ resected was the vagina (ten patients), followed by seminal vesicles (six patients), prostate (four patients), ovary (three patients), and ureter (one patient). R0 resection was achieved in 182 (93.8%) patients. One hundred and six (54.4%) patients had pathologically confirmed lymph node metastasis in the mesorectum or lateral pelvis, and 86 (44.1%) patients underwent postoperative chemotherapy.
Table 1.
Patient, Surgical, and Pathological Characteristics.
| All | No visible LPLN | SAD <5 mm | SAD 5-7 mm | SAD ≥7 mm | p value | |
|---|---|---|---|---|---|---|
| n = 195 | n = 94 | n = 56 | n = 28 | n = 17 | ||
| Age, years | 60 (53-67) | 60 (52-67) | 59 (52-66) | 62 (55-67) | 57 (47-72) | 0.915 |
| Sex, male (%) | 130 (66.7) | 58 (61.7) | 39 (69.6) | 12 (75.0) | 12 (70.6) | 0.521 |
| Tumor size, cm | 5.0 (4.1-6.3) | 5.0 (4.0-6.1) | 5.5 (4.2-6.4) | 5.5 (4.5-6.4) | 6.0 (4.9-6.5) | 0.423 |
| Histology, n (%) | 0.454 | |||||
| tub1/tub2 | 181 (92.8) | 89 (94.7) | 50 (89.3) | 27 (96.4) | 15 (88.2) | |
| por/muc/sig | 14 (7.2) | 5 (5.3) | 6 (10.7) | 1 (3.6) | 2 (11.8) | |
| Distance from AV, n (%) | 0.187 | |||||
| <5 cm | 124 (63.6) | 62 (66.0) | 28 (50.0) | 21 (75.0) | 13 (76.5) | |
| ≥5 cm | 70 (35.9) | 31 (33.0) | 28 (50.0) | 7 (25.0) | 4 (23.5) | |
| Missing | 1 (0.5) | 1 (1.1) | 0 | 0 | 0 | |
| AJCC cT classification, n (%) | 0.648 | |||||
| T3 | 175 (89.7) | 87 (92.6) | 49 (87.5) | 24 (85.7) | 15 (88.2) | |
| T4 | 20 (10.3) | 7 (7.4) | 7 (12.5) | 4 (14.3) | 2 (11.8) | |
| AJCC cN classification, n (%) * | 0.900 | |||||
| N0 | 121 (62.1) | 61 (64.9) | 32 (57.1) | 19 (67.9) | 9 (52.9) | |
| N1 | 51 (26.2) | 22 (23.4) | 17 (30.4) | 6 (21.4) | 6 (35.3) | |
| N2 | 23 (11.8) | 11 (11.7) | 7 (12.5) | 3 (10.7) | 2 (11.8) | |
| Surgical procedure, n (%) | 0.811 | |||||
| Sphincter preserving | 124 (63.6) | 61 (64.9) | 33 (58.9) | 18 (64.3) | 12 (70.6) | |
| Non-sphincter preserving | 71 (36.4) | 33 (35.1) | 23 (41.1) | 10 (35.7) | 5 (29.4) | |
| Multivisceral resection, n (%) | 0.216 | |||||
| Present | 24 (12.3) | 7 (7.4) | 10 (17.9) | 5 (17.9) | 2 (11.8) | |
| Absent | 171 (87.7) | 87 (92.6) | 46 (82.1) | 23 (82.1) | 15 (88.2) | |
| AJCC pT classification, n (%) | 0.047 | |||||
| T1 | 2 (1.0) | 2 (2.1) | 0 | 0 | 0 | |
| T2 | 52 (26.7) | 30 (31.9) | 10 (17.9) | 6 (21.4) | 6 (35.3) | |
| T3 | 129 (66.2) | 61 (64.9) | 41 (73.2) | 17 (60.7) | 10 (58.8) | |
| T4 | 12 (6.2) | 1 (1.1) | 5 (8.9) | 5 (17.9) | 1 (5.9) | |
| AJCC pN classification, n (%) | 0.457 | |||||
| N0 | 89 (45.6) | 49 (52.1) | 22 (39.3) | 13 (46.4) | 5 (29.4) | |
| N1 | 60 (30.8) | 28 (29.8) | 19 (33.9) | 7 (25.0) | 6 (35.3) | |
| N2 | 46 (23.6) | 17 (18.1) | 15 (26.8) | 8 (28.6) | 6 (35.3) | |
| R status, n (%) | 0.160 | |||||
| R0 | 182 (93.8) | 90 (95.7) | 51 (91.1) | 24 (85.7) | 17 (100.0) | |
| R1 | 13 (6.2) | 4 (4.3) | 5 (8.9) | 4 (14.3) | 0 | |
| Postoperative chemotherapy, n (%) | 0.119 | |||||
| Present | 86 (44.1) | 38 (40.4) | 28 (50.0) | 9 (32.1) | 11 (64.7) | |
| Absent | 109 (55.9) | 56 (59.6) | 28 (50.0) | 19 (67.9) | 6 (35.3) |
Values are presented as median (IQR).
