Abstract
Objectives
Although smoking is a well-recognized causative factor of urothelial bladder cancer and accounts for 50% of cases, less is known about the prognostic significance of smoking on non-muscle invasive bladder cancer (NMIBC) prognosis. This systematic review and meta-analysis aimed to evaluate the effect of smoking on the risk of NMIBC recurrence and progression.
Materials and methods
We systematically searched Medline, Web of Science and Scopus databases for original articles published before October 2021 regarding the effect of smoking on NMIBC recurrence and progression. Information about smoking status and the number of events or odds ratio or hazard ratio for event-free survival must have been reported to include the study in the analysis. Quality In Prognosis Studies tool was utilized for the risk of bias assessment.
Results
We selected 64 eligible studies, including 28 617 patients with NMIBC with available data on smoking status. In a meta-analysis of 28 studies with 7885 patients, we found that smokers (current/former) were at higher risk for recurrence (OR = 1.68; 95% CI 1.34–2.09; P < 0.0001) compared to never smokers. Subgroup analysis of 2967 patients revealed that current smokers were at a 1.24 higher risk of recurrence (OR = 1.24; 95% CI 1.02–1.50; P = 0.03) compared to former smokers. A meta-analysis of the hazard ratio revealed that smokers are at higher risk of recurrence (HR = 1.31; 95%CI 1.15–1.48; P < 0.0001) and progression (HR = 1.18; 95%CI 1.08–1.29; P < 0.001) compared to never smokers. Detrimental prognostic effect of smoking on progression, but not for recurrence risk was also noted in the subgroup analysis of high-risk patients (HR = 1.30; 95%CI 1.09–1.55; P = 0.004) and BCG-treated ones (HR = 1.15; 95%CI 1.06–1.25; P < 0.001).
Conclusion
In conclusion, patients with non-muscle invasive bladder cancer and a history of smoking have a worse prognosis regarding recurrence-free and progression-free survival compared to non-smokers.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-022-04464-6.
Keywords: Non-muscle-invasive bladder cancer, Smoking, Current smoker, Recurrence, Progression
Introduction
Urothelial bladder cancer (UBC) is ranked as the seventh most common malignancy worldwide in men and seventeenth in women. Smoking is a well-evidenced, strong causative factor responsible for the development of approximately 50% of bladder tumours (Burger et al. 2013). Cumulative exposure to tobacco smoke is associated with a strong risk of cancer development in the urinary tract (Zeegers et al. 2000; Brennan et al. 2000). Smoking cessation could mitigate the risk of bladder cancer development (Zeegers et al. 2000). On the other hand, one of the prospective surveys showed that only 36% of patients who presented to a urology clinic were aware of bladder cancer risk caused by smoking compared to 98% aware of the link between smoking and lung cancer (Nieder et al. 2006). Despite the detrimental effect of smoke on health including cardiovascular and pulmonary functions and the risk of other cancer development, approximately 30% of patients remain active smokers at UBC diagnosis (Grotenhuis et al. 2014). Strikingly a prospective observational study showed that only 34.5% of smokers have quit smoking after the diagnosis (Serretta et al. 2020).
Available meta-analysis of 17 studies on 13 777 patients indicates that response to neoadjuvant chemotherapy (NAC) and outcomes of radical cystectomy (RC) for muscle-invasive bladder cancer (MIBC) are compromised in smokers (Cacciamani et al. 2020 Oct). To date studies assessing the impact of smoke on non-muscle invasive bladder cancer (NMIBC) prognosis are not convincing, with several retrospective analyses showing conflicting results (Grotenhuis et al. 2014; Ogihara et al. 2016; D’Andrea et al. 2017; Ślusarczyk et al. 2019). Due to the following reasons, smoking status has not been included in the most widely used risk tables for NMIBC recurrence and progression (Fernandez-Gomez et al. 2009; Sylvester et al. 2006). Simultaneously, limited evidence impairs consecutive smoking cessation counselling in UBC patients and tailoring the treatment protocol in smokers. One meta-analysis including 11 studies on the cohort of 7210 patients showed a worse recurrence-free survival (HR = 1.27; 95% CI 1.09–1.46) in current compared to never smokers diagnosed with NMIBC (Osch et al. 2016). Due to the limited number of patients included in the risk assessment of PFS according to the smoking status, the meta-analysis failed to show a statistically significant association(Osch et al. 2016).
The aim of this systematic review and meta-analysis was to evaluate the effect of smoking on the risk of NMIBC recurrence and progression.
Materials and methods
Study selection
A systemic literature review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (PRISMA guideline, for details please see the Supplementary file) to identify studies published before October 2021. We searched Medline (Pubmed), Scopus and Web of Science databases using the following terms: “((progression OR progression-free survival OR muscle-invasive) AND (non-muscle invasive bladder cancer OR NMIBC) AND (Risk factors OR Smoking OR smoke OR cigarette OR tobacco)) OR ((progression OR recurrence-free survival OR muscle-invasive OR recurrence) AND (non-muscle invasive bladder cancer OR NMIBC) AND (Smoking OR smoke OR cigarette OR tobacco))” and “(recurrence OR progression OR survival) AND (non-muscle invasive bladder cancer OR NMIBC) AND (Smoking OR smoke OR cigarette OR tobacco)”, respectively.
Relevant citations of identified papers were manually searched to retrieve any further studies not found using algorithmic queries. Studies were included only if the information regarding smoking status (current, former, never smoker OR smoker vs non-smoker) and NMIBC recurrence and/or progression risk were available(for details see the selection flowchart Fig. 1).
Fig. 1.
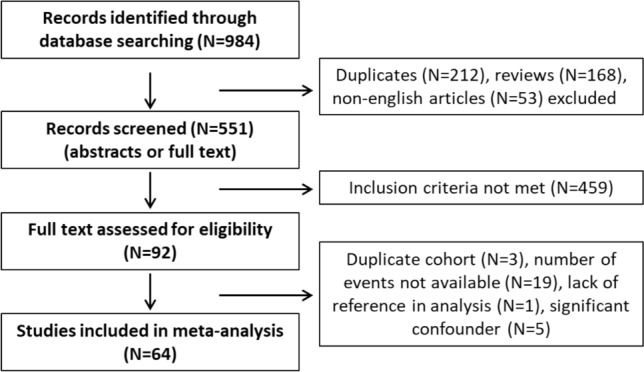
Flowchart for study selection process using Medline, Web of Science and Scopus databases to identify the original articles demonstrating the effect of smoking on prognosis in non-muscle-invasive bladder cancer
The inclusion criteria were as follows: provided data on smoking status (smoker/nonsmoker OR current/former/never smoker) and its association with the risk of recurrence and/or progression in non-muscle invasive bladder cancer. Either the number of events, event rate or odds ratio or hazard ratio for event-free survival must have been reported to include the study in the analysis. Studies in which the odds ratio for recurrence/progression according to the smoking status were available but the number of events were not reported and could not be recalculated were excluded (e.g., (Rausch et al. 2014; Pastore et al. 2015; Nerli et al. 2018; Holz et al. 2017)). In all studies the definition of the progression included the development of muscle-invasive disease (MIBC) and in the majority of studies any increase in T stage or grade was also regarded as progression. We excluded the studies reporting only smoking intensity or smoking time but not precisely the smoking status (former/current/never or smoker/nonsmoker) (Andrade et al. 2020). Studies with a single analysis for the cohort of both NMIBC and MIBC or studies in which the information on tobacco use was not precise were also excluded (Nerli et al. 2018; Zhang et al. 2021; Ahirwar et al. 2008).
Primary endpoints were the risk of tumour recurrence and/or progression. Abstracts were screened for eligibility and selected original papers were studied as full text. The data was retracted from prospective studies and retrospective studies including accepted manuscripts and their supplementary data files. Two independent investigators performed the screening and extracted data. In the event of duplicated analyzes of same cohorts in different publications, the study with more robust data was included.
Risk of bias assessment
Studies differently reported smoking status, which is the unavoidable bias of our meta-analysis. The reported smoking history is presented for each study in Table 1. Doubts regarding the smoking status interpretation occurred due to a lack of definitions and unclear reports in several studies. Several ways of reporting the smoking status were provided in different papers as follows: smokers vs non-smokers, smoking (yes/no) and smoking status (yes/no), and definitions were most commonly not available. History of smoking (yes/no) was also a common way of reporting and was interpreted as ever smoking vs never smoking (Shen et al. 2016; Yang et al. 2021; Mano et al. 2015). Some studies provided an explanation of the smoking status (yes vs no) (Kim et al. 2018; Ferro et al. 2020) as ever smoking (former/current) vs never smoking (Kim et al. 2018). Similarly, the variable “smoking (yes vs no)” and “smokers vs non-smokers” (Kang et al. 2014; Gangawar et al. 2010) was interpreted as a comparison between ever-smoking vs never-smoking but explained only in a few studies as cited. Only 30 studies provided an analysis of outcomes (hazard ratio or number or rate of recurrence/progression) according to detailed smoking status (never/former/current smoking). To assess the robustness of performed analysis, sensitivity analyses were performed including studies with the most reliable data on smoking status and the risk of recurrence and progression (pooled hazard ratio).
Table 1.
