Abstract
Significant improvements in the management of hepatocellular carcinoma (HCC) during the past three years have urged the timely update of clinical guidelines in China. In brief, aMAP score is newly recommended as an effective risk stratification tool to predict HCC occurrence especially for non-cirrhotic patients. Biomarker-based surveillance including 7 micro-RNA panel and GALAD score are advocated to assist early diagnosis. China liver cancer (CNLC) staging system proposed in the 2017 guideline continues to be the standard model for staging with modifications in the treatment allocations. Conversion therapies using multi-modal, high intensity strategies are advocated to facilitate subsequent resection for patients with technically unresectable CNLC stage Ia, Ib, IIa HCC, or technically resectable IIb, IIIa HCC. Super-selective transcatheter arterial chemoembolization (TACE) with the assistance of Cone-Beam CT if necessary is recommended to guarantee the efficacy of TACE. Hepatic arterial infusion chemotherapy (HAIC) using oxaliplatin, fluorouracil, and leucovorin (FOLFOX) regimen alone or in combination with systemic therapy is recommended for TACE-refractory patients or for patients with locally advanced HCC. The systemic treatments for HCC have evolved considerably since atezolizumab plus bevacizumab, and suntilimab plus bevacizumab analogue showing superior survival benefit to sorafenib, and donafenib with comparable efficacy with sorafenib are added to the first-line treatments. In addition to regorafenib, apatinib, camrelizumab and tislelizumab are added as the second-line systemic therapies for patients who progressed on sorafenib. Updates in the 2022 Barcelona Clinic Liver Cancer (BCLC) guidelines and Japanese Society of Hepatology (JSH) consensus statement are also introduced and compared with the 2022 Chinese guidelines.
Keywords: Clinical practice guidelines, hepatocellular carcinoma, diagnosis, treatment algorithm
Introduction
Liver cancer is the fourth most prevalent and the second most lethal cancer in China (1,2). It is estimated that, by 2025, approximately 431 thousand individuals will be newly diagnosed with primary liver cancer in China (gco.iarc.fr). Hepatocellular carcinoma (HCC) constitutes the majority of primary liver cancer, accounting for 75–85% of the cases (2). Most HCC cases have a background of chronic hepatitis derived from hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, alcohol abuse or non-alcoholic steatohepatitis (NASH). HBV-associated HCC, predominating in China, is molecularly enriched with a proliferation subclass featured by poor differentiation and high aggressiveness, whereas HCV-, alcoholic- or NASH related HCC in western countries tend to present with moderate to well differentiation and low frequency of vascular invasion (3). In China, approximately 9% and 55% of HCC cases were initially diagnosed at intermediate and advanced stages respectively, whereas in Japan and north America, the percentages of patients diagnosed at intermediate stage were 10% and 14%, and those diagnosed at advanced stage were 42% and 12% respectively according to the BRIDGE study (4).
Breakthroughs in HCC treatment during the past three years have urged the publication of the 2022 edition of Chinese guidelines. The Barcelona Clinic Liver Cancer (BCLC) group and the Japanese Society of Hepatology (JSH) have also updated recommendations on the staging and treatment allocations of HCC recently (5,6). Herein, we summarize the updated recommendations on the surveillance, diagnosis, staging and treatment algorithm of HCC in the 2022 version of Chinese guidelines. Discrepancies on the staging and treatment landscape of HCC among different practical guidelines are also discussed.
Surveillance and diagnosis
Guidelines across different regions agree that patients with liver cirrhosis are at a high-risk for HCC (7,8). Besides, patients with HBV or HCV infection, alcohol abuse, non-alcoholic steatohepatitis (NASH) or family history of HCC, especially men aged more than 40 years old, are also deemed as high-risk population for developing HCC in China. The aMAP (age-Male-ALBI-Platelets) scoring system (9) as an effective quantitative risk stratification tool to predict HCC occurrence irrespective of etiology and ethnicity is newly recommended in the updated Chinese guidelines. The high-risk group (aMAP score >60) with an annual incidence of HCC reaching 1.6–4% warrant intensive surveillance. Ultrasonography combined with α-fetoprotein (AFP) every 6 months remain the recommended surveillance strategy. Other blood-based biomarkers including lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), protein induced by vitamin K absence/antagonist-Ⅱ (PIVKA-II), 7-miRNA panel (10) involving miR-122, miR-192, miR-21, miR-223, miR-26a, miR-27a and miR-801, and GALAD score (11) based on sex, age, AFP, PIVKA-II and AFP-L3 are recommended to assist early detection of HCC, especially for AFP-negative patients. The diagnostic algorithms based on imaging examination in the 2022 Chinese guidelines remain identical to the previous versions. Briefly, for patients with liver cirrhosis or chronic hepatitis B/C, nodules >2 cm can be diagnosed as HCC based on typical features (hyperenhancement in arterial phase and washout in portal venous/delayed phases using contrast agents, or impaired hepatocyte function in hepatobiliary phase using hepatobiliary agents) on one of the three imaging techniques [contrast enhanced computed tomography (CECT)/magnetic resonance imaging (CEMRI)/gadolinium-ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced MRI (EOB-MRI)], or based on arterial phase hyperenhancement with late (≥60 s) washout on contrast-enhanced ultrasound (CEUS), whereas nodules ≤2 cm need confirmation by at least two imaging methods. For indeterminate nodules, especially nodules >2 cm, pathological evaluation via biopsy or definitive resection is necessary to make a diagnosis.
Staging
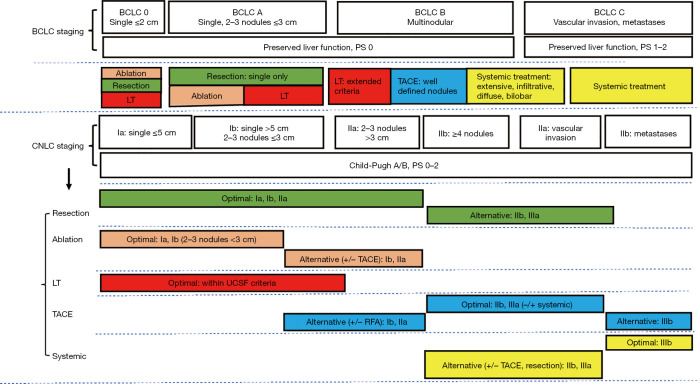
Both the China liver cancer (CNLC) staging and BCLC classification combine performance status (PS), liver function and tumor characteristics, including tumor size, tumor number, vascular invasion and metastasis, to stratify patient prognosis and allocate treatment. While Child-Pugh A/B continues to be set as a prerequisite for anti-tumor therapy in the CNLC staging, Child-Pugh classification as the only parameter to evaluate liver function has been abandoned since the 2018 version of BCLC staging system. Instead, preserved liver function is advocated and evaluated via comprehensive evaluation of Albumin-Bilirubin (ALBI) score (12), Child-Pugh score, model for end-stage liver disease (MELD) 3.0 points (13) as well as AFP level irrespective of tumor burden (14). PS scoring 0 is essential to classify patients as early- or middle-stage HCC, while the corresponding score is defined as 0–2 in the CNLC staging. Regarding tumor characteristic itself, both the BCLC and CNLC revised versions remain consistent with the previous ones. Differences in staging and treatment allocation between the CNLC and BCLC systems are detailed in Figure 1.
