Correction to: Journal of Neurology 10.1007/s00415-023-11560-1
The original version of this article unfortunately contained a mistake. The corrected details are given below for your reading.
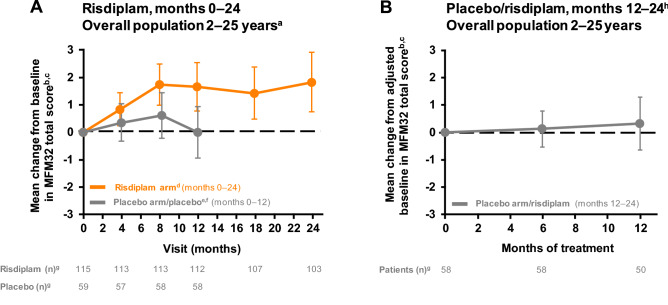
In figure 1, there is an error in the n numbers below the graph in Panel 1b for the placebo group. The n numbers underneath Panel 1b should be 58 58 50.
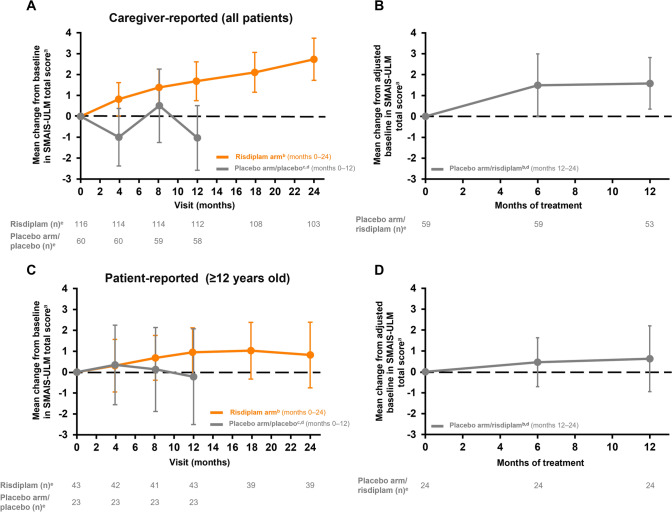
There is an error within Fig. 4. The dashed lines at ~ − 1.4 should be at 0. They have been moved downwards and are no longer in the correct place.
The corrected Figs. 1 and 4 are given in the following page.
Fig. 1.
Change from baseline in MFM32 total score in patients treated with risdiplam for up to 24 months and those who previously received placebo until study month 12. aThirty-one percent (55/180) of the SUNFISH intent-to-treat population were 2–5 years old at baseline. b± 95% CI. cBaseline is the last measurement prior to the first dose of risdiplam or placebo. dData cut-off: 30 Sep 2020. eData cut-off: 6 Sep 2019. fPatients in the placebo arm received placebo for 12 months followed by risdiplam treatment for 12 months. gNumber of patients with valid results = number of patients with an available total score (result) at respective time points. Intent-to-treat patients. hPatients in the placebo arm received placebo for 12 months followed by risdiplam treatment for 12 months. Placebo period not shown in this graph. CI confidence interval, MFM32 32-item motor function measure
Fig. 4.
Change in caregiver- and patient-reported SMAIS upper limb total score from baseline in patients receiving risdiplam for up to 24 months and those who previously received placebo up to study month 12. a± 95% CI. Baseline is the last measurement prior to the first dose of risdiplam or placebo. bData cut-off: 30 Sep 2020. cData cut-off: 6 Sep 2019. dPatients in the placebo arm received placebo for 12 months followed by risdiplam treatment for 12 months. Risdiplam period not shown in this graph. eNumber of patients with valid results = number of patients with an available total score (result) at respective time points. Intent-to-treat patients. SMAIS scores range from 0 to 44 following rescoring to a 0–2 response scale for each item. Higher scores indicate greater independence in completing daily activities. CI confidence interval, SMA spinal muscular atrophy, SMAIS SMA Independence Scale, SMAIS-ULM SMA Independence Scale-Upper Limb Module