Abstract
Introduction
This study describes the epidemiological, clinical, patient-reported and economic burden of inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), in Spain.
Methods
A systematic review was performed of observational studies reporting the epidemiological, clinical, patient-reported and economic burden of IBD in the Spanish population, from 2011 to 2021. Original articles and conference abstracts published in English or Spanish were eligible.
Results
A total of 45 publications were included in the review. The incidence of IBD in adults ranged from 9.6 to 44.3 per 100,000 inhabitants (4.6 to 18.5 for CD and 3.4 to 26.5 for UC). The incidence increased between 1.5- and twofold from 2000 to 2016 (regionally). Up to 6.0% (CD) and 3.0% (UC) IBD-associated mortality was reported. Disease onset predominantly occurs between 30 and 40 years (more delayed for UC than CD). Most frequently reported gastrointestinal manifestations are rectal bleeding in UC and weight loss in CD. Extraintestinal manifestations (EIM) have been described in up to 47.4% of patients with CD and 48.1% of patients with UC. Psychiatric comorbidities were frequently reported in both CD and UC (depression up to 20% and anxiety up to 11%). Reduced health-related quality of life (HRQoL) compared to the general population was reported. Significant symptomatology was associated with high levels of anxiety, depression, stress and lower HRQoL. Main healthcare resources reported were emergency department visits (24.0%), hospitalization (14.7%), surgery (up to 11%) and use of biologics (up to 60%), especially in CD. Direct and indirect annual costs per patient with UC were €1754.1 and €399.3, respectively.
Conclusion
Patients with CD and UC present a high disease burden which negatively impacts their HRQoL, leading to elevated use of resources.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02473-6.
Keywords: Adult, Burden, Costs, Crohn’s disease, Epidemiology, Inflammatory bowel disease, Paediatric, Systematic review, Ulcerative colitis
Key Summary Points
| The incidence of IBD in adults ranged from 9.6 to 44.3 per 100,000 inhabitants (4.6 to 18.5 for CD and 3.4 to 26.5 for UC), the most recent estimate being 16.2 per 100,000 inhabitants (7.4 CD and 8.1 UC). |
| Patients with IBD frequently present psychiatric comorbidities, in both CD and UC. |
| HRQoL impairment in patients with IBD is high compared to the general population. |
| The resource use related to IBD is high in both CD and UC. |
| IBD has high associated costs for the national healthcare system (mainly related to hospitalisations, surgeries and medication). |
Introduction
Inflammatory bowel disease (IBD) is a term for two conditions (Crohn’s disease and ulcerative colitis) that are characterized by chronic inflammation of the gastrointestinal tract, differentiated by location and depth of involvement in the bowel wall. Crohn’s disease (CD) can affect any portion of the gastrointestinal tract (most often in the ileum), causing transmural inflammation reaching through the multiple layers of the walls of the gastrointestinal tract; ulcerative colitis (UC) involves the colon and rectum, with inflammation limited to the mucosal layer of the colonic tissue [1]. Nonetheless, sometimes they can be indistinguishable and are classified as inflammatory bowel disease unclassified (IBDU) [2].
Typical gastrointestinal (GI) signs and symptoms of both CD and UC include diarrhoea, bowel urgency, abdominal pain, gastrointestinal bleeding, weight loss and malnutrition [1]. Complications may include stricture and blockage (bowel obstruction), perforation, fistula and abscess in CD and perforated bowel and toxic megacolon in UC. Besides the digestive symptoms and complications resulting from the disease, extraintestinal manifestations (EIM) affect up to 25% of patients with IBD [3, 4]. As a result of recurrent clinical manifestations of the disease, patients often report poor health-related quality of life (HRQoL).
IBD is characterised by heterogeneous clinical manifestations and a chronic relapsing–remitting course caused by multiple genetic and environmental factors [5, 6].
Induction and maintenance of remission are the main goals of IBD treatment. Nowadays, a wide range of therapies are available, including aminosalicylates, corticosteroids, immunosuppressive agents, antibiotics, advanced therapies (including biologics and small molecules) and surgery [7]. In recent years, the early introduction of immunosuppressive and biological therapies has become a frequent strategy with tailored therapeutic approaches to meet specific patients’ needs [8–10].
An estimated 2.5–3 million people in Europe are affected by IBD, with a direct healthcare cost of 4.6–5.6 billion euros/year in Europe, mostly due to hospitalisations and surgeries [11]. Furthermore, the prevalence of IBD continues to rise and thus the impact on healthcare budgets is expected to increase worldwide [11].
There is substantial published evidence on the disease burden in Spain; however, such studies are highly heterogeneous, showing geographical and temporal differences. Therefore, clinicians and decision-makers would benefit from the availability of a comprehensive overview of the latest published data about the burden of IBD in Spain.
We aim to review the existing literature to identify observational studies reporting the epidemiological, clinical, patient-reported and economic burden of IBD in Spain, as a whole, and separately for CD or UC.
Methods
A systematic review of observational studies reporting the epidemiological, clinical, patient-reported and economic burden of IBD (UC or CD) from the last 10 years (2011–2021) was carried out following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Cochrane Handbook for Systematic Reviews of Interventions [12, 13].
Data Sources and Search Strategy
The international databases PubMed/Medline and Cochrane library, and the national Medicina en Español (MEDES) and the Índice Bibliográfico Español en Ciencias de la Salud (IBECS) databases, were searched to identify relevant publications for review. Additionally, manual searches (Google and Google Scholar) were conducted to identify studies published during the last 2 years at key national and European congresses such as Asociación Española de Gastroenterología, Sociedad Española de Gastroenterología y Hepatología y Nutrición pediátrica, Congress of European Crohn´s and Colitis Organisation and Sociedad Española de Patología Digestiva. Finally, the bibliographic citations of the selected articles were also reviewed to retrieve relevant publications that had not been detected in the bibliographic search.
The different databases were searched using both MeSH (Medical Subject Headings) and free-text terms, combined with the Boolean connectors OR and AND. The list of MeSH and free-text terms and the search strategy used in the Cochrane PubMed/MedLine database, IBECS and MEDES are detailed in Table S1 of the supplementary material.
Study Selection
Two independent reviewers screened all identified references at two levels. Level 1 entailed a wide screen based on item titles and/or abstracts. Level 2 involved two reviewers independently reviewing the full-text articles and applying the inclusion/exclusion criteria. At both screening levels, discrepancies were resolved by consensus or by involving a third team member.
Eligibility Criteria
To obtain maximum records in the recent period of biological therapies era, original articles and conference abstracts of observational studies conducted in the Spanish population published in English or in Spanish between 2011 (February) and 2021 (October) were eligible. Studies performed outside Spain, not including/reporting data of the Spanish population, were excluded. Table S2 of the supplementary material lists the inclusion and exclusion criteria.
Data Extraction and Quality Assessment
Data extracted included disease epidemiology (prevalence, incidence and mortality), demographics (age and sex), clinical and treatment characteristics (age of onset, location of the lesion, flare-ups, EIM, disease behaviour and comorbidities), patient-reported outcomes (e.g. HRQoL), resource utilization, and costs (direct and indirect) of IBD overall and separately for CD and UC, where available. Additionally, from each selected publication, the data recorded included type and size of population, data collection period, geographic scope, study design and data source. Two independent reviewers extracted all data and resolved discrepancies by consensus. A standardised data extraction form was used to extract the data from the selected articles. No formal statistical analysis was performed. Frequencies and ranges have been reported to summarise the number of studies and publications. Some extracted data are represented graphically.
The quality of included observational studies was assessed using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [14]. Two independent reviewers assessed study quality, with discrepancies being resolved by consensus.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
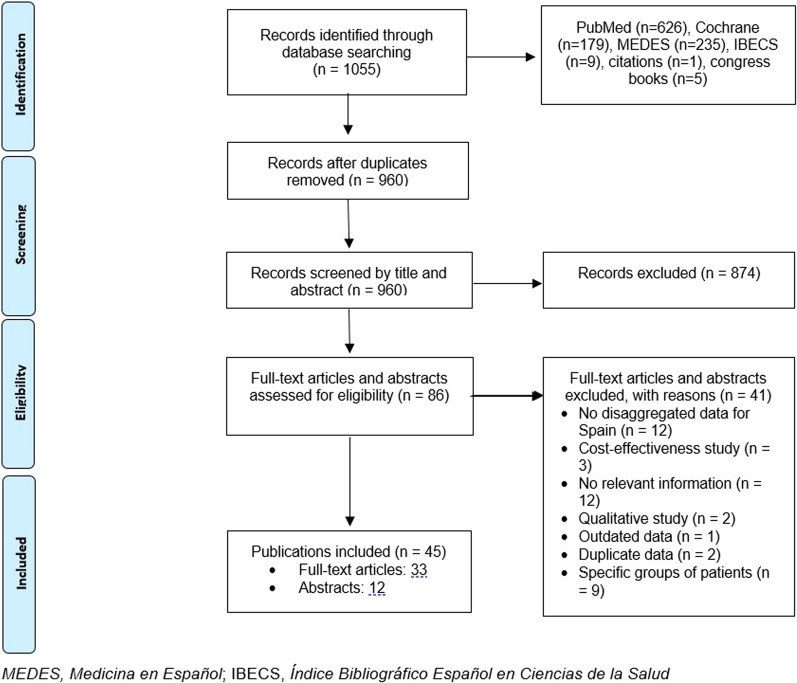
As shown in the PRISMA flow diagram (Fig. 1), 1055 records were identified through database searches. After duplicates removal and the established selection/inclusion criteria were applied, 45 publications (33 full-text articles and 12 abstracts) were included in the synthesis.
Fig. 1.
PRISMA flow diagram showing the selection process of the included publications
The characteristics of each publication are detailed in Table 1. Of note, the data described in the 45 publications are derived from 38 different studies. Out of 45 publications, 34 targeted patients with IBD, with different levels of granularity in reporting the data (IBD overall and/or separately for UC and CD), while five focused exclusively on CD and six on UC. A summary of the main characteristics of these studies is presented in Table 2.
Table 1.
