Abstract
Introduction
The efficacy and safety of upadacitinib in atopic dermatitis have been defined in clinical trials, but long-term real-life experience, essential for clinical decision-making, is still limited. We aimed to assess the effectiveness and tolerance of upadacitinib in a real-life cohort of adults and adolescents with severe atopic dermatitis in whom previous systemic therapies largely failed.
Methods
Retrospective cohort study collecting data from adults and adolescents treated with upadacitinib 15 or 30 mg per day between July 2021 to August 2022. The outcomes for effectiveness were evaluated by the percentage of patients who achieved a validated Investigator’s Global Assessment for atopic dermatitis (vIGA-AD) of 0 (clear) or 1 (almost clear) and/or an improvement of at least 75% on the Eczema Area and Severity Index (EASI 75) at the end of the follow-up. All treatment-emergent adverse events were collected.
Results
A total of 29 patients were included (22 adults and 7 adolescents), with a median follow-up of 54.4 weeks. At the end of the follow-up, 23 patients (79.3%) reached a vIGA-AD of 0/1, and 24 patients (82.7%) achieved EASI 75. Among patients treated with upadacitinib after initial failure of first- and/or second-line treatment with biologics or baricitinib, 5/7 patients (71.4%) reached a vIGA-AD score of 0/1. Disease control was slightly better in adults than in adolescents (81.8% vs 71.4% reached the efficacy endpoint, respectively). Response rate in patients with upadacitinib 15 mg seemed better than in clinical trials or network meta-analysis. Safety data were reassuring; lipid changes were the most frequent adverse event.
Conclusion
This real-life study confirms the effectiveness of upadacitinib, particularly for the treatment of atopic dermatitis recalcitrant to conventional systemic agents, biologics or baricitinib. Induced lipid changes require close follow-up.
Keywords: Atopic dermatitis, Real-life study, JAK inhibitor, Upadacitinib, Effectiveness, Tolerance
Key Summary Points
| This real-life study assessed the effectiveness and tolerance of upadacitinib in severe atopic dermatitis in a series of 29 adults and adolescents with a median follow-up of 54.4 weeks |
| Upadacitinib was effective, also in severe and recalcitrant atopic dermatitis after failure of treatment with biologics or baricitinib |
| Effectiveness of upadacitinib 15 mg suggests that it could be considered as a starting dose for older patients, in case of comorbidities, or as a tapered dose for responders |
| Induced lipid changes were the most frequent adverse events, requiring close follow-up |
Introduction
The emergence of new systemic treatments for atopic dermatitis (AD) and their large potential to change AD management have made them a current center of interest for many publications, conferences and updates in dermatology [1, 2]. The efficacy and safety of these molecules [biologics and/or Janus kinase (JAK) inhibitors] are being evaluated in clinical trials, and several of them have already received marketing authorization. However, clinical trials are conducted in controlled situations and with selected populations, which do not necessarily reflect prescribing conditions in daily practice. Therefore, the evaluation of these molecules in real-life settings is essential for clinical practice because they assess treatment outcomes within patient populations displaying a heterogeneity of AD phenotypes.
Upadacitinib is an oral JAK 1-selective inhibitor approved for the treatment of moderate-to-severe AD in adults and adolescents. Its efficacy and safety have been demonstrated in clinical trials [3–5].
The aim of this monocentric retrospective study was to assess effectiveness and tolerance of upadacitinib in real-life conditions in adolescents and adults with severe AD who failed treatment (because of inefficacy, contraindication or intolerance) with other systemic agents, i.e., cyclosporine, biologics (dupilumab, tralokinumab, nemolizumab or risankizumab) or baricitinib, a JAK-1 and 2 inhibitor.
Methods
Patients aged ≥ 12 years were included from July 2021 to August 2022. The primary outcome was evaluated by the percentage of patients who achieved a validated Investigator’s Global Assessment for AD (vIGA-AD) of 0 (clear) or 1 (almost clear) and/or an improvement of at least 75% of the Eczema Area and Severity Index (EASI 75) at the end of the follow-up. All adverse events were reported during the study. This study and data collection were conducted with the approval of the hospital and faculty institutional review board (Commission d’Ethique Biomédicale Hospitalo-Facultaire) of Université catholique de Louvain (UCLouvain), Belgium (registration no. B4032020000018). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. The patients reported in this article have given written informed consent to participate and for publication of their case details.