*Regional (mesorectal, intermediate, and IMA) N status
SAD: short-axis diameter, LPLN: lateral pelvic lymph node, AV: anal verge, AJCC: the American Joint Committee on Cancer, R: resection, IMA: inferior mesenteric artery
There were no differences according to LPLN SAD in age, sex, tumor size, histology, tumor location, AJCC cT/N classification, surgical procedures, AJCC pN classification, R status, and postoperative chemotherapy. AJCC pT classification was earlier in the no visible LPLN group compared to the SAD <5 mm and SAD 5-7 mm groups.
Associations between LPLN diameter and pathologic positivity
Overall, 101 (51.8%) and 94 (48.2%) patients had visible and no visible LPLNs in preoperative imaging. Of those with visible LPLNs, 56 (28.7%), 28 (14.4%), and 17 (8.7%) patients had SADs of <5 mm, 5-7 mm, and ≥7 mm, respectively. The median (IQR) LPLN SAD for patients with visible LPLNs was 4.7 (3.7-6.4) mm.
Of all 195 patients, 46 (23.6%) had pathologically confirmed LPLN metastasis. The incidence of LPLN metastasis according to SAD in preoperative imaging is shown in Figure 1. Seventeen of 94 (18.1%) patients with no visible LPLN and 12 of 56 (21.4%) patients with LPLNs of less than 5 mm in preoperative imaging had positive LPLNs. Meanwhile, patients who had positive LPLNs remained 8 of 20 (28.6%) in patients with LPLNs of 5-7 mm, and 9 of 17 (52.9%) in patients with LPLNs of 7 mm or more in preoperative imaging.
Figure 1.
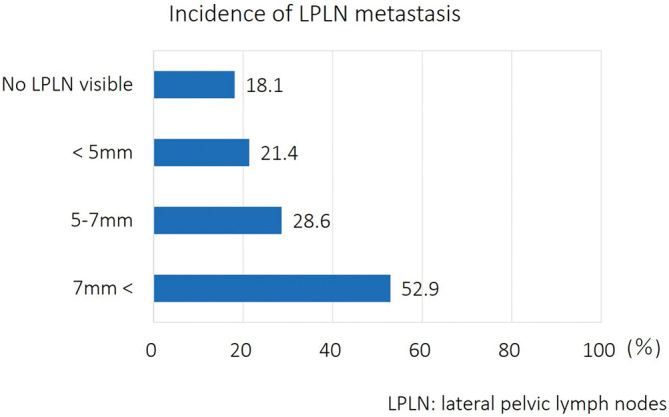
Lateral pelvic lymph node metastasis according to short-axis diameter in preoperative imaging.
The results of univariate and multivariate analyses for LPLN metastasis are shown in Table 2. In univariate analysis, AJCC cT/N classification and LPLN diameter were significantly associated with the incidence of LPLN metastasis. After backward selection and development of the multivariate model, AJCC cN classification and LPLN SAD remained significant predictors of LPLN metastasis, along with sex.