Main characteristics of selected publications/studies
| Author (year)/study acronyma | Geographic scope | Study design | Collection time | Population type and size | Population age, years | Data source | Recovered variables |
|---|---|---|---|---|---|---|---|
|
Nunes (2013) [75] ENEIDA registry |
Spain | Retrospective | 2013 | 3224 CD patients | NS | Registry |
Demographic characteristics Risk factors Disease severity Treatment utilization patterns |
|
Andreu (2014) [34] ENEIDA registry |
Spain | Retrospective | NS | 11,983 IBD patients: 6200 CD, 5783 UC | NS | Registry |
Demographic characteristics Risk factors Disease severity Age of onset |
|
Chaparro (2019) [48] ENEIDA registry |
Spain | Retrospective | 2007–2017 | 21,200 IBD patients: 10,480 CD, 10,025 UC | All | Registry |
Demographic characteristics Disease severity Treatment utilization patterns Resource use |
| Schreiber (2013) [76] | Spain | Cross-sectional | 2010 | 150 UC patients, 100 physicians |
Adult (> 18) |
Survey |
Demographic characteristics Disease severity Comorbidity Treatment utilization patterns |
|
Marín-Jiménez (2014) [35] Aquiles study |
Spain | Cross-sectional | 2008–2010 | 526 IBD patients: 300 CD, 218 UC |
Adult (> 18) |
Registry |
Demographic characteristics Comorbidity Disease severity |
|
Martín-de-Carpi (2014) [28] EXPERIENCE registry |
Spain | Retrospective | 1985–2009 | 2602 paediatric IBD patients: 278 CD, 198 UC, 19 IBDU |
Paediatric (< 18) |
Registry |
Incidence Demographic characteristics Age of onset |
|
López-Sanromán (2017) [43] UC-LIFE study |
Spain | Cross-sectional | 2014 | 436 UC patients |
Adult (> 18) |
Survey |
Demographic characteristics Disease severity PROs |
|
Panés (2017) [44] CRONICA-UC study |
Spain | Prospective | 2017 | 199 UC patients |
Adult (> 18) |
N/A (prospective) |
Demographic characteristics Disease severity Treatment utilization patterns PROs |
| Péntek (2017) [47] | Spain | Cross-sectional | 2016 | Questionnaire sent to an expert gastroenterologist (focus on CD) | NS | Survey |
Treatment utilization patterns Pharmacological cost |
|
Bastida G (2021) [77] VERNE study |
Spain | Retrospective | 2011–2013 | 310 IBD patients: 194 CD, 116 UC | Adult | Hospital clinical record |
Demographic characteristics Disease severity Treatment utilization patterns |
|
Chaparro M (2021) [19] EpidemIBD study |
Spain | Prospective | 2017 | 3611 IBD patients: 1647 CD, 1807 UC | Adult | N/A (prospective) |
Incidence Disease severity Demographic characteristics Treatment utilization patterns Resource use |
|
Guevara M (2021) [23] EPIC study |
Spain | Prospective | 1992–2016 | 32,663 healthy volunteers (32 CD and 57 UC) |
Adult (> 18) |
N/A (prospective) |
Incidence Risk factors Disease severity Age of onset |
|
Marín-Jiménez I (2018)[26] EPICURE study |
Spain | Retrospective | 2011 | 274,640 inhabitants (41,840 UC patients) |
Adult (> 18) |
Hospital clinical record |
Incidence Prevalence Resource use |
|
Martín-de-Carpi J (2013) [27] SPIRIT registry |
Spain | Retrospective | 1985–2009 | 2107 IBD patients: 1165 CD, 788 UC, and 154 IBDU |
Paediatric (< 18) |
Hospital clinical record |
Incidence Demographic characteristics Age of onset |
| Gómez-García (2013) [37] | Andalucía | Retrospective | 1996–2013 | 812 IBD patients: 419 CD, 393 UC | NS | Hospital clinical record |
Demographic characteristics Comorbidity Disease severity Age of onset Treatment utilization patterns |
| Chaaro-Benalla (2017) [18] | Andalucía | Retrospective | 1995–2014 | 2519 IBD patients: 1224 CD, 1295 UC |
Adult (> 14) |
Hospital clinical record |
Incidence Demographic characteristics Age of onset |
| *Diáz-Alcázar MM (2020) [78] | Andalucía | Retrospective | NS | 100 IBD patients: 28 CD, 70 UC, 2 IBDU | All | Hospital clinical record |
Demographic characteristics Disease severity |
| *Diáz-Alcázar MM (2020) [79] | Andalucía | Retrospective | NS | 50 IBD patients |
Adult (> 65) |
Hospital clinical record | Demographic characteristics |
| *Diáz-Alcazar MM (2021) [50] | Andalucía | Retrospective | NS | 100 IBD patients |
All (> 65, < 65) |
Hospital clinical record |
Demographic characteristics Disease severity Age of onset Treatment utilization patterns Resource use |
| Merino Gallego E (2021) [73] | Andalucía | Retrospective | NS | 122 IBD patients | NS | Hospital clinical record |
Disease severity Comorbidity |
| Abautret-Daly (2017) [42] | Asturias | Cross-sectional | 2017 | 18 IBD patients: 8 CD, 10 UC; 19 controls |
Adult (30–70) |
Hospital clinical record + survey |
Demographic characteristics Treatment utilization patterns PROs |
| Cordero Jorge V (2020) [32] | Canarias | Ambispectiveb | NS | 103 CD patients |
All (< 16, 17–40) |
Hospital clinical record |
Demographic characteristics Disease severity Resource use |
| Cordero Jorge V (2020) [49] | Canarias | Ambispectiveb | 1987–2019 | 102 UC patients |
All (< 16, 17–40) |
Hospital clinical record |
Demographic characteristics Disease severity Resource use |
| García (2020) [36] | Cantabria | Ambispectiveb | 2018 | 1448 IBD patients: 680 CD, 700 UC | NS | Hospital clinical record |
Demographic characteristics Comorbidity Disease severity Age of onset Treatment utilization patterns Resource use |
| Ramos A (2015) [53] | Castilla y León | Cross-sectional | 2012–2013 | 293 IBD patients: 151 CD, 142 UC |
Adult (18–67) |
Survey |
Prevalence Demographic characteristics Age of onset Treatment utilization patterns Productivity |
| Lucendo (2014) [25] | Castilla-La Mancha | Retrospective | 2000–2012 | 1047 IBD patients: 599 CD, 436 UC, 12 IBDU |
Adult (> 16) |
Hospital clinical record |
Incidence Prevalence Demographic characteristics Disease severity Age of onset Comorbidity |
| Casellas (2012) [38] | Cataluña | Cross-sectional | 2012 | 54 IBD patients: 43 CD, 11 UC | NS | Hospital clinical record + survey |
Demographic characteristics Disease severity Age of onset Treatment utilization patterns PROs |
| Marín (2013) [39] | Cataluña | Cross-sectional | 2013 | 355 IBD patients: 85 CD, 115 UC; 200 controls |
Adult (25–65) |
survey |
Demographic characteristics Comorbidity Treatment utilization patterns PROs Resource use |
| Aldeguer (2016) [31] | Cataluña | Retrospective | 2002–2012 | 285 UC patients |
Adult (> 18) |
Hospital clinical record |
Demographic characteristics Comorbidity Resource use Direct cost Productivity Absenteeism/presenteeism |
| Brunet (2018) [16] | Cataluña | Retrospective | 2011–2017 | IBD patients: number of patients NS | All | Registry |
Incidence Prevalence Mortality Demographic characteristics |
| Brunet (2020) [52] | Cataluña | Retrospective | 2011–2017 | CD patients: number of patients NS | NS | Registry |
Treatment utilization patterns Resource use |
| **Iglesias-Rey (2013) [33] | Galicia | Cross-sectional | 2009–2010 | 793 IBD patients: 323 CD, 470 UC | NS | Hospital clinical record + survey |
Demographic characteristics Disease severity Age of onset Comorbidity Treatment utilization patterns PROs Resource use |
| **Iglesias-Rey (2014) [40] | Galicia | Cross-sectional | 2009–2010 | 793 IBD patients: 323 CD, 470 UC |
Adult (> 18) |
Survey | PROs |
|
***Vegh (2014) [29] EpiCom study |
Galicia | Prospective | 2011 | 102 (2010) and 97 (2011) IBD patients |
Adult (> 15) |
N/A (prospective) | Incidence |
| ***Burisch J (2014) [17] EpiCom study | Galicia | Prospective | 2010 | 89 IBD patients | All | N/A (prospective) | Incidence |
|
***Fernández A (2015) [22] EpiCom study |
Galicia | Prospective | 2010 | 106 IBD patients (102 adult and 4 paediatric): 53 CD, 47 UC | All | N/A (prospective) |
Incidence Demographic characteristics Disease severity |
|
****De Castro ML (2020) [46] EC-IBD and EpiCOM studies |
Galicia | Retrospective | 1991–2011 | IBD patients: NA |
Adult (> 15) |
Registry | Treatment utilization patterns |
|
****De Castro ML (2020) [80] EC-IBD and EpiCOM studies |
Galicia | Retrospective | 1991–2011 | 102 IBD patients: 35 CD, 65 UC, 1 IBDU |
Adult (> 15) |
Registry |
Incidence Demographic characteristics Age of onset |
|
****De Castro Parga ML (2020) [21] EC-IBD and EpiCOM studies |
Galicia | Retrospective | 1991–2011 | 102 IBD patients: 35 CD, 65 UC, 1 IBDU |
Adult (> 15) |
Registry |
Incidence Demographic characteristics Disease severity |
|
Hernández V (2021) [24] Epi-IBD |
Galicia | Prospective | 2010 | 100 IBD patients: 34 CD, 49 UC, 17 IBDU | All | N/A (prospective) |
Incidence Demographic characteristics Disease severity Age of onset |
| Algaba (2013) [15] | Madrid | Prospective | 2005–2011 | 590 IBD patients: 313 CD, 256 UC, 21 IBDU | All | N/A (prospective) |
Demographic characteristics Comorbidity Disease severity Age of onset Treatment utilization patterns |
| Jijón-Andrade J (2020) [81] | Madrid | Retrospective | 2010–2020 | 57 IBD patients: 31 CD, 26 UC | Paediatric | Hospital clinical record | Treatment utilization patterns |
| López Cortés (2016) [41] | Navarra | Cross-sectional | 2014 | 100 IBD patients: 59 CD, 41 UC | NS | Hospital clinical record + Survey |
Demographic characteristics Disease severity Age of onset PROs |
| Ballester (2017) [30] | Valencia | Ambispectiveb | 2017 | 1211 IBD patients: 594 CD, 617 UC | NS | Hospital clinical record + patient interview |
Mortality Demographic characteristics Disease severity Age of onset Treatment utilization patterns |
| Rodríguez A (2021) [45] | Valencia | Prospective | NS | 122 CD patients |
Adult (18–40) |
N/A (prospective) | PROs |
IBD inflammatory bowel disease, IBDU inflammatory bowel disease unclassified, CD Crohn’s disease, UC ulcerative colitis, N/A not applicable, NS not specified, PROs patient-reported outcomes
*,**,***,***Data from same study
aStudy acronym is shown in italics
bTwo-way study (retrospective and prospective) from inception of the study
Table 2.
Summary of key characteristics of selected studies
| Target population by IBD type, % (n) | N = 38 |
| IBD (overall IBD, CD and/or UC) | 71.1 (27) |
| CD (exclusively) | 15.8 (6) |
| UC (exclusively) | 13.2 (5) |
| IBD population size | |
| Range | 18–41,840 |
| Median [IQR] | 405 [103–2313] |
| Age group (inclusion criteria), % (n) | |
| Adult | 47.4 (18) |
| Paediatric | 7.9 (3) |
| Both (adult and paediatric) | 21.1 (8) |
| NS | 23.7 (9) |
| Study design, % (n) | |
| Retrospective | 44.7 (17) |
| Cross-sectional | 26.3 (10) |
| Prospective | 18.4 (7) |
| Ambispective | 10.2 (4) |
| Geographic scope, % (n) | |
| National and/or all regions covered | 36.8 (14) |
| Regional | 63.2 (24) |
| Cataluña | 13.2 (5) |
| Galicia | 10.5 (4) |
| Andalucía | 10.5 (4) |
| Canarias | 5.3 (2) |
| Madrid | 5.3 (2) |
| Valencia | 5.3 (2) |
| Asturias | 2.6 (1) |
| Cantabria | 2.6 (1) |
| Castilla y León | 2.6 (1) |
| Castilla-La Mancha | 2.6 (1) |
| Navarra | 2.6 (1) |
IBD inflammatory bowel disease, CD Crohn’s disease, UC ulcerative colitis, IQR interquartile range, NS not specified
Epidemiology
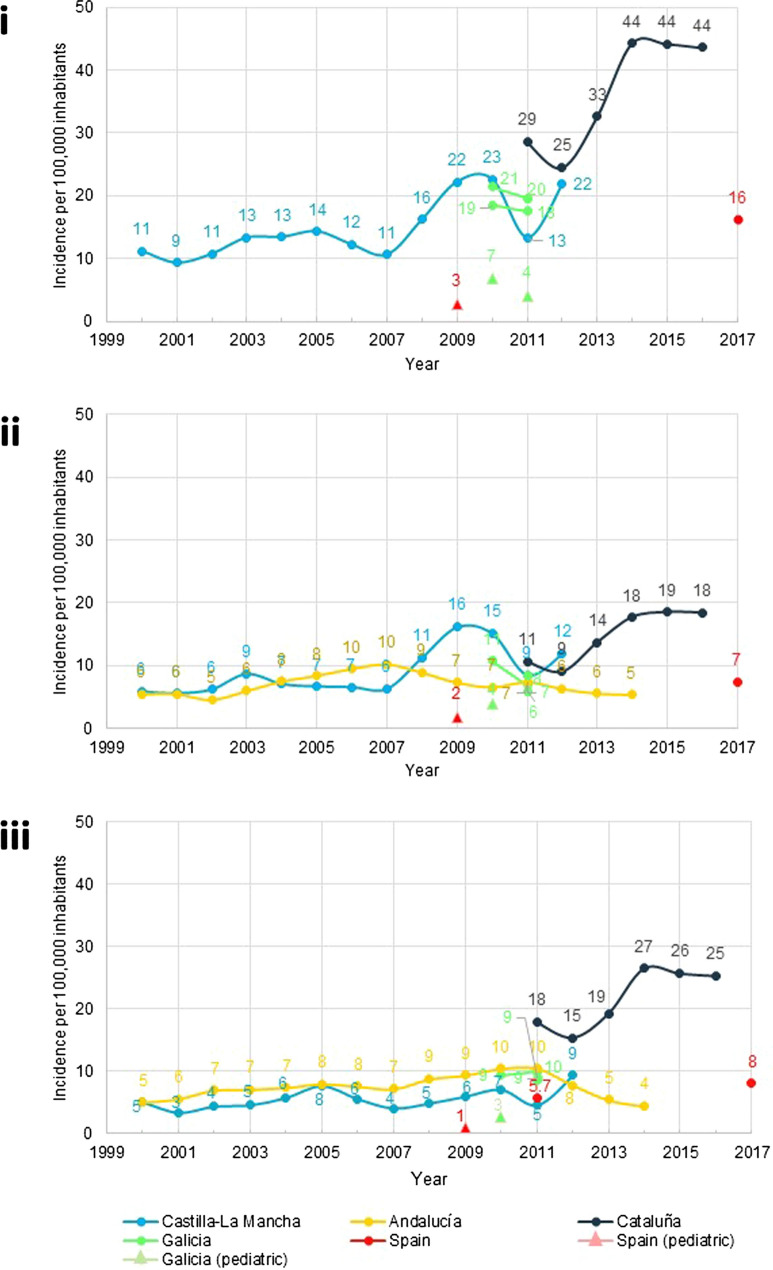
Incidence Fourteen publications reported data on IBD incidence [overall IBD (n = 10), CD (n = 13) and UC (n = 14)], 12 of them in adults (including studies involving patients of all ages) and two in paediatrics. The incidence of IBD, CD and UC varied greatly by study, region and year, ranging from 9.6 to 44.3 per 100,000 inhabitants (Fig. 2) [15–29]. The incidence ranges for CD and UC were 4.6 to 18.5 and 3.4 to 26.5 per 100,000 inhabitants, respectively.