Results
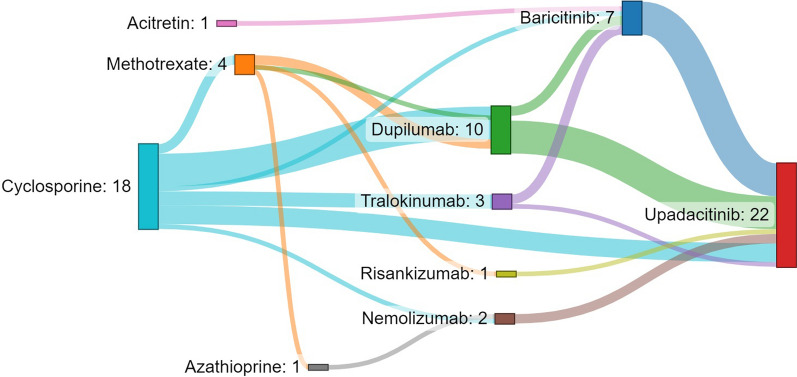
Twenty-two adults (treated with upadacitinib 30 mg/day except for three patients who received 15 mg/day) and seven adolescents (all treated with 15 mg/day) were enrolled, with a median ± interquartile range (IQR) follow-up duration of 54.4 ± 18.3 weeks. Patients’ characteristics are detailed in Table 1. All patients had severe disease at baseline with adults presenting median ± IQR vIGA-AD and EASI scores of 4 ± 1 and 22.0 ± 4.6, respectively, and adolescents presenting vIGA-AD and EASI scores of 4 ± 0 and 23.4 ± 3.8, respectively. All patients were previously treated with a mean of two systemic drugs before upadacitinib initiation (Fig. 1). At the first follow-up visit between 12 to 16 weeks, 23 patients (79.3%) achieved a vIGA-AD score of 0 or 1 and 17 patients (58.6%) an EASI 75. At the end of the follow-up, 23 patients (79.3%) reached a vIGA-AD of 0 or 1, 24 patients (82.7%) achieved an EASI 75 and 17 (58.6%) an EASI 90. Improvement of vIGA-AD and EASI was significant (P value < 0.001, using a pairwise Wilcoxon signed-rank test) when comparing baseline values with both week 12–16 and last follow-up visit scores. For two adolescents and three adults (one treated with 15 mg/day), disease remained inadequately controlled. Two adults rapidly improved when shifted at week 42 and 62, respectively, to abrocitinib (another JAK 1 inhibitor) and a third one after increasing upadacitinib dosage from 15 to 30 mg. Among patients treated with upadacitinib after initial failure of first- and/or second-line treatment with biologics or baricitinib (due to inefficacy except for one patient with severe dupilumab-induced conjunctivitis), 5/7 patients (71.4%) reached the primary outcome. Disease control was slightly better in adults than in adolescents (81.8% vs 71.4% reached the efficacy endpoint, respectively).
Table 1.
Demographics and clinical characteristics of patients included in the study
| Variable | Value |
|---|---|
| Total population | 29 patients |
| Median age, years (± IQR) | 37.0 ± 37.0 |
| Age group, n (%) | |
| < 18 years | 7 (24.1) |
| ≥ 18 years | 22 (75.9) |
| Sex, male, n (%) | 20 (69.0) |
| Age at onset of atopic dermatitis, n (%) | |
| Child | 22 (75.9) |
| Teenager | 1 (3.4) |
| Adult | 6 (20.7) |
| History of personal atopy, n (%) | |
| Allergic asthma | 8 (53.3) |
| Allergic rhino-conjunctivitis | 8 (53.3) |
| Missing, n/n total | 14/29 (48.3) |
| Previous topical treatments for atopic dermatitis, n (%) | |
| Emollients | 23 (100.0) |
| Topical corticosteroids | 23 (100.0) |
| Topical immunomodulators | 12 (52.2) |
| Missing, n/n total | 6/29 (20.7) |
| Previous systemic treatments for atopic dermatitis, n (%) | |
| Oral corticosteroids | 11 (37.9) |
| Cyclosporine | 18 (62.0) |
| Acitretin | 1 (3.4) |
| Phototherapy | 2 (6.9) |
| Methotrexate | 4 (13.8) |
| Dupilumab | 10 (34.5) |
| Failure of treatment, n/n total | 9/10 |
| Adverse events, n/n total | 1/10 |
| Tralokinumab | 3 (10.3) |
| Failure of treatment, n/n total | 3/3 |
| Nemolizumab | 2 (6.9) |
| Failure of treatment, n/n total | 2/2 |
| Baricitinib | 7 (24.1) |
| Failure of treatment, n/n total | 7/7 |
| Clinical severity at baseline, median (± IQR) | |
| v-IGA-AD | |
| Adults | 4 ± 1 |
| Adolescents | 4 ± 0 |
| EASI | |
| Adults | 22.0 ± 4.6 |
| Adolescents | 23.4 ± 3.8 |
v-IGA-AD validated Investigator’s Global Assessment for atopic dermatitis, EASI Eczema Area and Severity Index, IQR interquartile range
Fig. 1.
