The Society for Immunotherapy of Cancer (SITC) published clinical practice guideline on immunotherapy for hepatocellular carcinoma (HCC) (1). Many clinical practice guidelines for HCC have been published by academic societies worldwide (2), but the SITC guideline is the first to focus exclusively on immunotherapy.
Firstly, this guideline clearly states that immunotherapy for HCC should be handled by experts in cancer immunology and liver disease because HCC is often associated with liver cirrhosis. They state that the treatment plan for HCC should usually be determined by a multidisciplinary team whose members may include oncologists, hepatologists, surgeons, interventional radiologists, and pathologists. In the recommendations section, the guideline reiterates that when selecting immunotherapy for HCC, it is essential first to have a multidisciplinary tumor board determine the treatment plan. In addition, the guideline recommends the proactive use of liver biopsy to diagnose advanced HCC, which is commonly treated by systemic therapy. However, liver biopsy is unsuitable because of seeding risk in early HCC, commonly treated by resection or liver transplantation. They state that, for drug selection, it is essential to differentiate HCC from tumors with similar imaging findings, such as cholangiocarcinoma, combined hepatocellular and cholangiocarcinoma, and neuroendocrine carcinoma. Another essential step for drug selection is genomic analysis of biopsy tissue samples to search for genetic alterations. Subsequentially, tumor biopsy is essential because it enables enrollment in clinical trials even when no suitable drug is available.
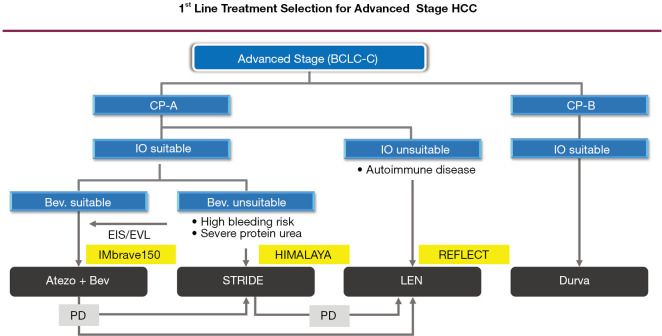
Next, regarding first-line immunotherapy for HCC, which is the main topic of this review, the guideline recommends atezolizumab plus bevacizumab (Atezo/Bev) (3). However, it is essential to note the general contraindications for bevacizumab, which are high risk of cardiac disease, stroke, hemorrhage, hemoptysis, gastrointestinal (GI) perforation, and non-healing wounds. Another point to note is that this guideline was published in 2021 and thus did not reflect the results of the HIMALAYA trial presented at the 2021 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (4). In other words, the guideline reflects the state of the field before positive results were presented for durvalumab plus tremelimumab (STRIDE regimen), an immune-oncology (IO) + IO combination of a programmed death-ligand 1 (PD-L1) antibody and cytotoxic T lymphocyte antigen-4 (CTLA-4) antibody. However, because both the HIMALAYA trial and IMbrave150 trial used sorafenib as the comparator, as is widely known, the relative efficacy and safety of each regimen can be evaluated objectively by comparing the hazard ratio (HR) for overall survival (OS) and the HR for progression-free survival (PFS). The STRIDE regimen had an HR for OS of 0.78 (95% CI: 0.66–0.92) and an HR for PFS of 0.90 (95% CI: 0.77–1.05), making it inferior to Atezo/Bev, which had an HR for OS of 0.58 (95% CI: 0.42–0.79) and an HR for PFS of 0.59 (95% CI: 0.47–0.79) (3,5) (Table 1) . The STRIDE regimen was also clearly inferior to Atezo/Bev in terms of objective response rate (ORR) (30% vs. 20%) and progressive disease (PD) rate (40% vs. 19%) (Table 1) (3,5). The STRIDE regimen also had an inferior safety profile: the rate of high-dose steroid use was 20.1% versus a systemic steroid use rate of just 12% for Atezo/Bev, and the rate of immune-related adverse events (irAEs) was also clearly higher (Table 1) (3,5). The above comparison suggests that Atezo/Bev should remain the first-line immunotherapy, even if the SITC guideline is revised in the future (Figure 1). Expert opinions by Llovet et al. and Bejjani et al. clearly state that Atezo/Bev is preferred over the STRIDE regimen as first-line immunotherapy (6,7). For PD after first-line Atezo/Bev, the guideline also recommends the anti-programmed cell death protein 1 (anti-PD-1) inhibitors nivolumab and pembrolizumab, which received accelerated approval by the US Food and Drug Administration, along with nivolumab plus ipilimumab. In addition, durvalumab plus tremelimumab has a different mode of action and is positioned as second-line immunotherapy but can be considered for first-line immunotherapy when tyrosine kinase inhibitor (TKI) or Anti-VEGF therapy is contraindicated (Figure 1). Immunotherapy rechallenge after initial immunotherapy has already been shown to have some effect (8).