Characteristics of studies included (N = 64) for meta-analysis
| Study | Smoking categories | Reported outcome | Continent/country | Study type | Years | Pts. no | Mean/median age | Pathological stage | Pathological grade | Adjuvant therapy | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abufaraj et al. (2017) | Never, former, current | HR | EU, USA | R | NR | 827 | 67 | Ta 56; T1 41.8; Tis 2.2 | G1 23.6; G2 32.3; G3 44.1 | BCG 16.3; CHT 2.7 | 55 |
| Adamkiewicz et al. (2021) | Smoking history (yes/no) | HR | EU | R | NR-2020 | 125 | 69 | Ta 42.4; T1 57.6 | LG 55; HG 45 | BCG 100 | 22–24 |
| Ajili et al. (2012) | Smoker or nonsmoker | HR, raw values | AFR | R | 2000–2007 | 112 | 63.9 | Ta 60.7; T1 39.3 | G1 39.2; G2 43.8; G3 17 | BCG 100 | 30 |
| Allard et al. (1995) | Never, former, current | HR, raw values | CA | P | 1990–1992 | 368 | 65 | Ta 78.8; T1 21.2 | G1 32.2; G2 53.8; G3 12 | BCG 17.4; CHT 2.2 | 23.7 |
| Blute et al. (2017) | Smoking history (yes/no) | HR | USA | R | 2000–2014 | 727 | 69.8 | Ta 70.7; T1 23.5; Tis 5.8 | LG 49; HG 51 | BCG 40.1; CHT 8.1 | 44.4 |
| Cao et al. 2016) | Never, former, current | HR, raw values | AS, EU | R | 2008–2013 | 242 | 64 | Ta 57; T1 43 | LG 61.2; HG 38.8 | BCG 0; CHT 100 | 21 |
| Cantiello et al. (2018) | Never, former, current | HR | EU, USA | R | 2002–2012 | 1155 | 71 | T1 100 | HG 100 | BCG 100 | 48 |
| Carta et al. (2018) | Never, former, current | Raw values | EU | R | 1997–2000 | 160 | NR | NR | LG 25.6; HG 74.4 | BCG NR, CHT NR | 55.6 |
| Chen et al. (2008) | Nonsmokers, former, quitters, continued smokers | HR, raw values | AS | R | 1997–2005 | 413 | 67 | Ta 58.4; T1 41.6 | LG 71; HG 29 | BCG 16; CHT 56.9; BCG/CHT 20.6 | 36 |
| Cheng et al. (2021) | Smoking history (yes/no) | HR | AS | R | 2013–2017 | 314 | 65 | Ta 56; T1 44 | LG 33.3; HG 66.7 | BCG or CHT 16.2 | 48 |
| Cheng et al. (1999) | Never, former, current | Raw values | USA | R | 1987–1992 | 83 | 71 | T1 100 | LG 34; HG 66 | BCG 13.2; CHT 19.7 | 62.4 |
| D’Andrea et al. (2017) | Nonsmoker, ever-smoker | HR | EU, USA, CA | R | NR | 918 | 66–67 | Ta 60.5; T1 39.5 | G1 20; G2 36.8; G3 43.2 | CHT 4.3; BCG 12.9 | 62 |
| Abd Elwahab et al. (2021) | Smoker or nonsmoker | Raw values | AFR | P | 2013–2020 | 65 | 61.5 | T1 100 | G3 100 | BCG 100 | 60 |
| Favilla et al. (2016) | Never, former, current | HR | EU | P | 2008–2014 | 178 | 69.3 | Ta 77.5; T1 22.5 | LG 70.8; HG 29.2 | BCG 10.7; CHT 73 | 53 |
| Zhu et al. (2019) | Smoker or nonsmoker | Raw values | AS | P | 2017–2018 | 95 | 65 | Ta 70; T1 25; Tis 5 | LG 39; HG 61 | BCG NR, CHT NR | 18 |
| Ferro et al. (2020) | Smoking status (current/former), never | HR | EU | R | 2002–2012 | 1172 | 70.3 | T1 100 | HG 100 | BCG 100 | 47 |
| Fukuokaya et al. (2020) | Smoking history (yes/no) | HR | AS | R | 2002–2018 | 582 | 73 | Ta 60; T1 36.9; Tis 2.9 | G1 6.3; G2 46.5; G3 47.2 | BCG 23.4 | 41.3 |
| Gangawar et al. (2010) | nonsmokers (never), smokers, chewers | HR, raw values | AS | R | 2006–2008 | 135 | 58 | NR | G1 50.4; G2/G3 49.6 | BCG 54.8; MMC 10.4 | 14 |
| Garczyk et al. (2021) | Never, former, current | HR, raw values | EU | R | 2008–2014 | 128 | 66 | Tis 19; Tis/TaT1 81 | HG 100 | BCG 70 | 66 |
| Garg et al. (2020) | Never, ever | HR | USA | R | 2003–2015 | 1485 | 73.5 | Ta 67.4; T1 26.3; Tis 5 | LG 47.5; HG 52.5 | BCG NR, CHT NR | 70.8 |
| Gee et al. (2009) | Current smoker, history of smoking | HR | USA | R | 1991–2003 | 43 | NR | Ta 9; Tis 84 | HG 100 | BCG 100 | 60 |
| Grotenhuis et al. (2014) | Never, ever | HR, raw values | EU | R | NR | 1269 | 64 | Ta 68; T1 26; Tis 4 | LG 61; HG 38 | BCG 20; CHT 31 | 60 |
| Hensley et al. (2021) | Never, ever | HR | USA | R | 2000–2018 | 518 | 66–69, NR | Ta 45; T1 47.5; Tis NR | LG 11; HG 89 | BCG 100 | 50 |
| Jobczyk et al.(2020) | Smoker or nonsmoker | HR | EU, USA | R | NR | 389 | 68 | Ta 63; Tis 3; T1 34 | G1 54; G2 35; G3 11 | BCG 29 | 48 |
| Huang et al.(2021) | Smoking history (yes/no) | HR | AS | R | 2011–2015 | 88 | 64.5 | Ta 72.7; T1 27.3 | LG 60.2; HG 39.8 | BCG NR, CHT NR | 60 |
| Hwang et al.(2011) | Smoker or nonsmoker | HR | AS | R | 2000–2010 | 251 | 67 | Ta 64; T1 36 | PUNLMP 5.6; LG 62.5; HG 32 | BCG 51; Epirubicin 14 | 34 |
| Kang et al.(2014) | Smoker (prior/current) or nonsmoker | Raw values | AS, USA | R | 1992–2009 | 135 | 65 | Ta 31.9; T1 68.1 | G1 23.7; G2 59.3; G3 17 | BCG 100 | 66.7 |
| Khan et al.(2014) | History of smoking vs never | Raw values | AS | R | 2008–2012 | 64 | 59.9 | NR | HG 100 | BCG 100 | 28.4 |
| Kim et al.(2018) | Smoking status (current/former), never | Raw values | AS | R | 1999–2014 | 64 | NR | Tis 100 | HG 100 | BCG 100 | NR |
| Kimura et al.(2018) | Never, former, current | HR | AS, EU, USA, CA | R | NR | 1117 | 67 | Ta 58; T1 40; Tis 2 | G1 21; G2 35; G3 44 | BCG 39.7; CHT 4 | 64 |
| Kobayashi et al.(2014) | Never, former, current | HR, raw values | AS | R | 1986–2016 | 190 | 62.9 | Ta 100 | LG 100 | BCG 37.4; MMC 6.3 | 101.5 |
| Kufukihara et al.(2021) | Never, former, current | HR | AS | R | 1999–2017 | 1097 | NR | Ta 70; T1 30 | G1 6; G2 54; G3 40 | BCG 41 | 60 |
| Lacombe et al.(2016) | Never, former, current | HR, raw values | CA | P | 1990–1992 | 189 | 62.8 | Ta 77.2; T1 22.8 | LG 31.2; HG 68.8 | BCG 100 | 67.2 |
| Lammers et al. (2011) | Never, former/current | HR | EU | P | 1998–2004 | 718 | 66.5 | Ta 78.7; T1 21.3 | G1 42.1; G2 47; G3 10.9 | BCG NR; CHT 100 | 30 |
| Li et al. (2017) | Never, former, current | HR, raw values | AS | R | 2007–2015 | 484 | 64 | Ta 83.5; T1 16.5 | G1 18.8; G2 65.3; G3 15.9 | CHT 71.7, BCG NR | 25 |
| Li et al. (2020) | Smoking (yes/no) | HR | AS | R | 2013–2017 | 115 | 64.5 | Ta 100 | PUNLMP 37; LG 37; HG 35 | CHT 100 | 24 |
| Li et al. (2020) | Smoking history (yes/no) | HR | AS | R | 2012–2015 | 206 | 62 | Ta 70.4; Tis 3.9; T1 25.7 | PUNLMP 8.2; LG 64.6; HG 27.2 | BCG NR, CHT NR | 42 |
| Lu et al. (2019) | Never, former, current | HR | AS | R | 2012–2016 | 477 | 64 | Ta 75.3; T1 24.7 | G1 67; G2 22; G3 11 | BCG NR | NR |
| Lunney et al. (2019) | Never, former, current | Raw values | USA | R | 2010–2016 | 70 | 65 | Ta 58.6; T1 28.6; Tis 9 | LG 44; HG 56 | BCG NR | 31.7 |
| Mano et al. (2015) | Smoking history (former/current vs never) | HR | AS | R | 2003–2010 | 122 | 68 | Ta 43; T1 57 | G1G2 39; G3 61 | BCG 50; CHT 25 | 40 |
| Matulewicz et al. (2021) | Never, former, current | HR | USA, CA | R | 2014–2020 | 723 | NR | NR-AUA risk groups | NR | BCG 56.6; CHT 17 | 23.9 |
| Mbeutcha et al. (2016) | Never, former, current | HR | EU, USA, CA | R | 1996–2007 | 1117 | 67 | Ta 60.3; T1 39.7 | G1 20.7; G2 35.6; G3 43.7 | BCG 26.9; CHT 4; | 64 |
