Take Home Message
Addition of topical corticosteroids to intermittent self-dilatation after direct vision internal urethrotomy can result in better short-term stabilisation of urethral strictures. The addition of topical corticosteroids appears safe, but further high-quality studies are needed to fully explore the utility of this approach in different treatment settings.
Keywords: Intermittent self-dilatation, Corticosteroids, Urethral stricture disease, Functional outcomes, Men
Abstract
Context
Intermittent self-dilatation (ISD) is a therapeutic strategy used to stabilise a urethral stricture and postpone or avoid further treatment. Adding corticosteroids to this mode of management might further enhance its outcomes by downregulation of collagen deposition and excessive scar tissue formation.
Objective
To explore whether a course of ISD with topical corticosteroids is superior at stabilising urethral stricture disease in men and improving functional outcomes over a course of ISD alone.
Evidence acquisition
This systematic review and meta-analysis was undertaken by the European Association of Urology Urethral Strictures Guideline Panel according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (CRD42021256744). The primary benefit outcome was successful stabilisation of the urethral stricture. Treatment-related complications were the primary harm outcome.
Evidence synthesis
In total, 978 records were screened for eligibility, ultimately leading to five included studies, all randomised controlled trials, comprising 250 patients, of whom 124 underwent a course of ISD with corticosteroids and 126 underwent a course of ISD alone, all after direct vision internal urethrotomy (DVIU). Successful stabilisation of the stricture was achieved in 77% and 64% of patients in the group with and without corticosteroids, respectively (p = 0.04). No extra complications related to the addition of corticosteroids to the ISD regimen were reported. The risk of bias of the included studies was generally unclear to high.
Conclusions
Based on the currently available data, a course of ISD with topical corticosteroids appears to be safe and superior at stabilising a urethral stricture after DVIU in the short term to a course of ISD alone. However, given the unclear to high risk of bias in the included studies, further high-quality studies are needed to fully underpin this.
Patient summary
This study shows that addition of topical corticosteroids to intermittent self-dilatation after direct vision internal urethrotomy can better stabilise the stricture in the short term.
1. Introduction
Urethral stricture disease involves a pathological condition where the urethral lumen narrows because of cicatrisation and fibrosis that occurs after damage to the urethral mucosa [1], [2]. As a result, the urinary outlet becomes obstructed, which may translate into symptoms that prompt men to seek medical help [1], [2].
The therapeutic options for urethral strictures in men vary, but mainly include the following: surgical dilatation, direct vision internal urethrotomy (DVIU), urethroplasty and urinary diversion [1], [3]. Urethroplasty generates the highest long-term success rates [3], [4], although endoluminal treatments such as surgical dilatation or DVIU are less invasive, and indicated for an isolated, short, primary stricture at the bulbar urethra or for patients unwilling or unfit to undergo an open reconstructive procedure [3].
Intermittent self-dilatation (ISD) is a therapeutic strategy in which the urethra is repeatedly dilated by the patient himself or a healthcare professional in order to stabilise the stricture and thus postpone or even avoid further treatment [3], [5]. It can be used as a treatment on its own or as an adjunct after surgical dilatation, DVIU, or urethroplasty to further optimise its outcomes [1], [3], [5], [6].
With ISD, the strictured segment of the urethra gets progressively stretched out and ultimately torn, resulting in a strip of urethral mucosa where re-epithelialisation is supposed to settle in faster than the formation of excessive scar tissue [1], [5]. In theory, addition of corticosteroids to this mode of management might further enhance its outcomes by a stimulating effect on endogenous collagenase and downregulation of collagen deposition [6], [7]. This may lead to healthier mucosal healing process, less excessive scar tissue formation, and thus lower chances of restenosis of the urethral lumen and better stabilisation of the disease [6], [7]. This application of corticosteroids can be achieved by an intralesional injection or by topical administration as an intraluminal instillation or as a lubricant on the catheter or dilatation instrument [6], [8].
In this context, the aim of this systematic review was to explore whether a course of ISD with topical corticosteroids is superior at stabilising urethral stricture disease in men and improving functional outcomes to a course of ISD alone.
2. Evidence acquisition
The a priori protocol for this systematic review was registered with the PROSPERO database (CRD42021256744).
2.1. Literature search and study selection process
The Medline, Embase, and Cochrane controlled trial databases were systematically searched for all relevant publications in March 2021 (English language, no restrictions in publication date). Additionally, the reference lists of similar reviews were screened separately for other potentially relevant publications. A second literature search was performed in May 2022.
Abstract and full-text screening was done from April 2021 until August 2021, data extraction from September 2021 until November 2021, and summary analysis and drafting of systematic review article from December 2021 until June 2022.
After deduplication, review authors (W.V., R.B., and G.C.) independently screened the titles and abstracts of the identified records for eligibility. The full texts of all potentially eligible records were retrieved and again screened independently for eligibility by the review authors (W.V., R.B., and G.C.) using a standardised eligibility form. Any disagreements were resolved by discussion or, if no consensus was reached, by consulting a senior review author (K.D.).