Table 2.
Univariate and Multivariate Analyses for Lateral Pelvic Node Metastasis.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age | ||||||
| <70 years | 1.000 | 1.000 | ||||
| ≥70 years | 2.118 | 0.948-4.732 | 0.067 | 2.050 | 0.861-4.882 | 0.105 |
| Sex | ||||||
| Male | 1.000 | 1.000 | ||||
| Female | 1.966 | 0.997-3.878 | 0.051 | 2.331 | 1.094-4.965 | 0.028 |
| Tumor size | ||||||
| <5 cm | 1.000 | |||||
| ≥5 cm | 1.272 | 0.631-2.563 | 0.501 | |||
| Distance from AV | ||||||
| <5 cm | 1.000 | |||||
| ≥5 cm | 1.845 | 0.884-3.861 | 0.103 | |||
| Histology | ||||||
| pap/tub | 1.000 | 1.000 | ||||
| por/muc/sig | 2.606 | 0.854-7.951 | 0.092 | 2.565 | 0.759-5.851 | 0.130 |
| AJCC cT classification | ||||||
| cT1-3 | 1.000 | 1.000 | ||||
| cT4 | 3.007 | 1.160-7.799 | 0.024 | 2.107 | 0.759-5.851 | 0.153 |
| AJCC cN classification* | ||||||
| cN0 | 1.000 | 1.000 | ||||
| cN1-2 | 2.116 | 1.081-4.143 | 0.029 | 2.613 | 1.252-5.453 | 0.011 |
| LPLN short-axis diameter | ||||||
| <5 mm | 1.000 | 1.000 | ||||
| ≥5 mm | 2.491 | 1.205-5.152 | 0.014 | 2.990 | 1.360-6.576 | 0.006 |
Values are presented as median (IQR).
*Regional (mesorectal, intermediate, and IMA) N status
AV: anal verge, AJCC: the American Joint Committee on Cancer, IMA: inferior mesenteric artery, LPLN: lateral pelvic lymph node
Correlations between short-axis diameter and oncologic outcomes
Median (IQR) follow-up durations for all patients were 44.0 (20.0 - 75.0) months for recurrence and 59.0 (35.0 - 87.0) months for survival. No perioperative mortality occurred within 30 days after surgery.
Overall, 55 (28.2%) patients developed recurrence during the follow-up period. Thirteen (6.7%) patients developed LR, yielding a five-year cumulative risk for LR of 7.4%. The most frequent pattern of LR was recurrence in the central pelvis (11 patients), followed by anastomosis (one patient) and lateral pelvis (one patient). Five-year RFS and five-year OS rates for all patients were 69.7% and 85.7%, respectively.