Figure 1.
Comparisons of staging and treatment algorithms of HCC between 2022 BCLC and 2022 Chinese guidelines. BCLC, Barcelona Clinic Liver Cancer; CNLC, China Liver Cancer Staging; LT, liver transplantation; TACE, transarterial chemoembolization; UCSF, University of California San Francisco; HC, hepatocellular carcinoma; PS, performance status.
Treatment
With a broad range of treatment options available for patients with HCC, a multidisciplinary team is required to allocate individual treatment. Improvements in early diagnosis, surgical techniques, locoregional interventions and systemic therapies have collectively contributed to a significantly prolonged patient survival. Currently, the expected survival attains >5, >2.5 and >2 years for patients with early, intermediate and advanced HCC respectively (5).
Hepatectomy
Hepatectomy, as a major curative treatment, is preferably indicated for patients with CNLC stage Ia, Ib and IIa HCC, the criteria of which is consistent with that in Japan. Preserved liver function, defined as Child-Pugh grade A, indocyanine green 15-min retention rate (ICG-R15) <30%, and future remnant liver volume >40% (for patients with liver fibrosis/cirrhosis) or >30% (for patients without liver fibrosis/cirrhosis) of the standardized liver volume, continues to be the prerequisite for hepatectomy in China. In experienced centers, minimally invasive laparoscopic or robot-assisted laparoscopic hepatectomy can achieve comparable oncological efficacy and operative safety to open hepatectomy (15). The indications for laparoscopic liver resection are expanded without strict restrictions on tumor size or tumor location, which become identical to that for open liver resection. Technically, fluorescence imaging with indocyanine green (ICG) facilitating tumor imaging and delineation of segmental boundaries has been advocated to secure R0 resection (16).
The JSH recommend either hepatectomy or ablation for patients with 1–3 tumors and tumor size ≤3 cm due to comparable recurrence-free survival (RFS) from the SURF trial (17), however, the Chinese experts prefer hepatectomy over ablation considering better overall survival (OS) (18). For selected HCC patients with confined portal vein or hepatic vein invasion, hepatectomy is suggested in Asian countries as propensity score matched (PSM) studies have shown improved survival benefit for patients undergoing resection compared to those receiving other treatments including intra-arterial therapies or chemotherapies (19,20). Notably, sorafenib had not become a standard treatment option during the study period and was not established as the control group in the above PSM studies. Recently, experiences from Europe and North America have verified the superiority of hepatectomy over sorafenib for patients with locally advanced HCC (21,22). For patients with ≥4 tumors, namely CNLC IIb HCC, hepatectomy can be implemented in case multiple tumors are localized in the same segment or lobe in China. A high recurrence rate can be anticipated for patients with CNLC IIb and IIIa after resection. Therefore, transcatheter arterial chemoembolization (TACE) and systemic therapy rather than surgical resection are recommended as the mainstay treatments for the majority of patients with CNLC IIb and CNLC IIIa HCC respectively. Under such circumstances, conversation therapy can also be considered (23).
For patients with well-preserved liver function and potentially resectable HCC, defined as technically unresectable CNLC stage Ia, Ib, IIa HCC, or technically resectable IIb, IIIa HCC, conversion therapy by multi-modal, high intensity strategies is advocated to facilitate subsequent resection in the updated Chinese guidelines. Systemic therapies like tyrosine kinase inhibitors (TKIs) plus programmed death-1 (PD-1) inhibitors (24-27), locoregional treatments including TACE, hepatic arterial infusion chemotherapy (HAIC) using oxaliplatin, fluorouracil, and leucovorin regimen (FOLFOX-HAIC), or TACE plus FOLFOX-HAIC (28), radiation plus fluorouracil-HAIC (29), and systemic therapies in combination with FOLFOX-HAIC (30) or TACE (31) have been explored as conversion therapies to induce tumor shrinkage or downstaging. Detailed research design, target population and resection rate of studies focusing on conversion therapy published in recent years are listed in Table 1. Nonetheless, the long-term survival benefit for hepatectomy after conversion therapy await to be evaluated. Comprehensive pathological evaluation of the resected tumor, including detailed description of the remnant alive tumor, the necrotic part and tumor stroma (inflammation and fibrous tissue), are proposed to be carried out to predict prognosis and allocate subsequent treatment for patients with conversion therapy. Major pathological response (MPR) defined as the remnant alive tumor ≤10% and complete pathological response (CPR) defined as complete necrosis of the tumor indicating favorable prognosis in other types of cancer may be equally applicable for HCC (32,33), the result of which need more studies for confirmation. For patients with unmet future liver reserve (FLR), portal vein embolization (PVE) and associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) are suggested as effective methods to induce liver regeneration. Recent studies recommend ALPPS over PVE in faster introduction of liver regeneration and fewer risk of tumor progression (34,35). On the other hand, the BCLC group insist on only patients with solitary HCC in the absence of clinically significant portal hypertension (CSPH) as optimal target population for hepatectomy (36).
