Platelet-Derived Growth Factor Receptor Beta (PDGFRB) is critically implicated in development. Constitutional pathogenic variants (CPVs) in the gene encoding this receptor result in several inherited syndromes, including primary familial brain calcifications (PFBC) or infantile myofibromatosis, but have not been associated with brain tumors. Here, we describe a 9-year-old male with a DCTN1-ALK fused inflammatory myofibroblastic tumor of the brain and an 11-year-old female with a TRIM24-MET fused occipital glioneural tumor. Both probands carry the same constitutive PDGFRB c.2959C>T (p. Arg987Trp) CPV, raising the possibility that this variant, previously described in PFBC, may also promote tumor formation including in the brain.
Platelet-derived growth factor receptors (PDGFR) are a family of receptor tyrosine kinases that exist as alpha and beta isoforms encoded by 2 genes, PDGFRA and PDGFRB, respectively.1 Somatic PDGFRA gain-of-function (GOF) variants, overexpression, and amplifications are commonly found in gliomas1 while constitutional GOF variants predispose to gastrointestinal stromal tumors (GISTs) and inflammatory fibroid polyps.2 Both somatic and constitutive pathogenic variants (PVs) and CPVs in PDGFRB have been described in myofibroma development3 and GOF CPVs were identified in several rare disorders such as infantile myofibromatosis and Kosaki overgrowth syndrome among others,1 but alterations in this receptor have not been reported in either GISTs or brain tumors. Loss-of-function (LOF) PDGFRB CPVs predispose to primary familial brain calcifications (PFBC), a rare autosomal dominant disease, with unknown penetrance.4 This includes a specific constitutive LOF variant, PDGFRB c.2959C>T (p. Arg987Trp), hereafter referred to as R987W, which was first identified in a sporadic case of PFBC (ie, no CT scans of family members available to confirm or refute segregation).5 Here, we describe 2 children with brain tumors who carry constitutive R987W PDGFRB CPV and have no PFBC.
The study was approved by the Quebec Research Ethics Board. Informed consent was obtained for both cases. DNA/RNA were extracted using standard protocols. RNA sequencing (RNAseq) on tumor samples was performed using TruSeq Stranded Total RNA libraries and the Ribo-Zero Gold Kit according to Illumina’s protocol. Libraries were sequenced (~70–175 million reads, paired-end 2 × 100 bp) on a Novaseq6000. All fusions and constitutional PDGFRB variants were validated in a CLIA-certified laboratory. Sanger sequencing was performed on all samples to validate specifically the PDGFRB R987W mutation and resulting chromatograms were visualized using SnapGene software. DNA methylation were performed using the EPIC platform and analyzed using the DKFZ DNA methylation classifier of CNS tumors. The version used is specified for each case.
