Abstract
Background
Medulloblastoma in adults is rare and treatment decisions are largely driven from pediatric literature. We sought to characterize recurrent medulloblastoma in adults.
Methods
From a single-institution dataset of 200 adult patients diagnosed with medulloblastoma during 1978–2017, those with recurrence were analyzed for clinical features, treatment, and outcome.
Results
Of the 200 patients, 82 (41%) with median age of 29 years (18–59) had recurrence after a median follow-up time of 8.4 years (95% CI = 7.1, 10.3). Of these, 30 (37%) were standard-risk, 31 (38%) were high-risk, and 21 (26%) had unknown-risk diseases at the time of initial diagnosis. Forty-eight (58%) presented with recurrence outside the posterior fossa, of whom 35 (43%) had distant recurrence only. Median Progression-free survival (PFS) and OS from initial surgery were 33.5 and 62.4 months, respectively. Neither PFS nor OS from initial diagnosis differed between the standard-risk and high-risk groups in those who experience recurrence (P = .505 and .463, respectively). Median OS from first recurrence was 20.3 months, also with no difference between the standard-risk and high-risk groups (P = .518). Recurrences were treated with combinations of re-resection (20 patients; 25%), systemic chemotherapy (61 patients; 76%), radiation (29 patients; 36%), stem cell transplant (6 patients; 8%), and intrathecal chemotherapy (4 patients; 5%). Patients who received radiation at recurrence had better OS (32.9 months) than those who did not (19.2 months) (P = .034).
Conclusions
Recurrent medulloblastoma in adults has a poor prognosis irrespective of initial risk stratification. Recurrence commonly arises outside the posterior fossa years after initial diagnosis.
Keywords: adult medulloblastoma, recurrent medulloblastoma, risk stratification, treatment at recurrence
Key Points.
Medulloblastoma in adults frequently recurs outside the primary site and has poor prognosis.
Clinical risk stratification at initial diagnosis does not affect survival after first recurrence.
Radiation at recurrence is associated with improved survival compared to other modalities.
Importance of the Study.
Data on medulloblastoma in adults are scarce, particularly with regard to long-term natural history and recurrence, and there is substantial variation in treatment modalities and reported survival. This is the largest retrospective single-institution series of recurrent adult medulloblastoma reported to date. We characterized clinical patterns, treatment modalities, responses to treatment, and survival outcomes. These results inform understanding of the natural history of adult medulloblastoma, the impact of treatments, and appropriate endpoints for future clinical trials. Fifty-eight percent of recurrences occurred outside the posterior fossa as leptomeningeal disease or distant metastasis including outside the central nervous system (CNS), suggesting the importance of CNS and systemic restaging at recurrence. Overall survival (OS) from the time of recurrence did not differ between patients with standard risk and those with high-risk disease according to the initial clinical risk stratification. Importantly, radiation at the time of recurrence was associated with improved OS.
Medulloblastoma is a highly cellular malignant embryonal tumor that most frequently arises in the posterior fossa of children, in whom it represents 8.4% of all primary central nervous system (CNS) neoplasms.1 Medulloblastoma arising after the age of 15 years is rare, with approximately 150 new cases in the United States annually.1 Most of these cases occur in adolescents and young adults; fewer than 8% of all medulloblastoma cases occur in patients aged 40 years and older.1,2 Owing to the low incidence of adult medulloblastoma and limited data from prospective trials, treatment paradigms for adult medulloblastoma continue to be informed by pediatric data and expert opinion. To date, there are no definitive therapy recommendations for treatment in adults3 and outcomes for adults have improved slightly over the past 2 decades,4 in contrast to pediatric patients in whom long-term survival has been increasing.5 A growing body of evidence suggests that adult medulloblastoma can behave differently from medulloblastoma in the pediatric population, including worse outcomes within molecular subgroups,6–8 and that further clinical characterization of adult medulloblastoma is required to optimize treatment strategies and improve outcomes.
Patients with medulloblastoma are typically stratified into standard-risk and high-risk groups according to traditional pediatric paradigms, such as the Packer staging system, based on clinical criteria including patient age, extent of resection, histology, and degree of tumor dissemination. Standard-risk patients are those older than 3 years with <1.5 cm2 of residual disease and no metastasis; all others are considered high-risk.9 This stratification has been useful for determining treatment intensity as well as estimating prognosis. Histologic phenotype also impacts survival with large cell/anaplastic (LC/A) portending a poor prognosis while desmoplastic/nodular and especially medulloblastoma with extensive nodularity (MBEN) correspond with a better prognosis.10 Additionally, research over the past decade has revealed at least 4 molecular subgroups of medulloblastoma with distinct clinical and molecular characteristics and DNA methylation statuses variably impacting survival. In adults, these subgroups are Sonic Hedgehog (accounting for approximately 60% of cases), group 4 (20%–25%), Wingless/Int1 (15%), and group 3 (extremely rare in adults).11
Across these subgroups, the standard of care for both standard-risk and high-risk patients generally includes maximal safe resection followed by craniospinal irradiation with a boost to the posterior fossa or tumor bed and sites of focal CNS metastasis when present,12 with the radiation dose depending on risk status.12,13 The benefit of first-line chemotherapy for standard-risk adult patients has been controversial, although recent studies, including one prospective trial, have demonstrated the feasibility and survival benefit of adjuvant chemotherapy.12,14–16 It has been previously demonstrated that adjuvant chemotherapy led to superior outcomes and neoadjuvant chemotherapy is associated with inferior outcomes in adult medulloblastoma.17,18
Long-term survival is possible in adults; however, outcome after recurrence tends to be poor across all ages, with failure in the posterior fossa being the most common.19 Relapse occurs in approximately 30% of pediatric cases and is associated with very high mortality despite a variety of attempted treatments, including re-resection, re-irradiation, high-dose chemotherapy with autologous stem cell transplant (SCT), and experimental therapy in clinical trials.20–22 Late relapse is more common in adults than in the pediatric population,23 particularly in standard-risk patients.
