Abstract
Healthy chickens are necessary to meet the ever-increasing demand for poultry meat. Birds are subjected to numerous stressful conditions under commercial rearing systems, including variations in the environmental temperature. However, it is difficult to counter the effects of global warming on the livestock industry. High environmental temperature is a stressful condition that has detrimental effects on growth and production performance, resulting in decreased feed intake, retarded growth, compromised gut health, enhanced oxidative stress, and altered immune responses. Traditional approaches include nutritional modification and housing management to mitigate the harmful effects of hot environments. Currently, broiler chickens are more susceptible to heat stress (HS) than layer chickens because of their high muscle mass and metabolic rate. In this review, we explored the possibility of in ovo manipulation to combat HS in broiler chickens. Given their short lifespan from hatching to market age, embryonic life is thought to be one of the critical periods for achieving these objectives. Chicken embryos can be modulated through either temperature treatment or nourishment to improve thermal tolerance during the rearing phase. We first provided a brief overview of the harmful effects of HS on poultry. An in-depth evaluation was then presented for in ovo feeding and thermal manipulation as emerging strategies to combat the negative effects of HS. Finally, we evaluated a combination of the two methods using the available data. Taken together, these investigations suggest that embryonic manipulation has the potential to confer heat resistance in chickens.
Keywords: Embryo, Thermal manipulation, In ovo feeding, Heat stress, Poultry
1. Introduction
Commercially grown chickens are exposed to several stressful conditions that affect production performance and ultimately cause economic losses (Saeed et al., 2019). Increased ambient temperature is an environmental stressor that influences the production capacity of chickens (Al-Saffar et al., 2002; Awad et al., 2020; Quinteiro-Filho et al., 2010). Owing to global warming, the poultry industry is more likely to face extreme temperatures in the future due to extreme weather variations. With elevated ambient temperatures, heat stress (HS) can lead to significant economic losses due to reduced growth rate, feed intake, egg production, meat quality, and enhanced mortality (Mashaly et al., 2004; Quinteiro-Filho et al., 2010).
The capacity of chickens to withstand high ambient temperatures is low due to the absence of sweat glands compelling them to depend on panting to keep themselves cool (Ensminger et al., 1990). With high metabolic rates, these negative factors make chickens more susceptible to high temperatures. Various strategies have been employed in attempt to alleviate the effects of HS in poultry, including traditional approaches such as feed supplementation (Niu et al., 2009) but their usefulness is limited. Traditional approaches to mitigating the adverse effects of HS to some extents have already been well documented (Goel et al., 2021c; Shlomo, 2000) and are beyond the scope of this review. This means that it is essential to adopt new strategies to overcome the negative effects of HS. The current review explores the possibility of improving thermotolerance in commercial chickens by adopting strategies such as in ovo supplementation, embryonic thermal manipulation, and a combination of both strategies.
As the market age continues to decrease with progress in genetic and nutritional understanding, broilers spend 30% to 40% of their life span inside the egg (Hulet et al., 2007). Thus, the importance of the embryonic life stage has significantly increased. Anything that can promote the reprogramming of the developing embryo may have the ability to transform the life of chickens. In light of the above information, manipulation during embryonic development has also been attempted over the last few decades. Thermal manipulation (TM) during incubation has been shown to mitigate the effects of HS (Al-Zghoul et al., 2019a; Barri et al., 2011; Loyau et al., 2015; Piestun et al., 2009). Another technique comprises in ovo feeding, by which substances such as nutrients are administered to the eggs of chickens (Das et al., 2021; Kadam et al., 2013; Retes et al., 2018; Uni et al., 2004, 2005) to improve their performance and immunity (Goel et al., 2016, 2017). Extensive work has been conducted to supplement nutrients inside the eggs during embryogenesis in chickens (Das et al., 2021; Kadam et al., 2013; Retes et al., 2018; Uni et al., 2004, 2005). Recently, in ovo supplementation was tested to mitigate the effects of HS in chickens (Han et al., 2017, 2018; Ncho et al., 2021a, 2021b).
The objective of this review was to explore the possibility of in ovo manipulation to improve the thermotolerance of broiler chickens. To this end, we first briefly overview the harmful effects of HS in poultry and then provide an in-depth evaluation of in ovo feeding and thermal manipulation as emerging strategies to combat the negative effects of heat stress. Finally, a combination of the two methods was assessed using available data.
2. Detrimental impact of high ambient temperature on chickens
To perform optimally at their ideal capacity, birds must maintain homeostasis with a thermal balance between themselves and their environment. Thermo-balance is an equilibrium between ambient heat and heat produced by living organisms, and should always be in the thermoneutral range of any given species for optimal production. Chickens perform best in the thermoneutral zone, which ranges between 18 and 22 °C (Daghir, 2009). Disturbance in thermo-balance leads to HS, adversely affecting bird performance via behavioral, biochemical, and physiological adaptations. To maintain normal body temperature under HS conditions, birds exhibit panting behavior to cool off (Ensminger et al., 1990). However, panting has detrimental effects on performance during the rearing phase.
Modern poultry genotypes, which result from extreme selection, produce more body heat because of their higher metabolic activity (Deeb et al., 2002). The simplest way to evaluate this is through rectal temperature. For example, acute HS of 32.0 °C for 6 h on the 33rd day of age resulted in enhanced rectal temperature in chickens (Lin et al., 2006). Several studies have reported a reduction in feed intake, body weight, and feed conversion ratio in chickens exposed to HS (Awad et al., 2020; Niu et al., 2009; Quinteiro-Filho et al., 2010, 2012). Furthermore, panting behavior and enhanced energy loss have been observed under such conditions (Ensminger et al., 1990; Kang et al., 2020; Sohail et al., 2012). Thus, an increase in temperature during growth is unfavorable for birds and may cause economic losses to the industry. These effects can therefore be attributed to behavioral, metabolic, and physiological alterations under high ambient temperatures. To enhance the present understanding of this sensitive issue, it is important to determine the detrimental effects of high ambient temperatures in chickens.
For optimum functioning of the living system, the essential nutrients are digested, absorbed by the intestine, and transported to other tissues. Epithelial permeability and mucosal surface area are responsible for nutrient absorption (Ferrer et al., 2003). The gut microbiota also play an important role and can be modified by nutrient supplementation. For instance, supplementation with a fiber-rich diet for three weeks enhanced the abundance of fibrolytic bacterial genera on the 28th day of broiler age (Goel et al., 2021b). The single-cell epithelial layer lining the gut lumen forms an important barrier between the internal and external environments of birds and plays a major role in the digestion and absorption of nutrients. The intestinal barrier, which is responsible for gut integrity, consists of tight junctions and is negatively affected by high ambient temperatures (Goo et al., 2019; Lambert, 2009). Extensive damage to the epithelial surface caused by HS increases the intestinal permeability associated with massive generalized sloughing and affects the morphological parameters of the duodenal, jejunal, and ileal mucosa (Santos et al., 2015). As the transfer of nutrients occurs through the intestinal epithelial membrane, nutrient uptake is altered by the modified expression of nutrient transporter genes under HS (Habashy et al., 2017a, 2017b). The same was true for pathogens in the gut under HS conditions. Acute heat stress increases pathogenic permeability through an enhanced Shannon diversity index (Goel et al., 2021a), thus impairing intestinal integrity, which can have negative effects, such as infection and mortality (Alhenaky et al., 2017).
Heat shock proteins (HSP) are associated with regulatory mechanisms of HS. The expression of HSP genes increases when birds are exposed to HS (Wang et al., 1998). Breeds with higher expression of the HSP70 gene suffered less during HS compared to commercial lines, suggesting a protective role of the HSP70 gene in chicken (Cedraz et al., 2017).
Oxidative stress is another concern because of the production of reactive oxygen species (ROS) under HS. The activity of antioxidant enzymes increases under HS (Yang et al., 2010). Therefore, it is critical to control ROS production during HS. The assessment of oxidative stress through antioxidant enzymes can prove to be an effective method for predicting cellular stress levels in chickens, but the interpretation of antioxidant enzyme activity should be performed with the caution.
The chicken immune system is well developed and activated to protect against adverse environmental conditions. Several studies have evaluated the effects of HS on the immune response through different immunological aspects in chickens (Mashaly et al., 2004; Puthpongsiriporn et al., 2001; Zhang et al., 2014; Zulkifli et al., 2000). For instance, the lymphoid organ weight (thymus, bursa, and spleen) was decreased when broiler chickens were exposed to 36.0 °C for six days from 37th to the 42nd day of age (Quinteiro-Filho et al., 2010). Furthermore, the white blood cell count and serum antibody titer were decreased when commercial laying hens were kept at 35.0 °C continuously for 5 weeks from the 31st to 35th week of age (Mashaly et al., 2004).
3. Embryonic manipulation to afford thermotolerance
Variations in the incubation temperature affected the performance of chicks after hatching. The optimum incubation temperature is defined as the temperature required to achieve maximum hatching potential (Hulet et al., 2007). Incubation temperature disparity can act as a stressor for the developing embryo. The commercial poultry industry demands high-yield, fast-growing broilers. Today's fast-growing broilers have a relatively shorter market age than in the past. Thus, in terms of total lifespan, it has increased the fraction of the embryonic life span, creating enormous pressure on the embryo, which consequently lowers hatchability and chick quality (Hulet et al., 2007). A slight inflection in the incubation temperature acts as a stress on the developing embryo, which modulates overall development by altering embryo size, organ development, skeletal growth, and hatchability (Salahi et al., 2012; Yalcin et al., 2003). However, a careful TM can result in thermal conditioning (Al-Zghoul et al., 2013, 2019c; Goel et al., 2022; Vinoth et al., 2018). Furthermore, in ovo feeding has shown promising results in improving the immune status of chickens during the rearing phase (Goel et al., 2016). Supplementing a variety of nutrients using the in ovo approach can also render adaptability to HS (Slawinska et al., 2019). To cope with the aftereffects of environmental stress, embryonic manipulation has recently been attempted using TM or nutrient supplementation.