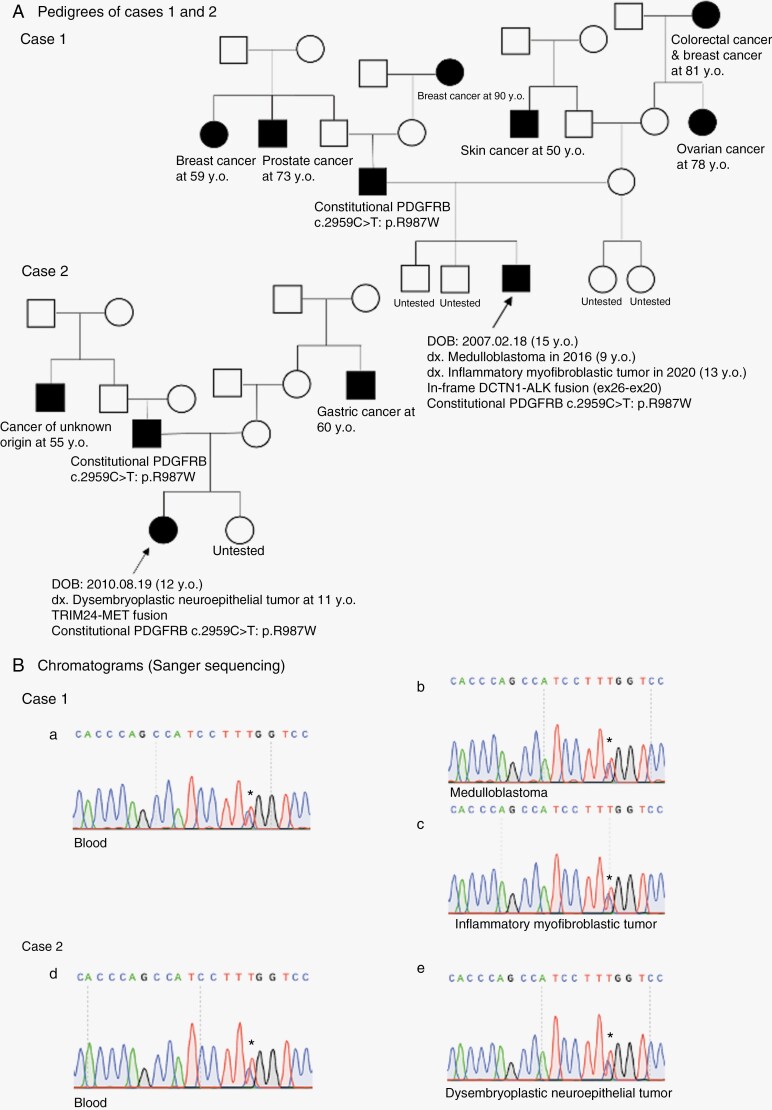
Case 1: a 9-year-old French-Canadian male was treated in 2016 for a WNT-activated medulloblastoma, diagnosed on typical β-catenin nuclear staining, monosomy 6, and DNA methylation classification (score of 0.99 using v11b4). Four years after remission, a new left frontal mass was identified on a control MRI. Following gross total resection, pathology indicated an inflammatory myofibroblastic tumor (IMT) of the brain. The patient is currently in remission 2 years post-surgery. Family history indicated that parents are non-consanguineous, and apparently healthy as are his other siblings. A wide range of different primary cancers were reported in relatives from both the paternal and maternal sides (Figure 1A). RNAseq performed on the IMT revealed an in-frame Dynactin subunit 1 - Anaplastic lymphoma kinase (DCTN1-ALK) fusion (ex26–ex20), validated by Fluorescence In Situ Hybridization (FISH), and a heterozygous PV, c.2959C>T: p.R987W PDGFRB mutation. Sequencing of the proband’s blood DNA revealed the PDGFRB R987W variant to be constitutive (Figure 1B). Further familial testing showed the father to be also a carrier of this variant (Figure 1A).
Figure 1.
(A) Family pedigrees of cases 1 and 2. Diagnosis and genetic analysis results are provided for the probands of each family. (B) Sanger sequencing results of all tumor and blood samples from cases 1 and 2 are shown via chromatograms using SnapGene software. PDGFRB c.2959C>T: p.R987W variant is confirmed in all samples.
Case 2: an 11-year-old French-Canadian female was admitted with generalized seizures following a 3-month story of headaches. MRI showed a hemorrhagic heterogenous right occipital lesion and the initial Computed tomography (CT) scan of the brain identified intra-tumoral calcifications. Following gross total resection, a diagnosis of glioneural tumor was made, which was further classified by DNA methylation as dysembroplastic neuro-epithelial tumor (DNET, score of 0.90 v12.5). Copy number variation profile indicated a gain in whole chromosome 7 and no other alterations. Parents were non-consanguineous, and in good health. An uncle of the mother and an uncle of the father died of cancer (Figure 1A). The patient is currently nine months post-surgery with no clinical/radiological sign of disease. A Tripartite motif-containing 24 (TRIM24)-Mesenchymal epithelial transition (MET) fusion and the PDGFRB R987W mutation were identified by RNAseq in the DNET, and this PDGFRB mutation was also detected in the blood (Figure 1B). Family testing indicated that the otherwise healthy father and sister also carry the same PDGFRB variant.