To date, only small series have been published regarding characteristics and outcomes of recurrent adult medulloblastoma.23,24 In this single-institution retrospective study, we described the clinical characteristics, treatment strategies, and survival outcomes of 82 adults with recurrent medulloblastoma, a subgroup of 200 adults with medulloblastoma whose data were previously published by our group.18
Methods
Patient Selection
A total of 200 adults with medulloblastoma initially diagnosed at 18 years of age or older were seen in adult neuro-oncology, neurosurgery, and radiation oncology clinics at The University of Texas MD Anderson Cancer Center (Houston, TX) from January 1978 through April 2017. We selected for further analysis the subset of patients who experienced at least one recurrence during the follow-up period.
Data Collection
The protocol for data collection were approved by the MD Anderson Cancer Center Institutional Review Board. A comprehensive data collection instrument were built in the REDCap database, in which we recorded patient demographics; vital status; date of death or last follow-up; clinical characteristics, including risk stratification at initial diagnosis; and recurrence data, which included symptoms, location of recurrence, treatment, and survival following recurrence.
The extent of resection was categorized as gross total resection (no residual disease), near-total resection (≤1.5 cm2 of residual disease), subtotal resection (>1.5 cm2 of residual disease), biopsy, or unknown. Extent of resection was obtained from radiology reports or clinical notes, and when the extent of resection was not clear from these, postoperative magnetic resonance imaging studies were reviewed, when available. Medulloblastoma was confirmed pathologically at MD Anderson in almost all patients. Leptomeningeal disease was determined radiographically and/or with cerebrospinal fluid analysis.
Statistical Analysis
Descriptive statistics (frequency distribution, mean [± standard distribution], and median [range]) were used to summarize patient characteristics. Progression-free survival (PFS) was defined as the time from initial surgery to the time of disease progression or death, whichever occurred first. Overall survival (OS) was defined as (1) the time from initial surgery to the time of death, or (2) the time from first recurrence to the time of death. First recurrence was defined as first histological confirmation of recurrence (or imaging demonstration of recurrence if histological confirmation was not obtained). For events that had not occurred by the time of data analysis, times were censored at the time of last contact at which the patient was known to be progression-free (for PFS) or the last time the patient was known to be alive (for OS). The distributions of the time-to-event outcomes were estimated using the Kaplan–Meier method.25 Log-rank test26 was performed to test the difference in survival between groups.
Results
Clinical Characteristics
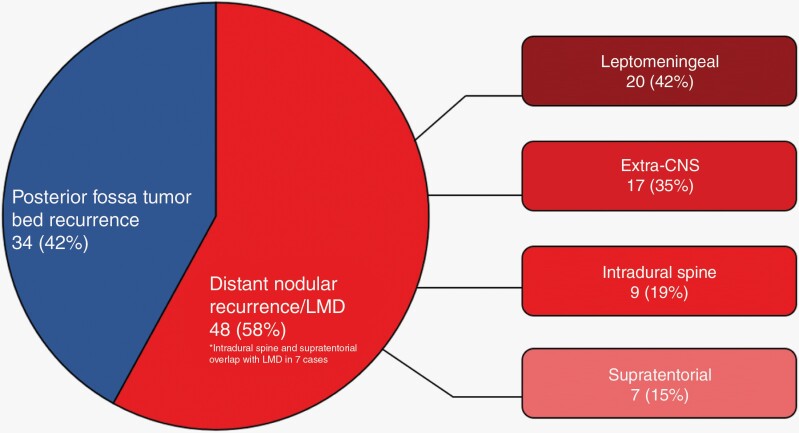
Eighty-two of the two hundred patients (41%) were identified as having experienced at least one recurrence; the median follow-up time for the 200 patients was 8.4 years (95% CI = 7.1, 10.3). The median follow-up time for the 82 patients with recurrence was 17.2 years (95% CI = 11.6, not reached). Clinical characteristics are summarized in Table 1. Thirty patients (37%) had been classified as standard-risk and 31 (38%) as high-risk diseases; risk status was unknown in 21 patients (26%). The most frequent symptoms reported at the time of recurrence were headache (61 patients; 74%), nausea (31 patients; 38%), vomiting (29 patients; 35%), ataxia (24 patients; 29%), and dizziness (18 patients; 22%) (Supplementary Table 1). Among histologic subtypes, there were 30 patients (37%) with classic medulloblastoma, 28 patients (34%) with desmoplastic, 3 patients (4%) with LC/A, and 21 (26%) with unknown histology. At initial diagnosis, one LC/A case was classified as high-risk based on extra-CNS metastasis and LMD, one was standard-risk, and one was unknown risk. Thirty-four patients (42%) had recurrence within the posterior fossa only, while 48 patients (58%) presented with distant recurrence (Figure 1), including 20 patients (42%) with leptomeningeal disease, 17 (35%) with extra-CNS disease (bone, bone marrow, lung, pleura, lymph nodes, and subcutaneous soft tissue), 9 (19%) with recurrence intradurally in the spine, and 7 (15%) with supratentorial recurrence. Five patients (6%) presented with leptomeningeal disease and a distant dominant metastatic nodule. Thirteen patients (16%) had simultaneous local and distant recurrence, while 35 patients (43%) had distant recurrence only.