Table 1. Comparison between IMbrave150 and HIMALAYA trials.
| Variables | IMbrave150 trial (update) | HIMALAYA trial | ||||
|---|---|---|---|---|---|---|
| Atezolizumab plus bevacizumab | Sorafenib | Durvalumab plus tremelimumab | Durvalumab | Sorafenib | ||
| Indication | Unresectable HCC (including Vp4) | Unresectable HCC (excluding Vp4) | ||||
| Hepatic functional reserve | Child-Pugh A | Child-Pugh A | ||||
| Number | 326 | 159 | 393 | 389 | 389 | |
| mOS (months) | 19.2 | 13.4 | 16.4 | 16.6 | 13.8 | |
| OS hazard ratio | 0.66 | 0.78 | 0.86 | |||
| mPFS (months) | 6.9 | 4.3 | 3.8 | 3.7 | 4.1 | |
| PFS hazard ratio | 0.65 | 0.90 | 1.02 | |||
| Response rate, n (%) | 97 (29.8) | 18 (11.3) | 79 (20.1) | 66 (17.0) | 20 (5.1) | |
| CR | 25 (7.7) | 1 (<1) | 12 (3.1) | 6 (1.5) | 0 | |
| PR | 72 (22.1) | 17 (10.7) | 67 (17.0) | 147 (37.8) | 20 (5.1) | |
| SD | 144 (44.2) | 69 (43.4) | 157 (39.9) | 176 (45.2) | 216 (55.5) | |
| PD | 63 (19.3) | 40 (25.2) | 157 (39.9) | 213 (54.8) | 153 (39.3) | |
| DCR, n (%) | 241 (73.9) | 87 (54.7) | 236 (60.1) | 213 (54.8) | 236 (60.7) | |
| Systemic steroid use (%) | 12.6 | N/A | 20.1* | 9.5* | N/A | |
*, high-dose steroid: predonizolone 40 mg/day. mOS, median overall survival; OS, overall survival; mPFS, median progression-free survival; PFS, progression-free survival; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate; HCC, hepatocellular carcinoma; Vp4, tumor thrombus at the main portal vein; NA, not applicable.
Figure 1.
1st line treatment selection for advanced stage HCC. BCLC, Balcerona Clinic Liver Cancer; CP, Child-Pugh grade; IO, immune-oncology; Bev., bevacizumab; EIS, endoscopic injection sclerotherapy; EVL, endoscopic variceal ligation; Atezo+Bev, atezolizumab plus bevacizumab; STRIDE, durvalumab plus tremelimumab; LEN, lenvatinib; Durva, durvalumab; PD, progressive disease; HCC, hepatocellular carcinoma.
Immunotherapy is indicated for patients with Child-Pugh A liver function but is also known to be beneficial for selected patients with Child-Pugh B liver function. The SITC guideline recommends anti-PD-1 antibody monotherapy for patients with Child-Pugh B liver function because efficacy and adverse events were comparable between Child-Pugh B (B7-B8) and Child-Pugh A patients in the CheckMate 040 trial of nivolumab monotherapy (9). Monotherapy with the anti-PD-L1 antibody durvalumab was non-inferior to sorafenib in the HIMALAYA trial, and thus durvalumab is recommended for Child-Pugh B7 and B8 HCC in practice (Figure 1).
Interestingly, the SITC guideline emphasizes the efficacy of combined locoregional-immunotherapy. For example, they note that transcatheter arterial chemoembolization (TACE) and drug-eluting bead TACE may produce a synergistic effect with PD-1/PD-L1 antibodies by inducing immunogenic cell death and infiltration of CD8-positive cells into the tumor. This combination therapy may induce antigen-specific CD4+ T cell and GPC3-specific cytotoxic T cell responses (10). Based on this rationale, the guideline states that combined locoregional-immunotherapy shows promise as a synergistic and effective combination strategy that will produce a strong therapeutic effect. This concept is also being demonstrated in clinical practice. Recent data shows that Atezo/Bev plus locoregional therapy resulted in curative conversion in 35% of patients and cancer-free status in 23% of patients (11-14).
The recommendations in the SITC guideline also discuss the importance of biomarkers. Specifically, they note the importance of identifying which patient populations will benefit from single-agent anti-PD-1/PD-L1 antibodies rather than combination immunotherapy. Evaluating tissue and blood samples obtained before and during treatment for changes in immune cells could help clarify the mechanisms of efficacy and resistance to combination immunotherapy and single-agent immunotherapy and is critical for establishing biomarkers to predict the efficacy of and resistance to immunotherapy.
The description of irAEs in the guideline is also helpful. The description of immune-mediated hepatotoxicity is particularly important. It is crucial to note that immune-mediated hepatotoxicity is extremely important in HCC with underlying cirrhosis and can easily be fatal in severe cases. Of course, monitoring for irAEs at other sites such as the skin, GI tract, lungs, thyroid, adrenal glands and pituitary glands, kidneys, nervous system, and blood and bone marrow is also necessary. It is essential to educate patients and their families about specific symptoms that may indicate various irAEs. This explanation is valuable information for real-world practice.