3.1. Thermal manipulation
3.1.1. Effect on hatchability
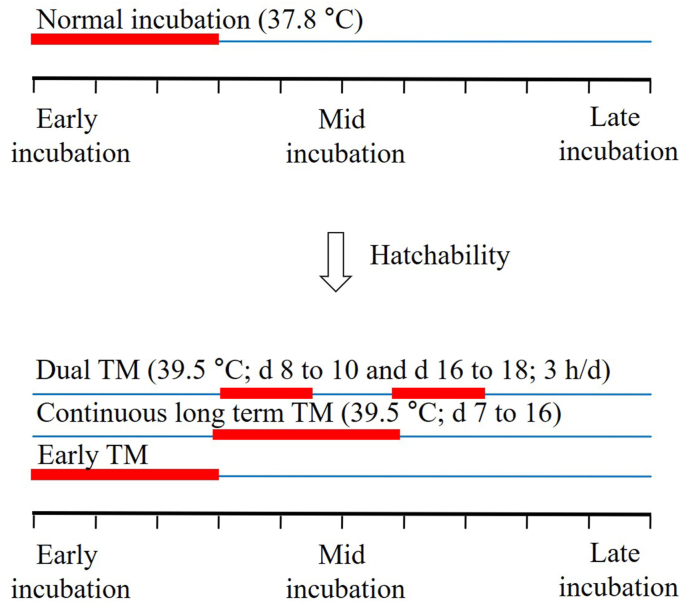
Modulation of the incubation temperature has been attempted in the last few decades. However, the results have been inconsistent. Hatchability is an important element for evaluating the sensitivity of embryos to TM (Fig. 1). The evaluation of hatching parameters is required before determining the HS-alleviating effects of TM. Hatchability was improved when eggs were acclimatized at 39 °C from the 16th to 18th day of incubation (Yahav et al., 2004). In contrast, hatchability was either reduced or unaffected when eggs were incubated at 39.6 or 39 °C from the 10th to 18th day for 6 or 18 h daily (Narinç et al., 2016; Saleh et al., 2020). The exposure time and duration of TM could pose as the main attributes that resulted in the observed inconsistency.
Fig. 1.
The relationship of hatchability with thermal manipulation (TM) during incubation. The blue line indicates normal incubation conditions while the red line indicates TM during incubation. The hatchability was reduced when TM was done during early incubation (Narinç et al., 2016) or done twice during the incubation period (Collin et al., 2007). Long-term continuous TM also resulted in decreasing hatchability (Piestun et al., 2008).
Embryonic age: The selection of embryonic age for TM should be carefully performed, as it has a direct impact on hatching rates. However, opinions differ among the research groups. Narinç et al. (2016) proposed the idea that long-term TM could reduce hatchability. In confirmation, an experiment was conducted in which hatchability decreased when eggs were incubated at 39.6 °C during late incubation (10th to 18th day) in comparison to early incubation (0 to 8th day) (Narinç et al., 2016). In contrast, no variations were reported in hatchability when eggs were incubated at 39.0 °C for 18 h daily for the same period (Al-Zghoul et al., 2019a). This indicates that temperature plays a more important role than exposure time. In correlation, a decrease in the hatchability was reported when eggs were exposed to either 39.5 or 40.0 °C from the 12th to 18th day for 18 h daily in comparison to 37.8, 38.5, and 39.0 °C respectively (Al-Zghoul and El-Bahr, 2019).
Single vs. multiple TM: Dual TM, even for a shorter period is more harmful to the developing embryo. This is evident from a study in which the eggs incubated for 3 h daily at 39.5 °C from the 8th to 10th day and from the 16th to 18th day had reduced hatchability (75.5%), compared with the control (88.2%) (Collin et al., 2007). Furthermore, it is important to monitor the type of thermal exposure during incubation. Continuous thermal exposure has a more drastic effect on hatchability than cyclic exposure. For instance, when incubated at 39.5 °C from the 7th to 16th day, eggs that were continuously exposed for 24 h daily had lower hatchability (50.6%) in comparison to eggs exposed to heat for 12 h daily (79.4%) for the same period (Piestun et al., 2008). Thus, it can be postulated that temperature modulation during incubation is an important physiological factor that can sometimes act as a stressor, but this can be overcome by careful selection of embryonic age, temperature, duration, and type of exposure.
Incubation temperatures: It was found that the hatchability was reduced from 91% to 87% when the incubation temperature was decreased from 37.8 to 36.6 °C (Joseph et al., 2006). In contrast, no changes were observed in the hatchability and performance of chicks produced from the eggs incubated at higher temperatures of approximately 39.5 °C (Barri et al., 2011). This implies that chicken embryos are more sensitive to lower temperatures than to higher ones. The complex nature of the embryo responds differently to changing environmental conditions at specific temperatures and times. Furthermore, not affecting hatchability performance at higher temperatures during incubation can be viewed as a window of opportunity to create thermotolerance in chicks.
3.1.2. Effects on chick quality
Chick weight at hatching is the major parameter that determines the quality of the chick. The viability of an effective TM depends on the hatchling quality. Genetic selection and breeder nutrition are major factors. The amount of residual yolk is important for nurturing the embryo to the last stage of incubation but has decreased over the last few decades (van der Wagt et al., 2020). Thus, it is essential to maintain high-quality chicks that can survive the transition period from hatchery to farm. Furthermore, body weight at hatching comprises a vital factor in determining the final chicken weight at slaughter; therefore, it is essential to ascertain the quality of chicks through hatchling weight (Sklan et al., 2003b). In contrast, no relationship has been found between hatchling weight and slaughter weight in male broilers (Molenaar et al., 2008). Although chick length could be a better indicator (Molenaar et al., 2008), it has been suggested as a crude parameter for measuring chick quality (Deeming, 2005). Therefore, the chick weight is still a popular parameter that indicates chick quality. Similarly, the effects of TM on body weight are somewhat controversial depending on studies, in which incubation temperatures are above or below the standard. When eggs were incubated at 39.5 °C either from the 8th to 10th day, 16th to 18th day, or both for 3 h daily, TM did not affect the body weight at the hatch (Collin et al., 2007). This indicates that embryonic TM has no adverse effects on body weight or chick quality. On the contrary, a recent study found that eggs incubated at 38.5 and 40.0 °C from the 12th to 18th day for 18 h daily had higher body weight at hatching in comparison to the control (37.8 °C) (Al-Zghoul and El-Bahr, 2019). When eggs were incubated at 39.5 °C from the 7th to 16th embryonic day for 12 h daily, hatching weight was reduced (Zaboli et al., 2017). This indicated a lower tolerance capacity of the embryo in the early stages of development before the 10th day of incubation. The role of the hypothalamic–pituitary–thyroid axis in thermal programming is expected to be established during the last phase of incubation (Jenkins et al., 2004; Morita et al., 2016). In conclusion, mid- or late-term incubation might be more effective in improving hatchling quality than the early incubation period.
3.1.3. Modulation of rectal temperature
Conditioning before hatching may help improve the heat resistance of chickens by lowering rectal temperature. Rectal temperature is considered an effective indicator of heat resistance (De Basilio et al., 2003). Thermal manipulation may improve thermotolerance acquisition by lowering the cloacal temperature in broiler chickens exposed to acute HS, making them more adaptable to changing environmental temperatures (Al-Zghoul et al., 2019c). The effectiveness of TM can be assessed by evaluating rectal temperature in newly hatched chicks. Recent studies have found that rectal temperature decreases after embryonic TM. For instance, TM at 39.5 °C for 3 h daily during the early (8th to 10th day) or late (16th to 18th day) incubation period reduced rectal temperature in chickens by approximately 0.3 °C (Collin et al., 2007). To demonstrate the stability of TM, the reduction in body temperature at hatching should be maintained consistently until the age of slaughter. The heat-manipulated group with an incubation temperature of 39.0 °C for 18 h daily from the 10th to 18th day had continuously lower rectal temperatures during most of the rearing period until the 35th day after hatching in Hubbard and Cobb chickens (Al-Zghoul et al., 2019b). It is obvious that the rectal temperature is elevated by HS, but chicks produced from heat-manipulated eggs should possess a lower temperature to reduce the aftereffects of HS. Thus, maintaining lower rectal temperatures consistently in thermally manipulated Hubbard and Cobb chicks during acute HS conditions, compared to controls, confirms its role in suppressing the deleterious effects of HS in chickens. In addition, a higher increase in rectal temperature was recorded in the control group than in the TM group under chronic HS conditions (Al-Zghoul et al., 2019a). Lower rectal temperatures indicated a decrease in the metabolic rate of the chickens. Thermal manipulation during incubation may be involved in reducing the effect of HS by acclimatizing to environmental conditions and modifying the thermoregulatory threshold response. However, the exact mechanism underlying this phenomenon remains unclear.
3.1.4. Effects on gut functions
Digestion and absorption comprise important processes for the availability of nutrients to different tissues. The small intestine is the initial site of nutrient absorption, but exposure to stressful conditions may influence the morphological parameters of the duodenal, jejunal, and ileal mucosa (Santos et al., 2015). Body weight is directly correlated with feed intake, and HS exposure tends to decrease feed intake and daily weight gain (Awad et al., 2020; Nawab et al., 2018; Quinteiro-Filho et al., 2010). Deprived feed intake may lead to a reduced supply of energy and nutrients such as carbohydrates, amino acids, and fatty acids. In addition to nutrient transport, the gastrointestinal tract prevents the introduction of harmful substances into the body. Enterocytes, mucus, and tight junctions are the major components of the intestinal barrier and ensure its integrity (Lambert, 2009). Heat stress results in intestinal barrier dysfunction by reducing the expression of genes involved in tight junctions (Goo et al., 2019). The uptake of endotoxins is also increased by leaks in the intestines, which can cause inflammation. A study conducted on thermal conditioning (36.0 ± 1 °C) for 24 h on the 3rd day of age reported higher villus volume on the 6th and 7th days of age in chicks (Uni et al., 2001). To date, no information is available regarding the effect of TM on gut integrity and the cause of hypophagia under HS conditions in chickens, which can be of interest to future studies.