Table 1.
Clinical Characteristics of Patients With Recurrent Medulloblastoma
| Patients With Recurrence (n = 82) | ||
|---|---|---|
| Characteristic | n | % |
| Sex | ||
| Male | 52 | 63.4 |
| Female | 30 | 36.6 |
| Age, years | ||
| ≤29 | 43 | 52.4 |
| >29 | 39 | 47.6 |
| Metastasis * | ||
| M+ | 11 | 13.4 |
| M0 | 37 | 45.1 |
| Unknown | 34 | 41.5 |
| Risk group * | ||
| Standard | 30 | 36.6 |
| High | 31 | 37.8 |
| Unknown | 21 | 25.6 |
| Histology | ||
| Classic | 30 | 36.6 |
| Desmoplastic | 28 | 34.1 |
| Large cell/anaplastic | 3 | 3.7 |
| Unknown | 21 | 25.6 |
| Initial localization | ||
| Vermis | 22 | 26.8 |
| Fourth ventricle | 8 | 9.8 |
| Left cerebellum | 26 | 31.7 |
| Right cerebellum | 24 | 29.3 |
| Other | 9 | 11.0 |
| Unknown | 7 | 8.5 |
| First-line treatment | ||
| Initial surgery | ||
| Gross total resection | 41 | 50.0 |
| Near-total resection | 3 | 3.7 |
| Subtotal resection | 30 | 36.6 |
| Biopsy | 1 | 1.2 |
| Unknown | 7 | 8.5 |
| Radiation therapy | 78 | 95.1 |
| Chemotherapy | ||
| Neoadjuvant | 16 | 19.5 |
| Concurrent | 9 | 11.0 |
| Adjuvant | 27 | 32.9 |
| Intrathecal | 12 | 14.6 |
*At initial diagnosis.
Figure 1.
Locations of recurrence. LMD – leptomeningeal disease; Extra-CNS – extradural disease including bone, bone marrow, lung, pleura, lymph nodes, and subcutaneous soft tissue; Intradural spine – nodular intradural disease involving spinal cord; Supratentorial – nodular intradural disease involving brain.
Seventy-seven (93.9%) patients had received craniospinal irradiation at initial diagnosis (2 received whole brain radiation, 1 received only posterior fossa radiation, and 2 were unknown). All patients had data available on treatment received at the time of first recurrence (Table 2). Twenty patients (25%) underwent re-resection, and 61 (76%) were treated with salvage chemotherapy. A variety of cytotoxic therapies were utilized, including cyclophosphamide plus etoposide–based regimens; nitrogen mustard, vincristine, procarbazine, and prednisone; cisplatin plus lomustine–based regimens; temozolomide; and ifosfamide, carboplatin, and etoposide. Twenty-nine patients (36%) underwent various re-irradiation treatments, which included Gamma Knife radiosurgery, local photon irradiation, and craniospinal irradiation. Six patients (8%) underwent a first or second SCT, and 4 (5%) received intrathecal chemotherapy. Thirty-eight patients (48%) underwent treatment with more than one modality at recurrence.
Table 2.
Summary of Survival by Risk Status and Treatment Modality From the Time of Recurrence*
| Risk Group | N | Events | Median Survival (Months) | 1-Year Survival Rate | 5-Year Survival Rate | 10-Year Survival Rate |
|---|---|---|---|---|---|---|
| PFS from time of initial surgery | ||||||
| All | 81 | 81 | 33.5 (28.1–37.1) | 0.81 (0.71–0.88) | 0.17 (0.1–0.26) | 0.02 (0–0.08) |
| Standard risk | 30 | 30 | 29.9 (19.6–38.8) | 0.77 (0.57–0.88) | 0.17 (0.06–0.32) | 0.00 |
| High risk | 30 | 30 | 32.4 (19.0–44.6) | 0.80 (0.61–0.9) | 0.13 (0.04–0.28) | 0.03 (0–0.15) |
| Unknown risk | 21 | 21 | 35.4 (23.6–57.7) | 0.90 (0.67–0.98) | 0.24 (0.09–0.43) | 0.05 (0–0.2) |
| OS from time of initial surgery | ||||||
| All | 82 | 70 | 62.4 (52.3–77.8) | 0.98 (0.91–0.99) | 0.53 (0.41–0.63) | 0.15 (0.07–0.24) |
| Standard risk | 30 | 24 | 54.7 (41.0–82.4) | 0.97 (0.79–1) | 0.44 (0.25–0.62) | 0.05 (0–0.21) |
| High risk | 31 | 30 | 61.3 (44.6–84.1) | 0.97 (0.79–1) | 0.50 (0.31–0.66) | 0.17 (0.06–0.32) |
| Unknown risk | 21 | 16 | 83.4 (48.8–116.9) | 1.00 | 0.66 (0.42–0.82) | 0.22 (0.06–0.45) |