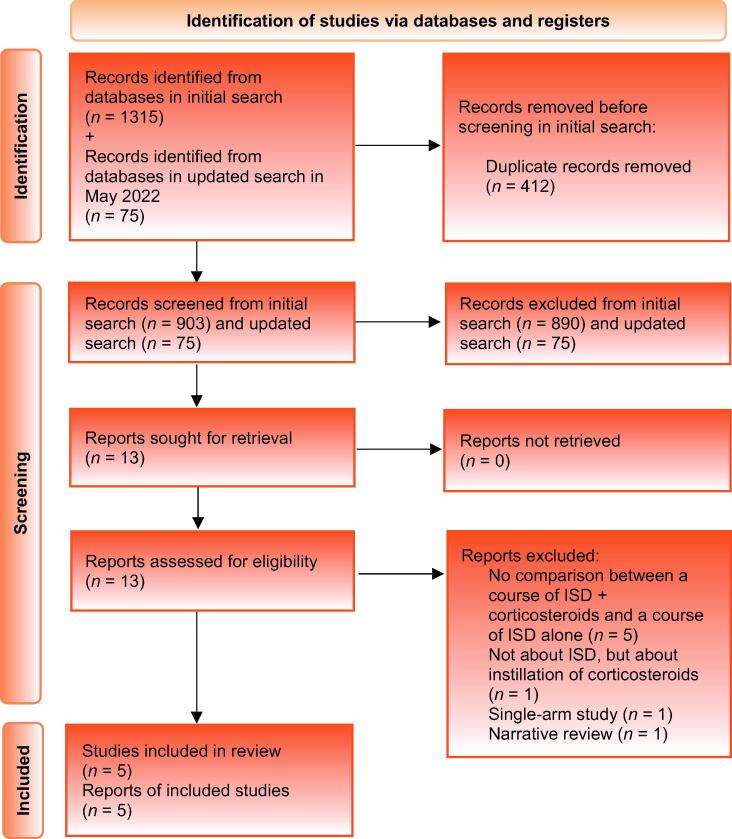
The literature search strategy is provided in the Supplementary material and a visual overview of the study selection process, according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram [9], is presented in Figure 1.
Fig. 1.
PRISMA flow diagram. ISD = intermittent self-dilatation; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses.
2.2. Types of studies
Randomised controlled trials (RCTs) and nonrandomised comparative studies, both prospective and retrospective, were included. A minimum of ten or more patients per group and a follow-up of ≥6 mo (counted from the end of the ISD course with or without corticosteroids) were required for inclusion. Narrative or systematic reviews, single-arm studies, case reports, abstracts only, conference abstracts, and letters to the editor were excluded.
2.3. Types of participants
Adult (≥18 yr) men with a diagnosis of urethral stricture disease were included. Both primary strictures, defined as strictures with no prior treatment, and recurrent strictures, defined as strictures previously treated with dilatation (any kind), urethrotomy (any kind), or urethroplasty (any kind), were allowed. Transmen and patients who underwent urinary diversion (any type, including definitive perineal urethrostomy, suprapubic cystostomy, or ileal conduit) were excluded.
2.4. Types of interventions
The intervention group was defined as patients who underwent a course of ISD with topical corticosteroids (all types of corticosteroids used as a catheter lubricant were allowed, as defined by trialists; the maximum duration of treatment was 6 mo) either as a stricture management on its own or as an adjunct after any of the following: surgical dilatation, urethrotomy, or urethroplasty (timing, frequency, catheter size, and protocol of dilatation as defined by trialists).
The comparator group was defined as patients who underwent a course of ISD alone (maximum duration of treatment 6 mo) either as a stricture management on its own or as an adjunct after any of the following: surgical dilatation, urethrotomy or urethroplasty (timing, frequency, catheter size, and protocol of dilatation as defined by trialists).
The panel felt that a duration of ISD of longer than 6 mo should be considered a palliative form of treatment in which the primary treatment effect of adding corticosteroids would be diluted or cancelled out. This served as the rationale for limiting the duration of ISD to a maximum of 6 mo.
2.5. Outcome measures
The primary benefit outcome was successful stabilisation of the urethral stricture, defined as no need for further management (return to ISD, surgical dilatation, DVIU, urethroplasty, or any form of urinary diversion such as definitive perineal urethrostomy, suprapubic cystostomy, or ileal conduit).
Treatment-related complications (such as pain, burning sensation, urethral irritation, episodes of urinary tract infections, bleeding, or false passage) were the primary harm outcomes.
Secondary outcomes included the following:
-
1.
Change in lower urinary tract symptoms (LUTS) from baseline, as defined by trialists
-
2.
Change in maximum urinary flow rate (Qmax) from baseline
-
3.
Change in quality of life from baseline, as defined by trialists
-
4.
Patient satisfaction, as defined by trialists
-
5.
Time to further management (return to ISD, surgical dilatation, DVIU, urethroplasty, or any form of urinary diversion such as definitive perineal urethrostomy, suprapubic cystostomy, or ileal conduit), as reported by trialists
Outcomes are reported at a 6, 12, 24, and 36 mo time points after cessation of ISD. For studies where outcomes were not reported at the prespecified time points, a descriptive text was provided.
The outcomes selected for presentation in the summary of findings table were successful stabilisation of the urethral stricture, change in LUTS from baseline, change in Qmax from baseline, and time to further management.
2.6. Data extraction and management
The review authors independently extracted outcome data (W.V., R.B., and G.C.)—two review authors per record. Study characteristics were extracted by one review author (W.V.) and checked for accuracy by a second review author (R.B. or G.C.). Any disagreements were resolved by discussion or, if no consensus was reached, by consulting a senior review author (K.D.). A standardised data extraction form was developed and piloted before use.
Data were stored in Excel files on Dropbox. Basic quality control checks were carried out on the database from each study in order to assess the quality of the data. A separate analysis was done for each study and compared with the results in the original publication of the study.
2.7. Risk of bias assessment
The risk of bias of each included study was assessed by two review authors working independently (W.V., R.B., and G.C.). Any disagreements were resolved by discussion or, if no consensus was reached, by consulting a senior review author (F.C.J. or K.D.). The risk of bias in RCTs was assessed by using the recommended tool in the Cochrane Handbook for Systematic Reviews of Interventions [10]. This includes the assessment of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias.
2.8. Summary measures
For categorical outcomes, we used odds ratios with 95% confidence intervals (CIs) and p values where available.
For continuous outcomes, we used mean difference or standardised mean difference with corresponding 95% CIs where available.