Fig. 2.
Incidence of IBD according to year of analysis: overall (i), CD (ii) and UC (iii) according to year
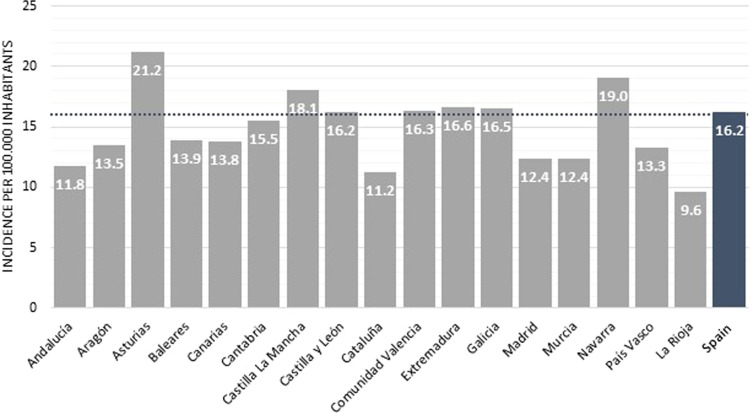
The most recent data at the national level identified in this review estimated adult overall IBD incidence to be 16.2 per 100,000 inhabitants, 7.4 for CD and 8.1 for UC in 2017, with higher rates reported in Asturias and Navarra (Fig. 3).
Fig. 3.
Incidence of adult IBD at regional level in 2017
Two studies conducted at the regional level (in Castilla-La Mancha and Cataluña) described an increase in IBD incidence between 1.5- and twofold in the period from 2000 to 2016 [16, 25] (Fig. 2i). A similar trend was reported for CD and UC at the national and regional levels, except in Andalucía, where incidence remained more stable (Fig. 2ii, iii) [16, 18, 25].
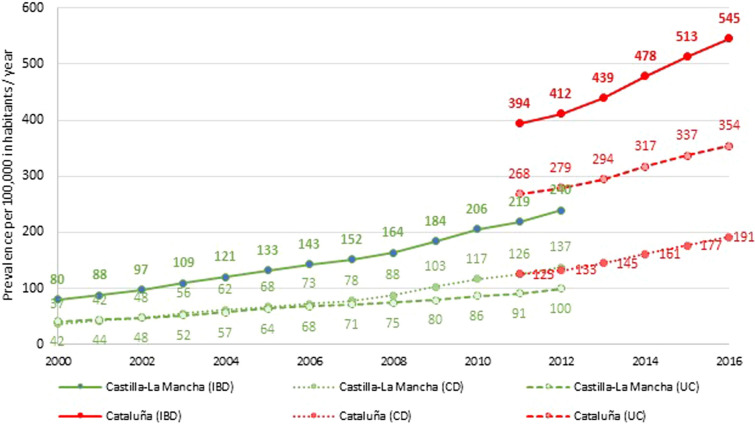
Prevalence Four publications reported data on adult IBD prevalence [overall IBD (n = 3), CD (n = 2) and UC (n = 3)]. Paediatric prevalence data were not available. Adult prevalence ranged from 79.8 to 545.3 per 100,000 inhabitants for IBD, from 37.2 to 191.4 for CD and from 41.5 to 354.0 for UC (Fig. 4).
Fig. 4.
Longitudinal trend of IBD, CD and UC prevalence in two Spanish regions
The most recent prevalence estimate at the national level identified in the search was 88.7 per 100,000 inhabitants [26] in 2011. However, other studies with a regional scope reported a higher prevalence (> 200) in the same year [16, 25], and an increasing trend over time (Fig. 4).
According to sex, data from Castilla-La Mancha showed a higher prevalence of UC in men than in women (115.39 vs. 84.54; p = 0.015), with no significant differences in CD [25].
Mortality Two publications reported IBD-associated mortality data [overall IBD (n = 2), CD (n = 2) and UC (n = 2)] [16, 30].
Over the period 2006–2015, CD and UC mortality rates of up to 6.0% and 3.0%, respectively, were reported [30]. More recently, an increase in mortality rates in Spain (expressed per 1000 inhabitants) was observed from 2011 to 2016 for IBD (14.7–18.6; 27% increase), CD (12.5–17; 36% increase) and UC (15.7–19.4; 24% increase) [16]. Compared to patients without IBD, the age and sex-adjusted odds ratio for death due to IBD were significantly higher for patients with CD (RR 1.63; 95% CI 1.39–1.89) and UC (RR 1.22; 95% CI 1.11–1.36) [16].
Demographic Characteristics
Seventeen publications reported data on the age of IBD onset [overall IBD (n = 11), CD (n = 14) and UC (n = 12)], 15 of them in adults and 2 in the paediatric population.
Disease onset predominantly occurs between 30 and 40 years of age in adult patients and seems to be more delayed for UC than CD (Table 3). Interestingly, the opposite trend was described in the two publications focused on the paediatric population, with an earlier diagnosis for patients with UC than for patients with CD (11.5 vs. 12.7 years; p < 0.001) [28].
Table 3.
Data reporting sex, age, and age at disease onset
| Author, year | Collection time | Geographic scope | Population type | Sex (female), % | Age, years | Age at disease onsetb Mean (SD)/median [range] |
|
|---|---|---|---|---|---|---|---|
| Population age | Mean/median/[range]a | ||||||
| Casellas (2012) [38] | NS | Cataluña | IBD | NS | NS | NS | NS |
| CD | 48.3 | 33 |
A1: 20.9% A2: 74.4% A3: 4.7% |
||||
| UC | 83.3 | 34 | 25 [23–31] | ||||
| Algaba (2013) [15] | 2005–2011 | Madrid | IBD | 51.7 | NS | 43.4 | NS |
| CD | NS | NS |
A1: 7.7% A2: 66.5% A3: 25.9% |
||||
| UC | NS | NS | NS | ||||
| Gómez-García (2013) [37] | 1996–2013 | Andalucía | IBD | 45.6 | NS | NS | 35.2 (16.5) |
| CD | NS | NS |
A1: 9.3% A2: 67.3% A3: 23.4% |
||||
| UC | NS | NS | NS | ||||
| Iglesias-Rey (2013) [33] | 2009–2010 | Galicia | IBD | 52.8 | NS | 44.6 | 36.2 (NS) |
| CD | 57.6 | 39.9 | 31.3 (NS) | ||||
| UC | 49.8 | 47.8 | 39.4 (NS) | ||||
| Marín (2013) [39] | NS | Cataluña | IBD | 56.9 | Adult (25–65) | 42.7/46.5 | NS |
| Martín-de-Carpi (2013) [27] | 1985–2009 | Spain | IBD | 43.6 | Paediatric (< 18) | [< 5] | 12.3 [9.7–14.6] |
| [6–12] | |||||||
| [13–17] | |||||||
| CD | 40.7 | [< 5] | 12.9 [10.7–15] | ||||
| [6–12] | |||||||
| [13–17] | |||||||
| UC | 47.2 | [< 5] | 12 [8.7–14.5] | ||||
| [6–12] | |||||||
| [13–17] | |||||||
| Nunes (2013) [75] | NS | Spain | CD | 50.1 | NS | NS | NS |
| Schreiber (2013) [76] | 2010 | Spain | UC | 55 | Adult (≥ 18) | 44.4 | NS |
| Andreu (2014) [34] | NA | Spain | IBD | 48.2 | NS | NS | 32 [24–44] |
| CD | 53.2 | NS | 29 [22–39] | ||||
| UC | 46.8 | NS | 36 [27–49] | ||||
| Lucendo (2014) [25] | 2000–2012 | Castilla-La Mancha | IBD | 46.0 | Adult (> 16) | 38.8 |
A1: 6.3% A2: 54.0% A3: 39.6% |
| CD | 48.6 | 35.9 |
A1: 8.4% A2: 59.2% A3: 32.4% |
||||
| UC | 42.2 | 42.4 |
A1: 3.5% A2: 48.2% A3: 48.2% |
||||
| Marín-Jiménez (2014) [35] | 2008–2010 | Spain | IBD | 52.7 | Adult (≥ 18) | 40.2 | NS |
| Martín-de-Carpi (2014) [28] | 1985–2009 | Spain | IBD | 43.6 | Paediatric (< 18) | NS | 12.4 [9.7–14.6] |
| CD | 41.7 | NS | 12.7 [NS] | ||||
| UC | 49.2 | NS | 11.5 [NS] | ||||
| Fernández A (2015) [22] | 2010 | Galicia | IBD | 42.5 | All | 39.5 | NS |
| CD | NS | 38 | NS | ||||
| UC | NS | 41 | NS | ||||
| Ramos A (2015) [53] | 2012–2013 | Castilla y León | IBD | 47 | Adult (18–67) | 45.5 | NS |
| CD | 46 | 43.1 |
A1: 4.6% A2: 74.8% A3: 20.5% |
||||
| UC | 47 | 48 | NS | ||||
| Aldeguer (2016) [31] | 2002–2012 | Cataluña | UC | 48.8 | Adult (≥ 18) | 44.5 | NS |
| López Cortés (2016) [41] | 2014 | Navarra | IBD | 45.0 | NA | 44.5 | 32.3 (13.6) |
| Abautret-Daly (2017) [42] | NS | Asturias | IBD | 61.1 | Adult (30–70) | NS | NS |
| CD | NS | NS | NS | ||||
| UC | NS | NS | NS | ||||
| Ballester (2017) [30] | 2006–2015 | Valencia | IBD | 47.3 | NS | NS | 32 [21] |
| CD | 49.3 | NS |
Sporadic: 28 [17] Familial: 26 [21] |
||||
| UC | 45.4 | NS |
Sporadic: 36 [22] Familial: 34 [20] |
||||
| Chaaro-Benalla (2017) [18] | 1995–2000 | Andalucía | CD | 42.0c | Adult (≥ 14) | NS |
A1: 10% A2: 70% A3: 20% |
| 2001–2014 | 43.0d | NS | |||||
| 1995–2000 | UC | 47.0c | NS |
A1: 5% A2: 55% A3: 40% |
|||
| 2001–2014 | 42.0d | NS | |||||
| López-Sanromán (2017) [43] | 2014 | Spain | IBD | 47.2 | Adult (≥ 18) | 46.2 | NS |
| Panés (2017) [44] | 2013 | Spain | UC | 55.8 | Adult (≥ 18) | 39 | NS |
| Brunet E (2018) [16] | 2011–2017 | Cataluña | IBD | NS | NS | NS | NS |
| CD | NS | 43.5 | NS | ||||
| UC | NS | 51.2 | NS | ||||
| Chaparro (2019) [48] | 2017 | Spain | IBD | 52.7 | All | 15e 39f | NS |
| CD | NS | NS | NS | ||||
| UC | NS | NS | NS | ||||
| García (2020) [36] | 2015–2018 | Cantabria | IBD | 49.2 | NS | 53.9 | 40.7 (16.1) |
| CD | 48.2 | 52.7 | 38.5 (16.6) | ||||
| UC | 50.9 | 55.0 | 42.4 (15.2) | ||||
| Diáz-Alcázar (2020) [78] | NS | Andalucía | IBD | 38 | Adult (> 65) | NS | NS |
| CD | NS | NS | NS | ||||
| UC | NS | NS | NS | ||||
| Cordero Jorge (2020) [32] | NS | Canarias | CD | 60 | Paediatric (< 16) | 11.3 | NS |
| 57 | Adult (17–40) | 29.3 | NS | ||||
| Cordero Jorge (2020) [49] | 1987–2019 | Canarias | UC | 47 | Paediatric (< 16) | 9.0 | NS |
| 54 | Adult (17–40) | 29.3 | NS | ||||
| De Castro (2020) [20] | 1991–2011 | Galicia | IBD | 39.6 | Adult (≥ 15) | 43.5 | NS |
| CD | 38.6 | 40.4 | NS | ||||
| UC | 35.2 | 46.1 | NS | ||||
| De Castro (2020) [80] | 1991–1993 | Galicia | IBD | NS | Adult (> 15) | 43.5 | 35.8 (16.2) |
| CD | NS | NS | 29.1 (15.7) | ||||
| UC | NS | NS | 39.2 (15.1) | ||||
| 2010–2011 | IBD | NS | 35.8 | 43.5 (16.4) | |||
| CD | NS | NS | 40.4 (15.8) | ||||
| UC | NS | NS | 46.1 (16.4) | ||||
| Bastida G (2021) [77] | 2011–2013 | Spain | IBD | 46.5 | Adult (≥ 18 with anti-TNF) | 44.0 | NS |
| CD | 46.9 | 43.0 | NS | ||||
| UC | 45.7 | 46.0 | NS | ||||
| Chaparro M (2021) [19] | 2017 | Spain | IBD | 47 | Adult (NS) | 42.0 | NS |
| CD | 50 | 41 | NS | ||||
| UC | 55 | 46 | NS | ||||
| Hernández V (2021) [24] | 2010 | Galicia | CD | 67.6 | All | 39.2 | Incidence peak: 15–24 and 35–44 |
| UC | 38.8 | 38.8 | Incidence peak: 25–34 and 55–65 | ||||
| Díaz-Alcázar (2021) [50] | NS | Andalucía | IBD | NS | All | 58.4 | > 65: 74.0% |
| Guevara (2021) [23] | 1992–2016 | Spain | IBD | NS | Adult (≥ 18) | NS | NS |
| CD | NS | NS | 60.7 [52–70] | ||||
| UC | NS | NS | 59.0 [55–65] | ||||
IBD inflammatory bowel disease, CD Crohn’s disease, UC ulcerative colitis, NS not specified, SD standard deviation, IQR interquartile range
aMean values in this column are upright; median values are in italics
bAge at diagnosis according to the Montreal classification (A1, < 16; A2, 17–40; A3, > 40)
cPeriod 1995–2000
dPeriod 2001–2014
eChildhood
fAdult
Clinical Characteristics
The clinical data hereby presented has been recorded at different times: 9 studies collected these data at diagnosis, 5 at study entry and 15 at any time in the course of the disease/study (Table 4).