Sankey diagram illustrating the sequence of systemic treatments for 22 adult patients with atopic dermatitis. The size of the box and width of the lines are proportional to the number of patients
At least one adverse event (AE) occurred in 13/29 (44.8%) patients, the most frequent being increased total cholesterol levels > 200 mg/dl [24.1%—6 adults, 1 adolescent; range, 207–330 mg/dl; normal value (NV) < 190 mg/dl]. Acne (20.7%—4 adults, 2 adolescents), lymphopenia (3.4%—1 adult, grade II), transitory neutropenia (6.9%—2 adults, grade II) and anemia (3.4%—1 adult, grade I) were also observed. One adult temporarily showed increased levels of aspartate aminotransferase (AST, 66 U/l, NV: 15–40 U/l) and lactate dehydrogenase (LDH, 439 U/l, NV < 250 U/l), one other had persistent increased gamma-glutamyl transpeptidase (GGT, 248 U/l, NV: 8–55 U/l), and one patient reported mild headaches (3.4%—1 adult). No thromboembolic, major cardiovascular AEs or severe infections were reported. No AEs led to treatment discontinuation.
Discussion
This real-life study demonstrates the 1-year effectiveness, safety and tolerance of upadacitinib in adults and adolescents, also in severe and recalcitrant AD after treatment failure with biologics or baricitinib. It also confirms the rapid clinical improvement of upadacitinib, with almost 80% of patients achieving vIGA-AD of 0 or 1 at week 12–16. These results are even better than those of the AD Up trial [4] where 40% and 59% of patients achieved a vIGA-AD of 0 or 1 at week 16, respectively, with upadacitinib 15 mg and 30 mg, combined with topical corticosteroids. The effectiveness and tolerance data are also comparable to those of other real-life studies with upadacitinib [6–11]. It adds further evidence to recent studies that have shown a superior efficacy of upadacitinib 30 mg to that of baricitinib 2 or 4 mg, dupilumab or tralokinumab [12–14]. However, the response rate with upadacitinib 15 mg in the present real-life series seemed better than in clinical trials or network meta-analysis, suggesting that lowering dosage to 15 mg could be an option in responders or as a starting dose for patients with moderate disease, for older patients or in case of comorbidities. The limitations of the study include the fact that it is a single-center study with a limited number of patients and the fact that it is a retrospective analysis with some missing data, notably in terms of scores (NRS-pruritus). Additionally, all patients included in this study were previously treated with systemic immunosuppressants and/or immunomodulators. This is in part explained by the fact that, in Belgium, access to upadacitinib is only granted as a third-line treatment in adults (after failure, contraindication or intolerance of ciclosporin and then of dupilumab and/or tralokinumab). However, in line with the objectives of our study, at present, this represents real-life usage of this medication for AD in Belgium.
Conclusion
Upadacitinib in real life is an effective and well-tolerated treatment, also for AD recalcitrant to classical immunosuppressive agents, biologic treatments and/or baricitinib. Safety data showed no new findings, although induced lipid changes require close follow-up. Upadacitinib longer term effectiveness, lower dose regimens as well as comparison with other JAK 1-selective inhibitors need further evaluation.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service and Open Access Fee were funded by the authors.
