Abstract
Exogenous estrogen is associated with reduced coronavirus disease (COVID) mortality in nonimmunosuppressed/immunocompromised (non-ISC) postmenopausal females. Here, we examined the association of estrogen or testosterone hormone replacement therapy (HRT) with COVID outcomes in solid organ transplant recipients (SOTRs) compared to non-ISC individuals, given known differences in sex-based risk in these populations. SOTRs ≥45 years old with COVID-19 between April 1, 2020 and July 31, 2022 were identified using the National COVID Cohort Collaborative. The association of HRT use in the last 24 months (exogenous systemic estrogens for females; testosterone for males) with major adverse renal or cardiac events in the 90 days post-COVID diagnosis and other secondary outcomes were examined using multivariable Cox proportional hazards models and logistic regression. We repeated these analyses in a non-ISC control group for comparison. Our study included 1135 SOTRs and 43 383 immunocompetent patients on HRT with COVID-19. In non-ISC, HRT use was associated with lower risk of major adverse renal or cardiac events (adjusted hazard ratio [aHR], 0.61; 95% confidence interval [CI], 0.57-0.65 for females; aHR, 0.70; 95% CI, 0.65-0.77 for males) and all secondary outcomes. In SOTR, HRT reduced the risk of acute kidney injury (aHR, 0.79; 95% CI, 0.63-0.98) and mortality (aHR, 0.49; 95% CI, 0.28-0.85) in males with COVID but not in females. The potentially modifying effects of immunosuppression on the benefits of HRT requires further investigation.
Keywords: transplantation, COVID-19, SARS-CoV-2, infection, hormone replacement therapy, estrogens, androgens, sex, exogenous hormones, immunity
1. Introduction
In the nonimmunosuppressed/immunocompromised (non-ISC) population, male sex has been associated with an increased risk of coronavirus disease 2019 (COVID-19)-attributable mortality.1 , 2 Immunocompetent females demonstrate a more robust antiviral immune response than males,3 which has been attributed at least in part to the role sex hormones play in immunity. Estradiol is typically immune enhancing whereas testosterone is immune suppressing.4 Thus, decreasing endogenous sex hormones over the lifespan (gradually in males and more rapidly in females at menopause) are associated with corresponding changes in the immune response.5
Sex-based differences in COVID-19 risk may be partially attributed to inflammaging, defined as diminishing adaptive immunity and dysregulation of the innate immune system with advancing age.6 This has been associated with an increased risk of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-triggered cytokine storm and, thereby, multiorgan failure and mortality with COVID-19.7 Although both males and females experience inflammaging, the impact is greater in older immunocompetent males than females as suppressive androgen levels decline.1
Exogenous estrogen supplementation in immunocompetent postmenopausal women may be protective against COVID-198 due to enhanced SARS-CoV-2 antiviral clearance9 and reduced immune dysregulation,9 among other mechanisms.10 , 11, 12, 13, 14, 15 Whether supplemental testosterone or androgen therapy modifies COVID-19 risk in the general population is unknown, nor is the role of progesterone.
Importantly, despite being at greater overall risk from COVID-19, the male mortality bias observed in the general population with COVID-19 is greatly attenuated in immunosuppressed solid organ transplant recipients (SOTRs).16 , 17 Maintenance immunosuppression may differentially impact males and females, suppressing the antiviral immune benefit in immunocompetent females versus males and mitigating the pathologic immune response and corresponding cytokine cascade that disproportionately impacts older males.17 The differential influence of exogenous sex hormone supplementation on infectious outcomes in immunosuppressed SOTRs compared with the immunocompetent population has not been examined. Therefore, using the National COVID Cohort Collaborative (N3C), the largest COVID-19 registry in the United States, we aimed to examine the association of sex-specific (estrogen/progesterone or testosterone) HRT with COVID-19 outcomes in SOTRs compared with the general, non-ISC population.
2. Methods
N3C is the largest data repository of SARS-CoV-2-infected persons in the United States and has been used to study COVID-19 outcomes in SOTRs.17, 18, 19, 20 N3C contains information from 76 US medical centers representing 6.2 million COVID-positive patients. Data are routinely transmitted to N3C and harmonized into the Observational Medical Outcomes Partnership (OMOP) 5.3.1 data model within an National Institutes of Health-maintained enclave. Inclusion criteria include any patients with suspected COVID-19 inpatient or outpatient diagnosis based on laboratory testing or diagnostic codes.21 Details of the N3C rationale, design, infrastructure, and data harmonization have been previously published.22 , 23 This retrospective cohort study received institutional review board approval from the University of Nebraska Medical Center (0853-21-EP). The N3C Data Access Committee approved this study (RP-B3442B), which operates under the authority of the National Institutes of Health institutional review board. No informed consent was obtained because the study used a limited data set. This study followed the Enhancing the Quality and Transparency of Health Research (EQUATOR) reporting guidelines: Reporting of Studies Conducted Using Observational Routinely Collected Health Data (RECORD).24 Data extraction was performed using PySpark and SQL, and statistical work utilized R version 4.1.3 within the N3C Enclave per N3C privacy22 and download review policies.
2.1. Design
Using the N3C Enclave, we conducted a cohort study of perimenopause or postmenopause/andropause males and females (aged ≥45 years) in the United States with a first diagnosis of COVID-19 (COVID+) between April 1, 2020 and July 31, 2022, with data extracted on November 10, 2022 (N3C release 100); patients were censored at the time of SARS-CoV-2 reinfection. Patients had a minimum follow-up time of 90 days based on the latest data deposited by participating center. COVID diagnosis was based on a positive test result from real-time polymerase chain reaction, antigen testing, or International Classification of Diseases diagnostic codes as previously reported.20 , 22 , 23 Our primary analysis was performed in COVID+ SOTRs, defined as having a kidney, liver, lung, heart, or pancreas transplant. For comparison, the analysis was repeated contemporaneously in the COVID+ immunocompetent peri- or postmenopause/andropause general population (non-ISC). The non-ISC population excluded patients with a diagnosis of autoimmune rheumatologic disease, prior bone marrow transplant, human immunodeficiency virus, multiple sclerosis, or malignant neoplasm, as per our earlier analyses, in addition to any individual identified to have taken an immunosuppressive medication (prednisone, tacrolimus, cyclosporin, mycophenolate mofetil, antithymocyte globulin, or basiliximab).19 , 20
2.2. Exposure
The primary exposure was sex-specific HRT use in the 24 months preceding COVID diagnosis; exogenous systemic estrogen and/or progesterone supplementation for females, and exogenous testosterone supplementation for males. A complete breakdown of the sex-specific HRT formulations in the HRT exposure variable is available on the project GitHub repository.25 Sex-specific HRT use was examined separately in males and females with and without solid organ transplant (SOT).
The OMOP common data model is designed to enable the analysis of healthcare data from disparate sources by standardizing the representation of healthcare concepts, including drugs, using standardized vocabularies.26 In the OMOP common data model, drugs are captured using RxNorm and National Drug Codes. Because data are harmonized across different health care systems, they can be reported as prescribed, dispensed, or administered. A comprehensive list of medication concepts and logic associated with medication capture is provided in Supplementary Methods.
2.3. Outcomes
As with our earlier analyses of COVID+ outcomes in SOTRs,17 , 18 , 20 the primary outcome was a composite of a major adverse renal or cardiac event (MARCE) in the 90 days post-COVID diagnosis, defined as acute kidney injury (AKI) with or without dialysis, acute myocardial infarction, angina, stent occlusion/thrombosis, stroke, transient ischemic attack, congestive heart failure, or death from any cause. Secondary outcomes included 90-day risk of major adverse cardiac event, AKI, death from any cause in the 90 days post-COVID-19 diagnosis, and hospitalization.
2.4. Data collection
In addition to the sex-specific HRT exposure variable, we also collected information on potential confounders including age, sex, race/ethnicity, time since transplant, type of organ transplant (kidney, liver, heart, lung, pancreas), comorbidities (chronic kidney disease [CKD], hypertension, diabetes, chronic obstructive pulmonary disease/asthma, cancer, coronary artery disease, congestive heart failure, peripheral vascular disease, liver disease, and obesity [body mass index >30 kg/m2]), immunosuppression (antithymocyte globulin induction, basiliximab induction, and maintenance therapy with prednisone, tacrolimus, cyclosporine, or mycophenolic acid), prior COVID vaccination status, and SARS-CoV-2 epoch based on US vaccine availability27 and variant dominance28 (prevaccination [before December 10, 2020], pre-Delta [December 10, 2020-June 14, 2021], Delta [June 15, 2021-December 21, 2021], and Omicron wave [December 22, 2021 or after]).20 Complete case analysis was performed for all primary and secondary analyses.