| OS from time of recurrence | ||||||
| All | 82 | 69 | 23.8 (19.0–28.4) | 0.74 (0.63–0.82) | 0.13 (0.06–0.22) | 0.02 (0–0.08) |
| Standard risk | 30 | 24 | 20.3 (10.6–26.0) | 0.64 (0.43–0.78) | 0.12 (0.02–0.3) | 0.00 |
| High risk | 30 | 29 | 25.7 (14.6–30.6) | 0.79 (0.6–0.9) | 0.10 (0.03–0.24) | 0.00 |
| Unknown risk | 21 | 16 | 28.4 (12.6–37.0) | 0.81 (0.57–0.92) | 0.19 (0.05–0.41) | 0.10 (0.01–0.33) |
| OS from time of recurrence, by treatment modality | ||||||
| Re-resection | 20 | 18 | 26.0 (12.6–30.6) | 0.84 (0.59–0.95) | 0.20 (0.06–0.4) | 0.00 |
| Chemotherapy | 61 | 52 | 24.4 (19.2–28.6) | 0.77 (0.64–0.85) | 0.13 (0.06–0.25) | 0.03 (0–0.12) |
| Radiation | 29 | 25 | 32.9 (23.8–42.2) | 0.86 (0.67–0.95) | 0.20 (.07–0.38) | 0.00 |
| SCT | 6 | 6 | 25.2 (19.2–37.0) | 1.00 | 0.00 | 0.00 |
| IT therapy | 4 | 3 | 11.6 (3.0–NR) | 0.50 (0.06–0.84) | 0.00 | 0.00 |
*Due to limited data on second recurrence, PFS from time of first recurrence was not analyzable.
Abbreviations: IT, intrathecal; OS, overall survival; PFS, progression-free survival; SCT, stem cell transplant.
Survival Outcomes
Survival from time of initial surgery.—
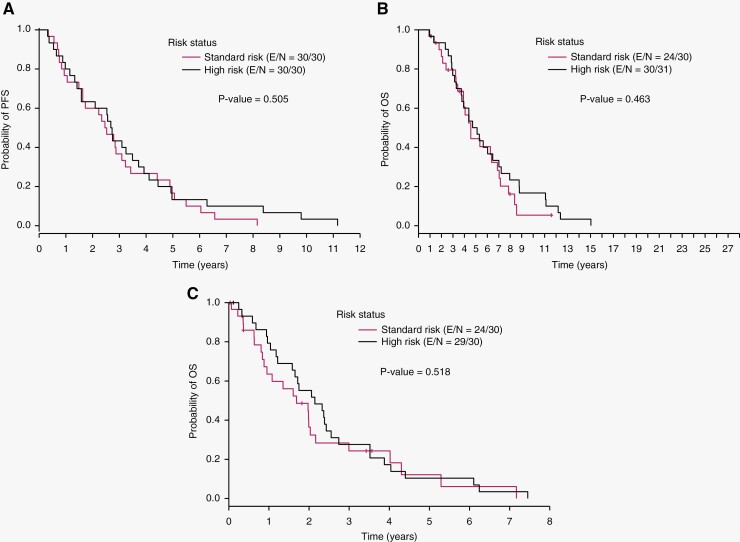
One high-risk patient with missing date of first recurrence was excluded from the PFS analysis. Among the 81 patients with known date of first recurrence, median PFS from the time of initial surgery was 33.5 months. Median PFS was 29.9 months for standard-risk patients, 32.4 months for high-risk patients, and 35.4 months for those with unknown risk (Table 2). There was no difference in median PFS between the standard-risk and high-risk groups (P = .505) (Figure 2A). Among the cases with LC/A histology, the patients classified as high-risk, standard-risk, and unknown risk based on clinical criteria experienced tumor recurrence at 4.0 months, 8.7 months, and 56.9 months respectively.
Figure 2.
A. Progression-free survival (PFS) from date of initial surgery. B. Overall survival (OS) from date of initial surgery. C. Overall survival (OS) from time of first recurrence. E/N – number of patients with event/ number of total patients.
Median OS from the time of initial surgery was 62.4 months. Median OS was 54.7 months for standard-risk patients, 61.3 months for high-risk patients, and 83.4 months for those with unknown risk (Table 2). A single patient surviving 27 years contributed to the trend in the unknown-risk group. There was no difference in median OS between the standard-risk and high-risk groups (P = .463) (Figure 2B).
Survival from time of recurrence.—
Sixty-nine of the eighty-two patients (84%) died during follow-up. Data on second recurrence was not available on sufficient patients to report PFS from the time of first recurrence. Median OS after recurrence was 20.3 months (95% CI, 10.6–26.0 months) for standard-risk patients, 25.7 months (95% CI, 14.6–30.6 months) for high-risk patients, and 28.4 months (95% CI, 12.6–37.0 months) for those with unknown risk (Table 2). There was no difference in OS from time of recurrence between the standard-risk and high-risk groups (P = 0.518) (Figure 2C). The 1-year OS rates for all, standard-risk, and high-risk patients were 74% (95% CI = 63%, 82%), 64% (95% CI = 43%, 78%), and 79% (95% CI= 60%, 90%), respectively; the 5-year OS rates for all, standard-risk, and high-risk patients were 13% (95% CI = 6%, 22%), 12% (95% CI = 2%, 30%), and 10% (95% CI = 3%, 24%), respectively; and the 10-year OS rates for all, standard-risk, and high-risk patients were 2% (95% CI = 0%, 8%), 0%, and 0%, respectively.