Table 1. Studies on conversion therapy and subsequent resection for initially unresectable HCC.
| Study | Study design | Target patient | Conversion therapy | ORR (RECIST 1.1) | Conversion rate | RFS post resection |
|---|---|---|---|---|---|---|
| Zhu et al. (24) | Retrospective | BCLC A (n=2), BCLC B (n=15), BCLC C (n=26) | PD-1 inhibitor + TKI (lenvatinib, apatinib) | NA | 23.3% | NA |
| Zhang et al. (25) | Non-randomized, open-lable | BCLC B/C (n=33) | PD-1 inhibitor + lenvatinib | 53.1% | 42.4% | NR |
| Wang et al. (26) | Prospective, single-arm, phase-II | BCLC B (n=12), BCLC C (n=14) | Sintilimab + lenvatinib | 35.0% | 27% | NR |
| Sun et al. (27) | Prospective, single-arm, phase-II | BCLC B (n=30) | Sintilimab + bevacizumab biosimilar (IBI305) | 23.3% | 43.3% | NR |
| Li et al. (28) | Retrospective | BCLC A/B (n=83) | FOLFOX-HAIC + cTACE (n=42) vs. cTACE (n=41) | 38.1% vs. 7.3% | 48.8% vs. 9.5% | NR vs. 9.2 months |
| Byun et al. (29) | Retrospective | BCLC C (n=637) | Fluorouracil-HAIC + radiation | NA | 10.7% | NA |
| Liu et al. (30) | Prospective, single-arm, phase-II | CNLC IIb (n=3), CNLC IIIa (n=16), CNLC IIIb (n=11) | FOLFOX-HAIC + sintilimab + IBI305 | 66.7% | 46.7% | NA |
| Zhang et al. (31) | Prospective, multi-center | BCLC B/C (n=38) | TACE + PD-1 inhibitor + TKI | NA | 50.0% | NR |
HCC, hepatocellular carcinoma; ORR, overall response rate; RECIST, Response Evaluation Criteria in Solid Tumors; RFS, recurrence-free survival; BCLC, Barcelona Clinic Liver Cancer; PD-1, programmed-death 1; TKI, tyrosine kinase inhibitor; NA, not available; NR, not reached; FOLFOX-HAIC, hepatic arterial infusion chemotherapy (HAIC) using oxaliplatin, fluorouracil, and leucovorin (FOLFOX) regimen; cTACE, conventional transarterial chemoembolization.
Postoperative recurrence occurs in up to 80% of patients (37). The STROM trial frustrates the usage of sorafenib as adjuvant therapy (38). The role of Immunotherapy as adjuvant therapy is under investigation (NCT03859128, NCT04639180, NCT03383458, NCT03867084, NCT04102098, and NCT03847428). For patients with intermediate risk (single nodule >5 cm without microvascular invasion) or high risk (single nodule >5 cm with microvascular invasion, multiple nodules) for recurrence, TACE has been established as an effective adjuvant therapy in China (39). And a recent study reported that 1–2 cycles of FOLFOX-HAIC also significantly improved postoperative RFS (27.0 vs. 11.3 months, P=0.001) for patients with microvascular invasion (40).
Transplantation
The expansion of criteria for liver transplantation (LT) beyond Milan criteria has been practiced with favorable survival results in China for years (41,42). Considering shortage of liver donors, University of California San Francisco (UCSF) criteria (solitary tumor ≤6.5 cm or ≤3 nodules ≤4.5 cm plus total tumor diameter ≤8 cm) continues to be advocated for wide application in China (43). There is a growing global consensus on that a minor increase in tumor size and number beyond Milan criteria does not withdraw the long-term survival benefit of LT (44-46). Patients with well-defined HCC nodules within BCLC B stage are newly suggested as target population for transplantation in the 2022 BCLC guidelines. Although the morphological criteria for defining transplant feasibility among this BCLC B subgroup has not been reached, AFP >1,000 ng/mL, as a surrogate reflecting invasive tumor biology, is set as a contraindication for LT in the 2022 BCLC guidelines (47,48). AFP has also been included in the eligibility criteria for LT in Japan. The 5-5-500 criteria (≤5 cm, ≤5 tumors, AFP ≤500 ng/mL) has been adopted as additional criteria to Milan criteria for insurance coverage in Japan since 2019 (49,50). For patients on the waiting list for LT, the efficacy of bridging therapies via locoregional or systemic therapies to prevent tumor progression remains controversial (51,52). On the other hand, for patients who are initially beyond the transplantation criteria, down-staging therapies via locoregional therapies to reduce tumor burden to within the Milan criteria have yielded favorable long-term outcomes (51,53). mTOR-inhibitor treatment with sirolimus for ≥3 months are recommended to prolong post-LT PFS and OS, especially for those with AFP ≥10 ng/mL (54).
Locoregional treatments
For patients with early-stage HCC inaccessible to resection or LT, ablation is another treatment modality for cure. Radiation can be considered as an alternative to ablation if there is a high probability of treatment failure or technical obstacles. According to several retrospective studies, stereotactic body radiation therapy (SBRT) showed comparable survival benefit with radiofrequency ablation (RFA) for early-stage HCC (55,56). A recent randomized controlled trial clarified that proton radiotherapy exhibited similar local tumor control rate (2-year local progression-free survival (PFS): 94.8% vs. 83.9%, P<0.001 for non-inferiority test) to RFA for patients with postoperative recurrent HCC (1–2 tumors, each <3 cm) (57).
TACE has been indicated mainly for patients with BCLC B HCCs. The heterogeneity of BCLB B HCC is highlighted and three subgroups are stratified in the 2022 BCLC guidelines. The application of TACE is restricted to the second subgroup who have defined tumor burden accessible to selective catheterization. TACE has been indicated for CNLC IIb, IIIa and some IIIb HCC since the 2017 Chinese guidelines. Technically, super-selective TACE with the assistance of Cone-Beam CT if necessary is emphasized to guarantee the efficacy of TACE in the updated guidelines (58,59). Besides the previous “six-and-twelve” scoring system (60), “Pre-TACE-Predict” model using tumor number, size, AFP, albumin, bilirubin, vascular invasion and etiology, and “Post-TACE-Predict” model using number, size, AFP, bilirubin, vascular invasion and imaging response are proposed to predict the efficacy of TACE (61). A tumoral and peritumoral radiomic signature (TPR-signature) in combination with clinical parameters including AFP, BCLC subclassification, tumor location and arterial hyperenchancement comprising a clinical-radiomic (CR) model can be served a robust tool to predict response to the first session of TACE (62).