2.5. Analysis
Baseline characteristics were reported for all SOT and non-ISC COVID+ patients in the study. Separately in the non-ISC and SOT cohorts, we examined the proportion of males with and without documented exogenous testosterone exposure (HRT) and the proportion of females with and without documented exogenous estrogen or progesterone exposure (HRT) in the preceding 24 months who developed each of the primary and secondary outcomes in the 90 days post-COVID. Significant differences in event rates separately in males and females with and without HRT exposure were determined using the chi-squared test.
Within the SOT cohort, we examined the adjusted risk of HRT exposure for each primary and secondary outcome separately in male and female transplant recipients with COVID-19. We used multivariable Cox proportional hazards models adjusting for the covariates listed above, including variant period, to examine the association of HRT with each outcome in males and females separately. Multivariable logistic regression was used for the binary outcome of hospitalization in the 90 days post-COVID diagnosis. The above analyses were then repeated in the non-ISC cohort for comparison.
2.6. Sensitivity analysis
We performed several sensitivity analyses by repeating our primary and secondary analyses with the following modifications:
-
1.
Including only those with HRT use in the preceding 6 months (instead of in the preceding 24 months).
-
2.
Restricting to adults ≥55 years, increasing the likelihood that the populations would represent menopause or andropause status.
-
3.
Examining only those adverse outcomes that happen within 90 days during the first post-COVID-19 hospitalization in that window (as opposed to all 90-day adverse outcomes).
-
4.
Examining 30-day outcomes after a COVID-19 diagnosis (as opposed to 90-day adverse outcomes).
-
5.
Adjusting for coadministration of outpatient statin therapy or anticoagulation (within the 24 months preceding COVID-19 diagnosis, Supplementary Methods), which may have been distributed differentially among SOT and non-ISC patients and associated with post-COVID outcomes.
-
6.
Examining an outcome of venous thromboembolism defined as a deep vein thrombosis or pulmonary embolism in the 90 days after COVID diagnosis.
-
7.
In female patients on sex-specific HRT, excluding those on progesterone monotherapy, given the less established role of progesterone on the immune response.
2.7. Propensity score matching
To account for potential unrecognized and unaccounted-for differences between non-ISC and SOT patients, we performed a propensity score (PS) matched analysis (using logistic regression with 1:1 matching for SOT status, matching on comorbidities [CKD, diabetes, coronary artery disease, congestive heart failure, obesity, and chronic obstructive pulmonary disease], age, sex, race, and SARS-CoV-2 variant period). We repeated our primary and secondary analyses in this PS-matched cohort of SOT and non-ISC individuals to examine the association of HRT exposure in the last 24 months with each primary and secondary outcome after COVID diagnosis and baseline difference between SOT and non-ISC individuals using multilevel modeling with SOT and non-ISC individuals.
3. Results
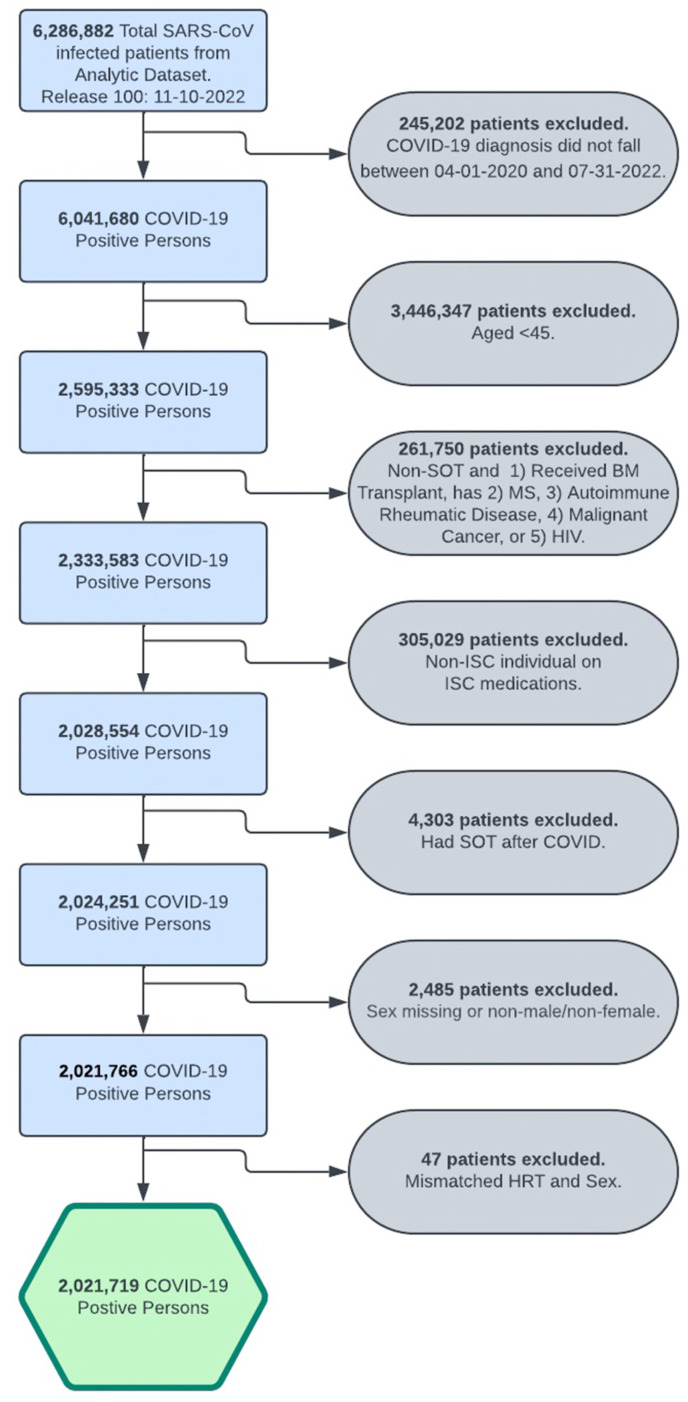
Over the study period, 29 066 SOTRs and 2.0 million non-ISC patients were diagnosed with COVID-19; 1135 (3.9%) SOTRs and 43 383 (2.6%) non-ISC were on sex-specific HRT within the 2 years prior to COVID diagnosis. Cohort derivation is demonstrated in Figure 1 . Baseline characteristics for the SOT and non-ISC cohorts stratified by sex are shown in Table 1 . Compared with the non-ISC population, COVID+ SOTRs were more likely to be male (60% vs 47%, P < .001) and Black (22% vs 11%, P < .001), to have received at least 2 doses of mRNA vaccination (41% vs 33%, P < .001), and had more comorbidities including CKD (66% vs 5.5%), hypertension (77% vs 31%), and diabetes (52% vs 15%, P < .001 for all).
Figure 1.
Cohort derivation. COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; HRT, hormone replacement therapy; ISC, immunosuppressed/immunocompromised; MS, multiple sclerosis; SARS-CoV, severe acute respiratory syndrome coronavirus; SOT, solid organ transplant.
Table 1.