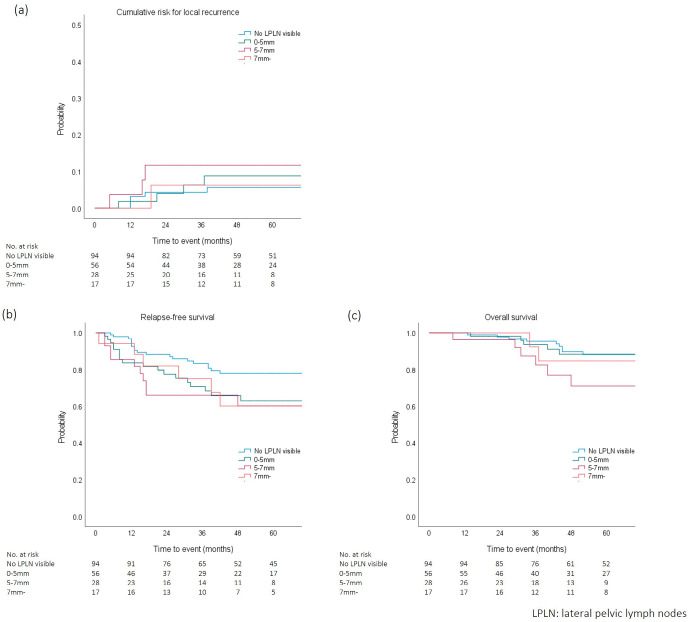
Stratified oncologic outcomes of cumulative risk for LR, RFS, and OS according to LPLN SAD in preoperative imaging are shown in Figure 2. Five-year cumulative risk for LR for no visible LPLN, SAD <5 mm, SAD 5-7 mm, and SAD ≥7 mm groups were 5.6%, 8.8%, 11.7%, and 6.2%, respectively. No differences were observed in the cumulative risk for LR between any pairs of groups. Five-year RFS rates for the no visible LPLN, SAD <5 mm, SAD 5-7 mm, and SAD ≥7 mm groups were 77.9%, 62.9%, 60.1%, and 60.1%, respectively. RFS for the no visible LPLN group was significantly better than those of the SAD <5 mm and SAD 5-7 mm groups (p = 0.043 and 0.048, respectively). No significant differences were observed in RFS between any other pairs of groups. Five-year OS rates for the no visible LPLN, SAD <5 mm, SAD 5-7 mm, and SAD ≥7 mm groups were 88.1%, 88.3%, 71.1%, and 84.6%, respectively. No differences were observed in OS between any pairs of groups.
Figure 2.
Cumulative risk for (a) local recurrence, (b) relapse-free survival, and (c) overall survival according to lateral pelvic lymph node diameter in preoperative imaging.
Discussion
The present study investigated the clinical relevance of LPLND for patients with low rectal cancer without preoperative treatment. Approximately 20% of patients with LPLN SAD <5 mm in preoperative imaging had pathologically confirmed LPLN metastasis. Conversely, nearly 50% of patients with LPLN SAD ≥7 mm had no LPLN metastasis. It is also noteworthy that, as opposed to a previous study reporting the limited impact of neoadjuvant chemoradiation for enlarged LPLNs[5], no significant difference was observed in the cumulative risk for LR regardless of LPLN SAD. Although the present study design had some limitations, the results highlight the good impact of LPLND on the prevention of lateral recurrence, as well as the difficulty of optimizing LPLND using only LPLN SAD in preoperative imaging.
An international multicenter pooled analysis from Western countries demonstrated an increased cumulative incidence of all and lateral LR with enlarged LPLNs in pretreatment imaging after preoperative chemoradiation followed by TME[5]. Meanwhile, the present results revealed an equivalent cumulative risk for LR in patients with LPLN SAD ≥7 mm and <7 mm after mesorectal excision and LPLND without preoperative chemoradiation. The former Western analysis also revealed a regrettable radiologic complete response rate of enlarged LPNs after chemoradiation of only about 5%[15]. These results suggest that LPLND can be an option for patients with apparently enlarged LPLNs in locally advanced rectal cancer.
Malakorn et al. reported that no patient with LPLN SAD ≤5 mm in post-neoadjuvant chemoradiation imaging had LPLN metastasis after TME and selective LPLND[16], and other studies from China also revealed only 3.8% of patients with LPLN SAD ≤7 mm in post-neoadjuvant chemoradiation imaging had LPLN metastasis[17]. Meanwhile, the present study found that about 20% of patients with LPLN SAD ≤5 mm in preoperative imaging had LPLN metastasis after ME and routine LPLND. These results suggest the treatment effect of preoperative chemoradiation for LPLN micrometastasis. A relatively high rate of LPLN micrometastasis and low rate after preoperative chemoradiation implies that preoperative chemoradiation might have a therapeutic effect toward micrometastases in LPLNs and, in other words, 20% of patients without enlarged LPLNs require LPLND in the absence of preoperative chemoradiation.