With the advent of a broad range of effective systemic therapies, patients who experience Untreatable Progression (UTP) under TACE defined as failure of TACE (63) by the BCLC group, or TACE refractoriness defined as no response after two TACE sessions (64) by the JSH are recommended to switch to systemic therapies (65). Even for treatment-naive patients with diffuse, infiltrative, bilobar multifocal HCC, namely the third BCLC B subgroup, systemic therapy is recommended as initial treatment, as this subgroup are unlikely to respond to TACE. Although the strict cutoff with regard to tumor morphological characteristics has not been raised, prognostic models to predict response to TACE may serve as useful tools to differentiate TACE-unsuitable subgroup. For TACE-unsuitable HCC, the JSH recommend a lenvatinib-TACE sequential therapy based on a proof-of-concept study (66,67). Various clinical trials have been conducted to evaluate the efficacy of TACE in combination with sorafenib for BCLC B HCC (68-71), but only one study warrant such combination therapy (71). According to the TACTICS trial which adapted the criteria of progression as time to UTP or to TACE refractoriness, sorafenib plus TACE was associated with a significant improvement in PFS (22.8 vs. 13.5 months, P=0.020) and a prolonged interval (21.1 vs. 16.9 weeks, p=0.018) between TACE sessions (71,72). Notably, median OS (mOS) failed to reach significant difference between the two groups (36.2 vs. 30.8 months, P=0.40) according to the recent report (72). Whether the synergistic effect of immunotherapy plus TACE in experimental research can also be attained in clinical practices is under investigation (NCT0426177, NCT03778957, and NCT04340193).
Hepatic arterial infusion chemotherapy (HAIC) using interferon, cisplatin or low-dose 5-FU plus cisplatin (FP) regimen, firstly developed in Japan, has been indicated for patients with major portal vascular invasion or with Child-Pugh B liver function. According to the SCOOP-2 trial (73) and the SILIUS trial conducted in Japan (74), neither HAIC (cisplatin regimen)-sorafenib sequential treatment (10.0 vs. 15.2 months, P=0.780) nor HAIC (FP regimen) plus sorafenib (11.8 vs. 11.5 months, P=0.955) improved mOS compared with sorafenib monotherapy. On the other hand, FOLFOX-HAIC developed in China was associated with a prolonged mOS (14.5 vs. 7.0 months, P<0.001) compared to sorafenib for locally advanced HCC (75). And FOLFOX-HAIC plus sorafenib showed a higher overall response rate (ORR) (40.8% vs. 2.46%, P<0.001) than sorafenib alone (76). According to two prospective studies recently, triple therapies involving FOLFOX-HAIC, PD-1 inhibitor and target agent were associated with improved ORR reaching 66.7% and 70.96% (30,77), but the long-term effect of which on liver function and OS needs to be clarified in future. Transarterial radioembolization (TRAE) as another intra-arterial therapy adopted in western countries has not been widely applied in China currently.
Systemic therapy
Systemic therapy is indicated for patients with advanced HCC and those ineligible for local therapies. The survival of patients treated with systemic therapy generally has significantly improved since 2017, partly due to a variety of agents available as post-line therapies. Detailed systemic treatments proposed in different guidelines are listed in Table 2. Updates of recommendations on systemic therapy in Version 2.2022 of National Comprehensive Cancer Network (NCCN) guidelines are also included in this table.
Table 2. Recommendations on systemic therapies in different guidelines.
| Guidelines | CNLC, 2022 | BCLC, 2022 | NCCN, 2022 | JSH, 2021 |
|---|---|---|---|---|
| First-line | Atezo-Bev, Suntilimab-bevacizumab analogue, lenvatinib, sorafenib, donafenib, FOLFOX chemotherapy | Preferred: Atezo-Bev, durvalumab-tremelimumab; other recommended regimen: sorafenib, lenvatinib | Preferred: Atezo-Bev; other recommended regimen: sorafenib, lenvatinib, durvalumab, pembrolizumab | Atezo-Bev |
| Second-line | Regorafenib, apatinib, camrelizumab, tislelizumab | Post-sorafenib: regorafenib, cabozantinib, ramucirumab (AFP ≥400 ng/mL); post-Atezo-Bev, durvalumab-tremelimumab, lenvatinib: clinical trial | Options: regorafenib, cabozantinib, ramucirumab (AFP ≥400 ng/mL), lenvatinib, sorafenib; other recommended regimen: nivolumab + ipilimumab, pembrolizumab | Lenvatinib, sorafenib |
| Third-line | NA | Cabozantinib | NA | Regorafenib, cabozantinib, ramucirumab (AFP ≥400 ng/mL) |
CNLC, China liver cancer; BCLC, Barcelona Clinic Liver Cancer; NCCN, National Comprehensive Cancer Network; JSH, Japanese Society of Hepatology; Atezo-Bev, Atezolizumab-Bevacizumab; AFP, α-fetoprotein; NA, not available.
Atezolizumab plus bevacizumab (Atezo-Bev), as the first regimen showing superior ORR (35% vs. 14%) and mOS (19.2 vs. 13.4 months, HR =0.66, P<0.001) over sorafenib, has been approved as first-line therapy for unresectable HCC worldwide (78). In the Chinese cohort, the survival benefit for patients receiving Atezo-Bev was more remarkable with mOS reaching 24.0 months. Sintilimab plus a bevacizumab biosimilar (IBI305) exhibiting a prolonged mOS (not reached vs. 10.4 months, P<0.001) compared with sorafenib (79), and donafenib (12.1 vs. 10.3 months, P=0.024) presenting comparable efficacy with sorafenib (80) are newly added to first-line therapies in China. Despite that Atezo-Bev, lenvatinib and sorafenib are widely accepted as first-line systemic therapies, different guidelines show varied recommendation priority. The 2022 BCLC guidelines recommend Atezo-Bev as the preferred first-line therapy over sorafenib and lenvatinib for Child-Pugh A patients without variceal bleeding risk, whereas current Chinese guidelines recommend the application of the six first-line therapies including Atezo-Bev, suntilimab-bevacizumab analogue, lenvatinib, sorafenib, donafenib and FOLFOX chemotherapy without priority. On the other hand, the JSH recommend Atezo-Bev as first-line therapy, and lenvatinib and sorafenib as second-line treatments. Nevertheless, there still lacks evidence whether the traditional first-line alternatives like lenvatinib or sorafenib can retain their effectiveness in patients initially receiving Atezo-Bev (81). According to the positive results of HIMALAYA trial (22), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) inhibitor tremelimumab plus programmed death ligand-1 (PD-L1) inhibitor durvalumab is recommended as an alternative first-line therapy to Atezo-Bev by the BCLC group, while durvalumab monotherapy is approved in the first-line setting by the NCCN panel. In fact, both the STRIDE group (tremelimumab plus durvalumab) and the durvalumab group showed improved ORR and survival benefit in the HIMALAYA study. On the other hand, lenvatinib plus pembrolizumab with encouraging results in the Ib study continued to render favorable OS benefit as first-line therapy for advanced HCC in the phase III LEAP-002 study (82,83). Unfortunately, it failed to meet pre-specified statistical significance (21.2 vs. 19.0 months, P=0.023) when compared to lenvatinib monotherapy (83). Notably, the mOS of the lenvatinib group attained 19.0 months, which was the longest ever reported among the control groups. Apart from the strong anticancer efficacy of lenvatinib itself, availability of various treatments after progression on lenvatinib also contributed to the prolonged OS. Although lenvatinib plus pembrolizumab may not be recommended with high strength in future guidelines, the clinically meaningful survival benefit still provides a rationale for its use as the first-line treatment. Meanwhile, the RESCUE trial evaluating the efficacy of camrelizumab plus apatinib versus sorafenib as fist-line therapy met dual primary endpoint and showed significant improvements in mOS (22.1 vs. 15.2 months, P<0.001) and PFS (5.6 vs. 3.7 months, P<0.001) in the combination arm (84). Currently, camrelizumab plus apatinib is the first PD-1/PD-L1 plus TKI regimen showing positive results, which can be expected to be included in future guidelines.