Baseline characteristics of COVID-positive nonimmunosuppressed/immunocompromised patients and solid organ transplant recipients, diagnosed April 1, 2020 to July 31, 2022
| Variable | Overall n (%) N = 2 021 719 | Non-ISC (Male) n (%) N = 930 715 | Non-ISC (Female) n (%) N = 1 061 938 | SOT (Male) n (%) N = 17 570 | SOT (Female) n (%) N = 11 496 |
|---|---|---|---|---|---|
| Hormone replacement therapy | |||||
| Total | 44 518 (2.2%) | 7991 (0.9%) | 35 392 (3.3%) | 322 (1.8%) | 813 (7.1%) |
| Female | 36 205 (1.8%) | 35 392 (3.3%) | 813 (7.1%) | ||
| Estrogen only | 31 262 (1.8%) | <30 550a | <730a | ||
| Progesterone only | 3958 (0.2%) | 3872 (0.4%) | 86 (0.7%) | ||
| Combined | 985 (<0.1%) | <980a | <20a | ||
| Male | 8313 (0.4%) | 7991 (0.9%) | 322 (1.8%) | ||
| Age, y (Median (IQR)) | 59 (52, 69) | 60 (52, 69) | 59 (52, 69) | 62 (55, 68) | 61 (54, 68) |
| Race | |||||
| White | 1 315 057 (65%) | 615 275 (66%) | 683 599 (64%) | 10 149 (58%) | 6034 (52%) |
| Black | 235 261 (12%) | 96 938 (10%) | 132 014 (12%) | 3541 (20%) | 2768 (24%) |
| Hispanic or Latino | 194 954 (9.6%) | 87 443 (9.4%) | 103 614 (9.8%) | 2274 (13%) | 1623 (14%) |
| Other/Unknown | 276 447 (14%) | 131 059 (14%) | 142 711 (13%) | 1606 (9.1%) | 1071 (9.3%) |
| Time since transplant (mo) | |||||
| <6 | 3068 (11%) | 1895 (11%) | 1173 (10%) | ||
| 6-24 | 7239 (25%) | 4405 (25%) | 2834 (25%) | ||
| >24 | 18 759 (65%) | 11 270 (64%) | 7489 (65%) | ||
| Organ type | |||||
| Kidney | 15 648 (54%) | 9181 (52%) | 6467 (56%) | ||
| Liver | 4386 (15%) | 2602 (15%) | 1784 (16%) | ||
| Lung | 2473 (8.5%) | 1423 (8.1%) | 1050 (9.1%) | ||
| Heart | 2827 (9.7%) | 2061 (12%) | 766 (6.7%) | ||
| Other/multiple | 3732 (13%) | 2303 (13%) | 1429 (12%) | ||
| Comorbidities | |||||
| CKD | 128 783 (6.4%) | 57 517 (6.2%) | 52171 (4.9%) | 11 649 (66%) | 7446 (65%) |
| Hypertension | 632 208 (31%) | 294 367 (32%) | 315447 (30%) | 13 643 (78%) | 8751 (76%) |
| Diabetes | 309 577 (15%) | 147 721 (16%) | 146 762 (14%) | 9423 (54%) | 5671 (49%) |
| Asthma/COPD | 150 747 (7.5%) | 57 233 (6.1%) | 88 376 (8.3%) | 2783 (16%) | 2355 (20%) |
| Cancer | 149 685 (7.4%) | 68 909 (7.4%) | 74 243 (7.0%) | 4264 (24%) | 2269 (20%) |
| CAD | 150 802 (7.5%) | 90 097 (9.7%) | 51 487 (4.8%) | 6260 (36%) | 2958 (26%) |
| CHF | 108 071 (5.3%) | 53 808 (5.8%) | 45 708 (4.3%) | 5467 (31%) | 3088 (27%) |
| PVD | 113 229 (5.6%) | 53 966 (5.8%) | 51 861 (4.9%) | 4624 (26%) | 2778 (24%) |
| Liver Disease | 84 479 (4.2%) | 37 414 (4.0%) | 39 725 (3.7%) | 4518 (26%) | 2822 (25%) |
| Obesity | 601 773 (30%) | 262 039 (28%) | 323 433 (30%) | 9694 (55%) | 6607 (57%) |
| Immunosuppression | |||||
| Prednisone | 20 196 (1.0%) | 12 070 (69%) | 8126 (71%) | ||
| Tacrolimus | 21 366 (1.1%) | 12 969 (74%) | 8397 (73%) | ||
| Cyclosporine | 2668 (0.1%) | 1508 (8.6%) | 1160 (10%) | ||
| MMF | 15 989 (0.8%) | 9947 (57%) | 6042 (53%) | ||
| ATG induction | 3090 (0.2%) | 1842 (10%) | 1248 (11%) | ||
| Basiliximab induction | 2177 (0.1%) | 1411 (8.0%) | 766 (6.7%) | ||
| Variant period | |||||
| Pre-Delta | 593 590 (29%) | 278 417 (30%) | 309 095 (29%) | 3761 (21%) | 2317 (20%) |
| Prevaccination | 448 759 (22%) | 209 765 (23%) | 233 373 (22%) | 3399 (19%) | 2222 (19%) |
| Delta | 404 808 (20%) | 190 670 (20%) | 208 671 (20%) | 3342 (19%) | 2125 (18%) |
| Omicron | 574 562 (28%) | 251 863 (27%) | 310 799 (29%) | 7068 (40%) | 4832 (42%) |
| 2+ vaccination status | 675 610 (33%) | 291 299 (31%) | 372 267 (35%) | 7151 (41%) | 4893 (43%) |
ATG, antithymocyte globulin; CAD, coronary artery disease; CHF, congestive heart disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; Delta (June 15, 2021-December 21, 2021); COVID, coronavirus disease; ISC, immunosuppressed/immunocompromised; MMF, mycophenolate mofetil; Omicron (after December 21, 2021); pre-Delta (December 10, 2020-June 14, 2021), prevaccination (before December 10, 2020); PVD, peripheral vascular disease; SOT, solid organ transplant.
<xx to prevent back calculation of remaining cells as per N3C small cell count policies.
3.1. HRT use reduced COVID event rates in non-ISC patients and SOT males
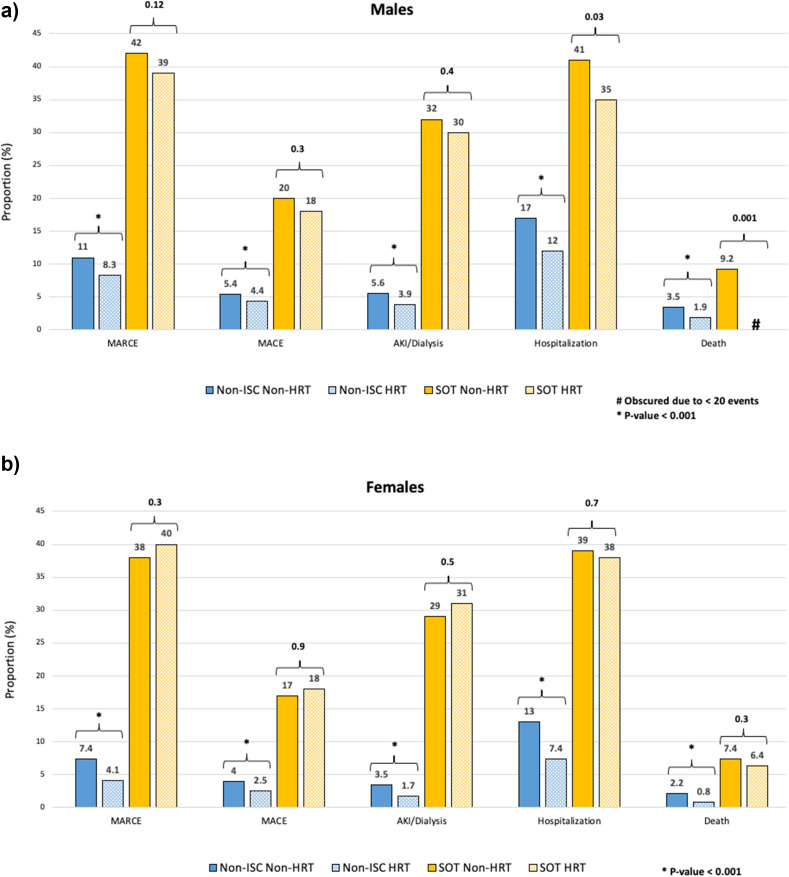
The overall risk of each outcome was significantly higher in the SOT than non-ISC populations (Table 2 ). The 90-day risk of each event (MARCE, major adverse cardiac event, AKI, death, hospitalization) after COVID+ by sex and HRT status in non-ISC individuals and SOTRs is shown in the bar graph in Figure 2 . SOTRs were at higher risk for all outcomes compared with non-ISC patients. In the non-ISC population, HRT use was associated with significantly fewer event rates, irrespective of patient sex. Among SOTRs, HRT use was associated with a significantly lower crude risk of hospitalization and death in male but not female recipients, with no difference for any other event.
Table 2.