2.9. Unit of analysis issues
The primary analysis was per participant (randomised). For studies with more than two intervention groups, only the intervention groups relevant to the review were selected.
2.10. Dealing with missing data
We conducted an intention-to-treat analysis when data were available; otherwise, we conducted an available case analysis. We did not impute missing data.
2.11. Assessment of publication bias
The review authors aimed to minimise the potential publication bias by conducting a comprehensive literature search for eligible studies. Funnel plots were not used because fewer than ten articles matched the inclusion criteria.
2.12. Synthesis of results
A meta-analysis was performed where there was more than one RCT reporting the same outcome. For studies with multiple publications, only the one with the most up-to-date or complete data for each outcome was used. A priori, a fixed-effect model was used to calculate pooled estimates of treatment effect across similar studies and their 95% CIs. Where clinical or methodological heterogeneity was expected, a random-effect model was used.
Dichotomous outcomes were combined using the Mantel-Haenszel method for odds ratios.
A narrative synthesis was used for outcomes inappropriate for a meta-analysis.
2.13. Assessment of heterogeneity
Heterogeneity between studies was assessed by visual inspection of plots of the data, the chi-square Q test for heterogeneity, and I2 statistics [11]. A range of 75–100% was defined as considerable heterogeneity.
3. Evidence synthesis
3.1. Results
A total of 978 records were screened for eligibility, ultimately leading to five included studies [12], [13], [14], [15], [16], all RCTs, comprising 250 patients of whom 124 underwent a course of ISD with corticosteroids and 126 underwent a course of ISD alone (Fig. 1).
3.1.1. Study characteristics
A tabulated overview of the study characteristics is presented in Table 1. All studies were prospective RCTs conducted at a single institution evaluating the effect of adding topical corticosteroids to a course of ISD as an adjunct therapy after DVIU [12], [13], [14], [15], [16]. Most included strictures were ≤2 cm long and confined to the bulbomembranous segment (two studies did not report stricture location), except in the study of Gücük et al. [13]. Stricture aetiology varied, and most of strictures were primary ones, as is shown in Table 1.
Table 1.
Baseline characteristics
| Reference | Country | Single/multicentre | Study type | Time frame | N total |
N I group |
N C group |
FU (mo), mean (SD)/median (IQR) | Age (yr), mean (SD)/median (IQR/range) | Stricture location, n (%) | Stricture length (cm), mean (SD)/median (IQR) | Stricture aetiology, n (%) | Primary/recurrent stricture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regmi (2018) [15] | Nepal | Single | Prospective RCT | 01/2015– 12/2017 |
60a | 27 | 28 | All had 6 mo of FU after termination of ISD | I: Mean (SD) = 37.2 (1.6) C: Mean (SD) = 36.0 yr (1.7) (p = 0.782) |
NR | I: Mean (SD) = 0.93 (0.33) C: Mean (SD) = 1.07 (0.32) (p = 0.973) |
I: Traumatic 12 (44.4); inflammatory 7 (25.9); other 8 (29.6) C: Traumatic 14 (50.0); inflammatory 5 (17.9); other 9 (32.1) |
NR |
| Ergün (2015) [12] | Turkey | Single | Prospective RCT | NR | 90 | 30 | 30 | All had 24 mo of FU after DVIU | Mean (range) = 59.6 (19–74) (I range: 60.7; C range: 61.2) |
Bulbomembranous | All ≤2 | I: Idiopathic 7 (23.3); iatrogenic 23 (76.7) C: Idiopathic 5 (16.7); Iatrogenic 25 (83.3) |
All primary |
| Yesil (2013) [16] | Turkey | Single | Prospective RCT | 2004–2007 | 41 | 22 | 19 | All had 36 mo of FU | I: Mean (SD) = 45.1 (8.8) C: Mean (SD) = 47.0 (8.8) (p = 0.47) |
Bulbar | All ≤1.5 | Instrumentation 12 (29); Trauma 3 (7); infection 7 (17); idiopathic 19 (46) | All primary |
| Gücük (2010) [13] | Turkey | Unclear | Prospective RCT | 03/2002– 02/2005 | 45 | 15 | 15 | Mean (SD) = 16.4 (2.97) | I: Mean (SD) = 31.2 (8.3) C: Mean (SD) = 35.2 (7.9) |
Bulbar | I: Mean (SD) = 8.3 (2.7) C: Mean (SD) = 8.3 (1.9) |
I: Trauma 4 (26.7); infection 2 (13.3); instrumentation 4 (26.7); idiopathic 5 (33.3) C: Trauma 5 (33.3); infection 2 (13.3); instrumentation 3 (20.0); idiopathic 5 (33.3) |
All primary |
| Hosseini (2008) [14] | Iran | Single | Prospective RCT | NR | 70b | 30 | 34 | All had 6 mo of FU after termination of ISD | I: Mean (SD) = 37.7 (17.1) C: Mean (SD) = 34.5 (13.3) |
NR | I: Mean (SD) = 0.85 (0.40) C: Mean (SD) = 0.90 (0.30) |
I: Urethral distraction 15 (50.6); straddle injury 4 (13.3); catheter 5 (16.7); other 6 (20.0) C: Urethral distraction 14 (41.2); straddle injury 8 (23.5); catheter 5 (16.7); other 7 (20.6) |
I: Prior urethroplasty 18 (60.0%) C: Prior urethroplasty 17 (50.0%) |
C = comparator; DVIU = direct vision internal urethrotomy; FU = follow-up; I = intervention; IQR = interquartile range; ISD = intermittent self-dilatation; NR = not reported; RCT = randomised controlled trial; SD = standard deviation.
Five patients were excluded post hoc because they were lost to follow-up.
Seventy patients were originally included; five were lost to follow-up in the intervention group, one was lost to follow-up in the comparator group, and thus 64 were eventually analysed.