Apart from regorafenib, targeted agent apatinib (80) and PD-1 inhibitors camrelizumab (85) and tislelizumab (86,87) are added as second-line systemic therapies for patients who progressed on sorafenib in the 2022 Chinese guidelines. Ramucirumab for patients with AFP >400 ng/ml and cabozantinib effective in the second-line setting are approved by western countries. These two agents have not been marketed in China currently. Cabozantinib is also effective in the third-line setting (88). In Japan, regorafenib, ramucirumab and cabozantinib are all accepted as third-line treatments. Although pembrolizumab vs. placebo as second-line treatment did not meet primary end point in western countries (89), a significant survival improvement was observed for patients treated with pembrolizumab in Asian countries (90). Considering a clinically meaningful superiority in ORR, pembrolizumab continues to be approved for sorafenib-treated patients in the updated NCCN guidelines. Besides, nivolumab plus ipilimumab with an ORR of 32% and a mOS of 22.8 months is also recommended as second-line treatment by the NCCN panel (91). Currently, optimal post-line treatment for patients who develop resistance to Atezo-Bev has not been clarified and comparative clinical trials are in urgent need to inform treatment selection.
After the approval of these new agents as the first-line or second-line therapies for advanced HCC, their efficacies especially the immune checkpoint (ICI)-based therapies in the neoadjuvant and adjuvant setting for early and intermediate HCC, or in combination with locoregional therapies for intermediate HCC, or as conversion approaches for intermediate or locally advanced HCC are being explored in phase III studies. With encouraging results from phase II studies (25-27) or real world practices (92), current Chinese guidelines recommend the integration of systemic therapies with local therapies, albeit the strength of the recommendation is rated as moderate.
Apart from anti-tumor therapies, antiviral therapies with entecavir, tenofovir or tenofovir alafenamide fumarate are recommended for patients with detectable HBV-DNA or positive hepatitis B surface antigen (HBsAg) through the entire duration of treatment for HCC (93). Direct-acting antiviral agents are indicated for patients with detectable HCV-RNA (94).
Treatment for HCC rupture
For patients with HCC rupture, treatment regimen should be individualized based on patient’s hemodynamical condition, liver function and tumor characteristics. Hepatectomy is recommended for patients with stable hemodynamics, well-preserved liver function and resectable HCC (95). For patients unsuitable for hepatectomy, transcatheter arterial embolization (TAE) is the first choice, especially for those with unstable hemodynamics (96). On follow-up evaluation, salvage resection can be performed for selected patients (95).
Conclusion and future perspectives
Due to a large population infected with HBV in China, optimal surveillance methods including new serum biomarkers are advocated to facilitate the identification of more patients at early-stage HCC. The advent of systemic treatments like Atezo-Bev with marked survival benefit encourages a shift of application to earlier stage in the BCLC guidelines, whereas more aggressive multimodal treatments like the addition of immunotherapy-based systemic treatment to local therapies are proposed in Chinese guidelines. Currently, more than 20 phase III trials are undergoing to identify the role of ICI-based therapies across all stages of HCC (97). With the release of their results, consensus on the implementation of immunotherapy-based systemic therapy to HCC at different stages can be anticipated. As a chance for cure, conversion therapies to transform initially unresectable HCCs to resectable ones have been advocated in the revised Chinese guidelines.
Other than PD-1/PD-L1 and CTLA-4 inhibitors, novel ICIs like lymphocyte-activation gene 3 (LAG-3) inhibitor relatlimab (98) and PD-1/CTLA-4 bi-specific antibody cadonilimab (99), which have been recently approved for use in patients with melanoma and cervical cancer respectively, are also perceived as promising opportunities for patients with HCC. Other immune strategies such as chimeric antigen receptor (CAR)-T/-NK transfer, vaccination or oncolytic virotherapy are under clinical development and may produce meaningful response in patients who become resistant to established ICIs. With the advent of more effective treatments, high-evidence trials are in need to illuminate the optimal sequence or the combination modality suitable for individual patients. Although various immune classifications and genomic signatures are identified to be associated with response to immunotherapy (100,101), currently no validated biomarker is available to guide clinical decision-making. Efforts to gap the molecular pathogenesis and therapeutic interventions via liquid biopsy, radiomics, or other techniques are promising with the updates in the future guidelines eagerly awaited.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (Nos. 82130077 and 81961128025), Basic Research Project from the Science and Technology Commission of Shanghai Municipality (Nos. 21JC1410100, 21JC1401200, 20JC1418900), and Natural Science Funds of Shanghai (No. 21ZR1413800).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-469/coif). All authors reports that this work was supported by National Natural Science Foundation of China (Nos. 82130077 and 81961128025), Basic Research Project from the Science and Technology Commission of Shanghai Municipality (Nos. 21JC1410100, 21JC1401200, 20JC1418900), and Natural Science Funds of Shanghai (No. 21ZR1413800). The authors have no other conflicts of interest to declare.