Carbohydrate absorption: The absorption of carbohydrates occurs from the intestinal lumen to maintain a constant energy supply (Sklan et al., 2003a). Several transporters are responsible for controlling carbohydrate movement in chickens. These transporter proteins are encoded by the SLC2 family. The transportation of fructose alone occurs through glucose transporter 5 (GLUT-5), while the transportation of glucose, galactose, and fructose is channelized through GLUT-2 across the membranes (Uldry et al., 2004). Na+-dependent uptake of glucose and galactose occurs through sodium–glucose-linked transporter-1 (SGLT-1) (Wright and Turk, 2004). Thermal manipulation did not adversely affect carbohydrate uptake after hatching. Interestingly, no significant variations were reported in the expression of the SGLT-1 and GLUT-5 genes at the time of hatching when eggs were exposed to 39.6 °C from the 13th to 21st embryonic day (Barri et al., 2011). In general, corticosterone levels increase under HS conditions. The release of corticosterone under HS conditions leads to the enhancement of glucose transport gene expression such as SGLT-1, in the cell membrane (Garriga et al., 2006). However, the expression of the GLUT-2 and SGLT-1 genes was highest on the 1st day of HS and decreased slowly in the TM group when chicks were exposed to chronic HS at 35 °C from the 28th to 35th day (Al-Zghoul et al., 2019a). The decreasing trend in SGLT-1 and GLUT-2 gene expression could be attributed to the reduction in stress markers such as corticosterone production under HS conditions, thus indicating the acquisition of thermotolerance in the TM group.
Amino acid absorption: Aspartate and glutamate have been elucidated as the primary fuels for enterocytes, and their transport takes place through the excitatory amino acid transporter-3 (EAAT3) (Kanai et al., 2004; Newsholme et al., 2003). The expression of the EAAT3 and PepT1 genes was upregulated in quails 10 days after hatching, when eggs were incubated at 38.5 and 39.5 °C in comparison to the control (37.5 °C) (Rashid et al., 2016). Chicks obtained from thermally manipulated embryos during incubation may have responded well to the availability of feed after hatching and may be correlated with improved gut health with intact enterocytes due to enhanced aspartate and glutamate absorption. Villus height and crypt depth were indicators of mucosal development. When embryos were incubated at standard (37.4 °C) or high (39.6 °C) temperatures from 13 to 21 days of incubation, villus height and crypt depth were increased with enhanced expression of the PepT1 gene 4 days after hatching (Barri et al., 2011). The higher absorption of nutrients from the yolk sac and externally available feed is most likely the reason for the improved gut health. The effect of HS on modulating the expression of nutrient transporter genes has already been described (Habashy et al., 2017a, 2017b) and digresses from the topic of present interest. However, to describe the role of embryonic TM, it is essential to evaluate the regulation of relevant nutrient transporter gene expression during or after HS, which should be addressed in future studies.
Fatty acid absorption: It is critical to maintain the fatty acid balance inside the body. The uptake of long-chain fatty acids from intestinal digesta occurs through fatty acid-binding proteins (FABP) (Prows et al., 1995). Heat stress accepts electrons from unsaturated fatty acids and produces free radicals, which can increase lipid peroxidation. This is evident from studies where the expression of FABP1 was decreased after 12 days of HS in broilers (Habashy et al., 2017b). However, the enhanced fatty acid uptake may diminish the negative effects of HS. Notably, the expression of fatty acid transporter genes was increased in the TM group (39 °C) against the control (37.8 °C) during HS at 35 °C for 7 days (Al-Zghoul et al., 2019a). This could be due to the effective binding of FABP to fatty acid metabolites generated by oxidation (Ek-Von Mentzer et al., 2001). Moreover, the probability of TM inactivating lipid peroxidation and preventing cell damage by oxidative degradation of lipids under HS exists.
3.1.5. Effects of heat-stress markers
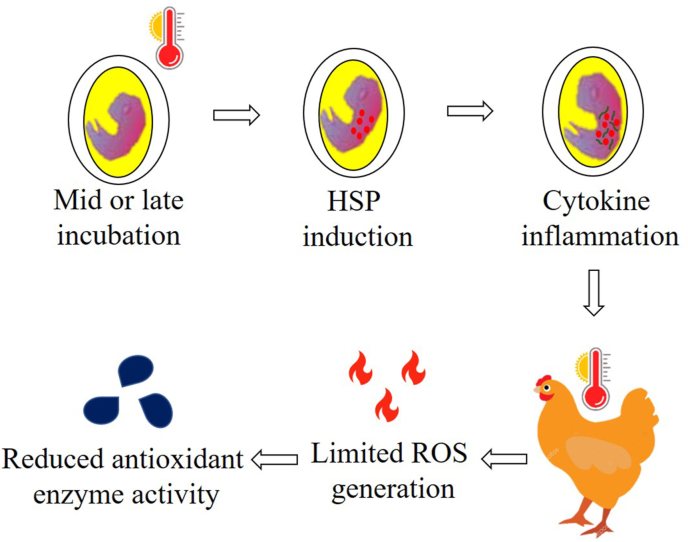
HSP gene family: The HSP gene family has been used as a marker to detect HS in chickens. However, there are contradictory observations regarding HSP gene expression in chickens. Although an early study showed reduced expression of HSP genes under HS conditions in chickens (Yahav et al., 1997), subsequent studies have been contradictory (Cedraz et al., 2017; Wang et al., 1998). Thus, the role of HSP in embryonic TM remains unclear. To address this, eggs were incubated at 38.8 °C for either 6 or 18 h a day from the 10th to 18th day, and found that the expression of the HSP70 gene was upregulated at various embryonic stages (Al-Zghoul et al., 2013). This suggests that the higher expression of HSP genes during the incubation period will help the embryos develop thermotolerance during the growing stages (Fig. 2). The next question was to identify the role of TM during the rearing phase under HS. Thermal exposure of eggs to 40.5 °C for 3 h on the 5th to 17th days of incubation decreased the expression of HSP genes when birds were exposed to 35 °C at 15 to 42 days of age. Later on, the authors explained that the reduced expression of HSP genes under HS in the TM group was beneficial for chickens (Vinoth et al., 2018) and this was in accordance with Yahav et al. (1997) and Rajkumar et al. (2015) wherein it was observed that thermal conditioning during early age-induced thermotolerance was due to enhanced adaptation, with the outcome of a lower degree of HSP expression. In contrast, Al-Zghoul et al. (2013, 2018) reported a higher expression of HSP genes when chickens were exposed to 41.0 °C for 6 h during different growing stages in thermally manipulated embryos. The authors suggested that the higher expression of HSP genes in chicks subjected to HS after TM treatment during incubation was due to the acquisition of thermotolerance. However, further studies are required to elucidate the exact mechanism underlying the acquisition of thermotolerance.
Fig. 2.
Mid or late-term thermal manipulation induces heat shock proteins (HSP) and cytokines during embryogenesis which helps the chicken to tolerate high ambient temperatures by decreasing reactive oxygen species (ROS) generation and antioxidant enzyme activity during the rearing phase. The diagram is drawn based on the articles (Al-Zghoul et al., 2013, 2019c; Saleh et al., 2020).
Oxidative stress and antioxidant enzymes: ROS production under high ambient temperature conditions comprises a major source of oxidative stress in chickens. Antioxidant enzymes prevent ROS production and can be used to determine stress intensity. During HS, the total antioxidant activity and gene expression of antioxidant enzymes increased (Kikusato et al., 2015; Yang et al., 2010), implying that a decrease in antioxidant activity could be used as an indicator of reduced oxidative stress and HS intensity. In a recent study, TM was shown to reduce the total antioxidant capacity and the expression of antioxidant enzyme-related genes such as nicotinamide adenine dinucleotide phosphate oxidase 4, superoxide dismutase, and catalase in HS chickens (Al-Zghoul et al., 2019c; Saleh et al., 2020). Therefore, it can be concluded that TM during incubation assists in the development of thermotolerance in chicks by reducing the oxidative stress levels in heat-exposed chickens (Fig. 2). Despite the essential role of antioxidant enzymes, studies on TM and HS in chickens are scarce. The role of TM during incubation in regulating antioxidant enzymes when birds are subjected to HS remains unclear and requires further investigation.
Immunomodulation: Immunity is evaluated to determine the defensive role of TM in HS. The expression of cytokines and toll-like receptors (TLR) is enhanced under HS (He et al., 2019; Varasteh et al., 2015). Duck embryos that were kept at a 0.5 °C higher temperature during the 1st to 21st days of incubation had higher expression of inflammatory cytokines on the 25th day of incubation (Shanmugasundaram et al., 2018), which could be related to HSP induction. Higher expression may help prepare chicks to withstand higher cellular concentrations of these genes in the presence of HS. In this regard, it was shown that higher expression of genes related to the induction pathway (HSP70, IL-6, IL-1β, TNF-α, NFκB50, TLR2, and TLR4) was reported in the TM group when chicks were exposed to acute HS at 40.0 °C for 7 h on the 28th day (Al-Zghoul et al., 2019b). Thus, it is assumed that the protective action of TM could be mediated through the enhanced expression of HSP and IL-6 genes in poultry under HS.
3.2. In ovo feeding
Extensive genetic selection and advances in feed technology continue to improve modern commercial chickens, allowing them to reach the market in approximately a month. Currently, much of the life of broilers lies within the frame of embryos (Hulet et al., 2007). This puts immense pressure on embryos to be viable and healthy. Due to the fixed nutritional supplements during laying, the perinatal period becomes critical, during which chicks can exposed to stressors, including nutrient deficiency. In ovo enrichment has been proposed to nourish the developing embryo and has the potential to increase development and immunity resistance in chickens (Goel et al., 2016, 2017). Many studies have explored various aspects of in ovo feeding and have been extensively reviewed previously (Das et al., 2021; Kadam et al., 2013). However, the use of in ovo feeding to develop heat tolerance is still in progress.