3.1.2. Synthesis of results
3.1.2.1. Successful stabilisation of the urethral stricture
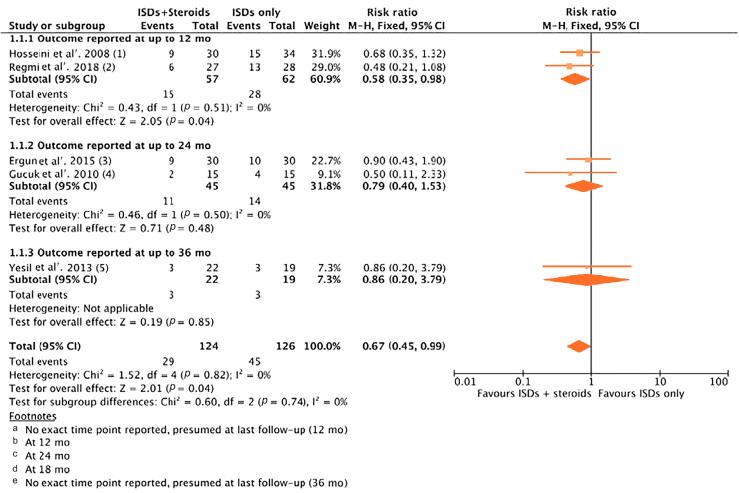
This outcome was reported in all five studies [12], [13], [14], [15], [16]. Individual study results can be found in Table 2. Data were pooled in a meta-analysis in which a fixed-effect model was used to calculate the pooled estimates of treatment effect, because of low clinical and methodological heterogeneity (Fig. 2). In total, 29/124 events (unsuccessful stabilisation of the urethral stricture) were observed in the ISD + corticosteroids group, while 45/126 were observed in the ISD-alone group (p = 0.04). Only studies reporting outcomes at 6 mo showed a significant benefit after pooling the respective results. Of note, the study of Yesil [16] incorrectly reported a p value of <0.05 for a very small sample size with only marginal differences in outcome.
Table 2.
Summary of findings
| Reference |
N I group |
ISD protocol I group |
N C group |
ISD protocol C group |
Specified time points | Successful stabilisation of stricture, n (%) |
Change in LUTS from baseline | Change in Qmax from baseline (ml/s) | Time to further management (months) |
|---|---|---|---|---|---|---|---|---|---|
| Regmi (2018) [15] | 27 |
|
28 |
|
6, 12 mo | I: 21/27 (77.78%; 12 mo) C: 15/28 (53.58%; 12 mo) p value: p = 0.04 |
NR | NR | I: Mean (SD) = 11.9 (3.0) C: Mean (SD) = 7.4 (4.5) p value: p = 0.16 |
| Ergün (2015) [12] | 30 |
|
30 |
|
1, 3, 6, 12, 24 mo | I: 21/30 (70.0%; 24 mo) C: 20/30 (66.7%; 24 mo) p value: p > 0.05 |
I: IPSS mean (SD): preop 23.2 (7.6), 24 mo 11.1 (4.1) C: IPSS mean (SD): preop 23.6 (7.2), 24 mo 11.4 (5.1) p value: p > 0.05 |
I: Mean (SD): preop 4.8 (4.1), 6 mo 13.3 (6.2), 12 mo 11.4 (8.4), 24 mo 9.6 (5.1) C: Mean (SD): preop 3.9 (3.2), 6 mo 12.1 (6.1), 12 mo 11.9 (9.4), 24 mo 8.9 (6.5) p value: p > 0.05 |
NR |
| Yesil (2013) [16] | 22 |
|
19 |
|
6, 12, 24, 36 mo | I: 19/22 (86%) (No time points reported) C: 16/19 (84%) (No time points reported) p value: p < 0.05 |
NR | I: Mean (SD): preop 8.2 (2.1), 6 mo 17.5 (4.4), 12 mo 18.0 (4.4), 24 mo 18.6 (4.6), 36 mo 17.9 (4.4) C: Mean (SD): preop 8.4 (1.8), 6 mo 18.0 (4.5), 12 mo 17.0 (4.7), 24 mo 17.6 (4.1), 36 mo 18.4 (4.3) p value: p > 0.05 |
NR |
| Gücü (2010) [13] | 15 |
|
15 |
|
2 wk and 3, 6, 12, 18 mo | I: 15/15 (100%; 6 mo), 14/15 (93%; 12 mo), 13/15 (87%; 18 mo), 12/15 (80%; >18 mo) C: 14/15 (93%; 6 mo), 13/15 (87%; 12 mo), 11/15 (73%; 18 mo), 8/15 (53%; >18 mo) p value: NR |
NR | I: Mean (SD): preop 6.95 (1.42), 6 mo 17.5 (3.0), 12 mo 15.7 (4.3), 18 mo 15.3 (4.6) C: Mean (SD): preop 7.23 (1.54), 6 mo 16.7 (3.7), 12 mo 14.5 (4.2), 18 mo 13.8 (4.8) p value: NR |
NR |
| Hosseini (2008) [14] | 30 |
|
34 |
|
1, 2, 3, 6, 9, 12 mo | I: 21/30 (70%) (No time points reported) C: 19/34 (56%) (No time points reported) p value: p = 0.24 |
NR | NR | NR |
C = comparator; I = intervention; IPSS = International Prostate Symptom Score; ISD = intermittent self-dilatation; LUTS = lower urinary tract symptoms; NR = not reported; Qmax = maximum urinary flow rate; SD = standard deviation.
Fig. 2.
Meta-analysis for successful stabilisation of the urethral stricture. CI = confidence interval; ISD = intermittent self-dilatation.
