Abstract
Introduction
The objective of this systematic literature review was to evaluate the available literature concerning the clinical, economic, and patient-reported benefits of insulin pen platforms, including connected insulin pens/caps/sleeves and insulin platforms, as well as mobile apps capable of receiving near real-time insulin dosing information.
Methods
Medline and Embase databases and the Cochrane Library were searched for published literature between January 2015 and May 2021, and manual searches for conference abstracts from 2018 to May 2021 were performed. These searches were supplemented by internet searches for relevant literature and clinical trials. Study selection involved the population, intervention, comparator, outcomes, time frame, and study design outline. Included studies investigated connected insulin systems or connected caps/sleeves enabling pens to be connected, or apps able to connect to these systems, in individuals of all ages with type 1 or type 2 diabetes mellitus.
Results
Searches identified a total of 26 publications (mostly observational studies and conference abstracts) for inclusion, representing ten unique, predominantly small studies. Evidence in this field is still in its early stages, and only two randomized controlled trials met our inclusion criteria. Available results showed that connected insulin pens and their systems potentially helped reduce suboptimal insulin use and may therefore improve glycemic control. Satisfaction of people with diabetes with the technologies used was high, and economic benefits were noted. Features of effective connected insulin pen devices include simplicity of use and data upload/sharing, useful “point-of-care” alerts, and simple and understandable data presentation to facilitate more effective consultations.
Conclusions
Connected insulin pen systems could be increasingly considered as part of routine clinical care for insulin-treated persons with diabetes who must manage the complexity of their daily insulin routine. Future research focusing on the way data obtained from these devices can be most effectively used alongside other information is urgently needed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02478-1.
Keywords: Connected insulin pens, Diabetes mellitus, Digital health, Economics, Insulin infusion systems, Patient-reported outcomes, Treatment outcome
Plain Language Summary
Digital health tools, like text message reminders and mobile apps, are being used more often to help people with diabetes improve their health in a way that works for them. For people who take insulin to treat their diabetes, what has been missing is a way to track insulin doses alongside other diabetes information in an app. Connected insulin pens, also called smart pens, are able to do this. In this article we have looked at the evidence available on the benefits of connected insulin pens. We found that while information on connected insulin pens is limited at the moment, what there is shows that using a connected insulin pen can help people remember to take their insulin and give themselves the right dose and that those who have used a connected insulin pen or related technology are happy with it. Useful features of connected insulin pens include being easy to use, having an alert function, and being able to share the insulin information with the user’s doctor. Connected insulin pens may also reduce diabetes-related costs. Connected insulin pens are likely to become more common for people with diabetes who take insulin, but there is a need for more research on how best to use them to improve the treatment of people with diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02478-1.
Key Summary Points
| Digital health tools, such as text message reminders, mobile apps, web-based glucose monitoring programs, and innovative insulin delivery platforms, are being increasingly used in diabetes, but an important “missing” aspect has been automatic capture of insulin dose data and integration of that data with other diabetes-related information |
| Connected insulin pens, also called smart pens, are simple to use and can record injections, provide a reminder for injections, facilitate accurate dosing, and often receive and integrate other data; through these functions, connected insulin pen systems may improve clinical decision-making, facilitate proactive, patient-centered management of diabetes, and improve outcomes |
| While evidence in this field is still in its early stages, studies report that connected insulin pens and their systems potentially help to reduce suboptimal insulin dosing and the number of missed insulin doses and improve measures of glycemic control |
| Available economic data suggest that the use of connected insulin pens and systems is potentially cost saving |
| Patient satisfaction with the technologies is high; features of effective connected insulin pen devices include simplicity of use and data upload/sharing, alert functions, and simple and clear data presentation |
Introduction
The International Diabetes Federation estimates that 537 million people were living with diabetes mellitus in 2021 and predicts that this will increase to 783 million by 2045 [1]. Diabetes is associated with a substantial economic burden on society [2]. People with diabetes also experience considerable burden associated with their disease, including pain, anxiety, inconvenience, and loss of health-related quality of life (QoL) [3, 4].