Survival from time of recurrence by treatment modality.—
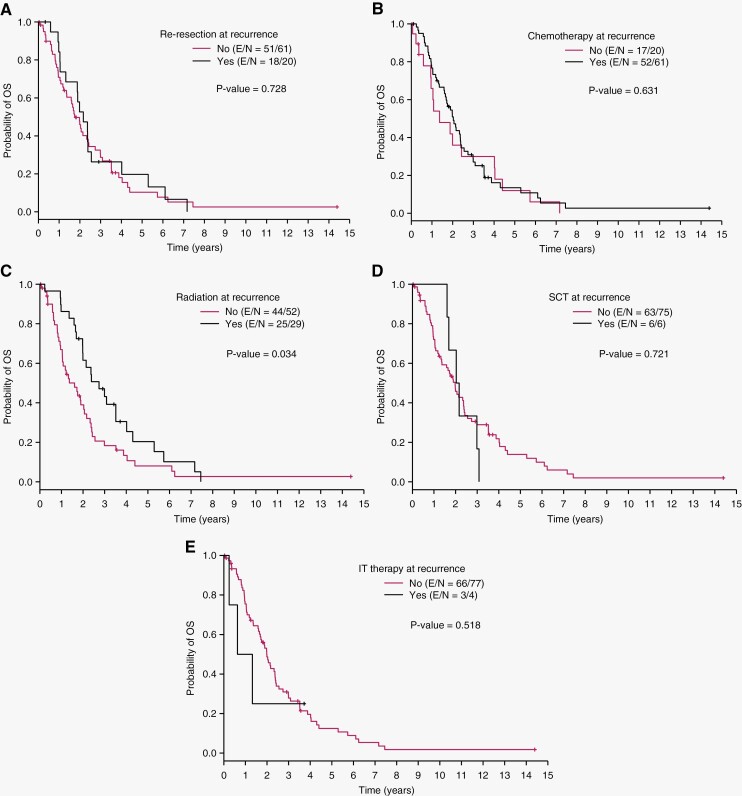
Median OS by modality of treatment at first recurrence was 26.0 months for re-resection, 24.4 months for chemotherapy, 32.9 months for radiation, 25.2 months for SCT, and 11.6 months for intrathecal chemotherapy (Table 2). Patients who received radiation at recurrence had significantly better OS than those who did not (32.9 months vs. 19.2 months; P = .034) (Figure 3). There was no difference in OS between patients who did and did not undergo re-resection, chemotherapy, SCT, or intrathecal chemotherapy at recurrence.
Figure 3.
Overall survival from time of first recurrence in patients who did and did not receive the following treatments at recurrence: (A) re-resection, (B) chemotherapy, (C) radiation, (D) stem cell transplant, and (E) intrathecal therapy. Patients who received radiation had significantly better OS than patients who did not receive radiation at recurrence (P = .034). E/N – number of patients with event/ number of total patients.
Discussion
This is the largest retrospective single-institution series of adult patients with recurrent medulloblastoma reported to date. Given that prospective clinical data for this population are scarce, retrospective analysis of well-annotated data with long-term follow-up continues to be important to inform our understanding of late outcomes, recurrence, and the impact of various treatments and to generate data to support future clinical trial endpoints.
In our previously published cohort of 200 patients initially diagnosed with medulloblastoma as adults (≥ 18 years of age), we observed a 5-year PFS rate of 55% and a median PFS of more than 6 years.18 PFS for the entire cohort was driven by patients who did not have a recurrence and who had less aggressive tumors and/or more appropriate therapy. In contrast, among the 82 patients in the present analysis, who experienced tumor recurrence during follow-up, median PFS from initial surgery was less than 3 years, with a 5-year PFS rate of 17%, and there was no significant difference in PFS between patients stratified as high-risk and those stratified as standard-risk on the basis of their clinical features at presentation. We also found that for the patients with recurrence, median OS was 5.2 years from initial surgery, compared to 8.8 years for the entire 200-patient cohort.