3.1.2.2. Treatment-related complications
None of the studies clearly reported on treatment-related complications, although three studies specifically mentioned that there were no extra complications related to the addition of corticosteroids to the ISD regimen [12], [14], [15].
3.1.2.3. Change in LUTS from baseline
Only one study reported on changes in LUTS from baseline after ISD with corticosteroids versus ISD alone [12]. In both groups, a similar decrease in the International Prostate Symptom Score (IPSS) was observed, with no statistically significant difference in treatment effect between those groups (Table 2).
3.1.2.4. Change in Qmax from baseline
Three studies reported on changes in Qmax from baseline for ISD with or without corticosteroids, and consistently reported an increase in Qmax postoperatively, with no statistically significant difference in treatment effect between ISD with or without corticosteroids (Table 2) [12], [13], [16].
3.1.2.5. Time to further management
Only Regmi et al. [15] reported on differences in time to further management for ISD + corticosteroids versus ISD alone. The mean (standard deviation) time intervals until further stricture treatment were 11.9 (3.0) and 7.4 (4.5) mo for the ISD + corticosteroids and ISD-alone groups, respectively (p = 0.16; Table 2) [15].
Change in quality of life from baseline and patient satisfaction was not studied in the five included articles.
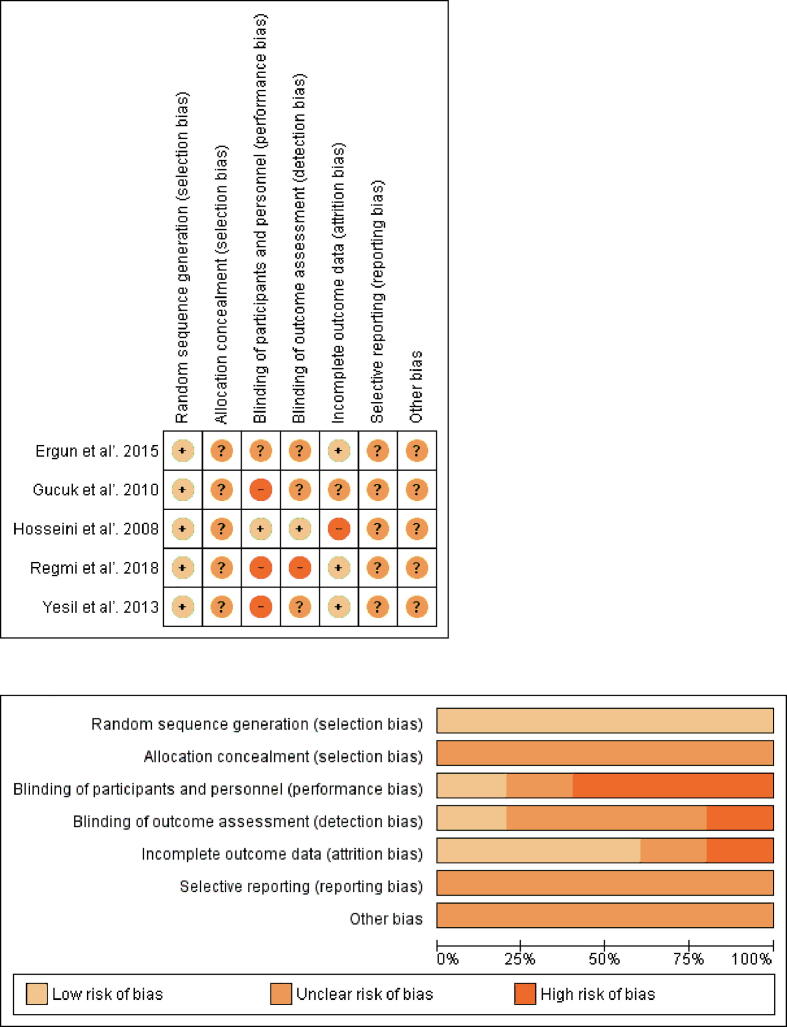
3.1.3. Risk of bias
The risk of bias assessment of all included studies is presented in Figure 3. Details on random sequence generation were not reported fully, especially with regard to allocation concealment. Only one study reported blinding of participants, personnel, and outcome assessors. The attrition bias was low in the majority of studies, while the reporting bias was unclear in all of these since none of the studies had an a priori protocol.
Fig. 3.
Risk of bias assessment.
3.2. Discussion
The current systematic review investigated whether a course of ISD with topical corticosteroids is superior at stabilising urethral stricture disease to a course of ISD alone. A total of five RCTs were included, in which 124 patients underwent a course of ISD with corticosteroids and 126 patients underwent a course of ISD alone [12], [13], [14], [15], [16]. Notably, in all of these studies, ISD was used as an adjunct after DVIU, which is probably driven by the fact that DVIU is more prone to recurrence than urethroplasty, especially in the bulbar segment, reflecting the greater degree of interest in adjunct therapies after DVIU than after urethroplasty [3], [4]. Successful stabilisation of the stricture was achieved in 77% and 64% of patients in the group with and without corticosteroids, respectively. The difference between groups was found to be statistically significant in the meta-analysis. This supports the use of corticosteroids when performing a course of ISD, especially since three of the included studies specifically reported that the addition of topical corticosteroids did not produce any additional side effects [12], [14], [15]. Notably, only studies reporting outcomes at 6 mo showed a statistically significant result, raising questions about the long-term effect of corticosteroids on tissue and scar remodelling. Regarding secondary outcomes, the application of corticosteroids did not lead to statistically significant benefit in terms of changes in Qmax [12], [13], [16], changes in LUTS [12], or time to further stricture treatment [15], although the latter two outcomes were reported only in one study each, both with relatively small sample sizes.