3.2.1. Nutrient supplementation
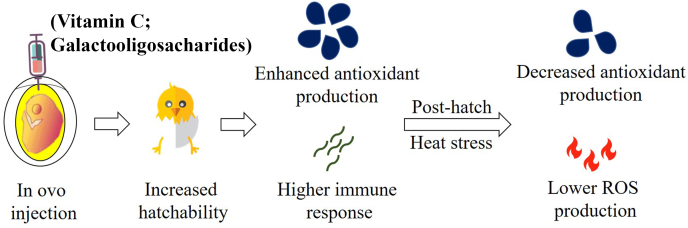
Initially, the need for in ovo supplementation was observed to obtain healthier chicks at hatching and sustain the energy demands required to transport chicks to distant locations in the early stages of life (Shinde et al., 2018). Healthy chicks at hatching may show characteristics of improved performance, immunity, and heat tolerance. For example, during in ovo feeding, carbohydrates improve immunity in chickens (Bhanja et al., 2015). However, the latter study did not evaluate heat tolerance and thus requires further investigation. Vitamins also play important roles in embryogenesis. In ovo, feeding chickens with various vitamins has the potential to improve growth and immunity (Goel et al., 2013). Vitamin C is a water-soluble vitamin used to mitigate heat stress by improving production performance through antioxidant modulation in chickens (Gouda et al., 2020). Recently, in ovo feeding of both water- and fat-soluble vitamins has been tested to modify nutrient uptake and antioxidant capacity in chickens. In ovo feeding of vitamin C (3 mg/egg) on the 15th day of incubation improved the antioxidant activity and immune function in chickens (Zhu et al., 2019). Vitamin C also plays a role in neurotransmitter modulation. Previous studies have suggested that vitamin C mitigates stress by regulating blood flow through the conversion of the neurotransmitter dopamine to norepinephrine (Harrison et al., 2009). However, further studies are warranted to explain the role of in ovo feeding of vitamin C in modulating neurotransmitters. Vitamin E has also been used to mitigate heat stress in chickens as it can protect epithelial membranes from peroxidation under heat stress (Oh et al., 2005). The role of vitamin E in combating heat stress in chickens by modulating immunity and antioxidant activity has already been elucidated (Habibian et al., 2014; Jiang et al., 2013). In ovo, vitamin E supplementation has been shown to improve chick quality, intestinal development, antioxidants at hatching, and immunity in heat-stressed chickens (Araújo et al., 2019; Heidari et al., 2021). The basic phenomenon for mitigating the adverse effects of heat stress by supplementing in ovo vitamins (vitamins C and E) might be by protecting the cells from oxidative stress through improved antioxidant status (Fig. 3). The role of these vitamins in improving immune tolerance cannot be ruled out.
Fig. 3.
In ovo supplementation improves performance at hatch and reduces antioxidant production when birds are exposed to heat stress during the rearing phase. In ovo manipulation during embryogenesis increased hatchability and had higher antioxidant enzyme activity and immune responses (Zhu et al., 2019). In ovo manipulated embryos when exposed to heat stress during the rearing phase showed lower antioxidant enzyme activity due to reduction in ROS generation in chickens (Slawinska et al., 2019). In parentheses are examples of in ovo injected materials. ROS = reactive oxygen species.
Amino acid requirements are critical for the development of chicken embryos. The benefits of in ovo amino acid supplementation in improving growth and immunity have already been documented (Bhanja et al., 2014; Gao et al., 2017). The concentration of free amino acids changes in different organs when chickens are exposed to heat stress (Chowdhury et al., 2014; Ito et al., 2014). Thus, exogenous supplementation with these amino acids may be beneficial under oxidative stress conditions. Recently, researchers have attempted to alleviate heat stress in chickens through the in ovo feeding of amino acids. A study conducted on in ovo feeding of L-leucine on the 7th embryonic day helped the chicken recover from chronic heat stress by modifying antioxidant levels (Han et al., 2022). Gamma-aminobutyric acid (GABA) is another amino acid that acts as an inhibitory neurotransmitter and thus has attracted the attention of researchers for its utility under stress conditions. Previous studies have suggested that dietary supplementation with GABA is beneficial for ameliorating the harmful effects of heat stress in chickens (Al Wakeel et al., 2017; Choi, 2019; El-Naggar et al., 2019). Recently, in ovo GABA feeding has been attempted to minimize the aftereffects of heat stress in chickens. Supplementation of GABA on the 17.5th day of incubation enhanced post-hatch heat tolerance by increasing antioxidant-related genes and decreasing the HSP-70 gene when birds were exposed to 38.0 °C for 3 h on the 10th day of age (Ncho et al., 2021b). Furthermore, in ovo GABA supplementation increased average daily gain and total antioxidant capacity while reducing ACC gene expression and corticosterone levels in broilers exposed to 33.0 °C for 6 h daily from the 28th to 31st day of age (Ncho et al., 2021a).
Trace minerals, such as zinc and selenium have been used to mitigate the adverse effects of heat stress in chickens (Calik et al., 2022; Gul et al., 2021; Sahin et al., 2003). During in ovo feeding, trace minerals can improve performance parameters, immune tolerance, and meat quality of chickens (Goel et al., 2016; Kim et al., 2022). However, they are rarely used in the direct context of heat stress after in ovo feeding. One of the studies evaluated the effect of in ovo feeding of nano-selenium and nano-zinc that was completed on the hatch day and concluded that these nano-minerals can alleviate oxidative stress by modifying antioxidants at hatching when the temperature of incubation was increased by 1.1 to 38.9 °C from the 19th to 21st day of embryogenesis (Shokraneh et al., 2020). Future studies are warranted in terms of in ovo feeding of trace minerals to correlate their roles under heat stress conditions.
3.2.2. Bioactive substances
There is a recent trend towards the use of prebiotics, probiotics, and synbiotics in poultry feeds. The use of such products as feed additives has been extensively explored. However, their use as feed additives is not the focus of the present study and has been presented elsewhere. The use of these products for embryonic feeding could be effective in improving gut health and reducing the destructive effects of stress in chickens. In general, probiotic products comprise favorable bacteria that colonize the gut either alone or in combination, whereas prebiotic products act as a substrate for the colonization of favorable bacteria to promote the utilization of nutrients more efficiently. Synbiotics comprise a combination of probiotics and prebiotics that have a synergistic effect on gut health. Due to the high vascularity of the chorioallantoic membrane, it is expected that probiotic substances will be able to migrate to the intestine after in ovo supplementation. The presence of microbiota in the gut of the embryo is limited, and its supplementation during incubation may augment the growth of beneficial bacteria to improve the gut health of chickens after hatching. This is evident from previous studies, where in ovo supplementation of probiotics augmented the growth of beneficial bacteria and reduced the colonization of pathogenic bacteria in the intestine of chicks (Majidi-Mosleh et al., 2017). The colonization of beneficial and pathogenic microbes in the gut is directly related to the growth performance of chickens. Body weight was found to be higher in chicks on the 34th day of age when eggs were injected with prebiotics or synbiotics on the 12th day of incubation (Pruszynska-Oszmalek et al., 2015). Improvements in gut-mediated growth performance may also be beneficial under challenging conditions. However, limited information is available on the effect of in ovo probiotic and synbiotic supplementation on the growth performance of chickens reared under HS conditions. Few studies have evaluated the effects of probiotic and prebiotic products on the antioxidant status of HS chickens and reported their beneficial effects. The reason behind this could be related to the reduced blood supply in tissues due to excessive deposition of fibrin in veins under HS, which causes intestinal hypoxia, ultimately resulting in oxidative stress (Leon et al., 2010). Another reason for this could be enhanced intestinal permeability due to tight junction dysfunction arising from extensive epithelial surface damage under HS (Goo et al., 2019; Lambert, 2009; Santos et al., 2015). In ovo feeding of prebiotics, such as galactooligosaccharides (GOS), has been explored comparatively in additional studies to evaluate its effects on performance, welfare, immunity, antioxidants, and meat quality in chickens. Evidently, in ovo feeding of GOS on the 12th day of incubation reduced antioxidant enzyme expression in chicks when they were subjected to HS at 30.0 °C for 8.5 h on the 32nd day of age (Slawinska et al., 2019). In this case, GOS supplementation during embryogenesis may act as a microbiome stimulator and protective agent to maintain intestinal integrity, decreasing the production of ROS, which is the most harmful sequel of HS. In another study, in ovo feeding of GOS on the 12th day of incubation did not affect the performance and meat quality of slow-growing broilers and showed higher resilience to enhanced temperatures (Tavaniello et al., 2022). Meat quality cannot be ignored because it is a good source of animal protein used for human consumption. In ovo feeding with GOS (3.5 mg/egg) can restore the nutritional value of meat by maintaining the fatty acid content of meat in heat-exposed chickens (Tavaniello et al., 2020).
The role of in ovo probiotic and prebiotic supplementation in immunity modulation cannot be ruled out. In general, the reduction in pathogenic penetration owing to intact intestinal integrity could be one of the major causes of improved immunity in in ovo probiotic-supplemented chicks. The role of genes related to TLR, along with their enhanced expression due to direct correlation with pathogen penetration, has already been explained previously (Goel et al., 2021c). The downregulation of genes related to TLRs during early age after in ovo supplementation with probiotics on the 18th day of incubation confirmed its beneficial effects (Pender et al., 2017). However, the role of interferons and interleukins after in ovo supplementation with probiotics and prebiotics remains unclear.
Pender et al. reported that in ovo supplementation with probiotics (a mixture of Lactobacillus acidophilus, Lactobacillus casei, Enterococcus faecium, and Bifidobacterium bifidum) on the 18th day of incubation was associated with the downregulation of interferon-γ, IL-4, and IL-13 genes in the ileum and cecal tonsils (Pender et al., 2017). In contrast, Alizadeh et al. reported that the expression of interferons and IL-13 genes was not affected when eggs were injected with probiotics (multi-strain lactobacilli mixture) on the 18th day of incubation (Alizadeh et al., 2020). The differences in the results could be attributed to the strain and dosage of probiotics used in these studies. Furthermore, it is critical to identify the effect of gut microbiota modifying products during stress conditions as heat tends to modify immune responses in chickens. A recent study suggested that in ovo prebiotic (GOS) supplementation on the 12th day of incubation tends to restore the enhanced gene expression of interleukins such as IL-2 and IL-4, to normal, which was otherwise increased due to HS (Slawinska et al., 2019). The HS mitigating effects of in ovo GOS feeding could be due to its ability to modulate the stress- and immune-related molecular pathways in slow-growing broilers (Pietrzak et al., 2020). Future studies are required to confirm the role of in ovo microbiota modifying products on interferons and interleukins in chickens.