References
- 1.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145-58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- 4.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-66. 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo M, Kawamura Y, Hasegawa K, et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021;10:181-223. 10.1159/000514174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver . Electronic address: easloffice@easloffice; . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 8.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 9.Fan R, Papatheodoridis G, Sun J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol 2020;73:1368-78. 10.1016/j.jhep.2020.07.025 [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Yu L, Gao X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol 2011;29:4781-8. 10.1200/JCO.2011.38.2697 [DOI] [PubMed] [Google Scholar]
- 11.Best J, Bechmann LP, Sowa JP, et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 2020;18:728-735.e4. 10.1016/j.cgh.2019.11.012 [DOI] [PubMed] [Google Scholar]
- 12.Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338-46. 10.1016/j.jhep.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 13.Kim WR, Mannalithara A, Heimbach JK, et al. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology 2021;161:1887-1895.e4. 10.1053/j.gastro.2021.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singal AG, Hoshida Y, Pinato DJ, et al. International Liver Cancer Association (ILCA) White Paper on Biomarker Development for Hepatocellular Carcinoma. Gastroenterology 2021;160:2572-84. 10.1053/j.gastro.2021.01.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu P, Liao W, Zhang WG, et al. A Prospective Study Using Propensity Score Matching to Compare Long-term Survival Outcomes After Robotic-assisted, Laparoscopic or Open Liver Resection for Patients with BCLC Stage 0-A Hepatocellular Carcinoma. Ann Surg 2023;277:e103-11. . 10.1097/SLA.0000000000005380 [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Teh CSC, Ishizawa T, et al. Consensus Guidelines for the Use of Fluorescence Imaging in Hepatobiliary Surgery. Ann Surg 2021;274:97-106. 10.1097/SLA.0000000000004718 [DOI] [PubMed] [Google Scholar]
- 17.Kudo M, Hasegawa K, Kawaguchi Y, et al. A multicenter randomized controlled trial to evaluate the efficacy of surgery versus radiofrequency ablation for small hepatocellular carcinoma (SURF trial): Analysis of overall survival. J Clin Oncol 2021;39:4093. 10.1200/JCO.2021.39.15_suppl.4093 [DOI] [Google Scholar]
- 18.Xu Q, Kobayashi S, Ye X, et al. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep 2014;4:7252. 10.1038/srep07252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokudo T, Hasegawa K, Matsuyama Y, et al. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: A Japanese nationwide survey. Hepatology 2017;66:510-7. 10.1002/hep.29225 [DOI] [PubMed] [Google Scholar]
- 20.Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938-43. 10.1016/j.jhep.2016.05.044 [DOI] [PubMed] [Google Scholar]
- 21.Famularo S, Donadon M, Cipriani F, et al. Hepatectomy Versus Sorafenib in Advanced Nonmetastatic Hepatocellular Carcinoma: A Real-life Multicentric Weighted Comparison. Ann Surg 2022;275:743-52. 10.1097/SLA.0000000000005373 [DOI] [PubMed] [Google Scholar]
- 22.Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol 2022;40:379. 10.1200/JCO.2022.40.4_suppl.379 [DOI] [Google Scholar]
- 23.Sun HC, Zhou J, Wang Z, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr 2022;11:227-52. 10.21037/hbsn-21-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu XD, Huang C, Shen YH, et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer 2021;10:320-9. 10.1159/000514313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Lu S, Hu B, et al. PD-1 inhibitor combined with lenvatinib for unresectable liver cancer as the conversion therapy: An open-label, non-randomized, phase IV study. J Clin Oncol 2021;39:e16173. 10.1200/JCO.2021.39.15_suppl.e16173 [DOI] [Google Scholar]
- 26.Wang L, Wang H, Cui Y, et al. Sintilimab plus lenvatinib as conversion therapy in patients with unresectable locally intermediate to advanced hepatocellular carcinoma: A single-arm, single-center, open-label, phase 2 study. J Clin Oncol 2022;40:449. 10.1200/JCO.2022.40.4_suppl.44934995105 [DOI] [Google Scholar]
- 27.Sun H, Zhu X, Gao Q, et al. Sintilimab combined with bevacizumab biosimilar as a conversion therapy in potentially resectable intermediate stage hepatocellular carcinoma (HCC): a phase II trial. Ann Oncol 2022;33:S323-S30. 10.1016/j.annonc.2022.07.835 [DOI] [Google Scholar]
- 28.Li B, Qiu J, Zheng Y, et al. Conversion to Resectability Using Transarterial Chemoembolization Combined With Hepatic Arterial Infusion Chemotherapy for Initially Unresectable Hepatocellular Carcinoma. Ann Surg Open 2021;2:e057. 10.1097/AS9.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byun HK, Kim HJ, Im YR, et al. Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother Oncol 2019;133:1-8. 10.1016/j.radonc.2018.12.025 [DOI] [PubMed] [Google Scholar]
- 30.Liu D, Mu H, Liu C, et al. Hepatic artery infusion chemotherapy (HAIC) combined with sintilimab and bevacizumab biosimilar (IBI305) for initial unresectable hepatocellular carcinoma (HCC): A prospective, single-arm phase II trial. J Clin Oncol 2022;40:4073. 10.1200/JCO.2022.40.16_suppl.4073 [DOI] [Google Scholar]
- 31.Zhang X, Zhu X, Liu C, et al. The safety and efficacy of transarterial chemoembolization (TACE) + lenvatinib + programmed cell death protein 1 (PD-1) antibody of advanced unresectable hepatocellular carcinoma. J Clin Oncol 2022;40:453. 10.1200/JCO.2022.40.4_suppl.453 [DOI] [Google Scholar]
- 32.Travis WD, Dacic S, Wistuba I, et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol 2020;15:709-40. 10.1016/j.jtho.2020.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein JE, Lipson EJ, Cottrell TR, et al. Pan-Tumor Pathologic Scoring of Response to PD-(L)1 Blockade. Clin Cancer Res 2020;26:545-51. 10.1158/1078-0432.CCR-19-2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li PP, Huang G, Jia NY, et al. Associating liver partition and portal vein ligation for staged hepatectomy versus sequential transarterial chemoembolization and portal vein embolization in staged hepatectomy for HBV-related hepatocellular carcinoma: a randomized comparative study. Hepatobiliary Surg Nutr 2022;11:38-51. 10.21037/hbsn-20-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Peng Y, Hu J, et al. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy for Unresectable Hepatitis B Virus-related Hepatocellular Carcinoma: A Single Center Study of 45 Patients. Ann Surg 2020;271:534-41. 10.1097/SLA.0000000000002942 [DOI] [PubMed] [Google Scholar]