Medical Writing and Editorial Assistance
We thank Dr. Mariana Andrade, MD (Andrade-Evrard SPRL), who provided editorial assistance.
Author Contributions
Axel De Greef: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Visualization; Pierre-Dominique Ghislain: Data Curation, Investigation, Writing—Review & Editing, Supervision; Laurence de Montjoye: Investigation, Data Curation; Marie Baeck: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Review & Editing, Supervision.
Disclosures
Pierre-Dominique Ghislain discloses his past participation as an investigator and as a scientific advisor for AbbVie. Pierre-Dominique Ghislain and Marie Baeck have previously participated as speakers in events sponsored by AbbVie. None of the authors has scientific or financial conflicts of interests to declare in the current reported study.
Compliance with Ethics Guidelines
This study and data collection were conducted with the approval of the hospital and faculty institutional review board (Commission d’Ethique Biomédicale Hospitalo-Facultaire) of Université catholique de Louvain (UCLouvain), Belgium (registration number B4032020000018). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. The patients in this manuscript have given written informed consent to participate and for publication of their case details.
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.McLornan DP, Pope JE, Gotlib J, Harrison CN. Current and future status of JAK inhibitors. Lancet. 2021;398(10302):803–816. doi: 10.1016/S0140-6736(21)00438-4. [DOI] [PubMed] [Google Scholar]
- 2.Schneider S, Li L, Zink A. The new era of biologics in atopic dermatitis: a review. Dermatol Pract Concept. 2021;11(4):e2021144. doi: 10.5826/dpc.1104a144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2150. doi: 10.1016/S0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 4.Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;398(10302):746. doi: 10.1016/S0140-6736(21)00589-4. [DOI] [PubMed] [Google Scholar]
- 5.Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the measure up 1 and measure up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404–413. doi: 10.1001/jamadermatol.2022.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanlerberghe J, Dezoteux F, Martin C, et al. Effectiveness and tolerance of Janus kinase inhibitors for the treatment of recalcitrant atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol. 2022 doi: 10.1016/j.jaad.2022.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Chiricozzi A, Gori N, Narcisi A, et al. Effectiveness and safety of upadacitinib in the treatment of moderate-severe atopic dermatitis: a multicentric, prospective, real-world, cohort study. Drugs R D. 2022;22(3):245–252. doi: 10.1007/s40268-022-00396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feraru G, Nevet MJ, Samuelov L, et al. Real-life experience of upadacitinib for the treatment of adult patients with moderate-to-severe atopic dermatitis—a case series. J Eur Acad Dermatol Venereol. 2022;36(10):e832–e833. doi: 10.1111/jdv.18311. [DOI] [PubMed] [Google Scholar]
- 9.Napolitano M, Fabbrocini G, Genco L, Martora F, Potestio L, Patruno C. Rapid improvement in pruritus in atopic dermatitis patients treated with upadacitinib: a real-life experience. J Eur Acad Dermatol Venereol. 2022;36(9):1497–1498. doi: 10.1111/jdv.18137. [DOI] [PubMed] [Google Scholar]
- 10.Pereyra-Rodriguez JJ, Herranz P, Figuras-Nart I, et al. Upadacitinib for the treatment of atopic dermatitis in a Spanish cohort-real life: fifty-two-week follow-up results. Dermatitis. 2022;33(6S):S124–S127. doi: 10.1097/DER.0000000000000958. [DOI] [PubMed] [Google Scholar]
- 11.Hagino T, Saeki H, Kanda N. The efficacy and safety of upadacitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2022;49(11):1158–1167. doi: 10.1111/1346-8138.16549. [DOI] [PubMed] [Google Scholar]
- 12.Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2022;158(2):219. doi: 10.1001/jamadermatol.2021.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drucker AM, Morra DE, Prieto-Merino D, et al. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022;158(5):523–532. doi: 10.1001/jamadermatol.2022.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Licata G, Gambardella A, Tancredi V, et al. Face atopic dermatitis resistant to dupilumab: a case series of three patients successfully treated with upadacitinib. J Eur Acad Dermatol Venereol. 2022;36(2):e150–e152. doi: 10.1111/jdv.17705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.