A possible explanation for these findings is that the corticosteroids are applied topically on the strip of torn mucosa that occurs after the dilatation of a stricture, and induce downregulation of collagen deposition and stimulate the endogenous collagenase enzymes [6], [7]. This can lead to a healthier mucosal healing process and helps the tissues to re-epithelialise fast enough to avoid excessive scar tissue formation and subsequent restenosis that might require further treatment [6], [7].
Our results are in line with the systematic review by Pang et al. [17] on the effect of different adjunct treatments after urethrotomy or surgical dilatation, although they differ from the findings of Zhang et al. [6] who published a systematic review on this subject in 2014. They found no significant differences in the stricture recurrence rates between the groups with and without corticosteroids, although they also included patients in whom corticosteroids were injected underneath the urethral mucosa during DVIU [6]. This single injection of corticosteroids might be insufficient to remodel the scar tissue in the long term, which may have led to a reduced treatment effect of adding corticosteroids in their study. However, they performed a subgroup analysis of patients where corticosteroids were applied as an ointment on the dilatation device. In this subgroup analysis, a trend towards a pooled treatment effect favouring addition of corticosteroids was observed, but this result was not statistically significant (p = 0.09). In our systematic review, addition of the data of two more recent RCTs has likely led to more statistical power, which probably explains our findings showing a statistically significant difference in favour of ISD with topical corticosteroids. This is also reflected in the more recent data of Soliman et al. [18], where DVIU with local corticosteroids (mix of submucosal injections and topical administration by ISD) led to fewer recurrences and a longer recurrence-free interval than DVIU alone.
It must be emphasised that in all included studies [12], [13], [14], [15], [16], the strictures were mostly primary, isolated, short, located at the bulbomembranous segment, and treated with DVIU only. Thus, no recommendations for other patient subgroups can be made based on the results of this systematic review and meta-analysis. It could be hypothesised that for distal strictures, this difference in treatment effect might be even more pronounced since the corticosteroid ointment can be better applied to the distal urethra than to the bulbar segment. The same hypothesis could be made for strictures where the fibrosis and cicatrisation are typically more severe (eg, lichen sclerosus cases or patients with a history of failed hypospadias repair) [19], [20]. Potts et al. [19] achieved a stabilisation rate of 89% (no need for escalation therapy) in lichen sclerosus patients after a median follow-up of 25 mo, although in their study, ISD with corticosteroids was continued indefinitely as a maintenance therapy and there was no comparison with an ISD-alone group. Unfortunately, the data retrieved in our systematic review were insufficient to allow for a subgroup analysis on lichen sclerosus patients.
In addition, it should be highlighted that this systematic review included only studies in which corticosteroids were used as a lubricant on the dilatation device. As such, we cannot comment on the outcomes of other corticosteroid application regimens, such as submucosal injection or urethral instillations, as described by Mazdak et al. [8]. In their study, 88% of the instillation group patients remained recurrence free versus 52% in the control group. Unfortunately, they did not report a mean or median follow-up for their groups, nor did they provide any details on how both groups were created. Even the use of oral corticosteroids has been investigated before, unfortunately with poorly reported results and no data on treatment safety [21]. In summary, whilst there are various ways to apply corticosteroids to the urethra, this systematic review is unable to advocate for one strategy over another.
Undeniably, there are several limitations to consider when interpreting the results of this systematic review. First of all, none of the included RCTs had an a priori protocol, a sample size calculation, or a power analysis. Second, the sample sizes of the included studies were small, leading to a relatively small overall sample size in the meta-analysis. Third, the risk of bias in the studies was generally high or unclear, often because of a poor methodology. Fourth, different time points were used to assess the outcomes across the different studies, and various treatment and follow-up strategies were used, which may have introduced some heterogeneity in the investigated sample. Fifth, only one study used a questionnaire in their follow-up, despite the fact that patient-reported outcome measures and validated questionnaires exist and are of utmost importance in the follow-up of urethral stricture patients. Unfortunately, the questionnaire used in this study was the IPSS questionnaire, which is an inadequate tool to use after urethroplasty [22]. Finally, the follow-up of the included studies ranged from 6 to 36 mo, and it is unclear whether the observed differences in treatment effect are sustained after a longer-term follow-up.
However, this systematic review and meta-analysis rigorously investigated the currently available literature on a very specific topic and included five prospective RCTs that investigated a rather homogeneous set of urethral stricture patients. All studies were critically assessed, and the remaining concerns and knowledge gaps were identified clearly, which may serve as a guide for future studies on this topic.
This project was undertaken by a multidisciplinary panel of clinical and methodological experts (European Association of Urology Urethral Strictures Guideline Panel) according to the PRISMA guidelines.
4. Conclusions
Based on the currently available data, a course of ISD with topical corticosteroids appears to be safe and superior at stabilising a urethral stricture after DVIU in the short term to a course of ISD alone. However, given the unclear to high risk of bias in the included studies, further high-quality studies are needed to fully underpin this and explore the effect of adding topical corticosteroids to ISD in other treatment settings.
This systematic review and meta-analysis is carried out under the auspices of the European Association of Urology Guidelines Office Board, European Association of Urology Urethral Stricture Guidelines Panel.
Author contributions: Wesley Verla had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Barratt, Dimitropoulos, Martins, Campos-Juanatey, Greenwell, Waterloos, Riechardt, Osman, Esperto, Ploumidis, Lumen, Chan, Verla.
Acquisition of data: Yuan, Barratt, Chan, Verla.
Analysis and interpretation of data: Barratt, Chan, Verla, Dimitropoulos.
Drafting of the manuscript: Barratt, Chan, Lumen, Verla, Dimitropoulos, Greenwell.
Critical revision of the manuscript for important intellectual content: Verla, Dimitropoulos, Lumen, Campos-Juanatey, Greenwell.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: Barratt, Chan, Verla, Dimitropoulos, Lumen.
Supervision: Dimitropoulos, Lumen.
Other: None.