Table 4.
Data reporting location, extent, behaviour, EIMs and clinical activity in patients with IBD, CD and UC
| Author, year | Population age, years | Clinical data collected at | Location (CD)a, row % |
Extent (UC)a, row % | Behavioura (CD), % | EIMs, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | E1 | E2 | E3 | B1 | B2 | B3 | P | IBD | CD | UC | |||
| Casellas (2012) [38] | NS | Any time | 32.6 | 20.9 | 44.2 | 2.3 | 0.0 | 27.3 | 72.7 | 30.2 | 16.3 | 20.9 | 32.5 | – | – | – |
| Algaba (2013) [15] | NS | Any time | 35.8 | 24.3 | 35.5 | 1.9 | 23.8 | 48.4 | 27.8 | 57.2 | 9.6 | 13.1 | 20.1 | – | – | – |
| Gómez-García (2013) [37] | NS | Any time | 44.0 | – | – | – | 21.2 | 32.5 | 3.1 | 62.1 | 15.5 | 22.4 | 21.0 | – | – | – |
| Iglesias-Rey (2013) [33] | NS | Study entry | 45.3 | 15.1 | 39.3 | 0.3 | 21.5 | 47.3 | 31.2 | 44.2 | 30.3 | 25.6 | 14.2 | 19.9 | 23.2 | 2.7 |
| Martín-de-Carpi (2013) [27] | Paediatric (< 5) | Diagnosis | 25.0 | 33.9 | 41.1 | – | 6.4 | 17.4 | 76.1 | – | – | – | – | – | – | – |
| Paediatric (6–12) | 22.6 | 16.3 | 61.1 | – | 7.5 | 25.1 | 67.4 | – | – | – | – | – | – | – | ||
| Paediatric (13–17) | 30.3 | 15.2 | 54.5 | – | 14.7 | 30.0 | 40.6 | – | – | – | – | – | – | – | ||
| Martín-de-Carpi (2014) [28] | Paediatric | Diagnosis | 26.6 | 16.4 | 57.0 | – | 10.0 | 27.1 | 62.9 | – | – | – | – | – | – | – |
| Nunes (2013) [75] | NS | Study entry | 29.3 | 8.2 | 39.1 | 10.6 | – | – | – | 57.4 | 20.1 | 19.2 | 27.3 | – | 21.6 | – |
| Andreu (2014) [34] | NS | Any time | 38.7 | 13.7 | 44.0 | 15.4 | 17.5 | 42.9 | 39.6 | 65.8 | 25.1 | 18.1 | 27.8 | – | 24.5 | 14.3 |
| Lucendo (2014) [25] | Adult | Diagnosis | 35.7 | 24.2 | 39.3 | 0.8 | 20.2 | 46.7 | 33.2 | 64.5 | 23.4 | 12 | – | – | – | – |
| Marín-Jiménez (2014) [35] | Adult | Any time | – | – | – | – | – | – | – | 61.3 | – | – | 23 | 16.2 | 20.3 | 10.6 |
| Ballester (2017) [30] | NS (sporadic) | Any time | 24.8 | 10.9 | 47.3 | 0.6 | 12.9 | 29.9 | 57.3 | 48.0 | 32.9 | 19.1 | 35.3 | – | 43.6 | 29.7 |
| NS (familial) | 32.9 | 12.7 | 35.4 | 0.0 | 8.6 | 34.6 | 56.8 | 41.8 | 32.9 | 25.3 | 33.3 | – | 47.4 | 48.1 | ||
| Panés (2017) [44] | Adult | Study entry | – | – | – | – | 19.1 | 41.7 | 39.2 | – | – | – | – | – | – | 22.6 |
| Chaparro (2019) [48] | All | Diagnosis | 40.4 | 17.2 | 38.4 | 7.4 | 31.0 | 39.1 | 25.8 | 87.7 | 7.3 | 5.0 | 21.9 | 13.3 | – | – |
| Paediatric | 26.0 | 14.0 | 59.0 | 15.4 | 50.8 | 35.6 | 13.6 | 94.0 | 3.7 | 2.3 | 16.4 | 12.0 | – | – | ||
| Adult | 43.0 | 18.0 | 39.0 | 7.0 | 32.0 | 41.0 | 27.0 | 87.5 | 7.5 | 5 | 10.8 | 13.8 | – | – | ||
| García (2020) [36] | NS | Study entry | 49.6 | 14.6 | 35.9 | 3.2 | 32.7 | 42.3 | 25.0 | 65.4 | 15.9 | 18.7 | 18.0 | – | – | – |
| Díaz-Alcázar (2020) [78] | < 65 | Diagnosis | – | – | – | – | – | – | – | 62 | 14 | 7 | – | – | – | – |
| ≥ 65 | 50 | 21 | 29 | 0 | 14 | 49 | 31 | 32 | 29 | 7 | – | – | – | – | ||
| Merino Gallego (2021) [73] | NS | Any time | – | – | – | – | – | – | – | – | – | – | – | – | 15 | – |
| Cordero Jorge (2020) [32] | Paediatric | Any time | – | – | 56 | – | – | – | – | 80 | – | – | 32 | – | 18 | – |
| Adult | 29.3 | 52.8 | – | 22.6 | – | – | – | 77 | – | – | 5 | – | 9 | – | ||
| Cordero Jorge (2020) [49] | Paediatric | Any time | – | – | – | – | 26 | 20 | 54 | – | – | – | – | – | – | 14 |
| Adult | – | – | – | – | 29 | 20 | 51 | – | – | – | – | – | – | 15 | ||
| De Castro Parga (2020) [20] | Adult (1991–1993) | Any time | 24.2 | 33.3 | 42.4 | 6.3 | 78.8 | 9.1 | 12.1 | 78 | 9.1 | 12.1 | – | 7 | – | – |
| Adult (2010–2011) | 53.1 | 19.8 | 23.5 | 9.9 | 65.1 | 22.9 | 12.0 | 65.1 | 22.9 | 12 | – | 7 | – | – | ||
| Díaz-Alcázar (2021) [50] | < 65 | Diagnosis | – | – | – | – | – | – | – | 62 | 15 | 24 | – | – | – | – |
| > 65 | – | – | – | – | – | – | – | 64 | 22 | 7 | – | – | – | – | ||
| Bastida (2021) [77] | Adult (with anti-TNF) | Any time | 37.3 | 15.5 | 44.6 | 2.6 | – | – | – | 44.6 | 20.7 | 17.6 | 17.1 | 28.7 | 26.3 | 32.8 |
| Chaparro (2021) [19] | Adult | Diagnosis | 55 | 19 | 26 | 3 | 31 | 31 | 38 | 82 | 11 | 7 | 11 | 9 | 12 | 6 |
| Guevara (2021) [23] | Adult | Any time | – | – | – | – | – | – | – | – | – | – | – | – | – | 9 |
| Hernández (2021) [24] | All | Any time | 41.2 | 17.6 | 20.6 | – | 36.7 | 24.5 | 38.8 | 67.6 | 17.6 | 11.9 | 4.7 | – | 5.9 | 6.1 |
| Fernández (2015) [22] | All | Diagnosis | 54 | 23 | 19 | – | 17 | 57 | 26 | 66 | 17 | 9.5 | 5 | 9 | – | – |
| Ramos A (2015) [53] | Adult | Study entry | 40.3 | 21.8 | 34.4 | 3.3 | 28.2 | 36.7 | 35.2 | – | – | – | – | – | – | – |
| Aldeguer (2016) [31] | Adult | Any time | – | – | – | – | 39.3 | 36.5 | 24.2 | – | – | – | – | – | – | – |
| Abautret-Daly (2017) [42] | Adult | Any time | 5.6 | 5.6 | 33.6 | – | 5.6 | 33.3 | 16.7 | – | – | – | – | – | – | – |
| Chaaro-Benalla (2017) [18] | Adult (1995–2000) | Diagnosis | 25.8 | 37.6 | 36.6 | – | 31.1 | 46.3 | 22.6 | – | – | – | – | – | – | – |
| Adult (2001–2014) | 26.6 | 35.5 | 37.7 | – | 34.0 | 35.0 | 31.0 | – | – | – | – | – | – | – | ||
IBDinflammatory bowel disease, CD Crohn’s disease, UC ulcerative colitis, NS not applicable/not available, EIM extraintestinal manifestations
aAccording to Montreal classification: Location (L1, terminal ileum; L2, colon; L3, illeocolon; L4, upper GI); UC location (E1, ulcerative proctitis; E2, left side UC; E3, extensive colitis); Behaviour (B1, inflammatory; B2, stenosing, B3, fistulising; P, concomitant perianal disease)
Gastrointestinal manifestations are present in 94.0% of patients with CD and 89.0% of patients with UC [19]. Despite this, they have only been described in one publication for UC, with rectal bleeding (88.8%), diarrhoea (80.0%), pain (69.1%) and rectal urgency (59.3%) being the most common signs/symptoms [31]. Weight loss in 34% of adults and 78% of children and vomiting in 3.8% of adults and 32% of children have been reported in CD [32].
On the other hand, EIMs were described in 16 publications, with their presence ranging from 7.0% to 28.7% for overall IBD (Table 4). A higher prevalence of EIM in patients with CD compared to patients with UC was observed in five out of seven publications with available data [19, 30, 33–35]. The most common EIM was osteoarticular manifestations, reported in over 10% of patients for both CD and UC.
Between 13.5% [35] and 26.6% [36] of adult patients with IBD in the studies had at least one other immune-mediated inflammatory disease (IMID)
Psychiatric comorbidities were frequently reported, with depression described in up to 20% and anxiety in up to 11% of patients with CD and UC [19, 30, 33–35].
The most frequently diagnosed IMID and non-IMID comorbidities are described in Table 5.
Table 5.