3.3. Temperature manipulation and in ovo feeding
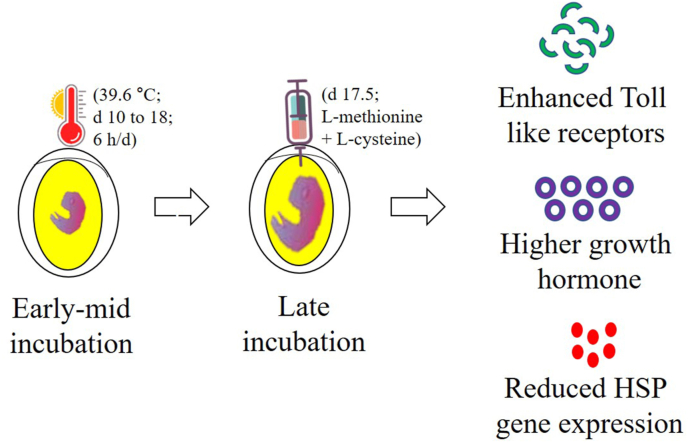
Incubation temperature is critical and any variation during different incubation phases can modify nutrient absorption during embryonic development. This is confirmed by studies demonstrating a decrease in the availability of free amino acids such as leucine, phenylalanine, and lysine in embryonic brain and liver tissues when eggs were incubated at 38.6 °C for 6 h daily from the 10th to 18th day of incubation (Han et al., 2017). Embryonic supplementation can help to meet the requirements for limiting nutrients during incubation. In ovo feeding is the only method used to modify the nutritional status of eggs after laying. Limited information regarding in ovo supplementation and thermal manipulation is available to mitigate the effects of HS in chickens. Several amino acids, such as leucine and methionine, have been investigated in TM before and after hatching. In ovo feeding of L-Leu at the 7th day of incubation reduced plasma T4 levels at hatching and improved thermotolerance by reducing the rectal temperature of 10th day chicks exposed to HS for 2 h (Han et al., 2019). Furthermore, Leu altered its metabolism in embryonic brain and liver tissues, stimulated O2 consumption, heat production, and lipid metabolism during embryogenesis, increased daily body temperature at hatching, increased food intake, and body weight gain, and improved the thermal tolerance of broiler chicks to HS (Han et al., 2017, 2018). In a recent study, in ovo feeding was performed on thermally manipulated embryos and it was found that TM and in ovo GABA feeding independently improved body weight at hatching, while TM regulated the expression of heat shock protein- and antioxidant-related genes in broilers (Goel et al., 2022). In another study, the hepatic expression of HSP genes and antioxidant-related genes was modified in cyclic heat-stressed broilers when embryos were thermally manipulated at 39.6 °C for 6 h daily from the 10th to 18th day and supplemented with in ovo GABA on 17.5th day of incubation (Ncho et al., 2022a). The importance of rectal temperature in heat-stressed chickens has been explained previously. A recent study suggested that rectal temperature decreases at the end of the cyclic heat stress stage, and the combination of thermal manipulation with in ovo GABA supplementation reduces the adverse effects of cyclic heat stress by enhancing the survival rates in broiler chickens (Ncho et al., 2022b). In ovo feeding of methionine on the 18th day of incubation of heat-exposed eggs resulted in enhanced GSH-Px, IGF-I, and TLR4 gene expression, thyroid hormone levels, antioxidant indices, reduced HSP70 and HSP90 gene expression, corticosterone concentrations, and lipid profile, and improved jejunal histomorphology in newly hatched chicks (Elnesr et al., 2019; Elwan et al., 2019). In another study, in ovo feeding of cysteine alone or in combination with lysine improved hatchability and growth performance of broilers when embryos were thermally manipulated at 39.6 °C for 6 h daily from the 10th to 18th day of incubation (Ajayi et al., 2022). These studies suggest that feeding amino acids in ovo can be used to overcome embryonic thermal stress by modifying the oxidative, antioxidant, immune, and metabolic pathways in chickens (Fig. 4). Thus, the role of phytogenic additives in overcoming stress cannot be ignored. During in ovo feeding, black cumin extract improved hatchability, performance, and antioxidant status of broilers when embryos were thermally manipulated at 39.6 °C for 6 h daily from the 10th to 18th day of incubation (Oke et al., 2021). In conclusion, in ovo feeding is attractive and could comprise a future technology that challenges global warming for poultry production.
Fig. 4.
In ovo feeding to thermally manipulated embryos increases toll-like receptors and growth hormone release and decreases the gene expression of heat shock proteins (HSP) in hatchlings. The diagram is drawn based on the articles (Elnesr et al., 2019; Elwan et al., 2019). Embryonic thermal manipulation during mid-term incubation and in ovo supplementation during late-term incubation may enhance hatching performance and reduce oxidative stress parameters in hatchlings. Such chickens are more resistant to heat stress as they possess lower oxidation and stress parameters during rearing conditions. In parentheses are examples of thermal manipulation or in ovo injection.
4. Conclusion
With the continuous improvement in genetic makeup, the productivity and feed efficiency of commercial chickens have greatly improved. Global warming has adversely affected the performance of modern poultry. Manipulation of embryos could be the future for creating heat-resistant chickens reared under high ambient temperatures. Recent methods used for embryonic modulation are useful for mitigating the detrimental effects of HS. Thermal manipulation during incubation helps attain heat tolerance by modifying chicken quality, rectal temperature, oxidative stress, and immune regulation. The lack of knowledge regarding the role of TM in terms of gut integrity, pathogen penetration, and hypophagia during HS requires further investigation. In ovo supplementation modifies nutrient uptake, metabolism, and antioxidant capacity to generate thermotolerance in chickens. In ovo, leucine feeding has been reported to increase prenatal metabolic rates and lipid metabolism (Han et al., 2018). Chicks produced after in ovo feeding with amino acids are expected to have lower body temperature along with higher body weight and feed intake during their early growth phase. Its efficacy during the later stages of HS still needs to be explored and requires further investigation. Furthermore, the expression of HS genes in response to HS needs to be elucidated as contradictory observations often occur. The correlation between pre- and post-hatch implementation requires further precision to enhance its accuracy towards the variability of results present in the different phases of incubation. To date, only a few prebiotics, probiotics, and amino acids have been explored. Additional work is required to fine-tune embryonic modulation for commercial application on farms.
Author contributions
YH Choi: Conceptualization, review, editing, supervision. AGoel: Conceptualization, methodology, investigation, data curation, original draft preparation. CM Ncho: Reviewing and editing. VGupta: Reviewing and editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported in part by the Brain Pool program funded by the Ministry of Science and ICT through the National Research Foundation of Korea (2019H1D3A1A01071142) and by the Forest Science and Technology R&D Program (2020193C10-2022-BA01) provided by Korea Forest Service (Korea Forestry Promotion Institute).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Ajayi O.I., Smith O.F., Oso A.O., Oke O.E. Evaluation of in ovo feeding of low or high mixtures of cysteine and lysine on performance, intestinal morphology and physiological responses of thermal-challenged broiler embryos. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.972041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saffar A., Rose S. Ambient temperature and the egg laying characteristics of laying fowl. World Poult Sci J. 2002;58:317–331. [Google Scholar]
- Al-Zghoul M.B. Thermal manipulation during broiler chicken embryogenesis increases basal mRNA levels and alters production dynamics of heat shock proteins 70 and 60 and heat shock factors 3 and 4 during thermal stress. Poult Sci. 2018;97:3661–3670. doi: 10.3382/ps/pey225. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Alliftawi A.R.S., Saleh K.M.M., Jaradat Z.W. Expression of digestive enzyme and intestinal transporter genes during chronic heat stress in the thermally manipulated broiler chicken. Poult Sci. 2019;98:4113–4122. doi: 10.3382/ps/pez249. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Dalab A.E., Ababneh M.M., Jawasreh K.I., Al Busadah K.A., Ismail Z.B. Thermal manipulation during chicken embryogenesis results in enhanced hsp70 gene expression and the acquisition of thermotolerance. Res Vet Sci. 2013;95:502–507. doi: 10.1016/j.rvsc.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., El-Bahr S.M. Thermal manipulation of the broilers embryos: expression of muscle markers genes and weights of body and internal organs during embryonic and post-hatch days. BMC Vet Res. 2019;15 doi: 10.1186/s12917-019-1917-6. 166-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Saleh K.M., Ababneh M.M.K. Effects of pre-hatch thermal manipulation and post-hatch acute heat stress on the mRNA expression of interleukin-6 and genes involved in its induction pathways in 2 broiler chicken breeds. Poult Sci. 2019;98:1805–1819. doi: 10.3382/ps/pey499. [DOI] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Sukker H., Ababneh M.M. Effect of thermal manipulation of broilers embryos on the response to heat-induced oxidative stress. Poult Sci. 2019;98:991–1001. doi: 10.3382/ps/pey379. [DOI] [PubMed] [Google Scholar]
- Al Wakeel R.A., Shukry M., Abdel A.A., Mahmoud S., Saad M.F. Alleviation by gamma amino butyric acid supplementation of chronic heat stress-induced degenerative changes in jejunum in commercial broiler chickens. Stress. 2017;20:562–572. doi: 10.1080/10253890.2017.1377177. [DOI] [PubMed] [Google Scholar]