Financial disclosures: Wesley Verla certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Nicolaas Lumen: receipt of grants/research supports—Bayer Sa Nv., Bayer, and Janssen; company speaker honorarium—Bayer and Janssen; trial participation—Janssen (Galamad), Roche (Ipatential), Pfizer Belgium, and AstraZeneca N.V. Felix Campos-Juanatey: company consultant—Boston Scientific; company speaker honorarium—ROVI; fellowship and travel grants—Rubio, SOBI, Janssen, and Almirall S.A. Tamsin Greenwell: participation in a company–sponsored speaker's bureau—Allergan; fellowship and travel grants—SPE Pharma, Astellas, and Medtronic; company speaker honorarium—Genesis Medical, Allergan, Astellas, and Colplast; trial participation: Medtronic and Axonics; fellowship and travel grants—Astellas; receipt of grants/research supports—Contura; educational meeting sponsorship towards the costs of the annual UCLH basics and advanced urodynamics courses—Laborie; sponsorship of surgical education—Boston Scientific; sponsorship of surgical education—Medtronic; education meeting sponsorship towards the running costs of the annual female urology and urogynaecology masterclass at UCLH—Boston Scientific, Allergan, Medtronic, Axonics, Contura, Laborie, Ferring, Pierre Fabre, Vesica Urology, and Aspire Pharma; educational meeting sponsorship for annual national service optimisation masterclass in botulinum toxin—Allergan and Laborie; other: immediate past chair BAUS FNUU Committee—demit 31/12/18, role in guiding BAUS policy on functional, neuro-urology and urodynamics and developing and organising FNUU offer at BAUS annual meeting; member of the editorial board of the journal of clinical urology—Responsibility for CPD commission. Mr. N.I. (Nadir) Osman: receipt of honoraria or consultation fees—Astellas; trial participation—Optilume. Francesco Esperto: database Manager—Biohealth Italia. Achilles Ploumidis: director or employee—Athens Private Practice of Robotics & Endoscopic Urology P.C.; company speaker honorarium—ESU, OLV Robotic Surgical Institute, and EUREP; fellowship and travel grants: Astellas; EAU/AUA exchange program; member of Hellenic Society of Reproductive Medicine (www.eeai.gr). Konstantinos Dimitropoulos, Francisco Martins, Silke Riechardt, Marjan Waterloos, Rachel Barratt, Garson Chan, Yuhong Yuan, and Wesley Verla: no conflicts of interest.
Funding/Support and role of the sponsor: None.
Acknowledgements: Names of all guideline panel members: Professor Dr. Nicolaas Lumen (Department of Urology, Ghent University Hospital, Ghent, Belgium), Dr. Felix Campos-Juanatey (Department of Urology, Hospital Universitario Marques de Valdecilla, Santander, Spain), Dr. Konstantinos Dimitropoulos (Department of Urology, Aberdeen Royal Infirmary, Aberdeen, Scotland, UK), Dr. Tamsin Greenwell (Department of Urology, University College London Hospital, London, UK), Dr. Francisco E. Martins (Department of Urology, Universidade de Lisboa, Hospital de Santa Maria, Lisbon, Portugal), Dr. Nadir Osman (Department of Urology, Royal Hallamshire Hospital, Sheffield, UK), Dr. Silke Riechardt (Department of Urology, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany), Dr. Marjan Waterloos (Department of Urology, AZ Maria Middelares, Ghent, Belgium); associates: Dr. Rachel Barratt (Department of Urology, University College London Hospital, London, UK), Dr. Garson Chan (Department of Urology, University of Saskatchewan, Saskatoon, Saskatchewan Canada), Dr. Francesco Esperto (Department of Urology, Campus Bio-Medico University, Rome, Italy), Dr. Achilles Ploumidis (Department of Urology, Athens Medical Centre, Athens, Greece), Dr. Wesley Verla (Department of Urology, Ghent University Hospital, Ghent, Belgium). Names and affiliations of guideline panel members involved in the review: Professor Dr. Nicolaas Lumen (Department of Urology, Ghent University Hospital, Ghent, Belgium), Dr. Felix Campos-Juanatey (Department of Urology, Hospital Universitario Marques de Valdecilla, Santander, Spain), Dr. Konstantinos Dimitropoulos (Department of Urology, Aberdeen Royal Infirmary, Aberdeen, Scotland, UK). Names and affiliations of review team members (senior associates, associates, clinical and methodological supervisors, information scientist): Professor Dr. Nicolaas Lumen (Department of Urology, Ghent University Hospital, Ghent, Belgium), Dr. Felix Campos-Juanatey (Department of Urology, Hospital Universitario Marques de Valdecilla, Santander, Spain), Dr. Konstantinos Dimitropoulos (Department of Urology, Aberdeen Royal Infirmary, Aberdeen, Scotland, UK), Dr. Wesley Verla (Department of Urology, Ghent University Hospital, Ghent, Belgium), Dr. Rachel Barratt (Department of Urology, University College London Hospital, London, UK), Dr. Garson Chan (Department of Urology, University of Saskatchewan, Saskatoon, Saskatchewan Canada), Dr. Yuhong Yuan (Department of Medicine, Health Science Centre, McMaster University, Hamilton, Ontario, Canada). Names and affiliations of other collaborators (including all other panel members): Dr. Tamsin Greenwell (Department of Urology, University College London Hospital, London, UK), Dr. Francisco E. Martins (Department of Urology, Universidade de Lisboa, Hospital de Santa Maria, Lisbon, Portugal), Dr. Nadir Osman (Department of Urology, Royal Hallamshire Hospital, Sheffield, UK), Dr. Silke Riechardt (Department of Urology, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany), Dr. Marjan Waterloos (Department of Urology, AZ Maria Middelares, Ghent, Belgium); associates: Dr. Francesco Esperto (Department of Urology, Campus Bio-Medico University, Rome, Italy), Dr. Achilles Ploumidis (Department of Urology, Athens Medical Centre, Athens, Greece).