Our findings regarding the timing of first recurrence in adults with medulloblastoma are generally in line with what has previously been reported. A multi-institutional series of 156 adults with medulloblastoma treated between 1975 and 1991 showed a 5-year event-free survival (EFS) rate of 61% and a median PFS of 30 months over a median follow-up of 5.9 years.27 The other large series of adults to date, which captured longer-term outcomes (with a median follow-up of 9 years) and better represents modern surgical and radiation techniques, included 29 patients, of whom 5 had recurrence at a median of 20.4 months after completing initial therapy.28 Small, prospective adult medulloblastoma trials including 10 and 11 patients showed 5-year PFS rates of 55% and 72%,13,17 and most recently, the NOA-07 study, which included 30 patients treated with up-front craniospinal irradiation with vincristine followed by eight cycles of cisplatin, lomustine, and vincristine, demonstrated a 3-year EFS rate of 66.6% after a median follow up of 58 months and with 83% of patients still without progression.16
As shown by our series and others, prognosis from the time of recurrence is universally poor in both children and adults with medulloblastoma. A study of 40 pediatric patients found that from the time of relapse, median EFS was 1 year, median OS was 19.2 months, and the 5-year OS rate was 8.2%.29 Previously published data on adult patients indicated a median OS of 21 months after first recurrence in a series of 17 patients.23 In our series, we found that median OS from time of first recurrence across all patients was 23.8 months; OS was similar between risk groups, at 20.3 months for standard-risk patients and 25.7 months for high-risk patients (P = .518). Although the difference between groups was not statistically significant, the fact that the median survival was numerically longer in the high-risk group than in the standard-risk group calls into question the utility of risk stratification at initial diagnosis based solely on clinical factors (extent of tumor resection, dissemination) in predicting prognosis upon recurrence. However, this finding may have been driven by some combination of (1) the relatively low number of patients with known risk status and the large number with unknown risk status, (2) patients who were classified as high-risk owing to less than gross total resection but who did not have metastatic disease at presentation, (3) differences in treatment received at initial diagnosis, as most standard-risk patients did not receive chemotherapy at initial diagnosis, whereas high-risk patients typically did, and (4) molecular factors not captured by this analysis that may contribute to variations in cancer behavior.
Indeed molecular subtyping, along with histological phenotype, is critical to the characterization of medulloblastoma biology including in the relapsed setting,30 representing a significant limitation of our study. It is likely that most patients included in the dataset had SHH tumors, similar to the overall population of adults with medulloblastoma, given the predominant location (cerebellar hemispheres) and histological subtypes most commonly found in our series (desmoplastic and classical). Despite these limitations posed by heterogeneity within our molecularly agnostic cohort, this aggregation of single-institution experience likely aligns with real-world practice in which molecular data are not consistently available, especially for patients who were diagnosed prior to the more recent incorporation of molecular analysis for Neuro-Oncology patients.
Treatment at the time of recurrence in our cohort was heterogeneous, and we found that any radiation therapy at recurrence improved OS compared to other modalities. It was previously shown, in a series of 38 pediatric patients with recurrent disease, that re-irradiation improved the OS rate from initial diagnosis to 55% at 5 years and 33% at 10 years versus 46% and 0% at 5 and 10 years, respectively, for patients who did not undergo re-irradiation (P = .036), although an increased rate of radiation necrosis was seen.31 A prospective pediatric study showed a non–statistically significant difference in EFS favoring re-irradiation following high-dose chemotherapy with autologous stem cell rescue.32 Other case series have demonstrated favorable long-term outcomes after re-irradiation, including 8-year PFS in an adult following re-irradiation without re-resection.33,34 Our data also suggest a survival benefit of re-irradiation at the time of recurrence; however, we note that this result may have been confounded by patient selection (for example, superior functional status or differences in location of recurrent tumor) or other unknown factors (for example, molecular subgroup), given the non-randomized origin of these data, and our analysis does not account for differing combinations and sequences of re-irradiation with other treatments.
The 6 patients in our cohort who received SCT at recurrence had a 100% 1-year OS rate with a median OS of 25.2 months after recurrence; 5 experienced documented re-recurrence. These patients were carefully selected for SCT and likely represent those with optimal functional status and lack of significant comorbid conditions. Further prospective studies will be useful in determining the utility of SCT and its optimal combination with other modalities. Surgery at recurrence did not appear to impact OS, although it remains an important option in well-selected cases, with anecdotal evidence supporting its use.35 Importantly, our data suggest that the treatment plan should be decided in the context of systematic restaging, given the high probability of out-of-field or leptomeningeal metastasis.
Median OS from time of recurrence was numerically longer in those who received chemotherapy at recurrence (24.4 months) than in those who did not (16.4 months), but the difference did not reach statistical significance. This may reflect selection bias if those who did not receive chemotherapy were not candidates owing to declining functional status. Analysis of outcomes following treatment with chemotherapy at recurrence was limited by heterogeneous selection of regimens, highlighting the need for further prospective studies to establish standard practices for treating recurrence with chemotherapy. Platinum- or cyclophosphamide-based therapies are commonly favored7 although less toxic regimens such as single-agent temozolomide and combinations of bevacizumab, irinotecan, and temozolomide have shown benefit for both children and adults in case reports.36–39 Intrathecal chemotherapy did not appear to be beneficial in our population, although selection of patients with advanced disease likely partially accounted for the poor OS in this group.
We found that 58% of recurrences arose outside of the posterior fossa, either as distant metastases or as leptomeningeal metastases, and that a significant proportion (43%) of patients presented with recurrence exclusively outside the primary site of disease. This indicates the need for magnetic resonance imaging of the spine during surveillance instead of performing magnetic resonance imaging of the brain only. Furthermore, our finding that 35% of patients developed extra-CNS metastases suggests a low threshold for systemic imaging via computed tomography or positron emission tomography scans if there are suspicious clinical findings. In line with previous reports of late recurrence in adults, we observed recurrences beyond 8 years from initial treatment, emphasizing the need for long-term follow-up even in patients with stable disease.
Further studies with larger combined datasets that account for molecular features and variation in treatment strategies are needed to better understand which factors are most contributory to prolonging PFS and OS in adults with medulloblastoma. With growing access to targeted therapies and chemotherapeutic clinical trials (SJMB12, NCT01878617; SJDAWN, NCT03434262; PersoMed-I, NCT04402073; and Alliance AMBUSH Trial40), and surgical trials (PBTC-053, NCT03904862) specifically including adults, subsequent studies must factor in optimal combinations of treatment modalities that include new targeted and immunotherapeutic approaches at recurrence.