Data reporting frequency of comorbidities and extraintestinal manifestations in patients with IBD, CD and UC
| Comorbidities | IBD | CD | UC | References |
|---|---|---|---|---|
| Cancer, % | 3.0 | NA | NA | [15] |
| Diabetes, % | NA | NA | 1.0a | [76] |
| Asthma, % | 6.6 | NA | 1.0a | [36, 76] |
| Osteoarticular manifestations, % | 11.8; 12.6 | 14.6 | 10.3 | [35, 73] |
| Spondyloarthropathies | 4.5; 8.9 | 11.7 | 5.5 | [35, 36] |
| Arthritis | 1.0a; 4.4 | NA | NA | [36, 76] |
| Osteoporosis | NA | NA | 11.6 | [31] |
| Skin disorders, % | 1.7; 3.9 | 2.3 | 0.9 | [25, 73] |
| Psoriasis | 3.4; 5.8 | 4.3 | 2.3 | [35, 36] |
| Pyoderma gangrenosum | 1.0 | 1.0 | 0.9 | [35] |
| Ocular disease, % | 1.4; 2.0 | 1.5 | 1.4 | [25, 73] |
| Uveitis | 2.1 | 2.7 | 1.4 | [35] |
| Ocular pain | NA | NA | 1.8 | [31] |
| Kidney disease, % | ||||
| Urinary calculus | NA | NA | 9.1 | [31] |
| Glomerulonephritis | NA | NA | 0.7 | [31] |
| Anemia, % | ||||
| Ferropenic anemia | NA | NA | 17.2 | [31] |
| Pernicious anemia | NA | NA | 2.8 | [31] |
| Venous thromboembolic disease, % | 0.5 | 0.2 | 0.9 | [25] |
| Thromboembolic events | 1.7 | NA | NA | [73] |
| Pulmonary embolism | NA | NA | 1.1 | [31] |
| Depression, % | 16.6; 20.1 | 20.4 | 1.0a–19.7 | [33, 39, 76] |
| Anxiety, % | 10.5 | 9.4 | 11 | [33] |
IBD inflammatory bowel disease, CD Crohn’s disease, UC ulcerative colitis, NA not applicable/not available
aReported by the patient
Although the overall risk of cancer did not significantly increase in patients with IBD [15, 37], the relative risk of developing some types of cancer has been reported: urothelial carcinoma (RR 5.23, 95% CI 1.95–13.87), appendiceal mucinous cystadenoma (RR 36.6, 95% CI 7.92–138.4), small intestine carcinoma (RR 13.1, 95% CI 1.82–29.7) and rectal carcinoid (RR 8.94, 95% CI 1.18–59.7) [15]. Two studies also evaluated the possible effect of thiopurines on the risk of extracolonic cancer [15] and overall neoplasm [37] but a clear association between variables was not found. Thus, it could not be concluded whether the risk of malignancy was attributable to a given drug, its combination with another, the time under treatment, doses or the disease itself. No CD- and UC-specific data were available.
Regarding the location of the CD lesion, heterogeneous data were observed according to the Montreal classification, which was adopted by all the publications (see description of mentioned disease classifications in the footnote of Table 4). However, L1 occurs more frequently in the adult and elderly population than in the paediatric population, whereas L3 shows the opposite trend (Table 4). Moreover, the paediatric population has been found to have the highest frequency of ileal involvement (L1) in 13–17-year-olds (30.3%) as compared to under 5 years (25%) and 6–12 years (22.6%) (p < 0.001) [27]. Disease behaviour in CD was described in 19 publications, with inflammatory type (B1) being the most frequently reported (14 out of 19 publications). Interestingly, the frequency of perianal disease was higher in the paediatric than in the adult population (Table 4).
Heterogeneous data were also observed in UC extent among the studies included in our review. Nonetheless, in the adult population E2 was the most frequent localization compared to E1 and E3. On the other hand, most studies in the paediatric population showed a higher prevalence of E3 than those carried out in the adult population (Table 4). In addition, E3 was significantly predominant in children under 5 years compared to 6–12-year-olds and 13–17-year-olds [27]. Disease severity in UC was only described in one publication [37], with most patients having S1 (51%) or S2 (41%).
Patient-Reported Outcomes
Nine of the publications reviewed assessed HRQoL in patients with IBD [overall IBD (n = 2), CD (n = 5) and UC (n = 6)] [33, 38–45]. Data for the paediatric population were not available.
Patients with IBD had lower HRQoL (as measured with the SF-36) than the reference values of the general population [40]. In addition, two studies in patients with IBD reported an association between high levels of anxiety, depression and stress (measured with the Hamilton Depression Rating Scale (HAM-D), Hospital Anxiety and Depression Scale (HADS), and Perceived Stress Scale (PSS) questionnaires) and low levels of HRQoL and more significant symptomatology, with no differences between CD and UC [40, 42]. Another study showed that patients with CD reported higher levels of sexual dysfunction than healthy controls (35% vs. 12%, p < 0.08) [45], with significant differences in erectile function, orgasm, sexual desire and global satisfaction (p < 0.05) [39, 45]. Among those patients who felt that intimacy had worsened because of IBD, fatigue was the leading complaint in men and women [39].
In general, no significant differences in HRQoL between patients with CD and UC have been described [38, 40, 42]. However, two studies reported a significantly poorer HRQoL in patients with CD compared to patients with UC, measured with the Inflammatory Bowel Disease Questionnaire (IBDQ-32): IBDQ-32 mean score 155.4 (SD 42.7) vs. 180.3 (SD 32.4), p = 0.005 [41]; IBDQ-36 functional domain 44.8 (39.9–47.6) vs. 46.9 (45.5–49.0), p = 0.02 and social affectations 39.6 (36.0–40.2) vs. 39.6 (39.6–40.8), p = 0.04 [38].
Women with IBD had poorer HRQoL than men [IBDQ-32 score 152.5 (SD 43.3) vs. 180.6 (SD 31.4), p = 0.001] [41], and a higher impact of UC on sleep quality and higher levels of anxiety and depression were reported in women than in men (p < 0.01) [43]. Data on the impact of CD on HRQoL in relation to sex were not available.
Other publications pointed out the role of age, EIMs, the presence of an exacerbation, the number of previous recurrences, disease activity and disease duration in HRQoL, suggesting a worse quality of life the more severe the disease is [38, 40, 43, 44].
The HRQoL among IBD treated patients was significantly improved in those who achieved remission after 1 year of biologic treatment while clinical activity decreased and normalization of HRQoL (IBDQ score > 209) was achieved in 74% of patients (67.4% CD vs. 100.0% UC, p < 0.05) [38].
Pharmacological Treatment Patterns
Twenty-three publications reported qualitative and quantitative data on drug treatment patterns [overall IBD (n = 8), CD (n = 11) and UC (n = 9)]. Twenty-two targeted the general or adult population and one the paediatric population. However, specific percentages of use (quantitative data) are reported in only 17 of these publications (Table 6).
Table 6.
Data reporting treatment utilization patterns in patients with IBD, CD and UC
| Author, year | Age selection criteria | Geographic scope | Collection time | Population size | Population type | Severity Median [IQR], mean, % |
ASAS % | CST % |
|---|---|---|---|---|---|---|---|---|
| Casellas (2012) [38] |
NA (on antiTNFα) |
Cataluña | 2012 |
54 patients (in remission) Basal |
IBD | – | – | – |
| CD | 0.0 [0.0–1.0], 4b | – | – | |||||
| UC | 1.0 [0.7–2–0], 2c | – | – | |||||
| Algaba (2013) [15] | All | Madrid | 2005–2011 |
590 patients Basal |
IBD | – | – | – |
| Gómez-García (2013) [37] | NA | Andalucía | 1996–2013 |
812 patients During illness |
IBD |
51.1 (S1)a 40.7 (S2)a 8.1 (S3)a |
– | – |
| Iglesias-Rey (2013) [33] | NA | Galicia | 2009–2010 |
799 patients Basal |
IBD | – | 42.3 | 14.3 |
| CD | 3.9b | 23.0 | 18.0 | |||||
| UC | 2.7d | 55.9 | 11.8 | |||||
| Marín (2013) [39] | Adult | Cataluña | 2013 |
355 patients (202 women, 123 men) During illness |
IBD women | – | – | 65 |
| IBD men | – | – | 52 | |||||
| Nunes (2013) [75] | NA | Spain | 2013 |
3224 patients Basal |
CD | – | – | 85.7 |
| Abautret-Daly (2017) [42] | Adult | Asturias | 2017 |
18 patients Basal |
IBD | – | 38.9 | – |
| Ballester (2017) [30] | NA | Valencia | 2017 |
1211 patients Basal |
IBD | – | – | – |
| CD sporadic | – | – | – | |||||
| CD familial | – | – | – | |||||
| UC sporadic | – | – | ||||||
| UC familial | – | – | – | |||||
| Panés (2017) [44] | Adult | Spain | 2017 |
199 patients Basal |
UC |
63.8 (Rc≤2) 36.2 (Ac>2) |
82.4 | 16.5 |
| Chaparro (2019) [48] | All | Spain | 2007–2017 |
21,200 patients Follow-up |
IBD adult | – | – | – |
| IBD paediatric | ||||||||
| García (2020) [36] | NA | Cantabria | 2018 |
1448 patients During illness |
IBD | – | – | – |
| CD | – | – | – | |||||
| UC | – | – | – | |||||
| Brunet (2020) [52] | NA | Cataluña | 2011 | NA—Basal | CD | – | 28.8 | 15.8 |
| 2017 | NA—Basal | – | 17.1 | 13.7 | ||||
| Jijón Andrade (2020) [81] | Paediatric (on biological therapy) | Madrid | 2010–2020 |
57 patients Follow-up |
IBD | – | – | – |
| Diáz-Alcázar (2021) [50] | All | Andalucía | NA |
100 patients At diagnosis |
IBD > 65 | – | – | 54 |
| IBD < 65 | – | – | 28 | |||||
| Bastida (2021) [77] | Adult (on biological therapy | Spain | 2011–2013 |
310 patients Pre-biological treatment |
IBD | – | 68.1 | 81.6 |
| CD | – | 54.1 | 76.3 | |||||
| UC | – | 91.4 | 90.5 | |||||
| Chaparro (2021) [19] | Adult | Spain | 2017 |
3611 patients First year after diagnosis |
IBD | – | – | 35 |
| CD | – | 38 | 71 | |||||
| UC | – | 73 | 38 | |||||
| Ramos (2015) [53] | Adult | Castilla y León | 2012–2013 |
293 patients Basal |
IBD | – | – | – |
| CD | – | – | – | |||||
| UC | – | – | – |
| Author, year | Immunomodulators, row % | Biological therapy, row % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | AZA | MTX | MYC | THIO | Total | ADA | IFX | GOL | VEDO | UST | TNFα | |
| Casellas (2012) [38] | 89.0 | 89.5 | 10.5 | – | – | 56.0 | 44.0 | – | – | – | – | |
| 88.4 | – | – | – | – | – | 60.4 | 39.6 | – | – | – | ||
| 90.9 | – | – | – | – | – | 36.4 | 63.6 | – | – | – | ||
| Algaba (2013) [15] | – | – | – | – | 43.9 | – | – | – | – | – | – | 14.2 |
| Gómez-García (2013) [37] | – | – | – | – | 52.8 | 21.2 | – | – | – | – | – | – |
| Iglesias-Rey (2013) [33] | 14.4 | – | – | – | – | 11.8 | – | – | – | – | – | – |
| 23.0 | – | – | – | – | 18.3 | – | – | – | – | – | – | |
| 8.6 | – | – | – | – | 6.9 | – | – | – | – | – | – | |
| Marín (2013) [39] | 59 | – | – | – | – | 23 | – | – | – | – | – | – |
| 64 | – | – | – | – | 20 | – | – | – | – | – | – | |
| Nunes (2013) [75] | 67.5 | – | – | – | – | 27.6 | – | – | – | – | – | – |
| Abautret-Daly (2017) [42] | – | 27.8 | 11.1 | – | – | – | – | 5.6 | – | – | – | – |
| Ballester (2017) [30] | – | – | – | – | – | – | – | – | – | – | – | – |
| 63.1 | – | – | – | – | 38.6 | – | – | – | – | – | – | |
| 79.9 | – | – | – | – | 54.4 | – | – | – | – | – | – | |
| 30.0 | – | – | – | 17.1 | – | – | – | – | – | |||
| 29.6 | – | – | – | – | 18.5 | – | – | – | – | – | – | |
| Panés (2017) [44] | – | – | – | – | 36.7 | – | – | – | – | – | – | 23.6 |
| Chaparro (2019) [48] | 47.0 | – | – | – | – | 29.2 | – | – | – | – | – | – |
| 73.2 | 53.0 | |||||||||||
| García (2020) [36] | 40.4 | – | – | – | – | 6.7 | – | – | – | – | – | – |
| 58.2 | – | – | – | – | 39.9 | – | – | – | – | – | – | |
| 24.3 | – | – | – | – | 15.0 | – | – | – | – | – | – | |
| Brunet (2020) [52] | 28.8 | – | – | – | – | 15 | – | – | – | – | – | – |
| 17.1 | – | – | – | – | 18.7 | – | – | – | – | – | – | |
| Jijón Andrade (2020) [81] | – | – | – | – | – | 100 | 26 | 47 | 2 | 3 | 3.5 | |
| Diáz-Alcázar (2021) [50] | – | – | – | – | – | – | – | – | – | – | – | – |
| – | – | – | – | – | – | – | – | – | – | – | – | |
| Bastida (2021) [77] | 78.4 | – | – | – | – | – | – | – | – | – | – | – |
| 82 | – | – | – | – | – | – | – | – | – | – | – | |
| 72.4 | – | – | – | – | – | – | – | – | – | – | – | |
| Chaparro (2021) [19] | 26 | – | – | – | – | 15 | – | – | – | – | – | – |
| 45 | – | – | – | – | 25 | – | – | – | – | – | – | |
| 10 | – | – | – | – | 7 | – | – | – | – | – | – | |
| Ramos (2015) [53] | 50 | – | – | – | – | – | – | – | – | – | – | 15 |
| 66 | – | – | – | – | – | – | – | – | – | – | 21 | |
| 32 | – | – | – | – | – | – | – | – | – | – | 8 | |
Bold values, percentage of the total; italic values, therapeutic group percentage
IBD inflammatory bowel disease, CD Crohn’s disease, UC ulcerative colitis, ASAS aminosalicylates, CST corticosteroids, AZA azathioprine, MTX methotrexate, MYC mycophenolate, THIO thiopurines, ADA adalimumab, IFX infliximab, GOL golimumab, VEDO vedolimumab, UST ustekinumab, TNFα tumour necrosis factor alpha
aAccording to Montreal classification: S0, clinical remission/asymptomatic; S1, mild UC; S2, moderate UC; S3, severe UC. A, active; R, remission
bHavery–Bradshaw (CD)
csCAI (UC)
dMayo index
Considerable variability in treatment patterns was observed across studies. In general, from 1996 to 2018 aminosalicylates were the most frequently used treatments in Spain followed by immunomodulators and corticosteroids, while biological drugs were the least prescribed (Table 6). However, a significant increase in topical salicylates, systemic steroids, immunosuppressive drugs, and biologics and a reduction in topical steroids and oral aminosalicylates were reported between 1991 and 2011 [46]. The most recent data on the use of biologics at the national level derives from the ENEIDA registry, estimating that 25% of patients with CD were treated with biologics in 2016 [47], with no reported data for UC.