- 36.Berzigotti A, Reig M, Abraldes JG, et al. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology 2015;61:526-36. 10.1002/hep.27431 [DOI] [PubMed] [Google Scholar]
- 37.Pinna AD, Yang T, Mazzaferro V, et al. Liver Transplantation and Hepatic Resection can Achieve Cure for Hepatocellular Carcinoma. Ann Surg 2018;268:868-75. 10.1097/SLA.0000000000002889 [DOI] [PubMed] [Google Scholar]
- 38.Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. 10.1016/S1470-2045(15)00198-9 [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Ren Z, Chen Y, et al. Adjuvant Transarterial Chemoembolization for HBV-Related Hepatocellular Carcinoma After Resection: A Randomized Controlled Study. Clin Cancer Res 2018;24:2074-81. 10.1158/1078-0432.CCR-17-2899 [DOI] [PubMed] [Google Scholar]
- 40.Li S, Mei J, Cheng Y, et al. Postoperative adjuvant hepatic arterial infusion chemotherapy (HAIC) with FOLFOX to improve outcomes of patients with hepatocellular carcinoma with microvascular invasion: A prospective multicenter, phase 3, randomized, controlled clinical trial. J Clin Oncol 2022;40:4013. 10.1200/JCO.2022.40.16_suppl.4013 [DOI] [Google Scholar]
- 41.Fan J, Yang GS, Fu ZR, et al. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol 2009;135:1403-12. 10.1007/s00432-009-0584-6 [DOI] [PubMed] [Google Scholar]
- 42.Li J, Yan LN, Yang J, et al. Indicators of prognosis after liver transplantation in Chinese hepatocellular carcinoma patients. World J Gastroenterol 2009;15:4170-6. 10.3748/wjg.15.4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. 10.1053/jhep.2001.24563 [DOI] [PubMed] [Google Scholar]
- 44.Mehta N, Bhangui P, Yao FY, et al. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020;104:1136-42. 10.1097/TP.0000000000003174 [DOI] [PubMed] [Google Scholar]
- 45.Kwong A, Mehta N. Expanding the Limits of Liver Transplantation for Hepatocellular Carcinoma: Is There a Limit? Clin Liver Dis 2021;25:19-33. 10.1016/j.cld.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 46.Ferrer-Fàbrega J, Sampson-Dávila J, Forner A, et al. Limited tumour progression beyond Milan criteria while on the waiting list does not result in unacceptable impairment of survival. J Hepatol 2021;75:1154-63. 10.1016/j.jhep.2021.06.015 [DOI] [PubMed] [Google Scholar]
- 47.Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018;154:128-39. 10.1053/j.gastro.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 48.Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945-51. 10.1002/lt.23904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimamura T, Akamatsu N, Fujiyoshi M, et al. Expanded living-donor liver transplantation criteria for patients with hepatocellular carcinoma based on the Japanese nationwide survey: the 5-5-500 rule - a retrospective study. Transpl Int 2019;32:356-68. 10.1111/tri.13391 [DOI] [PubMed] [Google Scholar]
- 50.Shinkawa H, Tanaka S, Takemura S, et al. Prognostic value of expanded liver transplantation criteria-the 5-5-500 rule-in patients with hepatic resection for intermediate-stage hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2020;27:682-9. 10.1002/jhbp.792 [DOI] [PubMed] [Google Scholar]
- 51.Lee S, Kim KW, Song GW, et al. The Real Impact of Bridging or Downstaging on Survival Outcomes after Liver Transplantation for Hepatocellular Carcinoma. Liver Cancer 2020;9:721-33. 10.1159/000507887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kulik L, Heimbach JK, Zaiem F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology 2018;67:381-400. 10.1002/hep.29485 [DOI] [PubMed] [Google Scholar]
- 53.Mazzaferro V, Citterio D, Bhoori S, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol 2020;21:947-56. 10.1016/S1470-2045(20)30224-2 [DOI] [PubMed] [Google Scholar]
- 54.Schnitzbauer AA, Filmann N, Adam R, et al. mTOR Inhibition Is Most Beneficial After Liver Transplantation for Hepatocellular Carcinoma in Patients With Active Tumors. Ann Surg 2020;272:855-62. 10.1097/SLA.0000000000004280 [DOI] [PubMed] [Google Scholar]
- 55.Kim N, Cheng J, Jung I, et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol 2020;73:121-9. 10.1016/j.jhep.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 56.Hara K, Takeda A, Tsurugai Y, et al. Radiotherapy for Hepatocellular Carcinoma Results in Comparable Survival to Radiofrequency Ablation: A Propensity Score Analysis. Hepatology 2019;69:2533-45. 10.1002/hep.30591 [DOI] [PubMed] [Google Scholar]
- 57.Kim TH, Koh YH, Kim BH, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase III trial. J Hepatol 2021;74:603-12. 10.1016/j.jhep.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 58.Miyayama S, Matsui O. Superselective Conventional Transarterial Chemoembolization for Hepatocellular Carcinoma: Rationale, Technique, and Outcome. J Vasc Interv Radiol 2016;27:1269-78. 10.1016/j.jvir.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 59.Pung L, Ahmad M, Mueller K, et al. The Role of Cone-Beam CT in Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. J Vasc Interv Radiol 2017;28:334-41. 10.1016/j.jvir.2016.11.037 [DOI] [PubMed] [Google Scholar]
- 60.Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol 2019;70:893-903. 10.1016/j.jhep.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 61.Han G, Berhane S, Toyoda H, et al. Prediction of Survival Among Patients Receiving Transarterial Chemoembolization for Hepatocellular Carcinoma: A Response-Based Approach. Hepatology 2020;72:198-212. 10.1002/hep.31022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M, Cao J, Hu J, et al. Clinical-Radiomic Analysis for Pretreatment Prediction of Objective Response to First Transarterial Chemoembolization in Hepatocellular Carcinoma. Liver Cancer 2021;10:38-51. 10.1159/000512028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Templeton AJ, Amir E, Tannock IF. Informative censoring - a neglected cause of bias in oncology trials. Nat Rev Clin Oncol 2020;17:327-8. 10.1038/s41571-020-0368-0 [DOI] [PubMed] [Google Scholar]
- 64.Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 2014;87 Suppl 1:22-31. 10.1159/000368142 [DOI] [PubMed] [Google Scholar]
- 65.Reig M, Darnell A, Forner A, et al. Systemic therapy for hepatocellular carcinoma: the issue of treatment stage migration and registration of progression using the BCLC-refined RECIST. Semin Liver Dis 2014;34:444-55. 10.1055/s-0034-1394143 [DOI] [PubMed] [Google Scholar]