| Nowak et al. (2021) | Never, former, current | HR | EU | R | 2001–2019 | 590 | 66.9 | T1 100 | G3 100 | BCG 100 | 40 |
| Mitrakas et al. (2019) | Never, former, current | Raw values | EU | R | NR | 80 | 67.5 | Ta 18.8; T1 81.2 | HG 100 | BCG 100 | 62.7 |
| Ogihara et al. (2016) | Smoking history (yes/no) | Raw values | AS | R | 1995–2012 | 634 | 68.5 | Ta 68.3; T1 31.7 | G1G2 62.7; G3 37.3 | BCG 45.6 | 68.1 |
| Ogihara et al. (2016) | Never, former, current | HR, raw values | AS | R | 1995–2013 | 605 | 68 | Ta 68.3; T1 31.7 | LG 60.7; HG 39.3 | BCG 47.8 | 68.8 |
| Osch et al. (2018) | Never, former, current | HR, raw values | EU | R | 2006–2011 | 210 | 71 | Ta 66; T1 33; Tis 1 | G1 30; G2 36; G3 34 | BCG NR; CHT NR | 4.21 |
| Özyalvaçlı et al. (2015) | Smoking (yes/no) | Raw values | AS | R | 2008–2013 | 722 | 67.5 | T1 100 | HG 100 | BCG 34 | 24.2 |
| Rink et al. (2012) | Never, former, current | HR | EU, USA, CA | R | 1987–2007 | 2043 | 67 | Ta 61; T1 39 | G1 23.6; G2 33.8; G3 42.6 | BCG 16.1; CHT 3.8 | 49 |
| Sawazaki et al. (2021) | Smoking history (yes/no) | HR | AS | R | 2014–2018 | 75 | 74.7 | Ta 38.6; T1 57.3; Tis 4 | LG 66.7; HG 33.3 | BCG 30.6 | 37 |
| Serretta et al. (2013) | Never, former/current | HR, event rate | EU | R | 2002–2003 | 395 | 68 | Ta 36.5; T1 63.5 | G1 35.9; G2 64.1 | CHT 100 | 48 |
| Sfakianos et al. (2011) | Never, former, current | HR | USA | R | 1994–2008 | 623 | 76 | Ta 35.2; T1 34.5; Tis 30.3 | LG 9.6; G3 90.4 | BCG 100 | 80.9 |
| Shen et al. (2016) | Smoking history (never vs former/current) | HR | AS | R | 2005–2011 | 318 | 65 | T1 100 | LG 48.8; HG 49.5; unk 1.7 | BCG 0; CHT 96.2 | 53.5 |
| Soria et al. (2018) | Never, former, current | HR | EU, AS, USA | R | 1996–2007 | 1117 | 68 | Ta 58; Tis 2; T1 40 | G1 20; G2 36; G3 44 | BCG 27; CHT 4 | 62.7 |
| Shiota et al. (2017) | Never, former/current | HR | AS | R | 2010–2013 | 228 | 70 | Ta 68.9; T1 21.9; Tis 9.2 | LG 44.3; HG 55.7 | BCG 29.8; CHT 47.8 | 3.6 |
| Temiz et al. (2021) | Never, former, current | Event rate | AS | R | 2015–2018 | 53 | 67 | Ta 41.5; T1 56.6; Tis 1.9 | LG 17; HG 83 | BCG 100 | 11.5 |
| Ucpinar et al. (2019) | Never, former, current | HR, raw values | AS | R | 2015–2018 | 231 | 64 | NR | NR | BGC NR | 24 |
| Wang et al. (2020) | Smoking (yes/no) | HR | AS | R | 2010–2014 | 606 | 70 | Ta 61.7; T1 29.4; Tis 8.9 | G1 18.6; G2 55; G3 26.4 | BCG 19.2; CHT 13 | 44.5 |
| Wyszynski et al. (2014) | Never, former, current | HR | AFR, EU | R | 1994–2001 | 726 | NR | TaT1 94; Tis 6 | LG 74; HG 26 | BCG NR | 72 |
| Yang et al. (2021) | Smoking history (Never vs former/current) | HR | AS | R | 2014–2018 | 235 | 66 | Ta 81.7; T1 18.3 | LG 57; HG 43 | BCG NR | 42 |
| Yonekura et al. (2019) | Never, former, current | HR | AS | R | 2011–2015 | 40 | 73 | Ta/Tis 75; T1 25 | LG 65.0; HG 35 | BCG 7.5 | 37.9 |
| Yuruk et al. (2017) | Never, former, current | Raw values | AS | R | 2005–2012 | 212 | 64.7 | Ta 50.3; T1 49.7 | LG 59.9; HG 40.1 | BCG 3.8; CHT 42.8 | 32 |
| Zeng et al. (2020) | Smoking (yes/no) | Raw values | AS | R | 2017–2019 | 40 | 65 | Ta 72.5; T1 27.5 | PUNLMP 25; LG 40; HG 35 | BCG NR; CHT 67.5 | 12 |
| Zhao et al. (2021) | Smoking history (yes/no) | HR | AS | R | 2011–2015 | 104 | 63.2 | Ta 71.3; T1 28.7 | LG 61.7; HG 38.3 | BCG NR | 43.6 |
HR hazard ratio, P prospective study, R retrospective study, NR not reported, LG low-grade, HG high-grade, PUNLMP papillary urothelial neoplasm of low malignant potential, BCG Bacillus Calmette-Guerin, CHT intravesical chemotherapy, MMC mitomycin, EU Europe, AS Asia, AFR Africa, USA Unites States of America, CA Canada
The quality of studies and the risk of bias were assessed using the Quality In Prognosis Studies tool (QUIPS). In the bias evaluation with QUIPS, the following six domains were analyzed: study participation (sampling bias), study attrition (attrition bias), prognostic factor measurement, outcome measurement (ascertainment bias), study confounding, and statistical analysis and reporting (Hayden et al. 2013). The overall risk of bias was assessed for each study. Robvis tool was used to visualize the risk of bias in all selected studies. Thirty-three out of sixty-four studies were characterized by a moderate to high-risk of bias (see Fig. 2 for details).
Fig. 2.
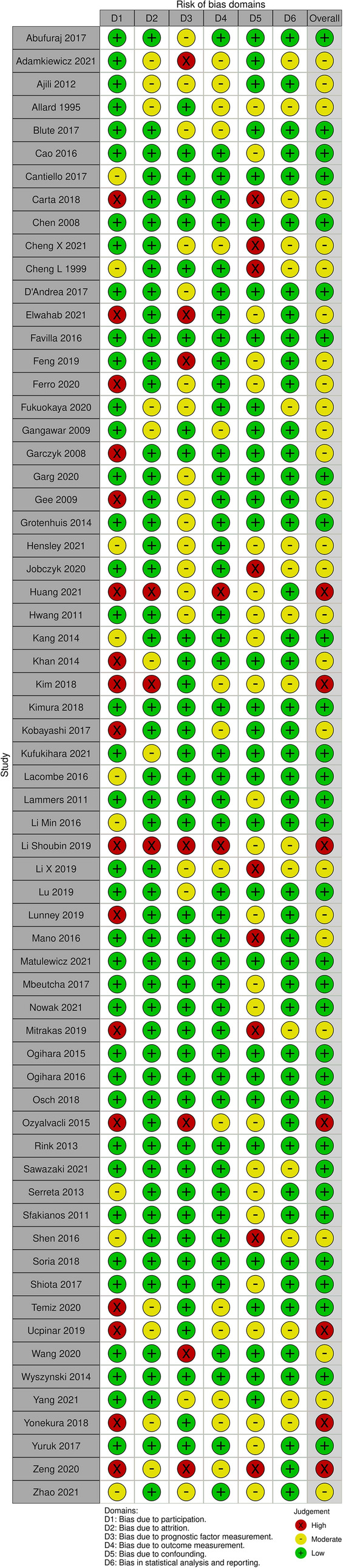
Risk of bias assessment using Quality In Prognosis Studies tool (QUIPS)
Statistical analysis
Groups of current smokers vs former smokers and former vs never smokers were compared in terms of NMIBC recurrence risk (odds ratio). Recurrence-free survival was compared between current vs never smokers and former vs never smokers (pooled hazard ratio). Groups of smokers (current or former) vs non-smokers (never smokers) were compared in terms of recurrence and progression risk (odds ratio and pooled hazard ratio). Odds ratios supplemented with 95% confidence intervals were generated based on contiguous tables containing raw data with the number of events in smokers/non-smokers or current/former/never smokers. Hazard ratios for time to recurrence or progression were retrieved from studies with respective 95% confidence intervals and the pooled hazard ratio was calculated. Adjusted hazard ratios were preferably extracted whenever available. We summarized the data using a random-effects model. Forest plots with 95% confidence intervals were generated. To assess the heterogeneity we used the Chi-square test for the degrees of freedom evaluation and the I2 statistic was calculated. I2 values of 25, 50, and 75% were regarded as a border of small, moderate, and large amounts of heterogeneity. All data were introduced and analyzed in the Review Manager (RevMan) version 5.4. software.
Results
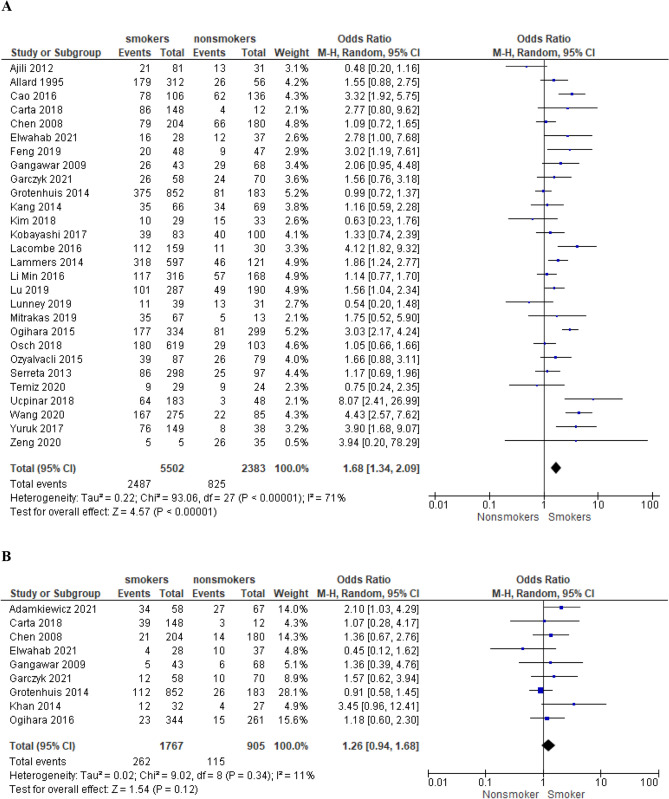
We selected 64 eligible studies, including 28,617 patients with non-muscle invasive bladder cancer and reported oncological outcomes according to the smoking status (Figs. 3, 4, 5, 6, 7, 8, 9). Thirty-one studies were eligible for the analysis of recurrence or progression rates among smokers and non-smokers. Among 8674 patients included in the above analysis, there were 2738 non-smokers (31.6%) and 5936 smokers (68.4%). Seventeen of included studies provided detailed information on the smoking status and 1490 (33.8%) were current, 1477 (33.5%) former and 1438 (32.6%) and never smokers, respectively. The median length of follow-up was 45.8 months (range between quartiles 30–60 months).
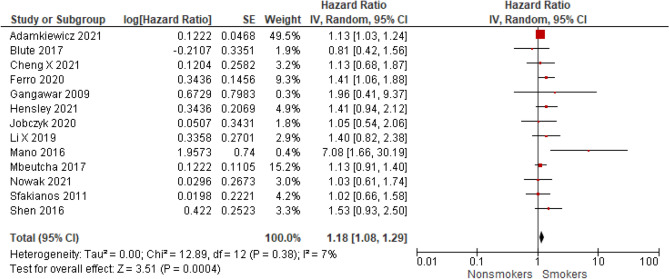
Fig. 3.