According to the IBD type, the use of biological treatment observed in the studies was more frequent in patients with CD (15.0–60.0%) than in patients with UC (6.9–36.0%) (Table 6). Moreover, data from the ENEIDA registry show that patients with CD had a higher risk of using immunosuppressants (HR 3.2 [95% CI 3.1–3.4]) and biological agents (HR 2.5 [95% CI 2.3–2.7]) than patients with UC [48].
The ENEIDA registry also reported that the use of immunomodulators and biologics was significantly higher in patients with childhood‐onset IBD than in those with adult-onset, [CD (85% vs. 66.2%, p < 0.001) and UC (56.1% vs. 28.3%, p < 0.001) and [CD (65% vs. 41.5%, p < 0.001) and UC (33% vs. 17.4%, p < 0.001), respectively] [48]. However, the median time from IBD diagnosis to the first biologic agent was similar in paediatric and adult‐onset patients (13 vs. 12 months, p > 0.05) [48].
Differences in treatment patterns between patients with familial and sporadic CD were also observed, showing a higher use of immunomodulators (79.9% vs. 63.1%) and biological therapy (54.4% vs. 38.6%) in the familial group [30]. Furthermore, in patients with IBD the presence of EIM was associated with a higher risk of using immunosuppressants or biological agents (1.2 [95% CI 1.1–1.3] and 1.7 [95% CI 1.6–1.7], respectively) [48].
Healthcare Resource Utilisation and Associated Costs
Resource use Twelve publications reported data on resource use [overall IBD (n = 4), CD (n = 5) and UC (n = 6)] [16, 19, 26, 31–33, 36, 39, 48–51].
A cross-sectional study conducted in Galicia from 2009 to 2010 on patients with IBD reported an emergency visit rate of 24.0% (CD 31.1%; UC 19.9%) and an annual hospitalisation rate of 14.7% (CD 20.3%; UC 10.6%) [33]. Interestingly, between 2011 and 2017, the rate of CD-related hospitalisations per 1000 patients/year decreased from 92.7 to 72.2 (p < 0.001) [52].
Spanish data also showed that paediatric patients with UC required more frequent hospitalisation than adults (72% vs. 40%; p = 0.004) [49], while there were no differences in patients with CD [32]. Likewise, a study conducted in two IBD cohorts (under 65 years and over 65 years) suggested that this trend might persist in adulthood, noting that the percentage of elderly patients who never required hospitalisation was higher than that of younger patients (54% vs. 36%)[50]. Concerning surgery, the results of a patient survey performed prior to 2013 among patients with IBD in Cataluña showed that 26–29% of patients required resection or colectomy, 4–7% transient or ostomy and 10–13% perianal surgery [39]. Interestingly, another retrospective study also conducted in Cataluña on patients with CD between 2011 and 2017 showed that the rate of ostomy surgical resection per 1000 patients/year decreased from 13.2 to 9.8 (p = 0.003), and from 24.1 to 18.0 (p = 0.001), respectively [52].
With respect to IBD type, the EpidemIBD study showed that in Spain, the cumulative incidence of surgery was higher in CD than in UC (11.0% vs. 1.3%, p < 0.01) [19]. In line, the ENEIDA study showed that patients with CD had a higher risk of undergoing surgery (HR 6.6 [95% CI 5.8–7.4]) than patients with UC [48]. In addition, the risk of needing surgery is higher in patients with CD with more severe forms of the disease structuring [HR 2.5 (95% CI 2.2–2.9)] and fistulising [4.1 (3.6–4.7)] compared to patients with inflammatory behaviour [48].
Cost Two studies provided economic data [overall IBD (n = 0), CD (n = 1) and UC (n = 1)] [31, 47], and two studies included information about productivity losses [overall IBD (n = 1), CD (n = 1) and UC (n = 2)] [31, 53]. Data for the paediatric population were not available.
The most comprehensive cost data were defined for UC, since for CD the cost of biologics was only estimated and the work disability ratio was described without associated costs.
The UC-associated costs from the societal perspective were estimated in Cataluña between 2002 and 2012. The mean direct cost per patient and year was €1754.1 [95% CI 1473.4–2034.8], with hospitalisations, medication and general practitioner visits as the main cost components [31].
Of the total of active workers with UC (n = 191), 33.5% had been on UC-related sick leave for a mean of 26.2 days (SD 37.4) per year. Furthermore, absenteeism due to medical visits caused a mean of 29.6 working hours lost (21.4) per year [31], with an associated cost of €88.2 per patient/year [31]. Regarding the UC indirect cost in Spain, the mean annual indirect cost was estimated at €399.3 [95% CI 282.3–422.7] per patient/year (expressed in euros 2012), mainly due to presenteeism and absenteeism [31].
Discussion
This systematic review provides a comprehensive overview of the epidemiological, clinical, patient-reported and economic burden of IBD in Spain. The scope of this review included publications reporting data on CD and UC separately, as well as IBD overall. This enabled us to characterize CD and UC as separate entities. Identified publications were linked to 38 different studies, heterogeneous in study design, temporal and geographic scope. Nearly half the publications were from years 2020–2021, revealing increasing research activity in recent years. Studies varied in size from approximately 37 to 275,000 subjects. Notably, large studies such as EpidemIBD, EPIC, EPICURE and registries such as ENEIDA are contributing significantly to the understanding of these diseases in our country.
High heterogeneity in IBD incidence has been observed among studies, with incidences as high as 44 cases per 100,000 inhabitants. Our review suggests that incidence and prevalence for both CD and UC have increased in Spain in recent years. The heterogeneity observed among regions and/or years originate from differences in the characteristics of study populations and study design.
The results of a recent worldwide systematic review found that incidence is stabilising in western countries, while burden remains high because of the increasing prevalence [54].
Although IBD is not considered a fatal disease, it may affect life expectancy. In fact, Spanish data show that mortality is higher in patients with CD and UC compared to patients without IBD and that mortality rate has increased in the past decade for both CD and UC [16]. Interestingly, while the number of IBD-related deaths worldwide increased by 67.0% from 1990 to 2017, the age-standardised death rate decreased by 16.4% over the same period [55]. The decline may reflect improved survival in patients with IBD, a trend which may be driven by the use of immunomodulators, early introduction of biological agents, and /or improvements in surgical techniques. Standardised data for Spain has not been found during this review and thus cannot be compared with global data; therefore mortality in Spain would require additional understanding. Nonetheless, on the basis of the age-standardised mortality rate estimated in a systematic global analysis, Spain had one of the lowest IBD-related death rates in the European Union in 2017 (0.2–0.4 per 100,000 inhabitants), while France (1.0–1.2) and Germany (1.6–1.8) had the highest rates [55].
Regarding demographic and clinical characteristics, the data included in our systematic review reveals that L3 is more frequent at a young age and L1 at an old age, which aligns with previous studies conducted on the non-Spanish population [56]. Furthermore, a different pattern of disease extension according to age is also observed in UC. Thus, findings indicate that E3 could be more prevalent in the paediatric population than in the adult population, which is consistent with studies conducted in other countries [56].
The most common comorbidities associated with IBD are described in this review. Although no overall cancer risk has been reported, a higher relative risk for certain cancer types has been described [15, 37], mainly resulting from the pro-neoplastic effects of chronic intestinal inflammation [57, 58]. Moreover, it appears to be related to the use of certain drugs, as some studies have suggested that patients exposed to immunosuppressive drugs such as thiopurines might suffer an increased risk of cancer, compared to those treated with biologicals [59]. Nonetheless, within the last few years, IBD-related cancer incidence has been decreasing, which might be attributed to better treatment options and surveillance strategies [59].
According to the European Crohn’s and Colitis Organisation (ECCO) consensus guideline [60], another aspect to consider in managing patients with IBD is EIM. In line with European cohorts [56], up to nearly 50% of patients with CD and UC in Spain might develop EIM, particularly in CD.
The impact of IBD symptoms on daily life contributes substantially to reduced HRQoL, suggesting the need for improved symptom mitigation strategies. Most of the studies included in our review show that patients with CD and UC experience a deterioration of HRQoL compared to the general population, with the presence of comorbid depression and anxiety. Various physical, psychological and sexual dimensions were also affected; however, none of the studies reported information on the impact of bowel urgency on HRQoL, which has recently been described as the most disruptive symptom in patients with UC, independently associated with lower HRQoL and worse long-term outcomes [61, 62]. In addition, an association between high symptomatology burden and poorer HRQoL has been demonstrated [40, 42]. Accordingly, a recent meta-analysis confirmed that HRQoL for individuals with CD and UC was poorer than healthy controls for mental and physical HRQoL both in adults and children [63]. In this regard, as a result of the emotional burden, the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) and the Association of Crohn’s Disease and Ulcerative Colitis Patients (ACCU) agreed on 15 recommendations to optimize the identification of psychological problems, the referral to mental health professionals and the management of psychological problems [64]. However, according to a national survey addressed to patients and specialists, only 50% of physicians would regularly ask about emotional issues in their consultations. On the other hand, only 25% of patients stated that these issues had been addressed in their consultations [65].
Currently, there are a wide range of treatment options for IBD, as evidenced by the considerable variability in treatment patterns observed across studies. Nonetheless, this heterogeneity could also be influenced by the year and region in which the studies were conducted.
The first biological drug authorized by the European Medicines Agency (EMA) for CD was infliximab, in 2001 [47]. Since then, several other biologicals have been launched and it was estimated that 25% of patients with CD in Spain received biological treatment in 2016, making it one of the countries with the highest use, only behind France (31% in 2017) and Poland (27.7% in 2015) [47].
Interestingly, coinciding with a significant increase in the number of patients treated with biologics, studies show that in Cataluña, the rate of CD-related hospitalisations decreased between 2011 and 2017 [52]. A recent meta-analysis corroborates these results reporting that patients with CD and UC diagnosed in the biological era (after 2000) had a lower cumulative incidence of hospitalisation [66]. Earlier and improved diagnosis of IBD, the introduction of biological agents and their early use could be driving the reduction of the hospitalisation rate observed in the last decades.