- 66.Kudo M, Ueshima K, Chan S, et al. Lenvatinib as an Initial Treatment in Patients with Intermediate-Stage Hepatocellular Carcinoma Beyond Up-To-Seven Criteria and Child-Pugh A Liver Function: A Proof-Of-Concept Study. Cancers (Basel) 2019;11:1084. 10.3390/cancers11081084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kudo M. Extremely High Objective Response Rate of Lenvatinib: Its Clinical Relevance and Changing the Treatment Paradigm in Hepatocellular Carcinoma. Liver Cancer 2018;7:215-24. 10.1159/000492533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64:1090-8. 10.1016/j.jhep.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 69.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011;47:2117-27. 10.1016/j.ejca.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 70.Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:565-75. 10.1016/S2468-1253(17)30156-5 [DOI] [PubMed] [Google Scholar]
- 71.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 2020;69:1492-501. 10.1136/gutjnl-2019-318934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kudo M, Ueshima K, Ikeda M, et al. Final Results of TACTICS: A Randomized, Prospective Trial Comparing Transarterial Chemoembolization Plus Sorafenib to Transarterial Chemoembolization Alone in Patients with Unresectable Hepatocellular Carcinoma. Liver Cancer 2022;11:354-67. 10.1159/000522547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kondo M, Morimoto M, Kobayashi S, et al. Randomized, phase II trial of sequential hepatic arterial infusion chemotherapy and sorafenib versus sorafenib alone as initial therapy for advanced hepatocellular carcinoma: SCOOP-2 trial. BMC Cancer 2019;19:954. 10.1186/s12885-019-6198-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kudo M, Ueshima K, Yokosuka O, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol 2018;3:424-32. 10.1016/S2468-1253(18)30078-5 [DOI] [PubMed] [Google Scholar]
- 75.Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol 2018;69:60-9. 10.1016/j.jhep.2018.02.008 [DOI] [PubMed] [Google Scholar]
- 76.He M, Li Q, Zou R, et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol 2019;5:953-60. 10.1001/jamaoncol.2019.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gu YK, Zhang TQ, Zuo MX, et al. Hepatic artery infusion chemotherapy (HAIC) combined with apatinib and camrelizumab for hepatocellular carcinoma (HCC) in BCLC stage c: A prospective, single-arm, phase II trial (TRIPLET study). J Clin Oncol 2022;40:4106. 10.1200/JCO.2022.40.16_suppl.4106 [DOI] [Google Scholar]
- 78.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 79.Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol 2021;22:977-90. 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- 80.Qin S, Bi F, Gu S, et al. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol 2021;39:3002-11. 10.1200/JCO.21.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol 2021;75:960-74. 10.1016/j.jhep.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 82.Llovet J, Shepard KV, Finn RS, et al. A phase Ib trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC): Updated results. Ann Oncol 2019;30:v286-7. 10.1093/annonc/mdz247.073 [DOI] [Google Scholar]
- 83.Finn RS, Kudo M, Merle P, et al. Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as firstline (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol 2022;33:S808-69. 10.1016/j.annonc.2022.08.031 [DOI] [Google Scholar]
- 84.Qin S, Chan LS, Gu S, et al. Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. Ann Oncol 2022;33:S808-69. 10.1016/j.annonc.2022.08.032 [DOI] [Google Scholar]
- 85.Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020;21:571-80. 10.1016/S1470-2045(20)30011-5 [DOI] [PubMed] [Google Scholar]
- 86.Ducreux M, Abou-Alfa G, Ren Z, et al. O-1 Results from a global phase 2 study of tislelizumab, an investigational PD-1 antibody, in patients with unresectable hepatocellular carcinoma. Ann Oncol 2021;32:S217. 10.1016/j.annonc.2021.05.005 [DOI] [Google Scholar]
- 87.Qin S, Kudo M, Meyer T, et al. Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann Oncol 2022;33:S808-69. 10.1016/j.annonc.2022.08.033 [DOI] [PubMed] [Google Scholar]
- 88.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63. 10.1056/NEJMoa1717002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merle P, Edeline J, Bouattour M, et al. Pembrolizumab (pembro) vs placebo (pbo) in patients (pts) with advanced hepatocellular carcinoma (aHCC) previously treated with sorafenib: Updated data from the randomized, phase III KEYNOTE-240 study. J Clin Oncol 2021;39:268. 10.1200/JCO.2021.39.3_suppl.268 [DOI] [Google Scholar]
- 90.Kudo M, Finn RS, Edeline J, et al. Updated efficacy and safety of KEYNOTE-224: a phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur J Cancer 2022;167:1-12. 10.1016/j.ejca.2022.02.009 [DOI] [PubMed] [Google Scholar]
- 91.El-Khoueiry AB, Yau T, Kang Y-K, et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): Long-term results from CheckMate 040. J Clin Oncol 2021;39:269. 10.1200/JCO.2021.39.3_suppl.26933275488 [DOI] [Google Scholar]
- 92.Xie D, Sun Q, Wang X, et al. Immune checkpoint inhibitor plus tyrosine kinase inhibitor for unresectable hepatocellular carcinoma in the real world. Ann Transl Med 2021;9:652. 10.21037/atm-20-7037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chinese Society of Infectious Diseases, Chinese Medical Association ; Chinese Society of Hepatology, Chinese Medical Association. The guidelines of prevention and treatment for chronic hepatitis B (2019 version). Zhonghua Gan Zang Bing Za Zhi 2019;27:938-61. [DOI] [PubMed] [Google Scholar]
- 94.European Association for the Study of the Liver . EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol 2018;69:461-511. 10.1016/j.jhep.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 95.Aoki T, Kokudo N, Matsuyama Y, et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg 2014;259:532-42. 10.1097/SLA.0b013e31828846de [DOI] [PubMed] [Google Scholar]
- 96.Shin BS, Park MH, Jeon GS. Outcome and prognostic factors of spontaneous ruptured hepatocellular carcinoma treated with transarterial embolization. Acta Radiol 2011;52:331-5. 10.1258/ar.2010.100369 [DOI] [PubMed] [Google Scholar]
- 97.Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 2022;19:151-72. 10.1038/s41571-021-00573-2 [DOI] [PubMed] [Google Scholar]
- 98.Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med 2022;386:24-34. 10.1056/NEJMoa2109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keam SJ. Cadonilimab: First Approval. Drugs 2022;82:1333-9. 10.1007/s40265-022-01761-9 [DOI] [PubMed] [Google Scholar]
- 100.Sia D, Jiao Y, Martinez-Quetglas I, et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017;153:812-26. 10.1053/j.gastro.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 101.Kurebayashi Y, Matsuda K, Ueno A, et al. Immunovascular classification of HCC reflects reciprocal interaction between immune and angiogenic tumor microenvironments. Hepatology 2022;75:1139-53. 10.1002/hep.32201 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as