The association of smoking with recurrence risk (A) and progression risk (B) in patients with non-muscle invasive bladder cancer
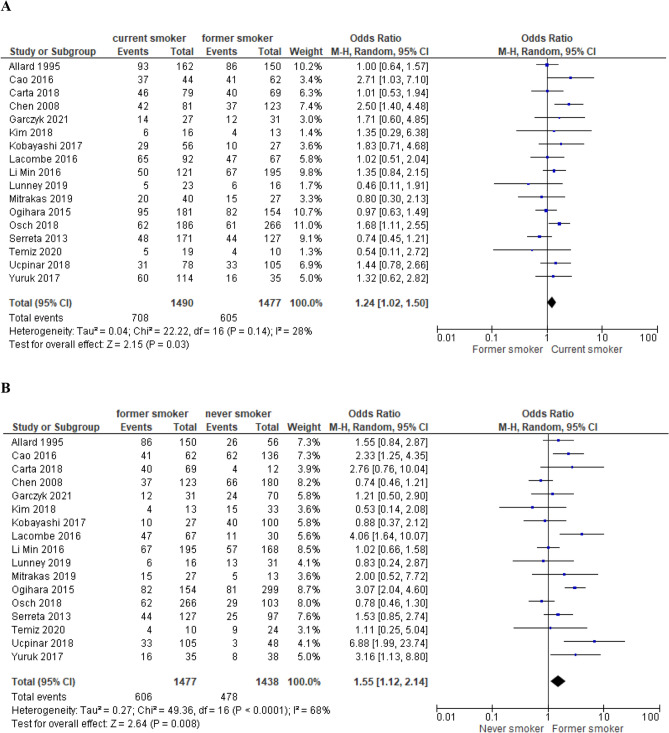
Fig. 4.
Recurrence risk in current compared to former smokers (A) and in former compared to never smokers (B) with non-muscle invasive bladder cancer
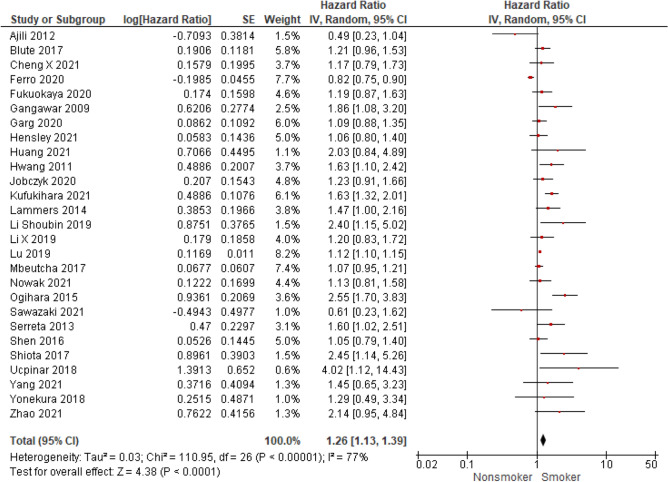
Fig. 5.
The association of smoking with recurrence-free survival in patients with non-muscle invasive bladder cancer
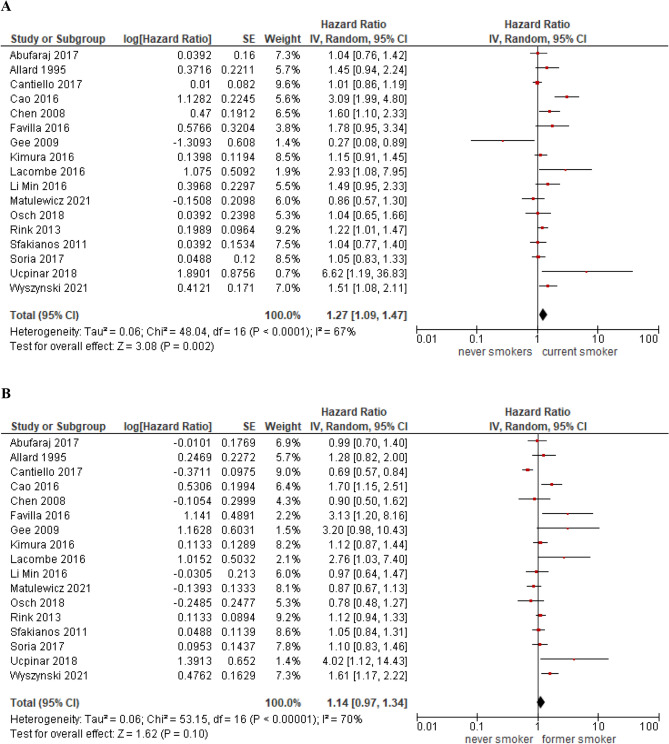
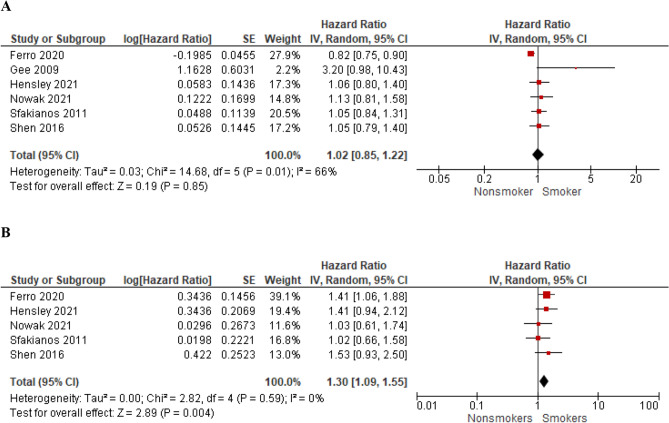
Fig. 6.
Recurrence-free survival in current compared to never smokers (A) and in former compared to never smokers (B) with non-muscle invasive bladder cancer
Fig. 7.
The association of smoking with progression-free survival in patients with non-muscle invasive bladder cancer
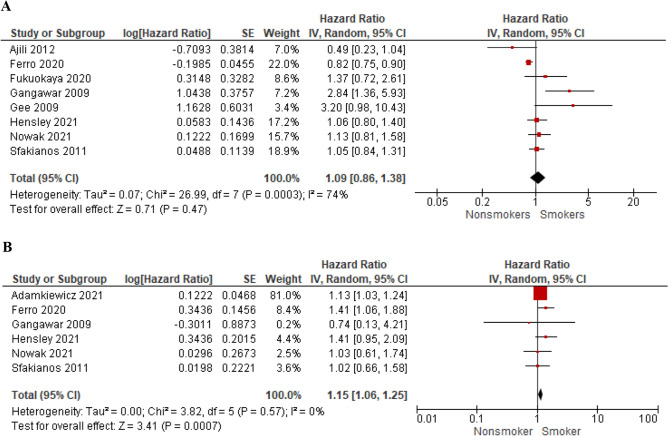
Fig. 8.
The association of smoking with recurrence-free survival (A) and progression-free survival (B) in high-risk non-muscle invasive bladder cancer
Fig. 9.
The association of smoking with recurrence-free survival (A) and progression-free survival (B) in BCG-treated patients with non-muscle invasive bladder cancer
The characteristics of included studies and patient cohorts are summarized in Table 1. Only a minor part of the studies focused exactly on the role of smoking on NMIBC prognosis, whereas the remaining studies included smoking in uni- or multivariate analysis as a potential confounding factor, but the smoking status was not the main subject of these studies. Among all 64 included studies, patients’ age, tumour pathologic characteristics and additional intravesical treatment (e.g. BCG) differed.
The conducted analyses (Figs. 3, 4, 5, 6, 7, 8, 9) included a different number of studies which provided data to investigate the association between smoking status (current/former/never smoker or smoker/non-smoker) and recurrence or progression. Necessary details, which explain the selection of studies for consecutive analyses were presented in Table 1.
Recurrence and progression risk in ever smokers
In a meta-analysis of 28 studies with 7885 patients we found that smokers (current/former) are at higher risk for recurrence (OR = 1.68; 95% CI 1.34–2.09; I2 = 71%; P < 0.0001) compared to never smokers (Fig. 3A). Among 5502 smokers, 2487 individuals (45.2%) developed tumour recurrence compared to 825 from 2383 patients classified as non-smokers (34.6%). Sensitivity analysis including only prospective studies confirmed that the risk of recurrence was higher for smokers compared to non-smokers (OR = 2.18 95% CI 1.57–3.02; I2 = 19%; P < 0.0001, Supp. Figure 1). Analysis of 9 studies including 2672 patients regarding the progression risk did not reveal an increased risk for smokers vs never smokers (progression rate 15.9% vs 13% in smokers and non-smokers, respectively; OR = 1.26; 95% CI 0.94–1.68; I2 = 11%; P = 0.12, Fig. 3B).
Recurrence risk in current smokers and former smokers
Seventeen studies (N = 4405) provided exact data on recurrences in the group of current smokers, former smokers and never smokers, respectively. The comparison between the risk of recurrence was also performed between the respective groups: current smokers vs former smokers and current vs never smokers (Fig. 4A, b). Current smokers were at 1.24 higher risk of recurrence (OR = 1.24; 95% CI 1.02–1.50; I2 = 28%; P = 0.03) compared to former smokers and former smokers had an increased risk compared to never (OR = 1.55; 95% CI 1.12–2.14; I2 = 68%; P = 0.008).
Many studies did not provide comprehensive data on recurrence/ progression rates or numbers in the subgroups of smokers and non-smokers, but provided hazard ratios supplemented with 95% confidence interval and those were used for RFS and PFS analysis. Fifty studies were available for analyses of the pooled hazard ratio for recurrence and/or progression.
Recurrence-free survival according to smoking status
Twenty-seven studies evaluated the effect of ever smoking on recurrence-free survival and seventeen provided more detailed information on the effect of current and former smoking compared to never smoking on RFS. RFS was worse in ever smokers compared to never smokers (HR = 1.26; 95%CI 1.13–1.39; I2 = 77%; P < 0.0001) (Fig. 5) and in current smokers vs never smokers (HR = 1.27; 95% CI 1.09–1.47; I2 = 67%; P = 0.002) (Fig. 6A). In the analysis of pooled hazard ratio, former smokers did not have an evident inferior RFS compared to those who never smoked (HR = 1.14; 95% CI 0.97–1.34; I2 = 70%; P = 0.10) (Fig. 6B). Sensitivity analysis was also performed and confirmed the higher risk of recurrence for ever smokers compared to never smokers (please see supplementary Fig. 2A to compare with Fig. 5.).
Progression-free survival according to smoking status
A meta-analysis of 13 studies showed that smokers have worse PFS (HR = 1.18; 95% CI 1.08–1.29; I2 = 7%; P < 0.001) compared to never smokers (Fig. 7). Sensitivity analysis was also performed and confirmed the higher risk of progression for ever smokers compared to never smokers (please see Supplementary Fig. 2B to compare with Fig. 7).