In addition to treatment, some patients require inpatient care and surgical interventions at some stages of the disease. Therefore, IBD management is associated with considerable medical costs. Several studies in Spain reveal that the use of healthcare resources by patients with IBD is substantial. Thus, between 19.9% and 31.1% of adult patients with IBD visit the emergency department and 40.0% require hospitalisation [33, 50, 52]. Nonetheless, a population-based study with data from different countries showed that in Spain the rate of IBD-related hospitalisation, 23.8%, was the fourth lowest in Europe, and far from the 50–60% rates reported in the countries with highest rates [67].
The UC direct and indirect cost per patient/year in Cataluña was estimated at €1754.1 and €399.3, respectively, between 2002 and 2012 [31]. Similarly, in the pre-biologic era, the European Collaborative Study Group of IBD estimated, in costs for 2004, a similar direct cost per patient-year in UC (€1524 in UC), with higher costs in patients with CD (€2548). The main cost drivers were hospitalisation and surgery [68]. By country, the cost per patient-year for IBD in Spain (€2090) was similar to the Netherlands (€2230), Israel (€2258) and Ireland (€2286), with the lowest cost in Norway (€888) and the highest in Denmark (€3705) [68].
In the biologic era, a prospective inception cohort involving 20 European countries estimated that first-year hospitalisations and diagnostic procedures accounted for more than 50% of the cost, while at 5-year follow-up, the expenditure on biologics accounted for 73% and 48% of the cost in CD and UC, respectively [69]. In addition, at 2015 values, a higher cost per patient/year in CD (€3542) compared to UC was also observed in this study (€2088) [69]. Another recent pan-European study raised the mean annual direct medical, direct non-medical and indirect costs for UC in Spain up to €4551, €1321 and €3061 respectively, resulting in a mean annual total cost of €8934 [70]. This cost was the highest among the 10 participating countries of the study, with an overall mean annual total cost of €7854, all in 2019 prices [70].
The introduction of biosimilars can be expected to reduce the cost associated with the use of the original biologics. Indeed, an economic model to simulate the introduction of biosimilars in IBD in the Dutch context (2017) estimated a reduction of 28% in total costs [71]. However, the economic impact will depend on local pricing, policies and therapeutic inertia. In addition, a recent probabilistic model showed that switching to biosimilar infliximab was less costly and less effective [72]. Thus, decision-makers need to consider the cost-effectiveness of these treatments.
The studies included herein did not analyse the impact of the disease according to severity. However, several studies suggested that more severe forms of the disease are associated with greater presence of EIMs and resource use [34, 39, 73]. In line with this, a pan-European study suggested that more severe phenotypes result in a significantly higher mean annual cost in both CD and UC [69]. Moreover, it has been described that pharmacological therapies (in particular biological agents) are the main cost driver in complex perianal CD [74].
This systematic review has also shown that data on survival/mortality, costs and data relating to the paediatric population are limited. It would be necessary to promote studies to assess the IBD costs (including CD and UC) in Spain, and the burden of IBD in the paediatric population.
Our review has some limitations. First, as previously mentioned, differences in the characteristics of study populations and study design lead to considerable heterogeneity between studies, hindering interpretation. Second, study quality was not an exclusion criterion. Third, the units in which data are reported are heterogeneous (e.g. crude rates, adjusted rates and time of data collection), which may hamper comparability. Finally, the number of studies conducted on the paediatric population is limited.
Conclusion
Patients with CD and UC present a high disease burden, with the presence of gastrointestinal symptoms (e.g. diarrhoea, bowel urgency), EIM, other IMID, and psychiatric comorbidities, which impact their HRQoL. This results in an elevated use of resources and associated costs for the national healthcare system (mainly related to hospitalisations, surgeries and medication). The review highlights the need for effective pharmacological interventions that help control symptoms, reduce related comorbidities, improve HRQoL and ultimately reduce the use of resources and associated costs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Verónica García, former student at Lilly, and Ana Causanilles received honoraria for her assistance.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Lilly.
Author Contributions
Manuel Barreiro de Acosta contributed to the data interpretation of the work and the critical revision of the manuscript. Alberto Molero contributed to the conception, design and data interpretation of the work, and the critical revision of the manuscript. Esther Artime and Silvia Díaz Cerezo contributed to the design and the data interpretation of the work, and the critical revision of the manuscript. Héctor D. de Paz contributed to the design, data acquisition and data analysis of the work, and drafting of the manuscript. Luis Lizán contributed to the design and data interpretation of the work and the critical revision of the manuscript. María D. Martín Arranz contributed to the data interpretation of the work and the critical revision of the manuscript.
Disclosures
Manuel Barreiro de Acosta reports honoraria from Abbvie, Takeda, Janssen, Pfizer, Lilly and Galapagos outside the submitted work. Alberto Molero, Esther Artime, Silvia Díaz Cerezo are employees of Lilly. Héctor D. de Paz and Luis Lizán work for an independent scientific consultancy (Outcomes’10) that has received honoraria for conducting the systematic review and writing the current manuscript. María D. Martín Arranz reports honoraria from Abbvie, Ferring and Janssen outside the submitted work.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analysed during this study are included in this published article/as supplementary information files.
References
- 1.Panaccione R. Mechanisms of inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2013;9(8):529–532. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou N, Chen WX, Chen SH, Xu CF, Li YM. Inflammatory bowel disease unclassified. J Zhejiang Univ Sci B. 2011;12(4):280–286. doi: 10.1631/jzus.B1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21(8):1982–1992. doi: 10.1097/MIB.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein C, Eliakim A, Fedail S, et al. Inflammatory bowel disease. World Gastroenterology Organisation Global Guidelines. Milwaukee: WGO; 2015. [DOI] [PubMed]
- 5.Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2010;6(5):339–346. [PMC free article] [PubMed] [Google Scholar]
- 6.Neuman MG, Nanau RM. Inflammatory bowel disease: role of diet, microbiota, life style. Transl Res. 2012;160(1):29–44. doi: 10.1016/j.trsl.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchgesner J, Lemaitre M, Rudnichi A, et al. Therapeutic management of inflammatory bowel disease in real-life practice in the current era of anti-TNF agents: analysis of the French administrative health databases 2009–2014. Aliment Pharmacol Ther. 2017;45(1):37–49. doi: 10.1111/apt.13835. [DOI] [PubMed] [Google Scholar]
- 9.Flamant M, Roblin X. Inflammatory bowel disease: towards a personalized medicine. Therap Adv Gastroenterol. 2018;11:1756283X17745029. doi: 10.1177/1756283X17745029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beard JA, Franco DL, Click BH. The burden of cost in inflammatory bowel disease: a medical economic perspective and the future of value-based care. Curr Gastroenterol Rep. 2020;22(2):6. doi: 10.1007/s11894-020-0744-z. [DOI] [PubMed] [Google Scholar]
- 11.Burisch J, Jess T, Martinato M, Lakatos PL, EpiCom E. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7(4):322–337. doi: 10.1016/j.crohns.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Algaba A, Guerra I, Castaño A, et al. Risk of cancer, with special reference to extra-intestinal malignancies, in patients with inflammatory bowel disease. World J Gastroenterol. 2013;19(48):9359–9365. doi: 10.3748/wjg.v19.i48.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunet E, Roig-Ramos C, Vela E, et al. Prevalence, incidence and mortality of inflammatory bowel disease in Catalonia. A population-based analysis. Ann Med. 2018;50(7):613–619. doi: 10.1080/07853890.2018.1523550. [DOI] [PubMed] [Google Scholar]
- 17.Burisch J. Crohn’s disease and ulcerative colitis occurrence, course and prognosis during the first year of disease in a European population-based inception cohort. Dan Med J. 2014;61(1):B4778. [PubMed] [Google Scholar]
- 18.Chaaro Benallal D, Guerra Veloz MF, Argüelles-Arias F, et al. Evolution of the incidence of inflammatory bowel disease in Southern Spain. Rev Esp Enferm Dig. 2017;109(11):757–760. doi: 10.17235/reed.2017.4739/2016. [DOI] [PubMed] [Google Scholar]
- 19.Chaparro M, Garre A, Núñez Ortiz A, et al. Incidence, clinical characteristics and management of inflammatory bowel disease in Spain: large-scale epidemiological study. J Clin Med. 2021;10(13):2885. [DOI] [PMC free article] [PubMed]
- 20.De Castro Parga ML, De Castro Luisa Hernández V, Pineda JR, et al. Incidence and clinical phenotype of inflammatory bowel disease in the north–west of Spain (Vigo) between 1991 and 2011. In: Congress of European Crohns and Colitis Organisation; Virtual. 2020.
- 21.De Castro Parga ML, De Castro Luisa Hernández V, Pineda JR, et al. P741 Incidence and clinical phenotype of inflammatory bowel disease in the north–west of Spain (Vigo) between 1991 and 2011. In: Congress of European Crohns and Colitis Organisation; Virtual. 2020.
- 22.Fernández A, Hernández V, Martínez-Ares D, et al. Incidence and phenotype at diagnosis of inflammatory bowel disease. Results in Spain of the EpiCom study. Gastroenterol Hepatol. 2015;38(9):534–540. doi: 10.1016/j.gastrohep.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Guevara M, Salamanca-Fernández E, Miqueleiz E, et al. Inflammatory potential of the diet and incidence of Crohn’s disease and ulcerative colitis in the EPIC-Spain Cohort. Nutrients. 2021;13(7):2201. [DOI] [PMC free article] [PubMed]
- 24.Hernández V, de Castro ML, Salinas-Rojo M, et al. Incidence of inflammatory bowel disease and phenotype at diagnosis in 2011: results of Epi-IBD 2011 study in the Vigo area. Rev Esp Enferm Dig. 2022;114(2):103–6. [DOI] [PubMed]
- 25.Lucendo AJ, Hervías D, Roncero Ó, et al. Epidemiology and temporal trends (2000–2012) of inflammatory bowel disease in adult patients in a central region of Spain. Eur J Gastroenterol Hepatol. 2014;26(12):1399–1407. doi: 10.1097/MEG.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 26.Marín-Jiménez I, Saro C, Díaz V, Acosta MB, Gómez-García M, Casbas AG. Epidemiology and hospital resources use in the treatment of ulcerative colitis at gastroenterology units in Spain (EPICURE study) Drugs Context. 2018;7:212505. doi: 10.7573/dic.212505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-de-Carpi J, Rodríguez A, Ramos E, Jiménez S, Martínez-Gómez MJ, Medina E. Increasing incidence of pediatric inflammatory bowel disease in Spain (1996–2009): the SPIRIT registry. Inflamm Bowel Dis. 2013;19(1):73–80. doi: 10.1002/ibd.22980. [DOI] [PubMed] [Google Scholar]
- 28.Martín-de-Carpi J, Rodríguez A, Ramos E, et al. The complete picture of changing pediatric inflammatory bowel disease incidence in Spain in 25 years (1985–2009): the EXPERIENCE registry. J Crohns Colitis. 2014;8(8):763–769. doi: 10.1016/j.crohns.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Vegh Z, Burisch J, Pedersen N, et al. Incidence and initial disease course of inflammatory bowel diseases in 2011 in Europe and Australia: results of the 2011 ECCO-EpiCom inception cohort. J Crohns Colitis. 2014;8(11):1506–1515. doi: 10.1016/j.crohns.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Ballester MP, Martí D, Tosca J, et al. Disease severity and treatment requirements in familial inflammatory bowel disease. Int J Colorectal Dis. 2017;32(8):1197–1205. doi: 10.1007/s00384-017-2791-y. [DOI] [PubMed] [Google Scholar]
- 31.Aldeguer X, Sicras-Mainar A. Costs of ulcerative colitis from a societal perspective in a regional health care area in Spain: a database study. Gastroenterol Hepatol. 2016;39(1):9–19. doi: 10.1016/j.gastrohep.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Cordero Jorge VHAA, Peñate Bolaños M, Peña Ferrera Luis, et al. Epidemiología e historia natural de la enfermedad de crohn diagnosticada en edad pediátrica y adulto joven Libro de trabajos de la Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica. 2020; p. 66.