Event rates in the 90 d post-COVID diagnosis in nonimmunosuppressed/immunocompromised patients and solid organ transplant recipients
| Outcomes | Non-ISC N (%) | SOT N (%) | P |
|---|---|---|---|
| MARCE | 156 695 (7.9%) | 10 728 (37%) | <.001 |
| MACE | 75 317 (3.8%) | 4739 (16%) | <.001 |
| AKI dialysis | 78 647 (3.9%) | 8039 (28%) | <.001 |
| Death | 27 790 (1.4%) | 1354 (4.7%) | <.001 |
| Hospitalization | 53 293 (2.7%) | 2441 (8.4%) | <.001 |
AKI, acute kidney injury; COVID, coronavirus disease; ISC, immunosuppressed/immunocompromised; MACE, major adverse cardiac event; MARCE, major adverse renal or cardiac event; SOT, solid organ transplant.
Figure 2.
Event rates in the 90 days post-COVID diagnosis in males and females on exogenous HRT. (A) Males. (B) Females. Numbers above brackets represent P values; ∗P < .001. COVID, coronavirus disease; HRT, hormone replacement therapy; ISC, immunocompromised/immunosuppressed; SOT, solid organ transplant.
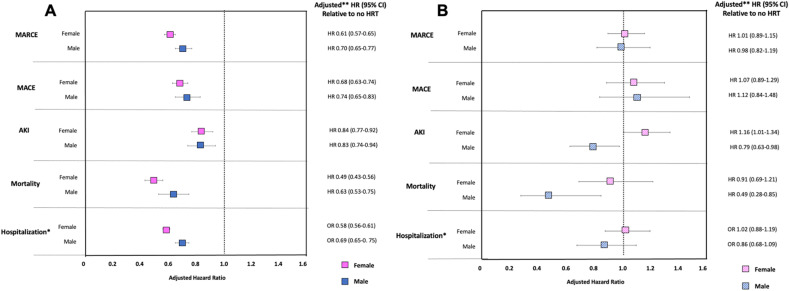
The adjusted hazard ratios (aHRs) (odds ratios for the outcome of hospitalization) for each primary and secondary outcome in males on exogenous testosterone supplementation and females on exogenous estrogen and/or progesterone supplementation in the 24 months preceding COVID diagnosis versus males and females without HRT exposure are shown in Figures 3 A (non-ISC) and 3B (SOT). Full regression models are available in Supplementary Table 1. In the non-ISC cohort, HRT use was associated with significantly lower risk of each outcome post-COVID, including MARCE (aHR, 0.61; 95% confidence interval (CI), 0.57-0.65 for females; aHR, 0.70; 95% CI, 0.65-0.77 for males), AKI (aHR, 0.84; 0.77-0.92 for females; aHR, 0.83; 95% CI, 0.74-0.94 for males), hospitalization (adjusted odds ratio [aOR], 0.58; 95% CI, 0.56-0.61 for females; aOR, 0.69; 95% CI, 0.65-0.75 for males), and especially mortality (aHR, 0.49; 95% CI, 0.43-0.56 for females; aHR, 0.63; 95% CI, 0.53-0.75 for males). In the non-ISC cohort, females derived significantly more benefit from HRT than males for the outcomes of MARCE and hospitalization.
Figure 3.
Adjusted risk of HRT (testosterone in males; estrogen/progesterone in females) on 90-day outcomes after COVID-19 diagnosis in (A) the nonimmunosuppressed general population and (B) solid organ transplant recipients. AKI, acute kidney injury; COVID-19, coronavirus disease 2019; HR, hazard ratio; HRT, hormone replacement therapy; MACE, major adverse cardiac event; MARCE, major adverse renal or cardiac event; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. ∗Odds ratio, ∗∗Adjusted for age, sex, race, time since transplant, type of organ transplant (kidney, liver, heart, lung, multiorgan), comorbidities (chronic kidney disease, hypertension, diabetes, chronic obstructive pulmonary disease/asthma, cancer, coronary artery disease, congestive heart failure, peripheral vascular disease, liver disease, and obesity [body mass index >30 kg/m2]), immunosuppression (antithymocyte globulin induction, basiliximab induction, and maintenance therapy with prednisone, tacrolimus, cyclosporine, or mycophenolic acid), prior COVID-19 vaccination status, and SARS-CoV-2 variant dominance period.
The relative benefit of HRT in the SOT cohort was attenuated for all outcomes in both males and females compared with the non-ISC population. In SOTRs, HRT significantly reduced the risk of AKI (aHR, 0.79; 95% CI, 0.63-0.98) and mortality (aHR, 0.49; 95% CI, 0.28-0.85) in males with COVID. Despite the larger sample size (813 female and 322 male SOTRs on HRT), HRT was not significantly associated with any outcome post-COVID in female SOTRs.
3.2. Sensitivity analyses
When HRT exposure was restricted to those taking HRT in the last 6 months (instead of the last 24 months), there was little effect on our results, Supplementary Figure 1a (non-ISC) and 1b (SOT). Similarly, results were impacted little by restricting our cohort to an older population ≥55 years (Supplementary Figure 2), those admitted with COVID-19 (Supplementary Figure 3), when examining 30 (rather than 90) day event rates after COVID diagnosis, Supplementary Figure 4, or when including baseline statin or anticoagulation use in the regression (Supplementary Figure 5). Among sensitivity analyses, the only notable difference was that HRT was not significantly associated with a reduced risk of AKI in non-ISC males or females when restricted to HRT use in the preceding 6 months or to those admitted with COVID-19. HRT use was associated with a lower risk of venous thromboembolism in non-ISC females, but not in non-ISC males or SOTR (Supplementary Figure 6). Excluding females on progesterone-only HRT (0.2%) did not change results, Supplementary Figures 7.
3.3. Propensity score matching
In total, 29 063 SOTRs were matched to 29 063 non-ISC patients. Baseline characteristics of the matched cohort are shown in Supplementary Table 2, and subsequent unadjusted and adjusted balances of matched variables are shown in Supplementary Figure 8. Case-mix was similar between SOT and non-ISC patients with selection of a cohort of increasingly comorbid non-ISC patients. The primary and secondary analyses were repeated in the new PS-matched cohorts; results are shown in Supplementary Figure 9. In the PS-matched cohorts, HRT use was not associated with outcomes of AKI or mortality, except in SOT males who had reduced risk (aHR, 0.79; 95% CI, 0.63-0.98 for AKI; aHR, 0.49; 95% CI, 0.28-0.85 for mortality). However, findings were limited by wide CIs reflecting low event rates and statistical uncertainty in the matched non-ISC population, which was <2% of the size of the original, unmatched cohort.
In a combined analysis, Supplementary Table 3, with SOT and non-ISC patients after PS-matching, HRT was protective against MARCE (aHR, 0.89; 95% CI, 0.82-0.98), mortality (aHR, 0.78; 95% CI, 0.63-0.96), and hospitalization (aOR, 0.84; 95% CI, 0.76-0.94), whereas SOT was associated with an increased risk of MARCE (1.05; 95% CI, 1.02-1.08), mortality (aHR, 1.39; 95% CI, 1.31-1.48), and hospitalization (aOR, 1.46; 95% CI, 1.41-1.51).
4. Discussion
In this study, we show for the first time that while sex-specific HRT is protective against an array of adverse outcomes for older males and females with COVID-19, the benefit of HRT in older immunosuppressed transplant recipients is effectively abolished. However, like their non-ISC counterparts, male (but not female) SOTRs on HRT experienced reduced COVID-related mortality and less AKI than those without hormone supplementation.
It is well established that in the general, non-ISC population, male sex is a significant risk factor for worse COVID-19 outcomes.1 , 2 Immunocompetent females have been shown to demonstrate a more robust antiviral immune response than males,3 including to SARS-CoV-2. Potential mechanisms include the higher burden of immune-related gene expression in female than male tissues on account of incomplete X inactivation, and estrogens are noted to be immune stimulating (and androgens immune suppressing), which results in a heightened immune response in females versus males that varies over the lifespan with changing sex hormone levels.3 , 4 , 29, 30, 31
However, the male mortality bias observed in the general population with COVID-19 is attenuated in immunosuppressed SOTRs.16 , 17 , 32 Maintenance immunosuppression may negatively impact females by suppressing their anti-SARS-CoV-2 viral response relative to males, and may benefit males by mitigating the cytokine storm that disproportionately impacts older males.17 Our earlier study of sex-based differences in the COVID-19 risk profile in the general population compared with those with SOT hinted at the changing role of sex hormones over the lifespan and how these differentially impacted the non-ISC and SOT populations.17 In the non-ISC population, the increased risk in male versus female patients with COVID-19 was most pronounced in the youngest (reproductive), then mid-age (perimenopause/andropause), then oldest age strata (postmenopause/andropause). Conversely, in SOTRs, age strata did not modify sex-based risk.