Achieving glycemic control is the cornerstone of disease management in diabetes [5, 6], with glycemic targets difficult to achieve, especially for those treated with insulin [7–9]. Evidence suggests that, despite numerous advances in insulin innovations, adequate glucose control in individuals with diabetes occurs less frequently than seen for other metabolic targets, such as blood pressure [10] or lipid levels [11]. In people requiring insulin therapy, key elements of this “failure” are barriers to appropriate dose adjustment and insulin omission/non-adherence (either intentional or unintentional); as such, these are important issues to address to improve diabetes management [12, 13]. Patient-centered care and engagement of the person with diabetes in self-care are increasingly important in the management of diabetes, and consideration of factors and preferences of the person with diabetes is crucial when tailoring treatment [6, 14].
Advice provided to a person with diabetes to help with their self-management is dependent on the quality of information, constructed from the data available to the clinician at each consultation. Thus, accurate and timely digital data collection can be a driver of more effective consultations and therefore better outcomes.
Digital health is the use of digital technologies and accessible data to help people achieve higher standards of health and access services to promote healthy lives and enhanced well-being [15]. The aims of digital health are to enhance the efficacy of health care delivery, reduce the burden on the person with diabetes, increase the personalization of treatment, and improve outcomes [16]. Digital health tools for diabetes are being increasingly used and include text message reminders, mobile applications (apps), web-based glucose monitoring programs, and innovative insulin delivery platforms [17, 18]. An important “missing” aspect of the digital health map in diabetes has been a mechanism to automatically capture insulin dose data and integrate these data with other diabetes-related information using an app or computer application. Connected insulin pens, also called smart pens, fill this void. They are simple to use and, depending on the product, can also record injections (timing and amount of insulin delivered), transmit data, assist patients with real-time insulin dosing, and provide downloadable reports that can be reviewed by the patient’s healthcare provider so that insulin dose can be adjusted if needed [19]; in addition, they can provide injection reminders, and, when used with a phone app or computer-based portal, can often receive and integrate other data (such as glycated hemoglobin [HbA1c] levels, glucose levels, exercise, and lifestyle factors) either manually or automatically. Through providing objective, integrated data, connected insulin pen systems may improve clinical decision-making and facilitate proactive, patient-centered management of diabetes, resulting in improved glycemic control and clinical outcomes as well as reduced health care costs [17, 18, 20, 21].
In addition to insulin pens, there are also pen caps and sleeves that can be used with a non-connected insulin pen to turn it into a connected device, and insulin platforms, which are websites or online portals capable of integrating with the connected pens to receive and/or transmit data from the pens. As availability and uptake of these technologies are growing, and digital solutions are increasingly being asked for by patients, payers and healthcare professionals, the number of publications on this topic is rapidly expanding. However, much of the literature on this topic is commentaries and expert opinions, and payers and healthcare professionals need primary clinical study results to make recommendations to stakeholders and treatment decisions with patients. The aim of this systematic literature review was to objectively evaluate the clinical, economic, and patient-reported benefits of insulin pens, including connected insulin caps/sleeves and insulin platforms, as well as mobile apps capable of receiving near real-time insulin dosing information in primary clinical studies of children and adults with T1DM or T2DM. This review will help clinicians, patients, and payers form opinions regarding connected insulin systems and enhance decision making regarding the use of these systems.
Methods
This systematic literature review was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions [22], the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23], and the systematic review methods specified by the National Institute for Health and Care Excellence (NICE) in Sect. 2.1 of the Single Technology Appraisal user guide [24]. This analysis used data from previously published studies already in the public domain; as such, ethics committee approval was not required.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Information Sources
Database Search
A published literature search of the Medline and Embase databases and the Cochrane Library was performed, via the Ovid platform, between January 2015 and May 2021. The start date of 2015 was chosen because before this time connected insulin pens were not available for general use. The Cochrane Library search included the Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews, and Database of Abstracts of Reviews of Effects (DARE); thus, all commonly used databases were searched to ensure the search was as broad as possible.
The literature search included disease- and intervention-related keywords; intervention-related keywords included general terms and specific brand names for the systems and apps of interest (Supplementary Material Table S1). The searches were limited to the period January 1, 2015, to May 26, 2021, and to English language articles. No geographic limits were applied.