- 33.Iglesias-Rey M, Barreiro-de Acosta M, Caamaño-Isorna F, et al. How do psychological variables influence coping strategies in inflammatory bowel disease? J Crohns Colitis. 2013;7(6):e219–e226. doi: 10.1016/j.crohns.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Andreu M, Márquez L, Domènech E, et al. Disease severity in familial cases of IBD. J Crohns Colitis. 2014;8(3):234–239. doi: 10.1016/j.crohns.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Marín-Jiménez I, García Sánchez V, Gisbert JP, et al. Immune-mediated inflammatory diseases in patients with inflammatory bowel disease baseline data from the Aquiles study. Gastroenterol Hepatol. 2014;37(9):495–502. doi: 10.1016/j.gastrohep.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 36.García MJ, Pascual M, Del Pozo C, et al. Impact of immune-mediated diseases in inflammatory bowel disease and implications in therapeutic approach. Sci Rep. 2020;10(1):10731. doi: 10.1038/s41598-020-67710-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gómez-García M, Cabello-Tapia MJ, Sánchez-Capilla AD, De Teresa-Galván J, Redondo-Cerezo E. Thiopurines related malignancies in inflammatory bowel disease: local experience in Granada. Spain World J Gastroenterol. 2013;19(30):4877–4886. doi: 10.3748/wjg.v19.i30.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casellas F, Robles V, Borruel N, et al. Restoration of quality of life of patients with inflammatory bowel disease after one year with antiTNFα treatment. J Crohns Colitis. 2012;6(9):881–886. doi: 10.1016/j.crohns.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Marín L, Mañosa M, Garcia-Planella E, et al. Sexual function and patients’ perceptions in inflammatory bowel disease: a case-control survey. J Gastroenterol. 2013;48(6):713–720. doi: 10.1007/s00535-012-0700-2. [DOI] [PubMed] [Google Scholar]
- 40.Iglesias-Rey M, Barreiro-de Acosta M, Caamaño-Isorna F, et al. Psychological factors are associated with changes in the health-related quality of life in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20(1):92–102. doi: 10.1097/01.MIB.0000436955.78220.bc. [DOI] [PubMed] [Google Scholar]
- 41.López Cortés R, Marín Fernández B, Hueso Montoro C, Escalada Hernández P, Sanz Aznarez AC, Rodríguez Gutiérrez C. Calidad de vida relacionada con la salud en pacientes con enfermedad inflamatoria intestinal. Anal Sis San Navarra. 2016;39(1):123–31. [DOI] [PubMed]
- 42.Abautret-Daly Á, Dempsey E, Riestra S, et al. Association between psychological measures with inflammatory and disease-related markers of inflammatory bowel disease. Int J Psychiatry Clin Pract. 2017;21(3):221–230. doi: 10.1080/13651501.2017.1306081. [DOI] [PubMed] [Google Scholar]
- 43.López-Sanromán A, Carpio D, Calvet X, et al. Perceived emotional and psychological impact of ulcerative colitis on outpatients in Spain: UC-LIFE survey. Dig Dis Sci. 2017;62(1):207–216. doi: 10.1007/s10620-016-4363-3. [DOI] [PubMed] [Google Scholar]
- 44.Panés J, Domènech E, Aguas Peris M, et al. Association between disease activity and quality of life in ulcerative colitis: results from the CRONICA-UC study. J Gastroenterol Hepatol. 2017;32(11):1818–1824. doi: 10.1111/jgh.13795. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez A, Herreros B, Muñoz R, et al. Disfunción sexual y factores de riesgo asociados en pacientes con enfermedad de crohn. Gastroenterol Hepatol. 2021;44:98. [Google Scholar]
- 46.de Castro ML, Hernández V, Pineda JR, et al. Evolución del manejo terapéutico en la enfermedad inflamatoria intestinal (EII): comparación de dos cohortes prospectivas en el periodo 1991–2001. Gastroenterol Hepatol. 2020;43:116. [Google Scholar]
- 47.Péntek M, Lakatos PL, Oorsprong T, et al. Access to biologicals in Crohn’s disease in ten European countries. World J Gastroenterol. 2017;23(34):6294–6305. doi: 10.3748/wjg.v23.i34.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaparro M, Garre A, Ricart E, et al. Differences between childhood- and adulthood-onset inflammatory bowel disease: the CAROUSEL study from GETECCU. Aliment Pharmacol Ther. 2019;49(4):419–428. doi: 10.1111/apt.15114. [DOI] [PubMed] [Google Scholar]
- 49.Cordero Jorge VHAA, Peñate Bolaños M, Ferrera Luis P, et al. Epidemiología y evolución de los pacientes pediátricos y adultos jóvenes afectados de colitis ucerosa. Libro de trabajos de la Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica. 2020; p. 72.
- 50.Díaz M, Benito Palma S, Martínez TP. Enfermedad inflamatoria intestinal en edad avanzada: experiencia de un centro. Rev Esp Enferm Dig. 2021;113:225. [Google Scholar]
- 51.López Cortés R, Marín Fernández B, Hueso Montoro C, Escalada Hernández P, Sanz Aznarez AC, Rodríguez GC. Quality of life in patients with inflammatory bowel diseases. An Sist Sanit Navar. 2016;39(1):123–131. doi: 10.4321/S1137-6627/2016000100014. [DOI] [PubMed] [Google Scholar]
- 52.Brunet E, Melcarne L, Vela E, et al. Evolución temporal de la enfermedad de crohn en Cataluña de 2011 a 2017. El aumento del tratamiento con fármacos biológicos se correlaciona con una menor necesidad de cirugía. Gastroenterol Hepatol. 2017;2020(46):93. [Google Scholar]
- 53.Ramos A, Calvet X, Sicilia B, et al. IBD-related work disability in the community: prevalence, severity and predictive factors. A cross-sectional study. United Eur Gastroenterol J. 2015;3(4):335–342. doi: 10.1177/2050640615577532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 55.GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30. [DOI] [PMC free article] [PubMed]
- 56.Zhao M, Gonczi L, Lakatos PL, Burisch J. The burden of inflammatory bowel disease in Europe in 2020. J Crohns Colitis. 2021;15(9):1573–1587. doi: 10.1093/ecco-jcc/jjab029. [DOI] [PubMed] [Google Scholar]
- 57.Stidham RW, Higgins PDR. Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal Surg. 2018;31(3):168–178. doi: 10.1055/s-0037-1602237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greuter T, Vavricka S, Konig AO, Beaugerie L, Scharl M, Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology Malignancies in inflammatory bowel disease. Digestion. 2020;101(Suppl 1):136–145. doi: 10.1159/000509544. [DOI] [PubMed] [Google Scholar]
- 59.Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol. 2016;22(20):4794–4801. doi: 10.3748/wjg.v22.i20.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harbord M, Annese V, Vavricka SR, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2016;10(3):239–254. doi: 10.1093/ecco-jcc/jjv213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sninsky JA, Barnes EL, Zhang X, Long MD. Urgency and its association with quality of life and clinical outcomes in patients with ulcerative colitis. Am J Gastroenterol. 2022;117(5):769–776. doi: 10.14309/ajg.0000000000001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carpio D, López-Sanromán A, Calvet X, et al. Perception of disease burden and treatment satisfaction in patients with ulcerative colitis from outpatient clinics in Spain: UC-LIFE survey. Eur J Gastroenterol Hepatol. 2016;28(9):1056–1064. doi: 10.1097/MEG.0000000000000658. [DOI] [PubMed] [Google Scholar]
- 63.Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses—part I. Inflamm Bowel Dis. 2018;24(4):742–751. doi: 10.1093/ibd/izx100. [DOI] [PubMed] [Google Scholar]
- 64.Barreiro-de Acosta M, Marín-Jiménez I, Panadero A, et al. Recomendaciones del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) y de la Confederación de Asociaciones de Enfermedad de Crohn y Colitis Ulcerosa (ACCU) para el manejo de los aspectos psicológicos en la enfermedad inflamatoria intestinal. Gastroenterol Hepatol. 2018;41(2):118–127. doi: 10.1016/j.gastrohep.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Marin-Jimenez I, Gobbo Montoya M, Panadero A, et al. Management of the psychological impact of inflammatory bowel disease: perspective of doctors and patients—the ENMENTE project. Inflamm Bowel Dis. 2017;23(9):1492–1498. doi: 10.1097/MIB.0000000000001205. [DOI] [PubMed] [Google Scholar]
- 66.Tsai L, Nguyen NH, Ma C, Prokop LJ, Sandborn WJ, Singh S. Systematic review and meta-analysis: risk of hospitalization in patients with ulcerative colitis and Crohn’s disease in population-based cohort studies. Dig Dis Sci. 2021;67:2451–2461. doi: 10.1007/s10620-021-07200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.King JA, Underwood FE, Panaccione N, et al. Trends in hospitalisation rates for inflammatory bowel disease in western versus newly industrialised countries: a population-based study of countries in the Organisation for Economic Co-operation and Development. Lancet Gastroenterol Hepatol. 2019;4(4):287–295. doi: 10.1016/S2468-1253(19)30013-5. [DOI] [PubMed] [Google Scholar]
- 68.Odes S, Vardi H, Friger M, et al. Cost analysis and cost determinants in a European inflammatory bowel disease inception cohort with 10 years of follow-up evaluation. Gastroenterology. 2006;131(3):719–728. doi: 10.1053/j.gastro.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 69.Burisch J, Vardi H, Schwartz D, et al. Health-care costs of inflammatory bowel disease in a pan-European, community-based, inception cohort during 5 years of follow-up: a population-based study. Lancet Gastroenterol Hepatol. 2020;5(5):454–464. doi: 10.1016/S2468-1253(20)30012-1. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz-Casas L, Evans J, Rose A, et al. The LUCID study: living with ulcerative colitis; identifying the socioeconomic burden in Europe. BMC Gastroenterol. 2021;21(1):456. doi: 10.1186/s12876-021-02028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Severs M, Oldenburg B, van Bodegraven AA, et al. The economic impact of the introduction of biosimilars in inflammatory bowel disease. J Crohns Colitis. 2017;11(3):289–296. doi: 10.1093/ecco-jcc/jjw153. [DOI] [PubMed] [Google Scholar]
- 72.Hughes A, Marshall JK, Moretti ME, Ungar WJ. A cost-utility analysis of switching from reference to biosimilar infliximab compared to maintaining reference infliximab in adult patients with Crohn’s disease. J Can Assoc Gastroenterol. 2021;4(1):48. doi: 10.1093/jcag/gwz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merino Gallego E, Gallardo SF. Evaluación de la prevalencia, los factores de riesgo y el tratamiento de las manifestaciones extraintestinales en los pacientes con enfermedad inflamatoria intestinal. Gastroenterol Hepatol. 2021;44:138. doi: 10.1016/j.gastrohep.2021.11.008. [DOI] [PubMed] [Google Scholar]
- 74.Chaparro M, Zanotti C, Burgueno P, et al. Health care costs of complex perianal fistula in Crohn’s disease. Dig Dis Sci. 2013;58(12):3400–3406. doi: 10.1007/s10620-013-2830-7. [DOI] [PubMed] [Google Scholar]
- 75.Nunes T, Etchevers MJ, Domènech E, et al. Smoking does influence disease behaviour and impacts the need for therapy in Crohn’s disease in the biologic era. Aliment Pharmacol Ther. 2013;38(7):752–760. doi: 10.1111/apt.12440. [DOI] [PubMed] [Google Scholar]
- 76.Schreiber S, Panés J, Louis E, Holley D, Buch M, Paridaens K. National differences in ulcerative colitis experience and management among patients from five European countries and Canada: an online survey. J Crohns Colitis. 2013;7(6):497–509. doi: 10.1016/j.crohns.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 77.Bastida G, Marín-Jiménez I, Forés A, et al. Treatment patterns and intensification within 5 year of follow-up of the first-line anti-TNFα used for the treatment of IBD: results from the VERNE study. Dig Liver Dis. 2021;54(1):76–83. doi: 10.1016/j.dld.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 78.Díaz M, Martínez Tirado P, Vidal Vílchez B, Zúñiga de Mora Figueroa B. Fenotipo de la enfermedad inflamatoria intestinal en mayores de 65 años: experiencia de un centro. Gastroenterol Hepatol. 2020;43:173. [Google Scholar]
- 79.Díaz M, Martínez Tirado P, Vidal Vílchez B, Zúñiga de Mora Figueroa B. Prevalencia de la enfermedad inflamatoria intestinal en mayores de 65 años: experiencia de un centro. Gastroenterol Hepatol. 2020;43:175. [Google Scholar]
- 80.de Castro ML, Hernández V, Pineda JR, et al. Incidencia y características clínicas de la enfermedad inflamatoria intestinal en el noroeste de España (Vigo) entre 1991–2011. Gastroenterol Hepatol. 2020;43:127. [Google Scholar]
- 81.Jijón Andrade MC, Peña Sainz-Pardo E, Gascón Galindo C, et al. Uso de biológicos en enfermedad inflamatoria intestinal pediátrica, situación actual en dos hospitales. Libro de trabajos de la Sociedad Española de Gastroenterología, Hepatología y Nutrición Pediátrica. 2020. p. 75.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article/as supplementary information files.