The role of exogenous sex hormones supplementation as a means of restoring sex hormone-mediated immune regulation in older immunocompetent males and females with COVID-19 has been previously explored. A Swedish study examining exogenous estrogen supplementation in postmenopausal women aged 50 to 80 years demonstrated a 53% reduction in the adjusted odds of mortality post-COVID-19 with HRT.8 Similarly, a propensity-matched study of immunocompetent women with COVID-19 >50 years showed a 67% reduction in mortality risk with exogenous estradiol supplementation.33 Although controversial, some studies have suggested the 100-fold increase in 17-estradiol levels in pregnancy may also be associated with reduced mortality.34 Multiple estradiol-mediated mechanisms have been proposed including an enhancement of the antiviral immune response through increased immune tolerance (expansion of regulatory T cells) and antibody production (stimulation of B cells),9 stabilization of the immune system and reduction in dysregulated innate immunity through interleukin 6, tumor necrosis factor α, and interleukin 1b inhibition,9 increased expression of the Mas-receptor with resultant protection against acute lung injury,10 and a corresponding downregulation of angiotensin-converting enzyme 2 receptor expression with corresponding diminished SARS-CoV-2 cell entry.11, 12, 13, 14 Progesterone has also been shown to regulate systemic inflammation in premenopausal women, and in a small randomized pilot trial of 42 hospitalized men hypoxemic with COVID-19, progesterone therapy + standard of care resulted in expeditious weaning of supplemental oxygen versus placebo.35
Conversely, androgens have been shown to have immune modulatory properties that suppress inflammatory cytokines and promote the synthesis of suppressive cytokines.36 High endogenous testosterone levels have been associated with poor humoral responses to influenza vaccination,37 and although controversial, low testosterone levels have been associated with greater COVID-19 severity.38, 39, 40, 41 Furthermore, testosterone deficiency has been associated with endothelial dysfunction, arterial stiffness and thrombocyte malfunction resulting in an increased risk of COVID-19 triggered thrombovascular events in hypogonadal males.42 , 43 However, other studies suggest a contradictory pathologic effect of androgens in patients with COVID-19. Testosterone has been proposed to upregulate the transmembrane serine protease 2 via the androgen receptor, potentially facilitating viral entry into cells via angiotensin-converting enzyme 2, with studies also showing increased COVID complications in men with high free testosterone levels,44 a higher incidence of androgenetic alopecia in patients admitted to ICU with COVID-19,45 , 46 and a 4-fold higher odds of SARS-CoV-2 infection in patients with prostate cancer not receiving androgen-deprivation therapy versus those on androgen-deprivation therapy.47 Thus, the effect of androgens on SARS-CoV-2 infection risk and post-COVID outcomes is unclear; there is a potential increased risk of infection via transmembrane serine protease 2 upregulation offset by a potential reduction in immune dysregulation and fatal cytokine storm. The effect of exogenous testosterone replacement therapy on outcomes after COVID-19 is unknown. One small study of 32 men on testosterone supplementation PS-matched to 63 men not on HRT showed no difference in complications after COVID-19.48
Our study is the largest by several orders of magnitude to examine sex-specific HRT in the general population with COVID-19, with nearly 8000 males on testosterone and over 35 000 females on sex hormone supplementation. It is also the first to examine sex-specific HRT in immunosuppressed SOTRs. Like earlier studies, we show that HRT use is associated with benefit in immunocompetent females with COVID-19. In our primary analysis, we defined HRT use in females as including estrogen and/or progesterone, but the results were nearly identical in a sensitivity analysis excluding women on progesterone monotherapy, suggesting estrogen was likely driving this effect. In addition, we show quite decisively that testosterone supplementation also associates with significant benefit in immunocompetent males with COVID-19. The benefit of HRT in transplanted females was abrogated, although in male SOTRs, HRT remained associated with a lower adjusted risk of AKI and death. Although there are many potential and as yet unknown explanations for this, this finding supports the hypothesis that the benefit of HRT in both males and females is via an immune-mediated pathway that is inhibited in immunosuppressed transplant recipients. This is an important mechanistic discovery that requires further investigation.
Although this study has a number of strengths including its size, its novelty, and our familiarity with the robust N3C Enclave data repository, there are also limitations. As with any retrospective analysis, this study was also at risk for miscoding and misclassification bias. We did not have information regarding the true SARS-CoV-2 variant of concern, and instead adjusted for time periods where a given variant demonstrated historical dominance. Another potential limitation is that in this study all systemic estrogen supplementation was examined together. Oral estrogen administration undergoes first-pass liver metabolism that has been shown to increase markers of inflammation and C-reactive protein.49 Earlier studies have suggested that perhaps transdermal estrogen supplementation has the greatest anti-inflammatory influence.49 Unfortunately, the cross-sectional nature of the N3C precludes detailed granularity around medication route or dosing and longitudinal use. It has been suggested that cardiovascular and stroke risk may be increased in women during the first year after HRT discontinuation50; if patients noted to be on HRT 24 months before their COVID diagnosis subsequently discontinued therapy, they may paradoxically be at an increased risk, despite being misclassified as “HRT exposed.” However, HRT discontinuation would not be expected to differ substantially between the SOT and non-ISC cohorts, and our results were reproduced in a cohort restricted to HRT use in the last 6 months. Similarly, we do not have detailed information regarding changes in immunosuppression, or differential therapies used in managing outpatient or hospitalized COVID patients. We acknowledge these are important limitations and areas for future study. Another limitation is that based on data availability, our observation window begins at the time of a positive COVID test. Thus, the current study cannot comment on the potential risk of acquiring SARS-CoV-2 infection in those with and without HRT exposure. Further, although this represents the largest study of its kind to date, the number of SOTRs on HRT with a diagnosis of COVID during the study period was relatively small (n=322 for males, n=813 for females). This resulted in greater instability around the true point estimate for the association of HRT versus no HRT for each outcome in males and females (particularly so in the smaller subgroup analyses). Finally, while we restricted our population to adults ≥45 years (peri- or postmenopausal/andropausal), we did not have access to measured sex hormones, and it is possible that patients not taking exogenous HRT still had adequate endogenous testosterone or estrogen reserve. This would attenuate the relative benefit observed with HRT but would not be anticipated to differentially impact the non-ISC and SOT populations. Therefore, the differential benefit of HRT in the general and SOT populations we demonstrate is unlikely to relate to differences in endogenous sex hormone levels. Importantly, our results persisted in a smaller sensitivity analysis restricted to adults >55 years of age and therefore more likely to be truly post-andropause or menopause. Finally, although we adjusted for potential confounders and confirmed our main results in a PS-matched cohort creating more balance between the SOT and non-ISC case-mix, as with all observational studies, there remains the potential for unmeasured confounding, and results should be interpreted with caution.
Expanding our understanding of the sex-based risk in COVID-19, including the potentially modifying effects of both male and female sex hormones on SARS-CoV-2 pathology, may result in novel strategies for treatment of patients with COVID-19. Whether these interventions benefit immunosuppressed transplant patients in addition to the general population will require targeted and prospective study in SOTRs. Furthermore, whether acute supplementation with estrogen or androgen therapies at the time of COVID-19 diagnosis modifies COVID-19 risk remains to be seen. Clinical trials are currently ongoing to examine this question in immunocompetent, but not immunosuppressed, populations.51
5. Conclusions
In conclusion, sex-specific HRT was associated with fewer adverse COVID-19 outcomes in older non-ISC males and females (exogenous testosterone supplementation in males; exogenous estrogen and/or progesterone supplementation in females). The benefit of HRT in SOTRs with COVID was limited to reduced AKI and mortality risk in SOT males in adjusted analyses. This is the largest study to examine sex-specific HRT in males and females with COVID-19 and the first to examine HRT use in SOTRs. We show for the first time that the beneficial effect of HRT in males and females with COVID-19 in the general population is not preserved in immunosuppressed transplant recipients. This provides further support to the hypothesis that the mechanism of benefit from HRT in both male and female patients with SARS-CoV-2 may be immune mediated. This, however, requires further investigation.