We describe here 2 children with brain tumors carrying the same constitutive PDGFRB R987W mutation. This variant has previously been reported in PFBC, a disease both patients do not have on imaging, and was shown to cause protein instability and to impair MAPK, AKT, and PLCγ signaling in in vitro studies.6 Both brain tumors reported herein had gene fusions involving DCTN1-ALK in Case 1 and TRIM24-MET in case 2. In IMT, 60% of cases carry Anaplastic lymphoma kinase (ALK) alterations,7 and the DCTN1-ALK fusion we identify leads to oncogenic constitutive ligand-independent ALK activation, in keeping with this diagnosis. Interestingly, data acquired in other ALK-mutant tumors indicate that PDGFRB inhibition led to prolonged survival and increased efficacy of an ALK-inhibitor,8 suggesting a possible crosstalk between both pathways. TRIM24-MET fusions are potent oncogenes commonly observed in cancer including in high-grade gliomas9 and its presence in a DNET is unusual and warrants close clinical follow-up. Notably, the highly vascular nature of both the IMT and the DNET further raises the possibility of a crosstalk between PDGFRB signaling, which is crucial in vasculogenesis, and the acquisition of the ALK or the MET fusions. These may represent a compensatory mechanism in specific cells where PDGFRB signaling is needed but not optimal based on the R987W mutation. This hypothesis remains speculative and warrants further in-depth investigation in appropriate model systems.
This report suggests that brain tumors can potentially be added to the list of disorders caused by PDGFRB CPVs. However, further work and more cases are required to confirm they promote tumor formation, and to assess penetrance as family members were carriers but otherwise asymptomatic. Last, although tyrosine kinase inhibitors such as imatinib remain effective against most PDGFRB GOF mutations,10 disorders such as PFBC with LOF CPVs in PDGFRB still require effective treatment methods. PDGFRB is a pleiotropic gene as evidenced by the wide spectrum of human diseases caused by both its GOF and LOF variants. This underscores the importance of additional functional characterization studies and the determination of specific pathways involved in PDGFRB-related tumorigenesis, as the exact mechanisms by which PDGFRB causes such a plethora of diseases remain uncertain.
Acknowledgments
We thank Dr. Régen Drouin for his contributions to this manuscript.
Contributor Information
HyeRim Han, Program in Molecular Mechanisms and Experimental Therapy in Oncology (Oncobell), IDIBELL, Hospitalet de Llobregat, Barcelona, Spain; Department of Human Genetics, McGill University Faculty of Medicine, Montreal, Québec, Canada.
Samuele Renzi, Department of Pediatric and Division of Hemato-Oncology, CHU de Québec-Université-Laval, Québec, Canada.
Valerie Larouche, Department of Pediatric and Division of Hemato-Oncology, CHU de Québec-Université-Laval, Québec, Canada.
Damien Faury, Department of Pediatrics, McGill University and McGill University Heath Centre Research Institute, Montreal, Quebec, Canada.
Sylvie Langlois, Division of Hematology-Oncology, Research Center, Centre Hospitalier Universitaire Sainte-Justine, Montréal, Québec, Canada.
Daniel Sinnett, Division of Hematology-Oncology, Research Center, Centre Hospitalier Universitaire Sainte-Justine, Montréal, Québec, Canada; Department of Pediatrics, University of Montreal, Faculty of Medicine, Montreal, Quebec, Canada.
Andrea Gomez, Department of Pathology, McGill University, Montreal, Quebec, Canada.
Jason Karamchandani, Department of Pathology, McGill University, Montreal, Quebec, Canada.