When preoperative chemoradiation is not performed, selecting 20% of patients who do not have enlarged LPLNs but have LPLN metastasis is not straightforward. Previous studies on the association between LPLN diameter and pathologic positivity without preoperative treatment are summarized in Table 3[18-25]. Most of these studies were retrospective studies that enrolled <100 patients, with accuracy in predicting LPLN metastasis of 59-87%. Some of them tried to define the optimal size criteria in their study for predicting LPLN metastasis; however, none of them could establish the practical cutoff values. In the present study setting, routine LPLND is performed for all patients with clinical stage II or III low rectal cancer instead of preoperative chemoradiation, given the difficulty of predicting LPLN metastasis. However, the fact remains that the other 80% of patients who have neither enlarged LPLNs nor LPLN metastasis were overtreated by invasive surgical procedures.
Table 3.
Previous and Present Studies on Associations between LPLN Short-Axis Diameter and Pathologic Positivity.
| Author | Year | N | Prevalence | Modality | Cut-off | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|---|---|---|
| Arii [18] | 2006 | 53 | 28% | MRI | LA 7 mm | 56% | 97% | 91% | 81% | 83% |
| Matsuoka [19] | 2007 | 51 | 29% | MRI | SAD 5 mm | 67% | 83% | N/A | N/A | 78% |
| Akasu [20] | 2009 | 104 | 14% | MRI | SAD 4 mm | 87% | 87% | 52% | 97% | 87% |
| Fujita [21] | 2009 | 210 | 22% | CT | LA 5 mm | 62% | 90% | 64% | 89% | 84% |
| Ogawa [22] | 2014 | 77 | 14.9% | MRI | LA 5 mm | 80.0% | 56.7% | 21.6% | 95.0% | 59.7% |
| Ishibe [23] | 2015 | 84 | 19.9% | MRI | SAD 10 mm | 75.0% | 69.1% | 36.4% | 92.2% | 70.2% |
| Komori [24] | 2019 | 351 | 7.3% | CT | SAD 5 mm | 37.5% | 89.1% | 21.4% | 94.8% | 85.3% |
| Hiyoshi [25] | 2020 | 78 | 11.5% | CT | SAD 5 mm | 66.7% | 79.7% | 30.0% | 94.8% | 78.2% |
| Present study | 2021 | 195 | 23.6% | CT | SAD 5 mm | 37.0% | 81.2% | 37.8% | 80.7% | 70.8% |
| SAD 7 mm | 19.6% | 94.6% | 52.9% | 79.2% | 76.9% |
LA: long-axis, SAD: short-axis diameter, PPV: positive predictive value, NPV: negative predictive value
In the present study, RFS for patients with no visible LPLNs was significantly better than those for patients with LPLN SAD <5 mm and SAD 5-7 mm. These results may be ascribed to the earlier AJCC pT classification in no visible LPLN group than SAD < 5 mm and SAD 5-7 mm groups. Schaap et al. reported the different impact that obturator node diameter has on survival based on whether LPLND is performed[26]. However, it remains unclear whether LPLND contributes to distant metastasis control and survival. Multidisciplinary treatment, including chemotherapy or total neoadjuvant therapy (TNT) in addition to local treatment, is desirable for patients with a high risk for distant metastasis[3,4].
The main limitation in the present study relates to the imaging modalities used for preoperative diagnosis. Although recommended for preoperative staging, pelvic MRI was not performed for all patients. However, Amano et al. reported in a retrospective study, albeit with a small number of patients, that MDCT and MRI had similar detection power for LPLN metastasis[27]. Only a few studies have compared the abilities of MDCT and MRI to detect LPLN metastasis, and the superior modality of the two remains controversial. Besides, MDCT is used more often than MRI for preoperative staging of locally advanced rectal cancer. Therefore, the present study design, which used MDCT, may be desirable from the perspective of generalizability of the results.