Funding
The project described was supported by the National Institute of General Medical Sciences, U54GM104942-05S2 and U54GM115458. R.B.M. is supported in part by the Dr Dennis Ross fund for Nephrology Research, the Nebraska Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the N3C program. The funders had no role in the design or conduct of the study. NCATS and N3C had a role in the review and approval of all results reported in the manuscript for public review.
Author contributions
Concept and design: A.J.V., R.B.M., A.J.A.
Acquisition, analysis, or interpretation of data: A.J.A., M.S., R.D.
Drafting of the manuscript: A.J.V.
Critical revision of the manuscript for important intellectual content: All authors
Supervision: R.B.M.
All authors approved the final manuscript.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by theAmerican Journal of Transplantation. A. Vinson has done consultancy work and received funding for a fellowship project grant through Paladin Labs Inc. R. Mannon reports grant funding from VericiDX, honoraria from Olaris Inc and Chinook Therapeutics and personal fees from Vitaerris as member of the IMAGINE Trial Steering committee, and personal fees from American Journal of Transplantation as Deputy Editor of the journal, outside the submitted work.
Review board approvals and consent to participate
National Institute of Health’s (NIH) National COVID Cohort Collaborative (N3C) Data Utilization Request Approval committee approved the data utilization request of this project (RP-CA3365), which is approved under the authority of the National Institutes of Health Institutional Review Board and with Johns Hopkins University School of Medicine serving as a central institutional review board. The study protocol was reviewed by the University of Nebraska Medical Center (0853-21-EP) IRB. The N3C data transfer to NCATS is performed under a Johns Hopkins University Reliance Protocol # IRB00249128 or individual site agreements with NIH. The N3C Data Enclave is managed under the authority of the NIH; information can be found at https://ncats.nih.gov/n3c/resources. No informed consent was obtained because the study used a limited data set.
Consortia authorship
CG Chute.
Data availability
The N3C Enclave is available for public research use. To access data, institutions must have a signed Data Use Agreement executed with the US National Center for Advancing Translational Sciences (NCATS), and investigators must complete mandatory training and submit a Data Use Request (DUR) to N3C. To request N3C data access, follow the instructions at https://covid.cd2h.org/onboarding. This project utilized data from N3C release 100, which can be replicated within the N3C Enclave by qualified N3C users. All concepts and definitions are provided in Supplementary Methods. All code used for analyses can be made available upon request. More than 4000 researchers currently have access to data in N3C, representing more than 300 US research institutions.
Acknowledgments
The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave covid.cd2h.org/enclave and supported by CD2H - The National COVID Cohort Collaborative (N3C) IDeA CTR Collaboration 3U24TR002306-04S2 NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data from participating organizations (covid.cd2h.org/dtas) and the organizations and scientists (covid.cd2h.org/duas) who have contributed to the on-going development of this community resource (cite this https://doi.org/10.1093/jamia/ocaa196).
The authors gratefully acknowledge the following core contributors to N3C:
Adam B. Wilcox, Adam M. Lee, Alexis Graves, Alfred (Jerrod) Anzalone, Amin Manna, Amit Saha, Amy Olex, Andrea Zhou, Andrew E. Williams, Andrew Southerland, Andrew T. Girvin, Anita Walden, Anjali A. Sharathkumar, Benjamin Amor, Benjamin Bates, Brian Hendricks, Brijesh Patel, Caleb Alexander, Carolyn Bramante, Cavin Ward-Caviness, Charisse Madlock-Brown, Christine Suver, Christopher Chute, Christopher Dillon, Chunlei Wu, Clare Schmitt, Cliff Takemoto, Dan Housman, Davera Gabriel, David A. Eichmann, Diego Mazzotti, Don Brown, Eilis Boudreau, Elaine Hill, Elizabeth Zampino, Emily Carlson Marti, Emily R. Pfaff, Evan French, Farrukh M Koraishy, Federico Mariona, Fred Prior, George Sokos, Greg Martin, Harold Lehmann, Heidi Spratt, Hemalkumar Mehta, Hongfang Liu, Hythem Sidky, J.W. Awori Hayanga, Jami Pincavitch, Jaylyn Clark, Jeremy Richard Harper, Jessica Islam, Jin Ge, Joel Gagnier, Joel H. Saltz, Joel Saltz, Johanna Loomba, John Buse, Jomol Mathew, Joni L. Rutter, Julie A. McMurry, Justin Guinney, Justin Starren, Karen Crowley, Katie Rebecca Bradwell, Kellie M. Walters, Ken Wilkins, Kenneth R. Gersing, Kenrick Dwain Cato, Kimberly Murray, Kristin Kostka, Lavance Northington, Lee Allan Pyles, Leonie Misquitta, Lesley Cottrell, Lili Portilla, Mariam Deacy, Mark M. Bissell, Marshall Clark, Mary Emmett, Mary Morrison Saltz, Matvey B. Palchuk, Melissa A. Haendel, Meredith Adams, Meredith Temple-O'Connor, Michael G. Kurilla, Michele Morris, Nabeel Qureshi, Nasia Safdar, Nicole Garbarini, Noha Sharafeldin, Ofer Sadan, Patricia A. Francis, Penny Wung Burgoon, Peter Robinson, Philip R.O. Payne, Rafael Fuentes, Randeep Jawa, Rebecca Erwin-Cohen, Rena Patel, Richard A. Moffitt, Richard L. Zhu, Rishi Kamaleswaran, Robert Hurley, Robert T. Miller, Saiju Pyarajan, Sam G. Michael, Samuel Bozzette, Sandeep Mallipattu, Satyanarayana Vedula, Scott Chapman, Shawn T. O'Neil, Soko Setoguchi, Stephanie S. Hong, Steve Johnson, Tellen D. Bennett, Tiffany Callahan, Umit Topaloglu, Usman Sheikh, Valery Gordon, Vignesh Subbian, Warren A. Kibbe, Wenndy Hernandez, Will Beasley, Will Cooper, William Hillegass, Xiaohan Tanner Zhang. Details of contributions available at covid.cd2h.org/core-contributors
The following institutions whose data is released or pending:
Available: Advocate Health Care Network — UL1TR002389: The Institute for Translational Medicine (ITM) • Boston University Medical Campus — UL1TR001430: Boston University Clinical and Translational Science Institute • Brown University — U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Carilion Clinic — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Charleston Area Medical Center — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • Children’s Hospital Colorado — UL1TR002535: Colorado Clinical and Translational Sciences Institute • Columbia University Irving Medical Center — UL1TR001873: Irving Institute for Clinical and Translational Research • Duke University — UL1TR002553: Duke Clinical and Translational Science Institute • George Washington Children’s Research Institute — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • George Washington University — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • Indiana University School of Medicine — UL1TR002529: Indiana Clinical and Translational Science Institute • Johns Hopkins University — UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • Loyola Medicine — Loyola University Medical Center • Loyola University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Maine Medical Center — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • Mayo Clinic Rochester — UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Medical University of South Carolina — UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • Montefiore Medical Center — UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Nemours — U54GM104941: Delaware CTR ACCEL Program • NorthShore University HealthSystem — UL1TR002389: The Institute for Translational Medicine (ITM) • Northwestern University at Chicago — UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • OCHIN — INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks • Oregon Health & Science University — UL1TR002369: Oregon Clinical and Translational Research Institute • Penn State Health Milton S. Hershey Medical Center — UL1TR002014: Penn State Clinical and Translational Science Institute • Rush University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Rutgers, The State University of New Jersey — UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Stony Brook University — U24TR002306 • The Ohio State University — UL1TR002733: Center for Clinical and Translational Science • The State University of New York at Buffalo — UL1TR001412: Clinical and Translational Science Institute • The University of Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Iowa — UL1TR002537: Institute for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine — UL1TR002736: University of Miami Clinical and Translational Science Institute • The University of Michigan at Ann Arbor — UL1TR002240: Michigan Institute for Clinical and Health Research • The University of Texas Health Science Center at Houston — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • The University of Texas Medical Branch at Galveston — UL1TR001439: The Institute for Translational Sciences • The University of Utah — UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center — UL1TR002544: Tufts Clinical and Translational Science Institute • Tulane University — UL1TR003096: Center for Clinical and Translational Science • University Medical Center New Orleans — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Alabama at Birmingham — UL1TR003096: Center for Clinical and Translational Science • University of Arkansas for Medical Sciences — UL1TR003107: UAMS Translational Research Institute • University of Cincinnati — UL1TR001425: Center for Clinical and Translational Science and Training • University of Colorado Denver, Anschutz Medical Campus — UL1TR002535: Colorado Clinical and Translational Sciences Institute • University of Illinois at Chicago — UL1TR002003: UIC Center for Clinical and Translational Science • University of Kansas Medical Center — UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • University of Kentucky — UL1TR001998: UK Center for Clinical and Translational Science • University of Massachusetts Medical School Worcester — UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Minnesota — UL1TR002494: Clinical and Translational Science Institute • University of Mississippi Medical Center — U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center — U54GM115458: Great Plains IDeA-Clinical & Translational Research • University of North Carolina at Chapel Hill — UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Oklahoma Health Sciences Center — U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • University of Rochester — UL1TR002001: UR Clinical & Translational Science Institute • University of Southern California — UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • University of Vermont — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • University of Virginia — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Washington — UL1TR002319: Institute of Translational Health Sciences • University of Wisconsin-Madison — UL1TR002373: UW Institute for Clinical and Translational Research • Vanderbilt University Medical Center — UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • Virginia Commonwealth University — UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • Wake Forest University Health Sciences — UL1TR001420: Wake Forest Clinical and Translational Science Institute • Washington University in St. Louis — UL1TR002345: Institute of Clinical and Translational Sciences • Weill Medical College of Cornell University — UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • West Virginia University — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI)
Submitted: Icahn School of Medicine at Mount Sinai — UL1TR001433: ConduITS Institute for Translational Sciences • The University of Texas Health Science Center at Tyler — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • University of California, Davis — UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, Irvine — UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, Los Angeles — UL1TR001881: UCLA Clinical Translational Science Institute • University of California, San Diego — UL1TR001442: Altman Clinical and Translational Research Institute • University of California, San Francisco — UL1TR001872: UCSF Clinical and Translational Science Institute
Pending: Arkansas Children’s Hospital — UL1TR003107: UAMS Translational Research Institute • Baylor College of Medicine — None (Voluntary) • Children’s Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • Cincinnati Children’s Hospital Medical Center — UL1TR001425: Center for Clinical and Translational Science and Training • Emory University — UL1TR002378: Georgia Clinical and Translational Science Alliance • HonorHealth — None (Voluntary) • Loyola University Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • Medical College of Wisconsin — UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • MedStar Health Research Institute — UL1TR001409: The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) • MetroHealth — None (Voluntary) • Montana State University — U54GM115371: American Indian/Alaska Native CTR • NYU Langone Medical Center — UL1TR001445: Langone Health’s Clinical and Translational Science Institute • Ochsner Medical Center — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • Regenstrief Institute — UL1TR002529: Indiana Clinical and Translational Science Institute • Sanford Research — None (Voluntary) • Stanford University — UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • The Rockefeller University — UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute — UL1TR002550: Scripps Research Translational Institute • University of Florida — UL1TR001427: UF Clinical and Translational Science Institute • University of New Mexico Health Sciences Center — UL1TR001449: University of New Mexico Clinical and Translational Science Center • University of Texas Health Science Center at San Antonio — UL1TR002645: Institute for Integration of Medicine and Science • Yale New Haven Hospital — UL1TR001863: Yale Center for Clinical Investigation.
Footnotes
Details of contributions available at covid.cd2h.org/core-contributors
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajt.2023.04.020.
Contributor Information
the National COVID Cohort Collaborative:
Adam B. Wilcox, Adam M. Lee, Alexis Graves, Alfred (Jerrod) Anzalone, Amin Manna, Amit Saha, Amy Olex, Andrea Zhou, Andrew E. Williams, Andrew Southerland, Andrew T. Girvin, Anita Walden, Anjali A. Sharathkumar, Benjamin Amor, Benjamin Bates, Brian Hendricks, Brijesh Patel, Caleb Alexander, Carolyn Bramante, Cavin Ward-Caviness, Charisse Madlock-Brown, Christine Suver, Christopher Chute, Christopher Dillon, Chunlei Wu, Clare Schmitt, Cliff Takemoto, Dan Housman, Davera Gabriel, David A. Eichmann, Diego Mazzotti, Don Brown, Eilis Boudreau, Elaine Hill, Elizabeth Zampino, Emily Carlson Marti, Emily R. Pfaff, Evan French, Farrukh M. Koraishy, Federico Mariona, Fred Prior, George Sokos, Greg Martin, Harold Lehmann, Heidi Spratt, Hemalkumar Mehta, Hongfang Liu, Hythem Sidky, J.W. Awori Hayanga, Jami Pincavitch, Jaylyn Clark, Jeremy Richard Harper, Jessica Islam, Jin Ge, Joel Gagnier, Joel H. Saltz, Joel Saltz, Johanna Loomba, John Buse, Jomol Mathew, Joni L. Rutter, Julie A. McMurry, Justin Guinney, Justin Starren, Karen Crowley, Katie Rebecca Bradwell, Kellie M. Walters, Ken Wilkins, Kenneth R. Gersing, Kenrick Dwain Cato, Kimberly Murray, Kristin Kostka, Lavance Northington, Lee Allan Pyles, Leonie Misquitta, Lesley Cottrell, Lili Portilla, Mariam Deacy, Mark M. Bissell, Marshall Clark, Mary Emmett, Mary Morrison Saltz, Matvey B. Palchuk, Melissa A. Haendel, Meredith Adams, Meredith Temple-O'Connor, Michael G. Kurilla, Michele Morris, Nabeel Qureshi, Nasia Safdar, Nicole Garbarini, Noha Sharafeldin, Ofer Sadan, Patricia A. Francis, Penny Wung Burgoon, Peter Robinson, Philip R.O. Payne, Rafael Fuentes, Randeep Jawa, Rebecca Erwin-Cohen, Rena Patel, Richard A. Moffitt, Richard L. Zhu, Rishi Kamaleswaran, Robert Hurley, Robert T. Miller, Saiju Pyarajan, Sam G. Michael, Samuel Bozzette, Sandeep Mallipattu, Satyanarayana Vedula, Scott Chapman, Shawn T. O'Neil, Soko Setoguchi, Stephanie S. Hong, Steve Johnson, Tellen D. Bennett, Tiffany Callahan, Umit Topaloglu, Usman Sheikh, Valery Gordon, Vignesh Subbian, Warren A. Kibbe, Wenndy Hernandez, Will Beasley, Will Cooper, William Hillegass, and Xiaohan Tanner Zhang
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Márquez E.J., Trowbridge J., Kuchel G.A., Banchereau J., Ucar D. The lethal sex gap: COVID-19. Immun Ageing. 2020;17:13. doi: 10.1186/s12979-020-00183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peckham H., de Gruijter N.M., Raine C., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 4.Giefing-Kröll C., Berger P., Lepperdinger G., Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelo-Branco C., Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014;30(1):16–22. doi: 10.3109/09513590.2013.852531. [DOI] [PubMed] [Google Scholar]
- 6.Franceschi C., Bonafè M., Valensin S., et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 7.Li G., Fan Y., Lai Y., et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sund M., Fonseca-Rodríguez O., Josefsson A., Welen K., Fors Connolly A.M. Association between pharmaceutical modulation of oestrogen in postmenopausal women in Sweden and death due to COVID-19: a cohort study. BMJ Open. 2022;12(2) doi: 10.1136/bmjopen-2021-053032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauvais-Jarvis F., Klein S.L., Levin E.R. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020;161(9) doi: 10.1210/endocr/bqaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erfinanda L., Ravindran K., Kohse F., et al. Oestrogen-mediated upregulation of the Mas receptor contributes to sex differences in acute lung injury and lung vascular barrier regulation. Eur Respir J. 2021;57(1) doi: 10.1183/13993003.00921-2020. [DOI] [PubMed] [Google Scholar]
- 11.Kalidhindi R.S.R., Borkar N.A., Ambhore N.S., Pabelick C.M., Prakash Y.S., Sathish V. Sex steroids skew ACE2 expression in human airway: a contributing factor to sex differences in COVID-19? Am J Physiol Lung Cell Mol Physiol. 2020;319(5):L843–L847. doi: 10.1152/ajplung.00391.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemes R.M.R., Costa A.J., Bartolomeo C.S., et al. 17beta-estradiol reduces SARS-CoV-2 infection in vitro. Physiol Rep. 2021;9(2) doi: 10.14814/phy2.14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stelzig K.E., Canepa-Escaro F., Schiliro M., Berdnikovs S., Prakash Y.S., Chiarella S.E. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2020;318(6):L1280–L1281. doi: 10.1152/ajplung.00153.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young M.J., Clyne C.D., Chapman K.E. Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2. J Endocrinol. 2020;247(2):R45–R62. doi: 10.1530/JOE-20-0260. [DOI] [PubMed] [Google Scholar]