- Alhenaky A., Abdelqader A., Abuajamieh M., Al-Fataftah A.R. The effect of heat stress on intestinal integrity and salmonella invasion in broiler birds. J Therm Biol. 2017;70:9–14. doi: 10.1016/j.jtherbio.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Alizadeh M., Shojadoost B., Astill J., Taha-Abdelaziz K., Karimi S.H., Bavananthasivam J., et al. Effects of in ovo inoculation of multi-strain lactobacilli on cytokine gene expression and antibody-mediated immune responses in chickens. Front Vet Sci. 2020;7:105. doi: 10.3389/fvets.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo I.C.S., Café M.B., Noleto R.A., Martins J.M.S., Ulhoa C.J., Guareshi G.C., et al. Effect of vitamin e in ovo feeding to broiler embryos on hatchability, chick quality, oxidative state, and performance. Poult Sci. 2019;98:3652–3661. doi: 10.3382/ps/pey439. [DOI] [PubMed] [Google Scholar]
- Awad E.A., Najaa M., Zulaikha Z.A., Zulkifli I., Soleimani A.F. Effects of heat stress on growth performance, selected physiological and immunological parameters, caecal microflora, and meat quality in two broiler strains. Asian Australas J Anim Sci. 2020;33:778–787. doi: 10.5713/ajas.19.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barri A., Honaker C.F., Sottosanti J.R., Hulet R.M., McElroy A.P. Effect of incubation temperature on nutrient transporters and small intestine morphology of broiler chickens. Poult Sci. 2011;90:118–125. doi: 10.3382/ps.2010-00908. [DOI] [PubMed] [Google Scholar]
- Bhanja S., Sudhagar M., Goel A., Pandey N., Mehra M., Agarwal S., et al. Differential expression of growth and immunity related genes influenced by in ovo supplementation of amino acids in broiler chickens. Czech J Anim Sci. 2014;59:399–408. [Google Scholar]
- Bhanja S.K., Goel A., Pandey N., Mehra M., Majumdar S., Mandal A.B. In ovo carbohydrate supplementation modulates growth and immunity-related genes in broiler chickens. J Anim Physiol Anim Nutr. 2015;99:163–173. doi: 10.1111/jpn.12193. [DOI] [PubMed] [Google Scholar]
- Calik A., Emami N.K., Schyns G., White M.B., Walsh M.C., Romero L.F., et al. Influence of dietary vitamin e and selenium supplementation on broilers subjected to heat stress, part ii: oxidative stress, immune response, gut integrity, and intestinal microbiota. Poult Sci. 2022;101 doi: 10.1016/j.psj.2022.101858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedraz H., Gromboni J.G.G., Garcia A.A.P.J., Farias Filho R.V., Souza T.M., Oliveira E.R., et al. Heat stress induces expression of hsp genes in genetically divergent chickens. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.-H. Effects of γ-aminobutyric acid on mortality in laying hens during summer time. J Agric Life Sci. 2019;53:131–139. [Google Scholar]
- Chowdhury V.S., Tomonaga S., Ikegami T., Erwan E., Ito K., Cockrem J.F., et al. Oxidative damage and brain concentrations of free amino acid in chicks exposed to high ambient temperature. Comp Biochem Physiol A Mol Integr Physiol. 2014;169:70–76. doi: 10.1016/j.cbpa.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Collin A., Berri C., Tesseraud S., Rodón F.E., Skiba-Cassy S., Crochet S., et al. Effects of thermal manipulation during early and late embryogenesis on thermotolerance and breast muscle characteristics in broiler chickens. Poult Sci. 2007;86:795–800. doi: 10.1093/ps/86.5.795. [DOI] [PubMed] [Google Scholar]
- Daghir N. Nutritional strategies to reduce heat stress in broilers and broiler breeders. Lohmann Inf. 2009;44:6–15. [Google Scholar]
- Das R., Mishra P., Jha R. In ovo feeding as a tool for improving performance and gut health of poultry: a review. Front Vet Sci. 2021;8 doi: 10.3389/fvets.2021.754246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Basilio V., Requena F., León A., Vilariño M., Picard M. Early age thermal conditioning immediately reduces body temperature of broiler chicks in a tropical environment. Poult Sci. 2003;82:1235–1241. doi: 10.1093/ps/82.8.1235. [DOI] [PubMed] [Google Scholar]
- Deeb N., Cahaner A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult Sci. 2002;81:293–301. doi: 10.1093/ps/81.3.293. [DOI] [PubMed] [Google Scholar]
- Deeming D.C. Yolk sac, body dimensions and hatchling quality of ducklings, chicks and poults. Br Poult Sci. 2005;46:560–564. doi: 10.1080/00071660500255042. [DOI] [PubMed] [Google Scholar]
- Ek-Von Mentzer B.A., Zhang F., Hamilton J.A. Binding of 13-hode and 15-hete to phospholipid bilayers, albumin, and intracellular fatty acid binding proteins. Implications for transmembrane and intracellular transport and for protection from lipid peroxidation. J Biol Chem. 2001;276:15575–15580. doi: 10.1074/jbc.M011623200. [DOI] [PubMed] [Google Scholar]
- El-Naggar K., El-Kassas S., Abdo S.E., Kirrella A.A.K., Al Wakeel R.A. Role of gamma-aminobutyric acid in regulating feed intake in commercial broilers reared under normal and heat stress conditions. J Therm Biol. 2019;84:164–175. doi: 10.1016/j.jtherbio.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Elnesr S.S., Elwan H.A.M., Xu Q.Q., Xie C., Dong X.Y., Zou X.T. Effects of in ovo injection of sulfur-containing amino acids on heat shock protein 70, corticosterone hormone, antioxidant indices, and lipid profile of newly hatched broiler chicks exposed to heat stress during incubation. Poult Sci. 2019;98:2290–2298. doi: 10.3382/ps/pey609. [DOI] [PubMed] [Google Scholar]
- Elwan H.A.M., Elnesr S.S., Xu Q., Xie C., Dong X., Zou X. Effects of in ovo methionine-cysteine injection on embryonic development, antioxidant status, IGF-I and TLR4 gene expression, and jejunum histomorphometry in newly hatched broiler chicks exposed to heat stress during incubation. Animals. 2019;9:25. doi: 10.3390/ani9010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger M.E., Oldfield J.E., Heinemann W.W. 1990. Feeds and nutrition digest: formerly, feeds and nutrition – abridged. [Google Scholar]
- Ferrer C., Pedragosa E., Torras-Llort M., Parcerisa X., Rafecas M., Ferrer R., et al. Dietary lipids modify brush border membrane composition and nutrient transport in chicken small intestine. J Nutr. 2003;133:1147–1153. doi: 10.1093/jn/133.4.1147. [DOI] [PubMed] [Google Scholar]
- Gao T., Zhao M., Zhang L., Li J., Yu L., Lv P., et al. Effect of in ovo feeding of l-arginine on the hatchability, growth performance, gastrointestinal hormones, and jejunal digestive and absorptive capacity of posthatch broilers. J Anim Sci. 2017;95:3079–3092. doi: 10.2527/jas.2016.0465. [DOI] [PubMed] [Google Scholar]
- Garriga C., Hunter R.R., Amat C., Planas J.M., Mitchell M.A., Moreto M. Heat stress increases apical glucose transport in the chicken jejunum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R195–R201. doi: 10.1152/ajpregu.00393.2005. [DOI] [PubMed] [Google Scholar]
- Goel A., Bhanja S.K., Mehra M., Majumdar S., Mandal A. In ovo silver nanoparticle supplementation for improving the post-hatch immunity status of broiler chickens. Arch Anim Nutr. 2017;71:384–394. doi: 10.1080/1745039X.2017.1349637. [DOI] [PubMed] [Google Scholar]
- Goel A., Bhanja S.K., Mehra M., Mandal A., Pande V. In ovo trace element supplementation enhances expression of growth genes in embryo and immune genes in post-hatch broiler chickens. J Sci Food Agric. 2016;96:2737–2745. doi: 10.1002/jsfa.7438. [DOI] [PubMed] [Google Scholar]
- Goel A., Bhanja S.K., Pande V., Mehra M., Mandal A. Effects of in ovo administration of vitamins on post hatch-growth, immunocompetence and blood biochemical profiles of broiler chickens. Indian J Anim Sci. 2013;83:916–921. [Google Scholar]
- Goel A., Kim B.-J., Ncho C.-M., Jeong C.-M., Gupta V., Jung J.-Y., et al. Dietary supplementation of shredded, steam-exploded pine particles decreases pathogenic microbes in the cecum of acute heat-stressed broilers. Animals. 2021;11:2252. doi: 10.3390/ani11082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Kim B.-J., Ncho C.-M., Jeong C.-M., Gupta V., Jung J.-Y., et al. Utilization of shredded steam-exploded pine particles as a dietary ingredient to modify cecal microbiota in broilers. Agriculture. 2021;11:1196. [Google Scholar]
- Goel A., Ncho C.M., Choi Y.-H. Regulation of gene expression in chickens by heat stress. J Anim Sci Biotechnol. 2021;12 doi: 10.1186/s40104-020-00523-5. 11–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A., Ncho C.M., Jeong C.-M., Choi Y.-H. Embryonic thermal manipulation and in ovo gamma-aminobutyric acid supplementation regulating the chick weight and stress-related genes at hatch. Front Vet Sci. 2022;8 doi: 10.3389/fvets.2021.807450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo D., Kim J.H., Park G.H., Delos Reyes J.B., Kil D.Y. Effect of heat stress and stocking density on growth performance, breast meat quality,and intestinal barrier function in broiler chickens. Animals. 2019;9:107. doi: 10.3390/ani9030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouda A., Amer S.A., Gabr S., Tolba S.A. Effect of dietary supplemental ascorbic acid and folic acid on the growth performance, redox status, and immune status of broiler chickens under heat stress. Trop Anim Health Prod. 2020;52:2987–2996. doi: 10.1007/s11250-020-02316-4. [DOI] [PubMed] [Google Scholar]
- Gul F., Ahmad B., Afzal S., Ullah A., Khan S., Aman K., et al. Comparative analysis of various sources of selenium on the growth performance and antioxidant status in broilers under heat stress. Braz J Biol. 2021;83 doi: 10.1590/1519-6984.251004. [DOI] [PubMed] [Google Scholar]
- Habashy W.S., Milfort M.C., Adomako K., Attia Y.A., Rekaya R., Aggrey S.E. Effect of heat stress on amino acid digestibility and transporters in meat-type chickens. Poult Sci. 2017;96:2312–2319. doi: 10.3382/ps/pex027. [DOI] [PubMed] [Google Scholar]
- Habashy W.S., Milfort M.C., Fuller A.L., Attia Y.A., Rekaya R., Aggrey S.E. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int J Biometeorol. 2017;61:2111–2118. doi: 10.1007/s00484-017-1414-1. [DOI] [PubMed] [Google Scholar]