This study has additional limitations. This study was retrospective in nature and had a small sample size. However, the present study included patients who underwent standardized follow-up in a single dedicated cancer center, enabling a detailed analysis of patient background and oncologic outcomes. Our data did not include CRM but pathological margin status due to the unique pathological diagnosis method for mesenteric lymph nodes in Japan. However, in the present study, there were no differences according to LPLN SAD in AJCC cT/N classification. Fewer patients received chemotherapy after surgery than indicated during the study period. However, the data used were real-world data, as the data were directly obtained from our clinical practice. Third, there were no uniform imaging protocols and slice thickness for preoperative CT imaging during the study period, resulting in the possible understaging of clinical LPLN status and a relatively high rate of LPLN metastasis compared to the previous studies (Table 3). However, enlarged LPLN of 5 mm or more seemed to be able to be detected by general imaging protocol with 5 mm slice thickness or less. Finally, the present results were obtained from a highly experienced, high-volume cancer center which routinely performs LPLND, and thus might not be generalizable to all practice settings.
In conclusion, the present study revealed no significant difference in the cumulative risk for LR and OS regardless of LPLN SAD, implying the good impact of LPLND on the prevention of lateral recurrence. Meanwhile, approximately 20% of patients with LPLN SAD <5 mm in preoperative imaging had pathologically confirmed LPLN metastasis, suggesting the difficulty of predicting LPLN metastasis using only LPLN SAD in preoperative imaging and further study requirement.
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
AO and KK were responsible for the study concept. However, all authors contributed to the study design. AO, KK, TK, and YuS created the prospectively collected database, and AO collected, assembled, and analyzed the data. AO wrote the initial draft, and all authors approved the final draft of the manuscript.
Approval by Institutional Review Board (IRB)
The present experimental protocols were approved by the institutional review committee at Aichi Cancer Center Hospital (2020-1-297).
Acknowledgements
We thank Grammarly Inc. (CA, US) and ProEdit Japan Inc. (Kyoto, Japan) for proofreading our draft.
References
- 1.Europe WHOROF. World Cancer Report 2019 December 2020. [Google Scholar]
- 2.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001 Aug; 345(9): 638-46. [DOI] [PubMed] [Google Scholar]
- 3.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021 Jan; 22(1): 29-42. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021 May; 22(5): 702-15. [DOI] [PubMed] [Google Scholar]
- 5.Ogura A, Konishi T, Cunningham C, et al. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol. 2019 Jan; 37(1): 33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hojo K, Koyama Y, Moriya Y. Lymphatic spread and its prognostic value in patients with rectal cancer. Am J Surg. 1982 Sep; 144(3): 350-4. [DOI] [PubMed] [Google Scholar]
- 7.Moriya Y, Sugihara K, Akasu T, et al. Nerve-sparing surgery with lateral node dissection for advanced lower rectal cancer. Eur J Cancer. 1995 Jul-Aug; 31A(7-8): 1229-32. [DOI] [PubMed] [Google Scholar]
- 8.Sugihara K, Kobayashi H, Kato T, et al. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum. 2006 Nov; 49(11): 1663-72. [DOI] [PubMed] [Google Scholar]
- 9.Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2019 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita S, Mizusawa J, Kanemitsu Y, et al. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg. 2017 Aug; 266(2): 201-7. [DOI] [PubMed] [Google Scholar]