High-risk subgroup- effect of smoking history on RFS and PFS
Subgroup analysis of RFS in high-risk NMIBC patients (> 80% of the cohort studied in each paper or subgroup analysis available) included six studies and showed that smoking status did not influence RFS (HR = 1.02; 95% CI 0.85–1.22; I2 = 66%; P = 0.85) (Fig. 8A). However, the data retrieved from five studies including the analysis of PFS in patients with high-risk NMIBC demonstrated that ever smokers had compromised PFS compared to never smokers (HR = 1.30; 95% CI 1.09–1.55; I2 = 0%; P = 0.004) (Fig. 8B).
BCG-treated subgroup- effect of smoking history on RFS and PFS
Subgroup analysis of RFS in BCG-treated patients (100% of the cohort studied in each paper or subgroup analysis available) included eight studies and showed that smoking status did not influence RFS (HR = 1.09; 95% CI 0.86–1.38; I2 = 74%; P = 0.47) (Fig. 9A). However, the data retrieved from six studies including the analysis of PFS in patients receiving BCG demonstrated that ever smokers had compromised PFS compared to never smokers (HR = 1.15; 95% CI 1.06–1.25; I2 = 0%; P < 0.001) (Fig. 9B).
Discussion
In the current meta-analysis, we sought to determine the effect of smoking on NMIBC recurrence and progression. Our meta-analysis shows that smokers (former/current) have a worse prognosis than non-smokers diagnosed with NMIBC. Both RFS and PFS are compromised in patients who have ever smoked compared to never smokers. A separate analysis of the recurrence rates confirmed an increased risk of recurrence but not progression in smokers compared to non-smokers.
The initial evidence of the smoking impact on bladder cancer prognosis comes from the cohorts treated with neoadjuvant chemotherapy (NAC) and radical cystectomy (RC), in which smoking status increased cancer-specific mortality (CSM) (Cacciamani et al. 2020; Crivelli et al. 2014). A meta-analysis by Cacciamani et al. showed that smoking affects the pathological response to NAC and CSM after NAC and RC. Active smokers had lower NAC response rates and the burden of CSM and OM and bladder cancer recurrences were higher compared to nonsmokers (Crivelli et al. 2014). Our meta-analysis was meant to provide the rationale for generalizing the previous observations on the detrimental effect of smoking on prognosis in locally advanced bladder cancer onto NMIBC.
To date several retrospective analyses presented conflicting results on the effect of smoking on NMIBC prognosis (Grotenhuis et al. 2014; Ogihara et al. 2016; D’Andrea et al. 2017; Ślusarczyk et al. 2019). It has been also recognized that former smokers perform better than those who continue smoking in NMIBC cohorts (Chen et al. 2007; Rink et al. 2013). Importantly, some studies show that smoking cessation might not only prevent recurrence and progression (Chen et al. 2007; Rink et al. 2013) but also impact survival as reported by the recent cross-sectional real-world experience study (Karlsson et al. 2021). In our meta-analysis, a subgroup analysis of 17 papers summarized by pooled hazard ratio demonstrated that current, but not former smoking is associated with unfavourable RFS compared to never smoking, which might suggest that smoking cessation improves the RFS. Moreover, the subgroup analysis of the other 17 papers, providing contingency tables for odds ratio calculation, revealed that current smokers are at increased risk for recurrence compared to former smokers. It has been already reported that smoking cessation more than 15 years before bladder cancer diagnosis reduces recurrences regardless of the intensity or duration of past smoking (Ogihara et al. 2016). On the other hand, a prospective trial by Seretta et al. did not confirm the current smoking as the risk factor for recurrence after TURBT as the observed 8.4% risk reduction in quitters failed to be statistically significant (Serretta et al. 2020). Noteworthy, the cessation time in this study has not exceeded 8.5 months. Thus, since the smoking-free interval might require to be longer to unsheathe the benefit in prognosis, our classification of patients as former smoking as simply individuals that stopped smoking before TURBT, might prevent the results from generalizing to some clinical settings.
Several studies suggest the relationship between smoking and the reduced effectiveness of intravesical adjuvant therapy (Abd Elwahab et al. 2021; Lammers et al. 2011; Rink et al. 2012). BCG immunotherapy was reported to be less effective in smokers compared to non-smokers (Ślusarczyk et al. 2019; Abd Elwahab et al. 2021; Lammers et al. 2011). Another study confirmed that greater lifetime smoke exposure (especially over 20 pack-years) confers a risk factor for recurrence and progression of NMIBC treated with BCG (Andrade et al. 2020 Aug). Such observation might reflect not only the carcinogenesis and mutagenesis in urothelial cells induced by smoke but also the immunomodulatory effects of tobacco smoking. Our meta-analysis of the NMIBC subgroup treated with BCG therapy showed worse PFS but not RFS in smokers compared to non-smokers, which emphasizes the importance of early smoking cessation to avoid progression and spare the bladder. The discrepancy between the clear effect of smoke on PFS despite no effect on RFS might come from high end-point prevalence (recurrence) despite a limited sample size. Noteworthy, in the majority of analyses high-grade and low-grade recurrences are not counted separately despite their completely different prognostic value. Since prospective evidence of the smoking effect on BCG therapy outcomes is lacking, clear recommendations in this area might be yet from clinical implementation. However, based on the results presented and the general harms related to smoking, patients with high-risk NMIBC should be strongly advised to quit smoking when qualified for BCG immunotherapy and informed about the potential increased risk of therapy failure when smoking. Implementation of novel immunotherapies targeting immune checkpoint (IC) raises the question of its applicability in certain groups of patients, including smokers. Current evidence from lung cancer suggests that due to higher mutational burden, smokers might achieve better responses to IC inhibitors than never smokers (Dai et al. 2021).
In summary, tobacco smoking has a detrimental effect on non-muscle invasive bladder cancer management. Smoking cessation should be counselled in each patient due to the general harm it causes and the worsening of bladder cancer prognosis. Bladder cancer was ranked as the most expensive cancer per capita to treat (Cost considerations in the management of bladder cancer [Internet]. 2021). High expenses for bladder cancer management come from several factors, one of which is the recurrent disease requiring repeated surgical procedures (e.g., TURBT) and intensive cystoscopic surveillance and intravesical therapy (Svatek et al. 2014). Smoking surely increases the cost of NMIBC treatment as according to our meta-analysis, the recurrence occurs 68% more commonly in ever smokers compared to never smokers. Current smoking at the time of UCB diagnosis is associated with an additional 24% increase in the recurrence risk compared to a history of smoking in the past. Urological advice and assistance in quitting smoking are suboptimal, despite the fact of their relevance and efficacy (Sosnowski et al. 2016). A prospective trial showed that a short 5 min brief smoking cessation intervention increased the rate of UBC patients who quit smoking (12.1 vs 2.6%) compared to patients under usual care (Bjurlin et al. 2013). Our study demonstrates the detrimental effect of smoking on further tumour recurrence risk and delivers another rationale for cessation intervention by urologists and proposal of nicotine replacement therapy or another quitting programme for highly addicted individuals. Other studies including a cessation group suggest that former smokers (especially those early quitting, 15 years before diagnosis) perform better than those who continue smoking (Ogihara et al. 2016; Chen et al. 2007; Rink et al. 2013). As this meta-analysis did not assess the effect of quitting to smoke after NMIBC diagnosis, no firm conclusions in that clinical setting can be made. Further prospective studies with cessation intervention are required to confirm a worse prognosis in continued smokers compared to those who quitted.
Our meta-analysis revealed several discrepancies that should be evaluated. Although progression-free survival was worse in smokers compared to non-smokers (P < 0.001), we failed to validate it in contingency tables evaluating progression risk itself (P = 0.12). The discrepancy between the evidenced risk of compromised PFS (HR) but not increased progression risk (OR) might signalize the bias in the selection of the studies. Significant differences in follow-up time and characteristics of cohorts analyzed in included studies might therefore explain the discrepancy. The extraction of the data for this meta-analysis from studies that did not aim to focus on the smoking effect on prognosis as the main research question and, therefore, did not show comprehensive data on smoking, must be considered as another limitation. Analyses of event-free survival and risk of an event do not lead to the same conclusions and, therefore, presented effects of smoking on progression should be interpreted with caution. Nevertheless, the detrimental effect of smoking on PFS was also observed in the sensitivity analysis (supplementary Fig. 2.) and subgroup analyses- in BCG-treated individuals as well as in the high-risk-only NMIBC cohort. Another issue is the lack of adjustment for other prognostic factors (e.g., grade, T category) when calculating recurrence/progression risk (odds ratio), but in the majority of studies providing RFS/PFS assessment, the hazard ratio was adjusted for other confounders. The higher prevalence of aggressive cancer in smokers than in non-smokers (Pietzak and Malkowicz 2014) is a non-negligible confounder, contributing to the potential bias in our analysis. On the other hand, some studies do not clearly confirm the association between baseline tumour characteristics and smoking status. In the study of Barbosa et al. analysing a large cohort of 1859 patients, smokers constituted 82% of low-risk and 82% of high-risk NMIBC (Barbosa et al. 2018). It should be, however, noted that among former smokers, duration and amount of smoking correlated with tumour aggressiveness (Barbosa et al. 2018). Another study by Pietzak et al. suggested that heavy smokers (≥ 30 pack-years) are at increased risk of high-grade tumour and MIBC at presentation compared to light smokers (< 30 pack-years) and non-smokers (Pietzak et al. 2015). Therefore, although higher aggressiveness of tumours in current smokers might result in certain confounding in itself, it seems that in the end what matters most is the smoke load.
Limitations of the above meta-analysis result from the inclusion of studies with moderate- to high-risk of bias (26 and 7 studies, respectively). Almost all included studies were retrospective and the majority of them did not focus directly on smoking status and did not address its prognostic value as a main research question. Consequently, smoking status reporting differed between studies (as shown in Table 1). Possible bias from not reporting the effect of smoking in other published studies, in which no effect of smoking was found, cannot be ruled out. The heterogeneity of studies included in the RFS analysis was moderate to high, which limits generalizing the results to every clinical setting. Noteworthy, a sensitivity analysis with only prospective studies was characterized by low heterogeneity and confirmed that smokers have a higher risk of recurrence than non-smokers (supp. Figure 1). To exclude another potential confounder, we have also performed a subgroup analyses for European/North American and Asian patients regarding RFS and PFS, which further confirmed that the effect of smoking remains significant in patients of different geographic origin (see Supp. Figure 3A–D). We also did not study the effect of smoking intensity and load on prognosis, because of the heterogeneity of reported data in single papers. Andrade et al. showed that smoke load, especially over 20 pack-years influenced recurrence and progression risk in BCG-treated pT1 NMIBC (Andrade et al. 2020). Rink et al. showed that heavy long-term smokers (≥ 20 years; ≥ 20 cigarettes per day) had the worst prognosis followed by light long-term (≥ 20 years; < 20 cigarettes per day), heavy short-term and light long-term smokers (Rink et al. 2013). Although a detailed analysis of smoking load or intensity would be more informative, we attempted to control this bias by providing a subgroup analysis.
