Clinical guidelines for the prevention, diagnosis and management of human disease are evidence-based tools that rely on quality of clinical research, including data from randomized controlled clinical trials (RCTs) and real-world evidence. The Society for Immunotherapy of Cancer (SITC) recently published a clinical practice guideline on immunotherapy for the treatment of hepatocellular carcinoma (HCC) (1).
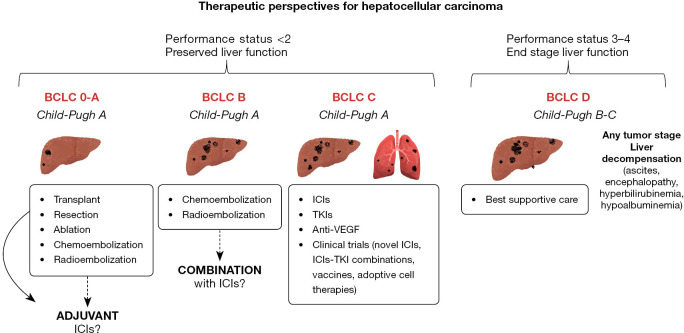
The topic is challenging considering that HCC is the most frequent form of primary liver cancer, and is among the top three causes of cancer-related deaths, with a number of new cases and deaths that is expected to rise over 55% by 2040 (2). The global epidemics of obesity and diabetes and effective antiviral treatments are driving an increasing prevalence of metabolic dysfunction-associated fatty liver disease (MAFLD) over viral-associated liver disease, with a radical change in the epidemiology of HCC. The lack of clear guidelines and effective surveillance strategies in non-cirrhotic MAFLD can potentially raise the number of tumors diagnosed at advanced stages, for which systemic treatment represents the standard of care (3). Fortunately, after a decade of stagnation following the approval of sorafenib in 2008, the number of systemic treatment options for advanced HCC has recently expanded, and since 2017 five immune checkpoint inhibitors (ICIs)-based regimens have been approved: atezolizumab plus bevacizumab, and durvalumab plus tremelimumab received full approval by Food and Drug Administration (FDA) for patients who have not received prior systemic therapy; in the second-line setting, nivolumab plus ipilimumab, nivolumab and pembrolizumab monotherapies received accelerated approvals, with only pembrolizumab being confirmed (1). The field of application of ICIs in HCC is constantly expanding, with positive preliminary results also in adjuvant settings (Figure 1) (4).
Figure 1.
Outline of hepatocellular carcinoma treatment modalities based on the stage of tumor and liver disease and potential use of ICI, TKI, VEGF agents. BCLC, Barcelona Clinic Liver Cancer; ICIs, immune checkpoint inhibitors; anti-VEGF, anti-vascular endothelial growth factor; TKIs, tyrosine kinase inhibitors.
Against this backdrop, SITC guidelines represent one of the first documents that attempt to rationalize the use of ICIs for the treatment of HCC. Clearly, they include recommendations derived from international guidelines on the diagnostic approach, but seeking to reopen a window that has long been closed, which is that of the histologic examination of the tumor. Indeed, HCC represents a peculiarity in oncology, since international guidelines call for the use of non-invasive, multiphasic imaging to obtain the diagnosis in cirrhotic patients, without histological confirmation. Such a strategy might have made sense when only sorafenib was available, but today liver tissue should become the magic box from which to extract valuable information to be combined into biomarkers of treatment response. This is especially relevant considering that ICIs monotherapy or combined therapy confer substantial benefit in only 20–30% of treated patients. Furthermore, circulating biomarkers are also increasingly seen as the tool that will allow prediction of response to different classes of drugs in the future, with the advantage of being non-invasive and therefore repeatable “on demand”.
However, the downside of the situation is that there is still no ideal biomarker to refine the prognosis of HCC treated with ICIs, despite advances in this field: tissue biomarkers assessed in other tumors, including PD-L1 expression or tumor mutational burden (TMB), have not proven their value yet. Recently, a 11-gene signature including interferon signaling and major histocompatibility complex-related genes showed to be predictive of response to first-line ICIs-based treatment (5). The recently published CRAFITY score, based on serum alpha-fetoprotein and C-reactive protein is able to predict survival and radiologic outcome, but still requires prospective validation before being implemented into clinical practice (6).