- Habibian M., Ghazi S., Moeini M.M., Abdolmohammadi A. Effects of dietary selenium and vitamin e on immune response and biological blood parameters of broilers reared under thermoneutral or heat stress conditions. Int J Biometeorol. 2014;58:741–752. doi: 10.1007/s00484-013-0654-y. [DOI] [PubMed] [Google Scholar]
- Han G., Cui Y., Shen D., Li M., Ren Y., Bungo T., et al. In ovo feeding of l-leucine improves antioxidative capacity and spleen weight and changes amino acid concentrations in broilers after chronic thermal stress. Front Vet Sci. 2022;9 doi: 10.3389/fvets.2022.862572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G., Yang H., Bahry M.A., Tran P.V., Do P.H., Ikeda H., et al. L-leucine acts as a potential agent in reducing body temperature at hatching and affords thermotolerance in broiler chicks. Comp Biochem Physiol A Mol Integr Physiol. 2017;204:48–56. doi: 10.1016/j.cbpa.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Han G., Yang H., Bungo T., Ikeda H., Wang Y., Nguyen L.T.N., et al. In ovo L-leucine administration stimulates lipid metabolisms in heat-exposed male, but not female, chicks to afford thermotolerance. J Therm Biol. 2018;71:74–82. doi: 10.1016/j.jtherbio.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Han G., Yang H., Wang Y., Haraguchi S., Miyazaki T., Bungo T., et al. L-leucine increases the daily body temperature and affords thermotolerance in broiler chicks. Asian Australas J Anim Sci. 2019;32:842–848. doi: 10.5713/ajas.18.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison F.E., May J.M. Vitamin c function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009;46:719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Yu Q., He Y., Hu R., Xia S., He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult Sci. 2019;98:6378–6387. doi: 10.3382/ps/pez471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari M., Mohebalian H., Hassanabadi A. The effects of in ovo injection of nanocurcumin and vitamin e on immune responses and growth performance of broiler chickens under heat stress. J Anim Vet Adv. 2021:20. [Google Scholar]
- Hulet R., Gladys G., Hill D., Meijerhof R., El-Shiekh T. Influence of egg shell embryonic incubation temperature and broiler breeder flock age on posthatch growth performance and carcass characteristics. Poult Sci. 2007;86:408–412. doi: 10.1093/ps/86.2.408. [DOI] [PubMed] [Google Scholar]
- Ito K., Erwan E., Nagasawa M., Furuse M., Chowdhury V.S. Changes in free amino acid concentrations in the blood, brain and muscle of heat-exposed chicks. Br Poult Sci. 2014;55:644–652. doi: 10.1080/00071668.2014.957653. [DOI] [PubMed] [Google Scholar]
- Jenkins S.A., Porter T.E. Ontogeny of the hypothalamo-pituitary-adrenocortical axis in the chicken embryo: a review. Domest Anim Endocrinol. 2004;26:267–275. doi: 10.1016/j.domaniend.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Jiang W., Zhang L., Shan A. The effect of vitamin e on laying performance and egg quality in laying hens fed corn dried distillers grains with solubles. Poult Sci. 2013;92:2956–2964. doi: 10.3382/ps.2013-03228. [DOI] [PubMed] [Google Scholar]
- Joseph N.S., Lourens A., Moran E.T., Jr. The effects of suboptimal eggshell temperature during incubation on broiler chick quality, live performance, and further processing yield. Poult Sci. 2006;85:932–938. doi: 10.1093/ps/85.5.932. [DOI] [PubMed] [Google Scholar]
- Kadam M.M., Barekatain M.R., Bhanja S.K., Iji P.A. Prospects of in ovo feeding and nutrient supplementation for poultry: the science and commercial applications – a review. J Sci Food Agric. 2013;93:3654–3661. doi: 10.1002/jsfa.6301. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Hediger M.A. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflug Arch. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- Kang S., Kim D.-H., Lee S., Lee T., Lee K.-W., Chang H.-H., et al. An acute, rather than progressive, increase in temperature-humidity index has severe effects on mortality in laying hens. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.568093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusato M., Yoshida H., Furukawa K., Toyomizu M. Effect of heat stress-induced production of mitochondrial reactive oxygen species on NADPH oxidase and heme oxygenase-1 mRNA levels in avian muscle cells. J Therm Biol. 2015;52:8–13. doi: 10.1016/j.jtherbio.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Kang H.K. Effects of in ovo injection of zinc or diet supplementation of zinc on performance, serum biochemical profiles, and meat quality in broilers. Animals. 2022;12 doi: 10.3390/ani12050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G.P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 2009;87:E101–E108. doi: 10.2527/jas.2008-1339. [DOI] [PubMed] [Google Scholar]
- Leon L.R., Helwig B.G. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109:1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Loyau T., Bedrani L., Berri C., Métayer-Coustard S., Praud C., Coustham V., et al. Cyclic variations in incubation conditions induce adaptive responses to later heat exposure in chickens: a review. Animal. 2015;9:76–85. doi: 10.1017/S1751731114001931. [DOI] [PubMed] [Google Scholar]
- Majidi-Mosleh A., Sadeghi A., Mousavi S., Chamani M., Zarei A. Effects of in ovo infusion of probiotic strains on performance parameters, jejunal bacterial population and mucin gene expression in broiler chicken. Braz J Poult Sci. 2017;19:97–102. [Google Scholar]
- Mashaly M.M., Hendricks G.L., 3rd, Kalama M.A., Gehad A.E., Abbas A.O., Patterson P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- Molenaar R., Reijrink I., Meijerhof R., Van den Brand H. Relationship between hatchling length and weight on later productive performance in broilers. World Poult Sci J. 2008;64:599–604. [Google Scholar]
- Morita V.d.S., de Almeida V.R., Matos J.B.J., Vicentini T.I., van den Brand H., Boleli I.C. Incubation temperature during fetal development influences morphophysiological characteristics and preferred ambient temperature of chicken hatchlings. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154928. e0154928-e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narinç D., Erdoğan S., Tahtabiçen E., Aksoy T. Effects of thermal manipulations during embryogenesis of broiler chickens on developmental stability, hatchability and chick quality. Animal. 2016;10:1328–1335. doi: 10.1017/S1751731116000276. [DOI] [PubMed] [Google Scholar]
- Nawab A., Ibtisham F., Li G., Kieser B., Wu J., Liu W., et al. Heat stress in poultry production: mitigation strategies to overcome the future challenges facing the global poultry industry. J Therm Biol. 2018;78:131–139. doi: 10.1016/j.jtherbio.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Ncho C.-M., Goel A., Jeong C.-M., Gupta V., Choi Y.-H. Effects of in ovo feeding of γ-aminobutyric acid on growth performances, plasma metabolites, and antioxidant status in broilers exposed to cyclic heat stress. Sustainability. 2021;13 [Google Scholar]
- Ncho C.M., Goel A., Gupta V., Jeong C.M., Choi Y.H. Embryonic manipulations modulate differential expressions of heat shock protein, fatty acid metabolism, and antioxidant-related genes in the liver of heat-stressed broilers. PLoS One. 2022;17 doi: 10.1371/journal.pone.0269748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ncho C.M., Goel A., Gupta V., Jeong C.M., Choi Y.H. Impact of embryonic manipulations on core body temperature dynamics and survival in broilers exposed to cyclic heat stress. Sci Rep. 2022;12 doi: 10.1038/s41598-022-19063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ncho C.M., Goel A., Jeong C.M., Youssouf M., Choi Y.H. In ovo injection of gaba can help body weight gain at hatch, increase chick weight to egg weight ratio, and improve broiler heat resistance. Animals. 2021;11 doi: 10.3390/ani11051364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P., Lima M.M., Procopio J., Pithon-Curi T.C., Doi S.Q., Bazotte R.B., et al. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36:153–163. doi: 10.1590/s0100-879x2003000200002. [DOI] [PubMed] [Google Scholar]
- Niu Z.Y., Liu F.Z., Yan Q.L., Li W.C. Effects of different levels of vitamin e on growth performance and immune responses of broilers under heat stress. Poult Sci. 2009;88:2101–2107. doi: 10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- Oh T.Y., Yeo M., Han S.U., Cho Y.K., Kim Y.B., Chung M.H., et al. Synergism of helicobacter pylori infection and stress on the augmentation of gastric mucosal damage and its prevention with alpha-tocopherol. Free Radic Biol Med. 2005;38:1447–1457. doi: 10.1016/j.freeradbiomed.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Oke O.E., Oyelola O.B., Iyasere O.S., Njoku C.P., Oso A.O., Oso O.M., et al. In ovo injection of black cumin (nigella sativa) extract on hatching and post hatch performance of thermally challenged broiler chickens during incubation. Poult Sci. 2021;100 doi: 10.1016/j.psj.2020.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender C.M., Kim S., Potter T.D., Ritzi M.M., Young M., Dalloul R.A. In ovo supplementation of probiotics and its effects on performance and immune-related gene expression in broiler chicks. Poult Sci. 2017;96:1052–1062. doi: 10.3382/ps/pew381. [DOI] [PubMed] [Google Scholar]
- Piestun Y., Harel M., Barak M., Yahav S., Halevy O. Thermal manipulations in late-term chick embryos have immediate and longer term effects on myoblast proliferation and skeletal muscle hypertrophy. J Appl Physiol. 2009;106:233–240. doi: 10.1152/japplphysiol.91090.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piestun Y., Shinder D., Ruzal M., Halevy O., Brake J., Yahav S. Thermal manipulations during broiler embryogenesis: effect on the acquisition of thermotolerance. Poult Sci. 2008;87:1516–1525. doi: 10.3382/ps.2008-00030. [DOI] [PubMed] [Google Scholar]