Conclusions
In conclusion, patients with non-muscle invasive bladder cancer and a history of smoking have a worse prognosis regarding recurrence-free and progression-free survival compared to non-smokers. Current smokers have a higher risk of recurrence than former smokers, so smoking cessation should be always counselled and referral for replacement nicotine therapy should be proposed. Smokers treated with intravesical adjuvant BCG have worse progression-free survival when compared to non-smokers. Prospective studies assessing the effect of smoking on NMIBC prognosis are required to confirm our findings.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Project development: A.S, P.R. Data collection: A.S., P.Z., L.Z. Data analysis: A.S., P.Z. Manuscript writing/editing: A.S., P.Z., L.Z., P.R.
Funding
This research did not receive any funding.
Data availability
The extracted data can be obtained from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aleksander Ślusarczyk, Email: aslusarczyk@wum.edu.pl.
Piotr Zapała, Email: pzapala@wum.edu.pl.
References
- Abd Elwahab KM, Desky EAE, Eldery MS, Mohammad FF, Seleem MM, El-Babouly IM. Apparent diffusion coefficient value can predict poor bacillus calmette-guérin responders in T1HG/NMIBC: prospective cohort study. Clin Genitourin Cancer. 2021;19(4):e248–e254. doi: 10.1016/j.clgc.2021.03.001. [DOI] [PubMed] [Google Scholar]
- Abufaraj M, Shariat SF, Haitel A, Moschini M, Foerster B, Chłosta P, et al. Prognostic role of N-cadherin expression in patients with non–muscle-invasive bladder cancer. Urol Oncol Semin Orig Investig . 2017;35(5):264–271. doi: 10.1016/j.urolonc.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Adamkiewicz M, Bryniarski P, Kowalik M, Burzyński B, Rajwa P, Paradysz A. Lymphocyte-to-monocyte ratio is the independent prognostic marker of progression in patients undergoing BCG-immunotherapy for bladder cancer. Front Oncol. 2021;26(11):655000. doi: 10.3389/fonc.2021.655000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahirwar D, Kesarwani P, Manchanda PK, Mandhani A, Mittal RD. Anti- and proinflammatory cytokine gene polymorphism and genetic predisposition: association with smoking, tumor stage and grade, and bacillus Calmette-Guérin immunotherapy in bladder cancer. Cancer Genet Cytogenet. 2008;184(1):1–8. doi: 10.1016/j.cancergencyto.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Ajili F, Manai M, Darouiche A, Chebil M, Boubaker S. Tumor multiplicity is an independent prognostic factor of non-muscle-invasive bladder cancer treated with bacillus calmette-guerin immunotherapy. Ultrastruct Pathol. 2012;36(5):320–324. doi: 10.3109/01913123.2012.681833. [DOI] [PubMed] [Google Scholar]
- Allard P, Fradet Y, Têtu B, Bernard P. Tumor-associated antigens as prognostic factors for recurrence in 382 patients with primary transitional cell carcinoma of the bladder. Clin Cancer Res. 1995;1(10):1195–1202. [PubMed] [Google Scholar]
- Andrade DL, Moretti TBC, Neto WA, Benedetti J, Reis LO. Smoke load prognostic impact on bacillus Calmette-Guérin (BCG) treated non-muscle invasive bladder cancer. Int Urol Nephrol. 2020;52(8):1471–1476. doi: 10.1007/s11255-020-02438-6. [DOI] [PubMed] [Google Scholar]
- Barbosa ALA, Vermeulen SHHM, Aben KK, Grotenhuis AJ, Vrieling A, Kiemeney LA. Smoking intensity and bladder cancer aggressiveness at diagnosis. PLoS ONE. 2018;13(3):e0194039. doi: 10.1371/journal.pone.0194039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjurlin MA, Cohn MR, Kim DY, Freeman VL, Lombardo L, Hurley SD, et al. Brief smoking cessation intervention: a prospective trial in the urology setting. J Urol. 2013;189(5):1843–1849. doi: 10.1016/j.juro.2012.11.075. [DOI] [PubMed] [Google Scholar]
- Blute ML, Kucherov V, Rushmer TJ, Damodaran S, Shi F, Abel EJ, et al. Reduced estimated glomerular filtration rate (eGFR <60 mL/min/1.73 m2) at first transurethral resection of bladder tumour is a significant predictor of subsequent recurrence and progression. BJU Int. 2017;120(3):387–393. doi: 10.1111/bju.13904. [DOI] [PubMed] [Google Scholar]
- Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000;86(2):289–294. doi: 10.1002/(SICI)1097-0215(20000415)86:2<289::AID-IJC21>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Burger M, Catto JWF, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Cacciamani GE, Ghodoussipour S, Mari A, Gill KS, Desai M, Artibani W, et al. Association between smoking exposure, neoadjuvant chemotherapy response and survival outcomes following radical cystectomy: systematic review and meta-analysis. J Urol. 2020;204(4):649–660. doi: 10.1097/JU.0000000000000813. [DOI] [PubMed] [Google Scholar]
- Cantiello F, Russo GI, Vartolomei MD, Farhan ARA, Terracciano D, Musi G, et al. Systemic inflammatory markers and oncologic outcomes in patients with high-risk non-muscle-invasive urothelial bladder cancer. Eur Urol Oncol. 2018;1(5):403–410. doi: 10.1016/j.euo.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Cao J, Xu R, Zhao X, Zhong Z, Zhang L, Zhu X, et al. Areca nut chewing and an impaired estimated glomerular filtration rate as significant risk factors for non-muscle-invasive bladder cancer recurrence. Sci Rep. 2016;6(1):29466. doi: 10.1038/srep29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta A, Pavanello S, Mastrangelo G, Fedeli U, Arici C, Porru S. Impact of occupational exposures and genetic polymorphisms on recurrence and progression of non-muscle-invasive bladder cancer. IJERPH. 2018;15(8):1563. doi: 10.3390/ijerph15081563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Shun CT, Huang KH, Huang CY, Tsai YC, Yu HJ, et al. Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer. BJU Int. 2007;100(2):281–286. doi: 10.1111/j.1464-410X.2007.06873.x. [DOI] [PubMed] [Google Scholar]
- Chen CH, Shun CT, Huang KH, Huang CY, Yu HJ, Pu YS. Characteristics of female non–muscle-invasive bladder cancer in taiwan: association with upper tract urothelial carcinoma and end-stage renal disease. Urology. 2008;71(6):1155–1160. doi: 10.1016/j.urology.2007.11.140. [DOI] [PubMed] [Google Scholar]
- Cheng L, Neumann RM, Weaver AL, Spotts BE, Bostwick DG. Predicting cancer progression in patients with stage T1 bladder carcinoma. JCO. 1999;17(10):3182–3187. doi: 10.1200/JCO.1999.17.10.3182. [DOI] [PubMed] [Google Scholar]
- Cheng X, Zhou X, Yi M, Xu S, Zhang C, Wang G. Preoperative aspartate transaminase/alanine transaminase ratio as a prognostic biomarker in primary non-muscle-invasive bladder cancer: a propensity score-matched study. BMC Urol. 2021;21(1):136. doi: 10.1186/s12894-021-00901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cost considerations in the management of bladder cancer [Internet]. Urology Times. [cited 2021 Nov 23]. Available from: https://www.urologytimes.com/view/cost-considerations-management-bladder-cancer
- Crivelli JJ, Xylinas E, Kluth LA, Rieken M, Rink M, Shariat SF. Effect of smoking on outcomes of urothelial carcinoma: a systematic review of the literature. Eur Urol. 2014;65(4):742–754. doi: 10.1016/j.eururo.2013.06.010. [DOI] [PubMed] [Google Scholar]
- D’Andrea D, Moschini M, Gust K, Abufaraj M, Özsoy M, Mathieu R, et al. Prognostic role of neutrophil-to-lymphocyte ratio in primary non–muscle-invasive bladder cancer. Clin Genitourin Cancer. 2017;15(5):e755–e764. doi: 10.1016/j.clgc.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Dai L, Jin B, Liu T, Chen J, Li G, Dang J. The effect of smoking status on efficacy of immune checkpoint inhibitors in metastatic non-small cell lung cancer: A systematic review and meta-analysis. eClinicalMedicine [Internet]. 2021 Aug 1 [cited 2022 Sep 25];38. Available from: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(21)00270-4/fulltext [DOI] [PMC free article] [PubMed]
- Favilla V, Castelli T, Urzì D, Reale G, Privitera S, Salici A, et al. Neutrophil to lymphocyte ratio, a biomarker in non-muscle invasive bladder cancer: a single-institutional longitudinal study. Int Braz J Urol. 2016;42(4):685–693. doi: 10.1590/S1677-5538.IBJU.2015.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol. 2009;182(5):2195–2203. doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Ferro M, Katalin MO, Buonerba C, Marian R, Cantiello F, Musi G, et al. Type 2 diabetes mellitus predicts worse outcomes in patients with high-grade T1 bladder cancer receiving bacillus Calmette-Guérin after transurethral resection of the bladder tumor. Urol Oncol Semin Orig Investig . 2020;38(5):459–464. doi: 10.1016/j.urolonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- Fukuokaya W, Kimura T, Miki J, Kimura S, Watanabe H, Bo F, et al. Red cell distribution width predicts time to recurrence in patients with primary non–muscle-invasive bladder cancer and improves the accuracy of the EORTC scoring system. Urol Oncol Semin Orig Investig. 2020;38(7):638.e15–638.e23. doi: 10.1016/j.urolonc.2020.01.016. [DOI] [PubMed] [Google Scholar]
- Gangawar R, Ahirwar D, Mandhani A, Mittal RD. Impact of nucleotide excision repair ERCC2 and base excision repair APEX1 genes polymorphism and its association with recurrence after adjuvant BCG immunotherapy in bladder cancer patients of North India. Med Oncol. 2010;27(2):159–166. doi: 10.1007/s12032-009-9187-y. [DOI] [PubMed] [Google Scholar]