Regarding the adjuvant setting, as of 2021, the SITC guideline stated that the results of clinical trials in the adjuvant setting are not yet known and “results are awaited”. However, in January 2023, the positive results of the IMbrave050 trial were announced in a press release. The trial proved that adjuvant Atezo/Bev effectively prevented disease recurrence after curative liver resection or ablation. The details were presented at the American Association for Cancer Research annual meeting in April 2023. Based on these results, future revisions of the SITC guideline will likely explain the results of the IMbrave050 trial in detail.
In addition, the SITC guideline states that immunotherapy is contraindicated in the posttransplant setting, but further investigation of the risks and benefits of immunotherapy in the pretransplant setting is warranted.
The expert panel further states that immunotherapy is effective regardless of etiology, even for HCC of non-viral etiology. In addition, although mRECIST is recommended for response assessment in patients receiving locoregional therapy, whether mRECIST or immune-related RECIST is a better criterion for immunotherapy remains inconclusive. The guideline also states that pseudoprogression is extremely rare in HCC, and hyperprogressive disease (HPD) is rare and unpredictable. Although HPD has been presumed to be linked to the expression of PD-1 on regulatory T cells, it remains impossible to predict HPD. The guideline also states that immunotherapy offers a better quality of life than past TKI therapies.
In the conclusion section of the guideline, the benefit of combined locoregional-immunotherapy is reemphasized. This statement is very much agreeable with the following facts:
The IMbrave050 trial showed positive results in the adjuvant use of Atezo/Bev after resection or ablation in early stage HCC.
Atezo/Bev followed by curative conversion (ABC conversion) (combination of locoregional therapy with Atezo/Bev) is effective for intermediate-stage HCC (14).
The combination of locoregional therapy with lenvatinib is effective, as shown in the positive results of the LAUNCH trial (15), which investigated lenvatinib plus TACE in patients with advanced HCC.
The SITC guideline is an excellent guideline that strongly suggests that combined locoregional-immunotherapy will come to play an important role in treatment for all stages of HCC.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-122/coif). The author reports consulting fees from Chugai, Roshe, Eisai, AstraZeneca, honoraria from Chugai, Eisai, Eli Lilly, Takeda, research funding for Institution from Otsuka, Taiho, Chugai, GE Healthcare, Eisai, AbbVie, EA Pharma. The author has no other conflicts of interest to declare.
References
- 1.Greten TF, Abou-Alfa GK, Cheng AL, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of hepatocellular carcinoma. J Immunother Cancer 2021;9:e002794. 10.1136/jitc-2021-002794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudo M, Kawamura Y, Hasegawa K, et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021;10:181-223. 10.1159/000514174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 4.Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid 2022;1:EVIDoa2100070. [DOI] [PubMed]
- 5.Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862-73. 10.1016/j.jhep.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 2022;19:151-72. 10.1038/s41571-021-00573-2 [DOI] [PubMed] [Google Scholar]
- 7.Bejjani AC, Finn RS. Hepatocellular Carcinoma: Pick the Winner-Tyrosine Kinase Inhibitor Versus Immuno-oncology Agent-Based Combinations. J Clin Oncol 2022;40:2763-73. 10.1200/JCO.21.02605 [DOI] [PubMed] [Google Scholar]
- 8.Scheiner B, Roessler D, Phen S, et al. Efficacy and safety of immune checkpoint inhibitor rechallenge in individuals with hepatocellular carcinoma. JHEP Rep 2022;5:100620. 10.1016/j.jhepr.2022.100620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudo M, Matilla A, Santoro A, et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol 2021;75:600-9. 10.1016/j.jhep.2021.04.047 [DOI] [PubMed] [Google Scholar]
- 10.Greten TF, Mauda-Havakuk M, Heinrich B, et al. Combined locoregional-immunotherapy for liver cancer. J Hepatol 2019;70:999-1007. 10.1016/j.jhep.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudo M. Atezolizumab plus Bevacizumab Followed by Curative Conversion (ABC Conversion) in Patients with Unresectable, TACE-Unsuitable Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer 2022;11:399-406. 10.1159/000526163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M. New treatment paradigm with systemic therapy in intermediate-stage hepatocellular carcinoma. Int J Clin Oncol 2022;27:1110-9. 10.1007/s10147-022-02166-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo M. A Novel Treatment Strategy for Patients with Intermediate-Stage HCC Who Are Not Suitable for TACE: Upfront Systemic Therapy Followed by Curative Conversion. Liver Cancer 2021;10:539-44. 10.1159/000519749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kudo M, Aoki T, Ueshima K, et al. Achievement of complete response and drug-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: A multicenter proof-of-concept study. Liver Cancer 2023. doi: . 10.1159/000529574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Z, Fan W, Zhu B, et al. Lenvatinib Combined With Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J Clin Oncol 2023;41:117-27. 10.1200/JCO.22.00392 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as