Aside from the need for predictors of response, the selection of patients for ICI treatment is another crucial point. We fully agree with SITC guideline that clinical and biochemical scores specifically designed to evaluate liver function before and during ICI treatment are urgently needed. The bottom line is the stage of the underlying chronic liver disease. Systemic therapies could be a trigger for hepatic decompensation in patients with “borderline” (Child-Pugh B) liver function. In a kind of vicious circle, liver function impairment affects the duration of systemic treatment itself, is tightly associated with a worse prognosis (7) and severely affects the likelihood of receiving a second line therapy. ICIs appear to be safe in patients with Child-Pugh class B cirrhosis, although there are no direct comparisons between tyrosine kinase inhibitors (TKIs) and ICIs in terms of risk of liver decompensation. Of note, hepatic decompensation is not an irreversible condition, and its resolution with appropriate treatment or removal of the precipitating cause can lead to an improvement in liver function, increasing the feasibility of ICIs treatment (7). This point remarks (I) the unavoidable need to manage the patient in a multidisciplinary setting (II) the utility of exploring the impact that specific etiological treatment (i.e., antiviral therapies for HBV and HCV infection) and disease-modifying agents (i.e., carvedilol, albumin, rifaximin, statins) could have in the management of patients treated with ICIs. It should also be considered that the coexistence of HCC with underlying cirrhosis predisposes to competing events related to tumor progression and/or hepatic decompensation (7). In ICIs RCTs, the reporting of safety data is severely affected by the lack of information on the risk of liver decompensation (ascites, portal hypertensive gastrointestinal bleeding and hepatic encephalopathy), that has never been prospectively assessed as a clinical endpoint in HCC studies. For patients with liver cirrhosis, compensated disease (Child A) at baseline is a necessary condition for enrolment, but changes in liver function parameters or the occurrence of liver decompensation during follow-up measured as time-to-event still need to be evaluated (7,8).
ICIs are also associated with a broad range of immune-related adverse events (irAEs). In a meta-analysis including nearly 3,000 treated patients, the most frequent target organs for irAEs during PD-(L)1 inhibition were skin, gastrointestinal tract and thyroid. Fatal events have been reported uncommonly. irAEs are typically manageable with corticosteroids that should be used with caution in cirrhotic patients due to the risk of infections and liver function worsening (9,10). Thus, toxicity remains a major challenge in patients’ care and a barrier for developing combinations that could be associated with higher efficacy.
The use of immunotherapy has also changed the way radiological images are interpreted. An accurate evaluation of radiological response is often challenging, because they can provide unconventional patterns of response and progression, with different radiological features compared with other locoregional or systemic treatments. In a minority of patients, the initial therapeutic effect could result in an increase in tumor diameter caused by immune cell infiltration, which is called pseudoprogression. To address this issue, immunotherapy RECIST (iRECIST) have been introduced to discriminate between progressive disease (PD) and pseudoprogression (PSPD) through a confirmation of a real PD by subsequent examinations (11). However, in most published ICIs RCTs, RECIST 1.1 criteria were used as primary reference, and the degree of surrogacy between true (i.e., OS) and surrogate radiological-based endpoints is a critical point when interpreting the results (8).
When progression is evident, what is the correct sequencing of systemic therapies is another hot topic. A therapeutic sequence should be established before starting systemic treatment, although evolutionary events during follow-up, such as cancer progression, toxicity and liver decompensation, should be kept in mind to guide the choice of the second-line. Potential treatment sequences, according to the available drug classes, are: (I) first-line ICIs-based combination, followed by second-line TKIs; (II) first-line TKIs followed by second-line TKIs; (III) first-line TKIs followed by second-line ICIs; (IV) first-line ICIs-based combination followed by second-line ICIs. Evidence on comparative efficacy is lacking, so the best sequential first-second line remains elusive. Therefore, choosing the best sequential treatment in clinical practice is difficult; according to patient’s characteristics at the time of first-line discontinuation, reasons for first-line discontinuation, specific contraindications to a drug class, and events occurring during the first-line treatment, it could be reasonable, whenever possible, to consider a shift in drug class (i.e., from ICIs to TKIs or from TKIs to ICIs) from first to subsequent lines of treatment. It should not be forgotten that prescribing regulations often limit clinicians’ decisions, so there is currently a huge gap to be filled on second- and third-line strategies. Last but not least, HCC diagnosis, symptoms, therapies, and potential adverse events deeply affect the quality of life (QoL) of patients and their caregivers; this should be systematically considered in the process of treatment selection, to enhance the benefits of a patient-tailored strategy. In fact, QoL comprehends not only clinical problems but also psychological, social and financial disease-related burdens: if QoL decreases, and liver function decompensation occurs, an upgrade of supportive treatment strategies is necessary. QoL should be an essential parameter for clinical outcomes in future prospective trials, particularly in those attempting the development of new therapeutic associations (12). We also strongly agree with SITC guideline on the institution of a more patient-centered medical environment, based on comprehensive counseling and education about expected or unexpected effects from treatment, and involvement in support groups.