- Garczyk S, Bischoff F, Schneider U, Golz R, von Rundstedt FC, Knüchel R, et al. Intratumoral heterogeneity of surrogate molecular subtypes in urothelial carcinoma in situ of the urinary bladder: implications for prognostic stratification of high-risk non-muscle-invasive bladder cancer. Virchows Arch. 2021;479(2):325–335. doi: 10.1007/s00428-021-03054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg T, Young AJ, O’Keeffe-Rosetti M, McMullen CK, Nielsen ME, Murphy TE, et al. Association between metabolic syndrome and recurrence of nonmuscle-invasive bladder cancer in older adults. Urol Oncol Semin Orig Investig. 2020;38(9):737.e17–737.e23. doi: 10.1016/j.urolonc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JR, Jarrard DF, Bruskewitz RC, Moon TD, Hedican SP, Leverson GE, et al. Reduced bladder cancer recurrence rate with cardioprotective aspirin after intravesical bacille Calmette-Guérin. BJU Int. 2009;103(6):736–739. doi: 10.1111/j.1464-410X.2008.08123.x. [DOI] [PubMed] [Google Scholar]
- Grotenhuis AJ, Dudek AM, Verhaegh GW, Witjes JA, Aben KK, van der Marel SL, et al. Prognostic Relevance of Urinary Bladder Cancer Susceptibility Loci. Black PC, editor. PLoS ONE. 2014;9(2):e89164. doi: 10.1371/journal.pone.0089164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- Hensley PJ, Bree KK, Brooks N, Matulay J, Li R, Nogueras González GM, et al. Time interval from transurethral resection of bladder tumour to bacille Calmette-Guérin induction does not impact therapeutic response. BJU Int. 2021;128(5):634–641. doi: 10.1111/bju.15413. [DOI] [PubMed] [Google Scholar]
- Holz S, Albisinni S, Gilsoul J, Pirson M, Duthie V, Quackels T, et al. Risk factor assessment in high-risk, bacillus Calmette–Guérin-treated, non-muscle-invasive bladder cancer. Res Rep Urol. 2017;26(9):195–202. doi: 10.2147/RRU.S143865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhao L, Wang K, Sun J, Tai S, Hua R, et al. Controlling nutritional status score evaluates prognosis in patients with non-muscle invasive bladder cancer. Cancer Control. 2021;1(28):107327482110210. doi: 10.1177/10732748211021078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang EC, Kim YJ, Hwang IS, Hwang JE, Jung SI, Kwon DD, et al. Impact of diabetes mellitus on recurrence and progression in patients with non-muscle invasive bladder carcinoma: a retrospective cohort study: DM and NMIBC. Int J Urol. 2011;18(11):769–776. doi: 10.1111/j.1442-2042.2011.02845.x. [DOI] [PubMed] [Google Scholar]
- Jobczyk M, Stawiski K, Fendler W, Różański W. Validation of EORTC, CUETO, and EAU risk stratification in prediction of recurrence, progression, and death of patients with initially non–muscle-invasive bladder cancer (NMIBC): A cohort analysis. Cancer Med. 2020;9(11):4014–4025. doi: 10.1002/cam4.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HW, Tchey DU, Yan C, Kim WT, Kim YJ, Yun SJ, et al. The predictive value of GSTT1 polymorphisms in predicting the early response to induction BCG therapy in patients with non–muscle invasive bladder cancer. Urol Oncol Semin Orig Investig. 2014;32(4):458–465. doi: 10.1016/j.urolonc.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Ellonen A, Irjala H, Väliaho V, Mattila K, Nissi L, et al. Impact of deep learning-determined smoking status on mortality of cancer patients: never too late to quit. ESMO Open. 2021;6(3):100175. doi: 10.1016/j.esmoop.2021.100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MK, Ahmed I, Raza SJ. Factors affecting recurrence and progression in intravesical BCG treated high grade non muscle invasive bladder cancer. Pak J Med Sci [Internet]. 2014 Jan 30 [cited 2021 Nov 23];30(2). Available from: http://pjms.com.pk/index.php/pjms/article/view/4117 [DOI] [PMC free article] [PubMed]
- Kim SJ, Nam W, You D, Jeong IG, Song C, Hong B, et al. Prognostic factors related to recurrence-free survival for primary carcinoma in situ of the bladder after Bacillus Calmette-Guérin: a retrospective study. Urol Int. 2018;101(3):269–276. doi: 10.1159/000492121. [DOI] [PubMed] [Google Scholar]
- Kimura S, Soria F, D’Andrea D, Foerster B, Abufaraj M, Vartolomei MD, et al. Prognostic value of serum cholinesterase in non–muscle-invasive bladder cancer. Clin Genitourin Cancer. 2018;16(6):e1123–e1132. doi: 10.1016/j.clgc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kikuchi E, Mikami S, Maeda T, Tanaka N, Miyajima A, et al. Long term follow-up in patients with initially diagnosed low grade Ta non-muscle invasive bladder tumors: tumor recurrence and worsening progression. BMC Urol. 2014;14(1):5. doi: 10.1186/1471-2490-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufukihara R, Kikuchi E, Ogihara K, Shigeta K, Yanai Y, Takamatsu K, et al. Role of previous malignancy history in clinical outcomes in patients with initially diagnosed non-muscle invasive bladder cancer. Ann Surg Oncol. 2021;28(9):5349–5359. doi: 10.1245/s10434-021-09750-0. [DOI] [PubMed] [Google Scholar]
- Lacombe L, Fradet V, Lévesque É, Pouliot F, Larue H, Bergeron A, et al. Phase II drug-metabolizing polymorphisms and smoking predict recurrence of non–muscle-invasive bladder cancer: a gene-smoking interaction. Cancer Prev Res. 2016;9(2):189–195. doi: 10.1158/1940-6207.CAPR-15-0069. [DOI] [PubMed] [Google Scholar]
- Lammers RJM, Witjes WPJ, Hendricksen K, Caris CTM, Janzing-Pastors MHC, Witjes JA. Smoking status is a risk factor for recurrence after transurethral resection of non–muscle-invasive bladder cancer. Eur Urol. 2011;60(4):713–720. doi: 10.1016/j.eururo.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Li HM, Azhati B, Rexiati M, Wang WG, Li XD, Liu Q, et al. Impact of smoking status and cumulative smoking exposure on tumor recurrence of non-muscle-invasive bladder cancer. Int Urol Nephrol. 2017;49(1):69–76. doi: 10.1007/s11255-016-1441-6. [DOI] [PubMed] [Google Scholar]
- Li X, Shu K, Zhou J, Yu Q, Cui S, Liu J, et al. Preoperative plasma fibrinogen and d-dimer as prognostic biomarkers for non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2020;18(1):11–19.e1. doi: 10.1016/j.clgc.2019.10.025. [DOI] [PubMed] [Google Scholar]
- Li S, Jia Y, Yu C, Xiao H, Guo L, Sun F, et al. Influences of different operative methods on the recurrence rate of non-muscle-invasive bladder cancer. Urol J. 2020;18(4):411–416. doi: 10.22037/uj.v16i7.5965. [DOI] [PubMed] [Google Scholar]
- Lu M, Chen S, Zhou Q, Wang L, Peng T, Wang G. Predicting recurrence of nonmuscle-invasive bladder cancer (Ta-T1): A study based on 477 patients. Medicine. 2019;98(28):e16426. doi: 10.1097/MD.0000000000016426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney A, Haynes A, Sharma P. Moderate or severe LUTS is associated with increased recurrence of non - muscle - invasive urothelial carcinoma of the bladder. Int Braz j Urol. 2019;45(2):306–314. doi: 10.1590/s1677-5538.ibju.2018.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano R, Baniel J, Shoshany O, Margel D, Bar-On T, Nativ O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non–muscle-invasive bladder cancer. Urol Oncol Semin Orig Investig . 2015;33(2):67.e1–67.e7. doi: 10.1016/j.urolonc.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Matulewicz RS, Ravvaz K, Weissert JA, Porten S, Steinberg GD. Association of smoking status and recurrence of non-muscle invasive bladder cancer among patients managed with blue light cystoscopy. Urol Oncol Semin Orig Investig. 2021;39(12):833.e19–833.e26. doi: 10.1016/j.urolonc.2021.04.028. [DOI] [PubMed] [Google Scholar]
- Mbeutcha A, Shariat SF, Rieken M, Rink M, Xylinas E, Seitz C, et al. Prognostic significance of markers of systemic inflammatory response in patients with non–muscle-invasive bladder cancer. Urol Oncol Semin Orig Investig. 2016;34(11):483.e17–483.e24. doi: 10.1016/j.urolonc.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Mitrakas L, Gravas S, Papandreou C, Koukoulis G, Karasavvidou F, Dimakopoulos G, et al. Primary high-grade non-muscle-invasive bladder cancer: high NFκB expression in tumor specimens distinguishes patients who are at risk for disease progression. Pathol Oncol Res. 2019;25(1):225–231. doi: 10.1007/s12253-017-0340-1. [DOI] [PubMed] [Google Scholar]
- Nerli RB, Ghagane SC, Shankar K, Sanikop AC, Hiremath MB, Dixit NS, et al. Low-grade, multiple, ta non-muscle-invasive bladder tumors: tumor recurrence and worsening progression. Indian J Surg Oncol. 2018;9(2):157–161. doi: 10.1007/s13193-018-0728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder AM, John S, Messina CR, Granek IA, Adler HL. Are patients aware of the association between smoking and bladder cancer? J Urol. 2006;176:2405–2408. doi: 10.1016/j.juro.2006.07.147. [DOI] [PubMed] [Google Scholar]
- Nowak Ł, Krajewski W, Moschini M, Chorbińska J, Poletajew S, Tukiendorf A, et al. Assessment of the oncological outcomes of three different bacillus Calmette-Guérin strains in patients with high-grade T1 non-muscle-invasive bladder cancer. Arab J Urol. 2021;19(1):78–85. doi: 10.1080/2090598X.2021.1874628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara K, Kikuchi E, Yuge K, Ito Y, Tanaka N, Matsumoto K, et al. Refraining from Smoking for 15 Years or More Reduced the Risk of Tumor Recurrence in Non-muscle Invasive Bladder Cancer Patients. Ann Surg Oncol. 2016;23(5):1752–1759. doi: 10.1245/s10434-015-5016-z. [DOI] [PubMed] [Google Scholar]
- Ogihara K, Kikuchi E, Yuge K, Yanai Y, Matsumoto K, Miyajima A, et al. The preoperative neutrophil-to-lymphocyte ratio is a novel biomarker for predicting worse clinical outcomes in non-muscle invasive bladder cancer patients with a previous history of smoking. Ann Surg Oncol. 2016;23(S5):1039–1047. doi: 10.1245/s10434-016-5578-4. [DOI] [PubMed] [Google Scholar]