Supplementary Material
Acknowledgments
We thank Emily Seyl and Stephanie Deming, Research Medical Library, MD Anderson Cancer Center, for editing the manuscript.
Contributor Information
Timothy A Gregory, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Maximilian Mastall, Department of Neurology, Clinical Neuroscience and Brain Tumor Center, University Hospital Zurich, Zurich, Switzerland.
Heather Lin, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Kenneth R Hess, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Ying Yuan, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Manuela Martin-Bejarano Garcia, Health Research Institute, Hospital Clinico San Carlos, Madrid, Spain.
Gregory N Fuller, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Kristin D Alfaro, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Maria K Gule-Monroe, Department of Neuroradiology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Jason T Huse, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Soumen Khatua, Department of Pediatric Neuro-Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Ganesh Rao, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, USA.
David I Sandberg, Department of Pediatric Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Jeffrey S Wefel, Department of Neuropsychology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Debra N Yeboa, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Arnold C Paulino, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Susan L McGovern, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Wafik Zaky, Department of Pediatric Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Anita Mahajan, Department of Radiation Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Dima Suki, Department of Pediatric Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Shiao-Pei Weathers, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Rebecca A Harrison, Department of Neuro-Oncology, BC Cancer Agency Vancouver Centre, Vancouver, British Columbia, Canada.
John F de Groot, Brain Tumor Center, UCSF Medical Center, San Francisco, California, USA.
Vinay K Puduvalli, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Marta Penas-Prado, Neuro-Oncology Branch, Center for Cancer Research, National Cancer Institute, Bethesda, Maryland, USA.
Nazanin K Majd, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Funding
Supported by the National Institutes of Health/National Cancer Institute under award number P30CA016672.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Authorship
Conceptualization of data collection: MPP and VKP. Provision of patient listings: AM and DS. Building the Research Electronic Data Capture (REDCap) database: KDA and MMBG. Collection of data on individual patients: MPP, NM, MM, and MMBG. Performance of the statistical analyses: HL, KRH, and YY. Contribution to the data analysis and significant portions of the manuscript writing: TAG, MPP, NM, HL. Participation in patient care: GF, MGM, JTH, GNR, JSW, DNY, ACP, SMG, AM, SK, DIS, WZ, SPW, RAH, JDG, NM, AM, VKP, and MPP. All authors were involved in writing, editing, and approval of the final manuscript.
References
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019;21(suppl_5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Truitt G, Gittleman H, Leece R, et al. Partnership for defining the impact of 12 selected rare CNS tumors: A report from the CBTRUS and the NCI-CONNECT. J Neurooncol. 2019;144(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franceschi E, Hofer S, Brandes AA, et al. EANO–EURACAN clinical practice guideline for diagnosis, treatment, and follow-up of post-pubertal and adult patients with medulloblastoma. Lancet Oncol. 2019;20(12):e715–e728. [DOI] [PubMed] [Google Scholar]
- 4. Penas-Prado M, Theeler BJ, Cordeiro B, et al. . Proceedings of the comprehensive oncology network evaluating rare CNS tumors (NCI-CONNECT) adult medulloblastoma workshop. Neuro-Oncol Adv. 2020;2(1):vdaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sedano P, Segundo CGS, De Ingunza L, et al. Real-world data for pediatric medulloblastoma: Can we improve outcomes? Eur J Pediatr. 2021;180(1):127–136. [DOI] [PubMed] [Google Scholar]
- 6. Zhao F, Ohgaki H, Xu L, et al. Molecular subgroups of adult medulloblastoma: A long-term single-institution study. Neuro Oncol. 2016;18(7):982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Majd N, Penas-Prado M.. Updates on management of adult medulloblastoma. Curr Treat Options in Oncol. 2019;20(8):64. [DOI] [PubMed] [Google Scholar]
- 8. Pham A, Wong K, Chang EL.. Adult medulloblastoma. In: Chang EL, Brown PD, Lo SS, Sahgal A, Suh JH, eds. Adult CNS Radiation Oncology. Cham, Switzerland: Springer International Publishing; 2018:377–397. [Google Scholar]
- 9. Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A children’s cancer group study. JCO. 1999;17(7):2127–2127. [DOI] [PubMed] [Google Scholar]
- 10. Ellison DW, Kocak M, Dalton J, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29(11):1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Remke M, Hielscher T, Northcott PA, et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29(19):2717–2723. [DOI] [PubMed] [Google Scholar]
- 12. Kocakaya S, Beier CP, Beier D.. Chemotherapy increases long-term survival in patients with adult medulloblastoma—a literature-based meta-analysis. Neuro Oncol. 2016;18(3):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brandes AA, Bartolotti M, Marucci G, et al. New perspectives in the treatment of adult medulloblastoma in the era of molecular oncology. Crit Rev Oncol Hematol. 2015;94(3):348–359. [DOI] [PubMed] [Google Scholar]