- Pietrzak E., Dunislawska A., Siwek M., Zampiga M., Sirri F., Meluzzi A., et al. Splenic gene expression signatures in slow-growing chickens stimulated in ovo with galactooligosaccharides and challenged with heat. Animals. 2020;10 doi: 10.3390/ani10030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prows D.R., Murphy E.J., Schroeder F. Intestinal and liver fatty acid binding proteins differentially affect fatty acid uptake and esterification in l-cells. Lipids. 1995;30:907–910. doi: 10.1007/BF02537481. [DOI] [PubMed] [Google Scholar]
- Pruszynska-Oszmalek E., Kolodziejski P.A., Stadnicka K., Sassek M., Chalupka D., Kuston B., et al. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult Sci. 2015;94:1909–1916. doi: 10.3382/ps/pev162. [DOI] [PubMed] [Google Scholar]
- Puthpongsiriporn U., Scheideler S.E., Sell J.L., Beck M.M. Effects of vitamin e and c supplementation on performance, in vitro lymphocyte proliferation, and antioxidant status of laying hens during heat stress. Poult Sci. 2001;80:1190–1200. doi: 10.1093/ps/80.8.1190. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sa L.R., et al. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Rodrigues M.V., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sa L.R., et al. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute hypothalamic-pituitary-adrenal axis activation. J Anim Sci. 2012;90:1986–1994. doi: 10.2527/jas.2011-3949. [DOI] [PubMed] [Google Scholar]
- Rajkumar U., Shanmugam M., Rajaravindra K., Vinoth A., Rao S. Effect of increased incubation temperature on juvenile growth, immune and serum biochemical parameters in selected chicken populations. Indian J Anim Sci. 2015;85:1328–1333. [Google Scholar]
- Rashid A., Sharma S.K., Tyagi J.S., Mohan J., Narayan R., Kolluri G., et al. Incubation temperatures affect expression of nutrient transporter genes in Japanese quail. Asian J Anim Vet Adv. 2016;11:538–554. [Google Scholar]
- Retes P.L., Clemente A.H.S., Neves D.G., Espósito M., Makiyama L., Alvarenga R.R., et al. In ovo feeding of carbohydrates for broilers-a systematic review. J Anim Physiol Anim Nutr. 2018;102:361–369. doi: 10.1111/jpn.12807. [DOI] [PubMed] [Google Scholar]
- Saeed M., Abbas G., Alagawany M., Kamboh A.A., Abd El-Hack M.E., Khafaga A.F., et al. Heat stress management in poultry farms: a comprehensive overview. J Therm Biol. 2019;84:414–425. doi: 10.1016/j.jtherbio.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Sahin K., Kucuk O. Zinc supplementation alleviates heat stress in laying Japanese quail. J Nutr. 2003;133:2808–2811. doi: 10.1093/jn/133.9.2808. [DOI] [PubMed] [Google Scholar]
- Salahi A., Khabisi M.M., Pakdel A., Baghbanzadeh A. Effects of cold stress during transportation on hatchability and chick quality of broiler breeder eggs. Turk J Vet Anim Sci. 2012;36:159–167. [Google Scholar]
- Saleh K.M.M., Tarkhan A.H., Al-Zghoul M.B. Embryonic thermal manipulation affects the antioxidant response to post-hatch thermal exposure in broiler chickens. Animals. 2020:10. doi: 10.3390/ani10010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos R.R., Awati A., Roubos-van den Hil P.J., Tersteeg-Zijderveld M.H., Koolmees P.A., Fink-Gremmels J. Quantitative histo-morphometric analysis of heat-stress-related damage in the small intestines of broiler chickens. Avian Pathol. 2015;44:19–22. doi: 10.1080/03079457.2014.988122. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram R., Wick M., Lilburn M.S. Effect of embryonic thermal manipulation on heat shock protein 70 expression and immune system development in pekin duck embryos. Poult Sci. 2018;97:4200–4210. doi: 10.3382/ps/pey298. [DOI] [PubMed] [Google Scholar]
- Shinde A., Goel A., Mehra M., Rokade J., Bhadauria P., Yadav A., et al. Delayed post-hatch feeding affects the performance and immunocompetence differently in male and female broiler chickens. J Appl Anim Res. 2018;46:306–313. [Google Scholar]
- Shlomo Y. Domestic fowl-strategies to confront environmental conditions. Avian Poult Biol Rev. 2000;11:81–95. [Google Scholar]
- Shokraneh M., Sadeghi A.A., Mousavi S.N., Esmaeilkhanian S., Chamani M. Effects of in ovo injection of nano-selenium and nano-zinc oxide and high eggshell temperature during late incubation on antioxidant activity, thyroid and glucocorticoid hormones and some blood metabolites in broiler hatchlings. Acta Sci Anim Sci. 2020;42 [Google Scholar]
- Sklan D., Geyra A., Tako E., Gal-Gerber O., Uni Z. Ontogeny of brush border carbohydrate digestion and uptake in the chick. Br J Nutr. 2003;89:747–753. doi: 10.1079/BJN2003853. [DOI] [PubMed] [Google Scholar]
- Sklan D., Heifetz S., Halevy O. Heavier chicks at hatch improves marketing body weight by enhancing skeletal muscle growth. Poult Sci. 2003;82:1778–1786. doi: 10.1093/ps/82.11.1778. [DOI] [PubMed] [Google Scholar]
- Slawinska A., Mendes S., Dunislawska A., Siwek M., Zampiga M., Sirri F., et al. Avian model to mitigate gut-derived immune response and oxidative stress during heat. Biosystems. 2019;178:10–15. doi: 10.1016/j.biosystems.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Sohail M.U., Hume M.E., Byrd J.A., Nisbet D.J., Ijaz A., Sohail A., et al. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult Sci. 2012;91:2235–2240. doi: 10.3382/ps.2012-02182. [DOI] [PubMed] [Google Scholar]
- Tavaniello S., Slawinska A., Prioriello D., Petrecca V., Bertocchi M., Zampiga M., et al. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult Sci. 2020;99:612–619. doi: 10.3382/ps/pez556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaniello S., Slawinska A., Sirri F., Wu M., De Marzo D., Siwek M., et al. Performance and meat quality traits of slow-growing chickens stimulated in ovo with galactooligosaccharides and exposed to heat stress. Poult Sci. 2022;101 doi: 10.1016/j.psj.2022.101972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldry M., Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflug Arch. 2004;447:480–489. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ferket P.R., Tako E., Kedar O. In ovo feeding improves energy status of late-term chicken embryos. Poult Sci. 2005;84:764–770. doi: 10.1093/ps/84.5.764. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ferket R. Methods for early nutrition and their potential. World Poult Sci J. 2004;60:101–111. [Google Scholar]
- Uni Z., Gal-Garber O., Geyra A., Sklan D., Yahav S. Changes in growth and function of chick small intestine epithelium due to early thermal conditioning. Poult Sci. 2001;80:438–445. doi: 10.1093/ps/80.4.438. [DOI] [PubMed] [Google Scholar]
- van der Wagt I., de Jong I.C., Mitchell M.A., Molenaar R., van den Brand H. A review on yolk sac utilization in poultry. Poult Sci. 2020;99:2162–2175. doi: 10.1016/j.psj.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinoth A., Thirunalasundari T., Shanmugam M., Uthrakumar A., Suji S., Rajkumar U. Evaluation of DNA methylation and mRNA expression of heat shock proteins in thermal manipulated chicken. Cell Stress Chaperones. 2018;23:235–252. doi: 10.1007/s12192-017-0837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Edens F.W. Heat conditioning induces heat shock proteins in broiler chickens and Turkey poults. Poult Sci. 1998;77:1636–1645. doi: 10.1093/ps/77.11.1636. [DOI] [PubMed] [Google Scholar]
- Wright E.M., Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004 Feb;447(5):510–518. doi: 10.1007/s00424-003-1063-6. Epub 2003 May 14. Erratum in: Pflugers Arch. 2004 Feb;447(5):813-5. PMID: 12748858. [DOI] [PubMed] [Google Scholar]
- Yahav S., Rath R.S., Shinder D. The effect of thermal manipulations during embryogenesis of broiler chicks (gallus domesticus) on hatchability, body weight and thermoregulation after hatch. J Therm Biol. 2004;29:245–250. [Google Scholar]
- Yahav S., Shamay A., Horev G., Bar-Ilan D., Genina O., Friedman-Einat M. Effect of acquisition of improved thermotolerance on the induction of heat shock proteins in broiler chickens. Poult Sci. 1997;76:1428–1434. doi: 10.1093/ps/76.10.1428. [DOI] [PubMed] [Google Scholar]
- Yalcin S., Siegel P.B. Exposure to cold or heat during incubation on developmental stability of broiler embryos. Poult Sci. 2003;82:1388–1392. doi: 10.1093/ps/82.9.1388. [DOI] [PubMed] [Google Scholar]
- Yang L., Tan G.-Y., Fu Y.-Q., Feng J.-H., Zhang M.-H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ros production and lipid peroxidation in broiler chickens. Comp Biochem Physiol C Toxicol Pharmacol. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Zaboli G.R., Rahimi S., Shariatmadari F., Torshizi M.A., Baghbanzadeh A., Mehri M. Thermal manipulation during pre and post-hatch on thermotolerance of male broiler chickens exposed to chronic heat stress. Poult Sci. 2017;96:478–485. doi: 10.3382/ps/pew344. [DOI] [PubMed] [Google Scholar]
- Zhang Z.W., Bi M.Y., Yao H.D., Fu J., Li S., Xu S.W. Effect of cold stress on expression of AMPKalpha-PPARalpha pathway and inflammation genes. Avian Dis. 2014;58:415–426. doi: 10.1637/10763-010814-Reg.1. [DOI] [PubMed] [Google Scholar]
- Zhu Y.F., Li S.Z., Sun Q.Z., Yang X.J. Effect of in ovo feeding of vitamin c on antioxidation and immune function of broiler chickens. Animal. 2019;13:1927–1933. doi: 10.1017/S1751731118003531. [DOI] [PubMed] [Google Scholar]
- Zulkifli I., Che Norma M.T., Israf D.A., Omar A.R. The effect of early age feed restriction on subsequent response to high environmental temperatures in female broiler chickens. Poult Sci. 2000;79:1401–1407. doi: 10.1093/ps/79.10.1401. [DOI] [PubMed] [Google Scholar]