Louis Crevier, Department of Surgery, CHU de Québec-Université-Laval, Québec, Canada.
William D Foulkes, Department of Human Genetics, McGill University Faculty of Medicine, Montreal, Québec, Canada; Gerald Bronfman Department of Oncology, McGill University, Montreal, Quebec, Canada; Department of Human Genetics, McGill University and McGill University Heath Centre Research Institute, Montreal, Quebec, Canada.
Nada Jabado, Department of Human Genetics, McGill University Faculty of Medicine, Montreal, Québec, Canada; Department of Pediatrics, McGill University and McGill University Heath Centre Research Institute, Montreal, Quebec, Canada.
Funding
This work was supported by Spanish funds through “Acció instrumental de formació de científics i tecnòlegs” (SLT017/20/000243) of the Department de Salut de la Generalitat de Catalunya, a predoctoral fellowship awarded to H.H. WDF is supported by a Canadian Institutes of Health Research grant (FDN-148390). N.J. and D.S. are jointly supported by the SIGNATURE project funded by the Charles Bruneau Cancer Foundation. D.S holds the FKV pediatric oncogenomics Chair position.
Authorship
Study conception and design: W.D.F. and N.J., data collection: S.R., V.L., L.C., analysis and interpretation of results: H.H., D.F., S.L., D.S., A.G., J.K., draft manuscript preparation: H.H., S.R. All authors have been involved in the writing of the manuscript at draft and any revision stages and have read and approved the final version.
Conflict of Interest
Authors declare there is no conflict of interest.
References
- 1. Guérit E, Arts F, Dachy G, Boulouadnine B, Demoulin JB.. PDGF receptor mutations in human diseases. Cell Mol Life Sci. 2021; 78(8):3867–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodan R, Charville GW, Ladabaum U.. Hereditary inflammatory fibroid polyps caused by germline pathogenic variants in PDGFRA: Refining PDGFRA-mutation syndrome. Cancer Genet. 2021; 256–257:106–109. [DOI] [PubMed] [Google Scholar]
- 3. Cheung YH, Gayden T, Campeau PM, et al. A recurrent PDGFRB mutation causes familial infantile myofibromatosis. Am J Hum Genet. 2013; 92(6):996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Westenberger A, Balck A, Klein C.. Primary familial brain calcifications: Genetic and clinical update. Curr Opin Neurol. 2019; 32(4):571–578. [DOI] [PubMed] [Google Scholar]
- 5. Nicolas G, Pottier C, Maltête D, et al. Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology. 2013; 80(2):181–187. [DOI] [PubMed] [Google Scholar]
- 6. Vanlandewijck M, Lebouvier T, Andaloussi Mäe M, et al. Functional characterization of germline mutations in PDGFB and PDGFRB in primary familial brain calcification. PLoS One. 2015; 10(11):e0143407e0143407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vidrine DW, Berry JF, Garbuzov A, et al. DCTN1-ALK gene fusion in inflammatory myofibroblastic tumor (IMT) of the CNS. Childs Nerv Syst. 2021; 37(7):2147–2151. [DOI] [PubMed] [Google Scholar]
- 8. Laimer D, Dolznig H, Kollmann K, et al. PDGFR blockade is a rational and effective therapy for NPM-ALK-driven lymphomas. Nat Med. 2012; 18(11):1699–1704. [DOI] [PubMed] [Google Scholar]
- 9. Hiemenz MC, Skrypek MM, Cotter JA, Biegel JA.. Novel TRIM24-MET fusion in a neonatal brain tumor. JCO Precis Oncol. 2019;3:1–6. [DOI] [PubMed] [Google Scholar]
- 10. Arts FA, Chand D, Pecquet C, et al. PDGFRB mutants found in patients with familial infantile myofibromatosis or overgrowth syndrome are oncogenic and sensitive to imatinib. Oncogene. 2016;35(25):3239–3248. [DOI] [PubMed] [Google Scholar]