Several ongoing trials are testing the efficacy and safety of novel treatments: (I) novel ICIs (tislezumab and camrelizumab); (II) ICIs-TKIs combinations (nivolumab-ipilimumab-cabozantinib, avelumab-axitinib); (III) vaccines (telomerase peptide vaccine, GPC3 peptide vaccines, AFP-based vaccines, tumor lysate-based vaccines), (IV) adoptive cell therapies (cytokine-induced killer cells and allogenic natural killers). Moreover, a few studies showed tolerable safety and better efficacy outcomes with a combination of ICIs and locoregional therapies (transarterial chemoembolization and radiofrequency ablation) but further studies are needed to assess the synergy and outcome of these strategies.
In conclusion, we are experiencing a major revolution in the systemic treatment of HCC, and this upheaval is related to the introduction of ICIs. The SITC guidelines not only include many of the key aspects for the use of these drugs in patients with HCC but conclude by emphasizing how crucial updating the recommendations will be in the future. Identification of effective biomarkers, management of liver disease and decompensation, establishment of an "ab initio" therapeutic strategy that is supported by viable second- and third-line alternatives, attention to QoL, and inclusion of the patient in the decision-making process are the pillars on which to base HCC systemic therapy in the future.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-97/coif). The authors have no conflicts of interest to declare.
References
- 1.Greten TF, Abou-Alfa GK, Cheng AL, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of hepatocellular carcinoma. J Immunother Cancer 2021;9:e002794. 10.1136/jitc-2021-002794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022;77:1598-606. 10.1016/j.jhep.2022.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vitale A, Svegliati-Baroni G, Ortolani A, et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002-2033: the ITA.LI.CA database. Gut 2023;72:141-52. 10.1136/gutjnl-2021-324915 [DOI] [PubMed] [Google Scholar]
- 4.Genentech’s Tecentriq plus Avastin is the first treatment combination to reduce the risk of cancer returning in people with certain types of early-stage liver cancer in a phase III trial. News Release. Genentech. January 18, 2023. Available online: https://www.nlm.nih.gov/bsd/uniform_requirements.html
- 5.Haber PK, Castet F, Torres-Martin M, et al. Molecular Markers of Response to Anti-PD1 Therapy in Advanced Hepatocellular Carcinoma. Gastroenterology 2023;164:72-88.e18. 10.1053/j.gastro.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 6.Scheiner B, Pomej K, Kirstein MM, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol 2022;76:353-63. 10.1016/j.jhep.2021.09.035 [DOI] [PubMed] [Google Scholar]
- 7.Cabibbo G, Aghemo A, Lai Q, et al. Optimizing systemic therapy for advanced hepatocellular carcinoma: the key role of liver function. Dig Liver Dis 2022;54:452-60. 10.1016/j.dld.2022.01.122 [DOI] [PubMed] [Google Scholar]
- 8.Cabibbo G, Celsa C, Enea M, et al. Progression-Free Survival Early Assessment Is a Robust Surrogate Endpoint of Overall Survival in Immunotherapy Trials of Hepatocellular Carcinoma. Cancers (Basel) 2020;13:90. 10.3390/cancers13010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721-8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang PF, Chen Y, Song SY, et al. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front Pharmacol 2017;8:730. 10.3389/fphar.2017.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou M, Zhang C, Nie J, et al. Response Evaluation and Survival Prediction Following PD-1 Inhibitor in Patients With Advanced Hepatocellular Carcinoma: Comparison of the RECIST 1.1, iRECIST, and mRECIST Criteria. Front Oncol 2021;11:764189. 10.3389/fonc.2021.764189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norman EML, Weil J, Philip J. Hepatocellular carcinoma and its impact on quality of life: A review of the qualitative literature. Eur J Cancer Care (Engl) 2022;31:e13672. 10.1111/ecc.13672 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as