- Özyalvaçlı ME, Ozyalvacli G, Kocaaslan R, Cecen K, Uyeturk U, Kemahli E, et al. Neutrophil-lymphocyte ratio as a predictor of recurrence and progression in patients with high-grade pT1 bladder cancer. CUAJ. 2015;9(3–4):126. doi: 10.5489/cuaj.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore AL, Palleschi G, Fuschi A, Silvestri L, Al Salhi Y, Costantini E, et al. Can daily intake of aspirin and/or statins influence the behavior of non-muscle invasive bladder cancer? A retrospective study on a cohort of patients undergoing transurethral bladder resection. BMC Cancer. 2015;15(1):120. doi: 10.1186/s12885-015-1152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzak EJ, Malkowicz SB. Does quantification of smoking history correlate with initial bladder tumor grade and stage? Curr Urol Rep. 2014;15(7):416. doi: 10.1007/s11934-014-0416-3. [DOI] [PubMed] [Google Scholar]
- Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology. 2015;86(5):968–972. doi: 10.1016/j.urology.2015.05.040. [DOI] [PubMed] [Google Scholar]
- Rausch S, Hennenlotter J, Todenhöfer T, Aufderklamm S, Schwentner C, Sievert KD, et al. Impaired estimated glomerular filtration rate is a significant predictor for non–muscle-invasive bladder cancer recurrence and progression—Introducing a novel prognostic model for bladder cancer recurrence. Urol Oncol Semin Orig Investig. 2014;32(8):1178–1183. doi: 10.1016/j.urolonc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Rink M, Xylinas E, Babjuk M, Pycha A, Karakiewicz PI, Novara G, et al. Smoking reduces the efficacy of intravesical bacillus calmette-guérin immunotherapy in non–muscle-invasive bladder cancer. Eur Urol. 2012;62(6):1204–1206. doi: 10.1016/j.eururo.2012.08.057. [DOI] [PubMed] [Google Scholar]
- Rink M, Xylinas E, Babjuk M, Hansen J, Pycha A, Comploj E, et al. Impact of Smoking on Outcomes of Patients with a History of Recurrent Nonmuscle Invasive Bladder Cancer. J Urol. 2012;188(6):2120–2128. doi: 10.1016/j.juro.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Rink M, Furberg H, Zabor EC, Xylinas E, Babjuk M, Pycha A, et al. Impact of smoking and smoking cessation on oncologic outcomes in primary non–muscle-invasive bladder cancer. Eur Urol. 2013;63(4):724–732. doi: 10.1016/j.eururo.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawazaki H, Arai Y, Ito Y, Sato K, Tsuda H, Yamaga T, et al. Expression of L-type amino acid transporter 1 is a predictive biomarker of intravesical recurrence in patients with non-muscle invasive bladder cancer. RRU. 2021;13:603–611. doi: 10.2147/RRU.S326249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretta V, Altieri V, Morgia G, Di Lallo A, Carrieri G, Allegro R. Cigarette smoking status at diagnosis and recurrence in intermediate-risk non–muscle-invasive bladder carcinoma. Urology. 2013;81(2):277–282. doi: 10.1016/j.urology.2012.09.040. [DOI] [PubMed] [Google Scholar]
- Serretta V, Di Maida F, Baiamonte D, Vella M, Pavone C, Cacciatore L, et al. Does smoking cessation at primary diagnosis reduce the recurrence risk of nonmuscle-invasive bladder cancer? Results of a prospective study. Urol Int. 2020;104(5–6):396–401. doi: 10.1159/000507122. [DOI] [PubMed] [Google Scholar]
- Sfakianos JP, Shariat SF, Favaretto RL, Rioja J, Herr HW. Impact of smoking on outcomes after intravesical bacillus Calmette-Guérin therapy for urothelial carcinoma not invading muscle of the bladder: impact of smoking after intravesical bcg therapy. BJU Int. 2011;108(4):526–530. doi: 10.1111/j.1464-410X.2010.09874.x. [DOI] [PubMed] [Google Scholar]
- Shen Z, Xie L, Chen T, Tian D, Xiaoteng L, Xu H, et al. Risk factors predictive of recurrence and progression for patients who suffered initial recurrence after transurethral resection of stage pt1 bladder tumor in Chinese population: a retrospective study. Medicine. 2016;95(5):e2625. doi: 10.1097/MD.0000000000002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota M, Kiyoshima K, Yokomizo A, Takeuchi A, Kashiwagi E, Dejima T, et al. Suppressed recurrent bladder cancer after androgen suppression with androgen deprivation therapy or 5α-reductase inhibitor. J Urol. 2017;197(2):308–313. doi: 10.1016/j.juro.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Ślusarczyk A, Zapała P, Zapała Ł, Piecha T, Radziszewski P. Prediction of BCG responses in non-muscle-invasive bladder cancer in the era of novel immunotherapeutics. Int Urol Nephrol. 2019;51(7):1089–1099. doi: 10.1007/s11255-019-02183-5. [DOI] [PubMed] [Google Scholar]
- Soria F, Pisano F, Gontero P, Palou J, Joniau S, Serretta V, et al. Predictors of oncological outcomes in T1G3 patients treated with BCG who undergo radical cystectomy. World J Urol. 2018;36(11):1775–1781. doi: 10.1007/s00345-018-2450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnowski R, Bjurlin MA, Verze P, De Nunzio C, Shariat SF, Brausi M, et al. Role of cigarette smoking in urological malignancies and clinical interventions for smoking cessation. Cent European J Urol. 2016;69(4):366–369. doi: 10.5173/ceju.2016.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svatek RS, Hollenbeck BK, Holmäng S, Lee R, Kim SP, Stenzl A, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014;66(2):253–262. doi: 10.1016/j.eururo.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Sylvester RJ, van der Meijden APM, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–465. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Temiz MZ, Colakerol A, Ulus I, Kilic E, Paslanmaz F, Sahin S, et al. Prediction of non-muscle-invasive bladder cancer recurrence during intravesical BCG immunotherapy by use of peripheral blood eosinophil count and percentage: a preliminary report. Cancer Immunol Immunother. 2021;70(1):245–252. doi: 10.1007/s00262-020-02673-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucpinar B, Erbin A, Ayranci A, Caglar U, Alis D, Basal S (2019) Prediction of recurrence in non-muscle invasive bladder cancer patients. Do patient characteristics matter? J BUON Off J Balk Union Oncol 24(4):1659–1665 [PubMed]
- van Osch FHM, Jochems SHJ, van Schooten FJ, Bryan RT, Zeegers MP. Significant role of lifetime cigarette smoking in worsening bladder cancer and upper tract urothelial carcinoma prognosis: a meta-analysis. J Urol. 2016;195(4 Pt 1):872–879. doi: 10.1016/j.juro.2015.10.139. [DOI] [PubMed] [Google Scholar]
- van Osch FHM, Jochems SHJ, Reulen RC, Pirrie SJ, Nekeman D, Wesselius A, et al. The association between smoking cessation before and after diagnosis and non-muscle-invasive bladder cancer recurrence: a prospective cohort study. Cancer Causes Control. 2018;29(7):675–683. doi: 10.1007/s10552-018-1046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gao W, Li J, Wang T, Zhu M, Duan Y. Development and validation of a novel recurrence risk stratification for initial non-muscle invasive bladder cancer in the han chinese population. J Cancer. 2020;11(7):1668–1678. doi: 10.7150/jca.38649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski A, Tanyos SA, Rees JR, Marsit CJ, Kelsey KT, Schned AR, et al. Body mass and smoking are modifiable risk factors for recurrent bladder cancer: BMI and smoking modify bladder cancer. Cancer. 2014;120(3):408–414. doi: 10.1002/cncr.28394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu C, Yan X, Li J, Yang X. Overnight continuous saline bladder irrigation after en bloc resection of bladder tumor does not improve oncological outcomes in patients who have received intravesical chemotherapy. Front Oncol. 2021;10(11):638065. doi: 10.3389/fonc.2021.638065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura S, Terauchi F, Hoshi K, Yamaguchi T, Kawai S. Androgen receptor predicts first and multiple recurrences in non-muscle invasive urothelial carcinoma of the bladder. Pathol Oncol Res. 2019;25(3):987–994. doi: 10.1007/s12253-018-0431-7. [DOI] [PubMed] [Google Scholar]
- Yuruk E, Tuken M, Colakerol A, Serefoglu EC. The awareness of patients with non - muscle invasive bladder cancer regarding the importance of smoking cessation and their access to smoking cessation programs. Int Braz j Urol. 2017;43(4):607–614. doi: 10.1590/s1677-5538.ibju.2016.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeegers MP, Tan FE, Dorant E, van Den Brandt PA. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89(3):630–639. doi: 10.1002/1097-0142(20000801)89:3<630::AID-CNCR19>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Zeng J, Zhang G, Chen C, Li K, Wen Y, Zhao J, et al. Alterations in urobiome in patients with bladder cancer and implications for clinical outcome: a single-institution study. Front Cell Infect Microbiol. 2020;15(10):555508. doi: 10.3389/fcimb.2020.555508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lai Q, Wang S, Meng Q, Mo Z. Clinical value of postoperative neutrophil-to-lymphocyte ratio change as a detection marker of bladder cancer recurrence. Cancer Manag Res. 2021;29(13):849–860. doi: 10.2147/CMAR.S289986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Sun J, Wang K, Tai S, Hua R, Yu Y, et al. Development of a new recurrence-free survival prediction nomogram for patients with primary non-muscle-invasive bladder cancer based on preoperative controlling nutritional status score. CMAR. 2021;13:6473–6487. doi: 10.2147/CMAR.S323844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Zhang Y, Shi L, Lei WC, Qi CS, Zheng H, et al. Gene mutation detection of urinary sediment cells for NMIBC early diagnose and prediction of NMIBC relapse after surgery. Medicine (baltimore) 2019;98(32):e16451. doi: 10.1097/MD.0000000000016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The extracted data can be obtained from the corresponding author upon reasonable request.