Associate Editor: Véronique Phé
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2023.01.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Verla W., Oosterlinck W., Spinoit A.-F., Waterloos M. A comprehensive review emphasizing anatomy, etiology, diagnosis, and treatment of male urethral stricture disease. Biomed Res Int. 2019;2019:9046430. doi: 10.1155/2019/9046430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latini J.M., McAninch J.W., Brandes S.B., Chung J.Y., Rosenstein D. SIU/ICUD consultation on urethral strictures: epidemiology, etiology, anatomy, and nomenclature of urethral stenoses, strictures, and pelvic fracture urethral disruption injuries. Urology. 2014;83(3 Suppl):S1–S7. doi: 10.1016/j.urology.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Lumen N., Campos-Juanatey F., Greenwell T., et al. European Association of Urology guidelines on urethral stricture disease (part 1): management of male urethral stricture disease. Eur Urol. 2021;80:190–200. doi: 10.1016/j.eururo.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Wong S.S.W., Aboumarzouk O.M., Narahari R., O’Riordan A., Pickard R. Simple urethral dilatation, endoscopic urethrotomy, and urethroplasty for urethral stricture disease in adult men. Cochrane Database Syst Rev. 2012;12 doi: 10.1002/14651858.CD006934.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Ivaz S.L., Veeratterapillay R., Jackson M.J., et al. Intermittent self-dilatation for urethral stricture disease in males: A systematic review and meta-analysis. Neurourol Urodyn. 2016;35:759–763. doi: 10.1002/nau.22803. [DOI] [PubMed] [Google Scholar]
- 6.Zhang K., Qi E., Zhang Y., Sa Y., Fu Q. Efficacy and safety of local steroids for urethra strictures: a systematic review and meta-analysis. J Endourol. 2014;28:962–968. doi: 10.1089/end.2014.0090. [DOI] [PubMed] [Google Scholar]
- 7.Lund L., Wai K.H., Mui L.M., Yeung C.K. Effect of topical steroid on non-retractile prepubertal foreskin by a prospective, randomized, double-blind study. Scand J Urol Nephrol. 2000;34:267–269. doi: 10.1080/003655900750042013. [DOI] [PubMed] [Google Scholar]
- 8.Mazdak H., Tolou Ghamari Z., Khorrami A. Investigation of triamcinolone instillation in the long-term rate of anterior urethral strictures’ recurrence. Curr Urol. 2020;14:206–210. doi: 10.1159/000499237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J.P.T., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta‐analyses. 2015. p. 243–96. https://handbook-5-1.cochrane.org/chapter_9/9_analysing_data_and_undertaking_meta_analyses.htm.
- 12.Ergün O., Güzel A., Armağan A., Koşar A., Ergün A.G. A prospective, randomized trial to evaluate the efficacy of clean intermittent catheterization versus triamcinolone ointment and contractubex ointment of catheter following internal urethrotomy: long-term results. Int Urol Nephrol. 2015;47:909–913. doi: 10.1007/s11255-015-0990-4. [DOI] [PubMed] [Google Scholar]
- 13.Gücük A., Tuygun C., Burgu B., Oztürk U., Dede O., Imamoğlu A. The short-term efficacy of dilatation therapy combined with steroid after internal urethrotomy in the management of urethral stenoses. J Endourol. 2010;24:1017–1021. doi: 10.1089/end.2009.0233. [DOI] [PubMed] [Google Scholar]
- 14.Hosseini J., Kaviani A., Golshan A.R. Clean intermittent catheterization with triamcinolone ointment following internal urethrotomy. Urol J. 2008;5:265–268. [PubMed] [Google Scholar]
- 15.Regmi S., Adhikari S.C., Yadav S., Singh R.R., Bastakoti R. Efficacy of use of triamcinolone ointment for clean intermittent self catheterization following internal urethrotomy. JNMA J Nepal Med Assoc. 2018;56:745–748. doi: 10.31729/jnma.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yesil S. The long-term effect of ureteral dilatation therapy combined with steroid after internal urethrotomy. Gazi Med J. 2013;24 [Google Scholar]
- 17.Pang K.H., Chapple C.R., Chatters R., et al. A systematic review and meta-analysis of adjuncts to minimally invasive treatment of urethral stricture in men. Eur Urol. 2021;80:467–479. doi: 10.1016/j.eururo.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Soliman C., Pan H.Y.C., Mulholland C.J., et al. Effect of local steroids on urethral strictures: A systematic review and meta-analysis. Investig Clin Urol. 2022;63:273–284. doi: 10.4111/icu.20210391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potts B.A., Belsante M.J., Peterson A.C. Intraurethral steroids are a safe and effective treatment for stricture disease in patients with biopsy proven lichen sclerosus. J Urol. 2016;195:1790–1796. doi: 10.1016/j.juro.2015.12.067. [DOI] [PubMed] [Google Scholar]
- 20.Hayden J.P., Boysen W.R., Peterson A.C. Medical management of penile and urethral lichen sclerosus with topical clobetasol improves long-term voiding symptoms and quality of life. J Urol. 2020;204:1290–1295. doi: 10.1097/JU.0000000000001304. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S., Roy S., Pal D.K. Efficacy of oral steroids after optical internal urethrotomy in reducing recurrence of urethral strictures. Turkish J Urol. 2018;44:42–44. doi: 10.5152/tud.2017.66564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam C.A., Elliott S.P., Voelzke B.B., et al. The International Prostate Symptom Score (IPSS) is an inadequate tool to screen for urethral stricture recurrence after anterior urethroplasty. Urology. 2016;95:197–201. doi: 10.1016/j.urology.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.