- 15.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinson A.J., Chong A.S., Clegg D., et al. Sex matters: COVID-19 in kidney transplantation. Kidney Int. 2021;99(3):555–558. doi: 10.1016/j.kint.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinson A.J., Dai R., Agarwal G., et al. Sex and organ-specific risk of major adverse renal or cardiac events in solid organ transplant recipients with COVID-19. Am J Transplant. 2022;22(1):245–259. doi: 10.1111/ajt.16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinson A.J., Agarwal G., Dai R., et al. COVID-19 in solid organ transplantation: results of the national COVID cohort collaborative. Transplant Direct. 2021;7(11):e775. doi: 10.1097/TXD.0000000000001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J., Zheng Q., Madhira V., et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182(2):153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinson A.J., Anzalone A.J., Sun J., et al. The risk and consequences of breakthrough SARS-CoV-2 infection in solid organ transplant recipients relative to non-immunosuppressed controls. Am J Transplant. 2022;22(10):2418–2432. doi: 10.1111/ajt.17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GitHub . 2021. National-COVID-Cohort-Collaborative/Phenotype_Data_Acquisition.https://github.com/National-COVID-Cohort-Collaborative/Phenotype_Data_Acquisition Published. [Google Scholar]
- 22.Haendel M.A., Chute C.G., Bennett T.D., et al. The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021;28(3):427–443. doi: 10.1093/jamia/ocaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett T.D., Moffitt R.A., Hajagos J.G., et al. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US national COVID cohort collaborative. JAMA Netw Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benchimol E.I., Smeeth L., Guttmann A., et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLOS Med. 2015;12(10) doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Github . 2022. COVID Hormone Replacement Therapy.https://github.com/National-COVID-Cohort-Collaborative/CS-ISC/tree/main/HRT Published 2022. [Google Scholar]
- 26.Informatics OHDSa . 2021. The Book of OHDSI.https://ohdsi.github.io/TheBookOfOhdsi/ Published. [Google Scholar]
- 27.Browne S.K., Beeler J.A., Roberts J.N. Summary of the Vaccines and Related Biological Products Advisory Committee meeting held to consider evaluation of vaccine candidates for the prevention of respiratory syncytial virus disease in RSV-naïve infants. Vaccine. 2020;38(2):101–106. doi: 10.1016/j.vaccine.2019.10.048. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) 2022. COVID Data Tracker.https://covid.cdc.gov/covid-data-tracker/ Published. [Accessed 3 January 2023] [Google Scholar]
- 29.Lepeytre F., Dahhou M., Zhang X., et al. Association of sex with risk of kidney graft failure differs by age. J Am Soc Nephrol. 2017;28(10):3014–3023. doi: 10.1681/ASN.2016121380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinson A.J., Zhang X., Dahhou M., et al. Age-dependent sex differences in graft loss after kidney transplantation. Transplantation. 2022;106(7):1473–1484. doi: 10.1097/TP.0000000000004026. [DOI] [PubMed] [Google Scholar]
- 31.Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev. 2012;11(6-7):A479–A485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Vart P., Duivenvoorden R., Adema A., et al. Sex differences in COVID-19 mortality risk in patients on kidney function replacement therapy. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-22657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seeland U., Coluzzi F., Simmaco M., et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 2020;18(1):369. doi: 10.1186/s12916-020-01851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zafari Zangeneh F., Sarmast Shoushtari M. Estradiol and COVID-19: does 17-estradiol have an immune-protective function in women against coronavirus? J Family Reprod Health. 2021;15(3):150–159. doi: 10.18502/jfrh.v15i3.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghandehari S., Matusov Y., Pepkowitz S., et al. Progesterone in addition to standard of care vs standard of care alone in the treatment of men hospitalized with moderate to severe COVID-19: a randomized, controlled pilot trial. Chest. 2021;160(1):74–84. doi: 10.1016/j.chest.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cattrini C., Bersanelli M., Latocca M.M., Conte B., Vallome G., Boccardo F. Sex hormones and hormone therapy during COVID-19 pandemic: implications for patients with cancer. Cancers (Basel) 2020;12(8):2325. doi: 10.3390/cancers12082325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furman D., Hejblum B.P., Simon N., et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111(2):869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rastrelli G., Di Stasi V., Inglese F., et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9(1):88–98. doi: 10.1111/andr.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroeder M., Schaumburg B., Mueller Z., et al. High estradiol and low testosterone levels are associated with critical illness in male but not in female COVID-19 patients: a retrospective cohort study. Emerg Microbes Infect. 2021;10(1):1807–1818. doi: 10.1080/22221751.2021.1969869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salonia A., Pontillo M., Capogrosso P., et al. Severely low testosterone in males with COVID-19: A case-control study. Andrology. 2021;9(4):1043–1052. doi: 10.1111/andr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Zeggeren I.E., Boelen A., van de Beek D., et al. Sex steroid hormones are associated with mortality in COVID-19 patients: level of sex hormones in severe COVID-19. Medicine (Baltimore) 2021;100(34) doi: 10.1097/MD.0000000000027072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreau K.L., Babcock M.C., Hildreth K.L. Sex differences in vascular aging in response to testosterone. Biol Sex Differ. 2020;11(1):18. doi: 10.1186/s13293-020-00294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mondal R., Lahiri D., Deb S., et al. COVID-19: are we dealing with a multisystem vasculopathy in disguise of a viral infection? J Thromb Thrombolysis. 2020;50(3):567–579. doi: 10.1007/s11239-020-02210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuel R.M., Majd H., Richter M.N., et al. Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell. 2020;27(6):876–889.e12. doi: 10.1016/j.stem.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wambier C.G., Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J Am Acad Dermatol. 2020;83(1):308–309. doi: 10.1016/j.jaad.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goren A., Vaño-Galván S., Wambier C.G., et al. A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain – a potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol. 2020;19(7):1545–1547. doi: 10.1111/jocd.13443. [DOI] [PubMed] [Google Scholar]
- 47.Montopoli M., Zumerle S., Vettor R., et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rambhatla A., Bronkema C.J., Corsi N., et al. COVID-19 infection in men on testosterone replacement therapy. J Sex Med. 2021;18(1):215–218. doi: 10.1016/j.jsxm.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mauvais-Jarvis F., Manson J.E., Stevenson J.C., Fonseca V.A. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev. 2017;38(3):173–188. doi: 10.1210/er.2016-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mikkola T.S., Tuomikoski P., Lyytinen H., et al. Increased cardiovascular mortality risk in women discontinuing postmenopausal hormone therapy. J Clin Endocrinol Metab. 2015;100(12):4588–4594. doi: 10.1210/jc.2015-1864. [DOI] [PubMed] [Google Scholar]
- 51.Okpechi S.C., Fong J.T., Gill S.S., et al. Global sex disparity of COVID-19: a descriptive review of sex hormones and consideration for the potential therapeutic use of hormone replacement therapy in older adults. Aging Dis. 2021;12(2):671–683. doi: 10.14336/AD.2020.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The N3C Enclave is available for public research use. To access data, institutions must have a signed Data Use Agreement executed with the US National Center for Advancing Translational Sciences (NCATS), and investigators must complete mandatory training and submit a Data Use Request (DUR) to N3C. To request N3C data access, follow the instructions at https://covid.cd2h.org/onboarding. This project utilized data from N3C release 100, which can be replicated within the N3C Enclave by qualified N3C users. All concepts and definitions are provided in Supplementary Methods. All code used for analyses can be made available upon request. More than 4000 researchers currently have access to data in N3C, representing more than 300 US research institutions.