- 11.Hajibandeh S, Hajibandeh S, Matthews J, et al. Meta-analysis of survival and functional outcomes after total mesorectal excision with or without lateral pelvic lymph node dissection in rectal cancer surgery. Surgery. 2020 Sep; 168(3): 486-96. [DOI] [PubMed] [Google Scholar]
- 12.Emile SH, Elfeki H, Shalaby M, et al. Outcome of lateral pelvic lymph node dissection with total mesorectal excision in treatment of rectal cancer: A systematic review and meta-analysis. Surgery. 2021 May; 169(5): 1005-15. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010 Jun; 17(6): 1471-4. [DOI] [PubMed] [Google Scholar]
- 14.Kanemitsu Y, Hirai T, Komori K, et al. Survival benefit of high ligation of the inferior mesenteric artery in sigmoid colon or rectal cancer surgery. Br J Surg. 2006 May; 93(5): 609-15. [DOI] [PubMed] [Google Scholar]
- 15.Ogura A, Konishi T, Beets GL, et al. Lateral Nodal Features on Restaging Magnetic Resonance Imaging Associated With Lateral Local Recurrence in Low Rectal Cancer After Neoadjuvant Chemoradiotherapy or Radiotherapy. JAMA Surg. 2019 Jul: e192172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malakorn S, Yang Y, Bednarski BK, et al. Who Should Get Lateral Pelvic Lymph Node Dissection After Neoadjuvant Chemoradiation? Dis Colon Rectum. 2019 Oct; 62(10): 1158-66. [DOI] [PubMed] [Google Scholar]
- 17.Zhou S, Jiang Y, Pei W, et al. Neoadjuvant chemoradiotherapy followed by lateral pelvic lymph node dissection for rectal cancer patients: A retrospective study of its safety and indications. J Surg Oncol. 2021 Sep; 124(3): 354-60. [DOI] [PubMed] [Google Scholar]
- 18.Arii K, Takifuji K, Yokoyama S, et al. Preoperative evaluation of pelvic lateral lymph node of patients with lower rectal cancer: comparison study of MR imaging and CT in 53 patients. Langenbecks Arch Surg. 2006 Sep; 391(5): 449-54. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka H, Nakamura A, Masaki T, et al. Optimal diagnostic criteria for lateral pelvic lymph node metastasis in rectal carcinoma. Anticancer Res. 2007 Sep-Oct; 27(5B): 3529-33. [PubMed] [Google Scholar]
- 20.Akasu T, Iinuma G, Takawa M, et al. Accuracy of high-resolution magnetic resonance imaging in preoperative staging of rectal cancer. Ann Surg Oncol. 2009 Oct; 16(10): 2787-94. [DOI] [PubMed] [Google Scholar]
- 21.Fujita S, Yamamoto S, Akasu T, et al. Risk factors of lateral pelvic lymph node metastasis in advanced rectal cancer. Int J Colorectal Dis. 2009 Sep; 24(9): 1085-90. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa S, Itabashi M, Hirosawa T, et al. Lateral pelvic lymph node dissection can be omitted in lower rectal cancer in which the longest lateral pelvic and perirectal lymph node is less than 5mm on MRI. J Surg Oncol. 2014 Mar; 109(3): 227-33. [DOI] [PubMed] [Google Scholar]
- 23.Ishibe A, Ota M, Watanabe J, et al. Prediction of Lateral Pelvic Lymph-Node Metastasis in Low Rectal Cancer by Magnetic Resonance Imaging. World J Surg. 2016 Apr; 40(4): 995-1001. [DOI] [PubMed] [Google Scholar]
- 24.Komori K, Fujita S, Mizusawa J, et al. Predictive factors of pathological lateral pelvic lymph node metastasis in patients without clinical lateral pelvic lymph node metastasis (clinical stage II/III): The analysis of data from the clinical trial (JCOG0212). Eur J Surg Oncol. 2019 Mar; 45(3): 336-40. [DOI] [PubMed] [Google Scholar]
- 25.Hiyoshi Y, Miyamoto Y, Kiyozumi Y, et al. Risk factors and prognostic significance of lateral pelvic lymph node metastasis in advanced rectal cancer. Int J Clin Oncol. 2020 Jan; 25(1): 110-7. [DOI] [PubMed] [Google Scholar]
- 26.Schaap DP, Boogerd LSF, Konishi T, et al. Rectal cancer lateral lymph nodes: multicentre study of the impact of obturator and internal iliac nodes on oncological outcomes. Br J Surg. 2021 Mar; 108(2): 205-13. [DOI] [PubMed] [Google Scholar]
- 27.Amano K, Fukuchi M, Kumamoto K, et al. Pre-operative Evaluation of Lateral Pelvic Lymph Node Metastasis in Lower Rectal Cancer: Comparison of Three Different Imaging Modalities. J Anus Rectum Colon. 2020; 4(1): 34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]