- 14. Kann BH, Lester-Coll NH, Park HS, et al. Adjuvant chemotherapy and overall survival in adult medulloblastoma. Neuro Oncol. 2017;19(2):259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franceschi E, Minichillo S, Mura A, et al. Adjuvant chemotherapy in average-risk adult medulloblastoma patients improves survival: A long term study. BMC Cancer. 2020;20(1):755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beier D, Proescholdt M, Reinert C, et al. Multicenter pilot study of radiochemotherapy as first-line treatment for adults with medulloblastoma (NOA-07). Neuro-Oncol. 2018;20(3):400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moots PL, O’Neill A, Londer H, et al. Preradiation chemotherapy for adult high-risk medulloblastoma: A trial of the ECOG-ACRIN cancer research group (E4397). Am J Clin Oncol. 2018;41(6):588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Majd NK, Mastall M, Lin H, et al. Clinical characterization of adult medulloblastoma and the effect of first-line therapies on outcome; The MD Anderson Cancer Center experience. Neurooncol Adv. 2021;3(1):vdab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fukunaga-Johnson N, Lee JH, Sandler HM, et al. Patterns of failure following treatment for medulloblastoma: Is it necessary to treat the entire posterior fossa? Int J Radiat Oncol Biol Phys. 1998;42(1):143–146. [DOI] [PubMed] [Google Scholar]
- 20. Hill RM, Richardson S, Schwalbe EC, et al. Time, pattern, and outcome of medulloblastoma relapse and their association with tumour biology at diagnosis and therapy: A multicentre cohort study. Lancet Child Adolesc Health. 2020;4(12):865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hill RM, Kuijper S, Lindsey JC, et al. Combined MYC and P53 defects emerge at medulloblastoma relapse and define rapidly progressive, therapeutically targetable disease. Cancer Cell. 2015;27(1):72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramaswamy V, Remke M, Bouffet E, et al. Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herrlinger U, Steinbrecher A, Rieger J, et al. Adult medulloblastoma: Prognostic factors and response to therapy at diagnosis and at relapse. J Neurol. 2005;252(3):291–299. [DOI] [PubMed] [Google Scholar]
- 24. Brandes AA, Franceschi E, Tosoni A, Blatt V, Ermani M.. Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer. 2007;110(9):2035–2041. [DOI] [PubMed] [Google Scholar]
- 25. Kaplan EL, Meier P.. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 26. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 27. Carrie C, Lasset C, Alapetite C, et al. Multivariate analysis of prognostic factors in adult patients with medulloblastoma. Retrospective study of 156 patients. Cancer. 1994;74(8):2352–2360. [DOI] [PubMed] [Google Scholar]
- 28. De B, Beal K, De Braganca KC, et al. Long-term outcomes of adult medulloblastoma patients treated with radiotherapy. J Neurooncol. 2018;136(1):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pizer B, Donachie PHJ, Robinson K, et al. Treatment of recurrent central nervous system primitive neuroectodermal tumours in children and adolescents: Results of a Children’s Cancer and Leukaemia Group study. Eur J Cancer. 2011;47(9):1389–1397. [DOI] [PubMed] [Google Scholar]
- 30. Kumar R, Smith KS, Deng M, et al. Clinical outcomes and patient-matched molecular composition of relapsed medulloblastoma. J Clin Oncol. 2021;39(7):807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wetmore C, Herington D, Lin T, et al. Reirradiation of recurrent medulloblastoma: Does clinical benefit outweigh risk for toxicity? Cancer. 2014;120(23):3731–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dunkel IJ, Gardner SL, Garvin JH, et al. High-dose carboplatin, thiotepa, and etoposide with autologous stem cell rescue for patients with previously irradiated recurrent medulloblastoma. Neuro Oncol. 2010;12(3):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bakst RL, Dunkel IJ, Gilheeney S, et al. Reirradiation for recurrent medulloblastoma. Cancer. 2011;117(21):4977–4982. [DOI] [PubMed] [Google Scholar]
- 34. Buglione M, Triggiani L, Grisanti S, et al. Retreatment of recurrent adult medulloblastoma with radiotherapy: A case report and review of the literature. J Med Case Rep. 2013;7(64). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balter-Seri J, Mor C, Shuper A, Zaizov R, Cohen IJ.. Cure of recurrent medulloblastoma: The contribution of surgical resection at relapse. Cancer. 1997;79(6):1241–1247. [DOI] [PubMed] [Google Scholar]
- 36. Cefalo G, Massimino M, Ruggiero A, et al. Temozolomide is an active agent in children with recurrent medulloblastoma/primitive neuroectodermal tumor: An Italian multi-institutional phase II trial. Neuro Oncol. 2014;16(5):748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Durando X, Thivat E, Gilliot O, et al. Temozolomide treatment of an adult with a relapsing medulloblastoma. Cancer Invest. 2007;25(6):470–475. [DOI] [PubMed] [Google Scholar]
- 38. Privitera G, Acquaviva G, Ettorre GC, Spatola C.. Antiangiogenic therapy in the treatment of recurrent medulloblastoma in the adult: Case report and review of the literature. J Oncol. 2009;2009:247873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levy AS, Krailo M, Chi S, et al. Temozolomide with irinotecan versus temozolomide, irinotecan plus bevacizumab for recurrent medulloblastoma of childhood: Report of a COG randomized Phase II screening trial. Pediatr Blood Cancer. 2021;68(8):e29031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mahajan A, Shih H, Penas-Prado M, et al. The alliance AMBUSH trial: Rationale and design. Cancers (Basel). 2022;14(2):414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.