Conference Proceedings and Clinical Trial Registries
Relevant conference abstracts published from 2018 to May 26, 2021, were identified through manual searches of conference archives from the following organizations: American Diabetes Association (ADA); Advanced Technologies & Treatments for Diabetes (ATTD); European Association for the Study of Diabetes (EASD); International Diabetes Federation (IDF); International Society for Pediatric and Adolescent Diabetes (ISPAD); International Society for Pharmacoeconomics and Outcomes Research (ISPOR); and American Association of Diabetes Educators (AADE—now known as the Association of Diabetes Care & Education Specialists [ADCES]).
To capture all relevant ongoing, discontinued, or completed clinical trials, ClinicalTrials.gov and the European Union Clinical Trials Register were searched. No time limits were applied to these searches.
Gray Literature and Unpublished Clinical Trial Data
A manual search was performed for literature related to outcome-based contracts, local intervention-specific gray literature, and presentations at local encore conferences. In these analyses, gray literature was defined as any literature in the public domain that was presented outside of peer-reviewed conferences or journals. Data from unpublished clinical trials sponsored by Eli Lilly and Co. were also included. An English language restriction was not applied to the gray literature.
Inclusion and Exclusion Criteria
Population, Intervention, Comparator, Outcomes, Time frame, Study design (PICOTS) outline was generally followed to select relevant studies for inclusion in this systematic literature review. Included studies were those that investigated connected insulin systems or connected caps/sleeves enabling pens to be connected, or apps able to connect to these systems, in individuals of all ages with type 1 or type 2 diabetes mellitus. Limits were not imposed on the type of devices so that the range of options available to study participants could be considered. Studies were included regardless of the comparator system or device (connected, nonconnected, or no comparator). Clinical trials of all types, questionnaires/surveys relating to real-world clinical practice, studies of the preference/satisfaction of people with diabetes, and economic evaluations were included. Any systematic reviews/meta-analyses found in the searches were checked for relevant references only.
Outcomes of interest included clinical outcomes (HbA1c, postprandial blood glucose, glycemic control, continuous glucose monitoring [CGM] outcomes, hypoglycemic events, insulin dose and missed doses, and long-term cardiovascular events), economic outcomes (direct and indirect costs and health care resource utilization), and patient-reported outcomes (PROs; QoL, utility, satisfaction, preference and empowerment, self-efficacy, and distress of the person with diabetes). Other outcomes included outcome-based contracts, adherence, health care provider preference or satisfaction, diabetes management, and attitudes toward technology.
Publications were excluded from the analysis if they were animal studies; if the main intervention of interest was nonconnected insulin pens, insulin pumps, oral treatments, or non-insulin injectables, or on-demand treatment/no planned regimen; if the insulin dosing data were blinded to participants, or if outcomes were any other than those listed in the inclusion criteria. Full details of the inclusion/exclusion criteria are listed in Supplementary Material Table S2.
Selection Process
The DistillerSR tool (Evidence Partners, Ottawa, Canada) was used for the screening process. DistillerSR is cloud-based literature review software, which manages the citations throughout the review. Screening consisted of an abstract review and a full-text review. Once citations were uploaded to DistillerSR, titles and abstracts were reviewed by two independent reviewers based on the eligibility criteria, with any conflicts resolved using a third independent reviewer. All citations included at the end of the abstract review were retained and downloaded for full-text review. Two independent reviewers assessed the full text against the eligibility criteria, using the same method for resolving reviewer conflict as for the abstract review. All publications included after the completion of the full-text review were retained for data extraction.
Data Extraction
The data extraction forms in DistillerSR were used for data extraction. For each included study, one reviewer entered the study design, characteristics of the people with diabetes, and outcomes of interest into the data extraction form in DistillerSR. The data were exported in Microsoft Excel for storage. Data extraction was quality checked by an independent reviewer.
Assessment of Quality and Bias
Study quality was assessed for randomized controlled trials (RCTs) and cohort studies only. RCTs were assessed using the NICE quality assessment checklist, which considers factors including randomization, blinding, similarity of baseline characteristics between groups, imbalances in study discontinuations, and the type of analysis [25]. Cohort studies were assessed using the Newcastle-Ottawa Scale [26]. Three factors were considered to score the quality of the included studies: selection; comparability; outcomes assessment.
Since this analysis did not include a network meta-analysis, no formal assessment of bias was performed. Comments on bias in publications describing real-world studies were noted.
Results
Study Selection
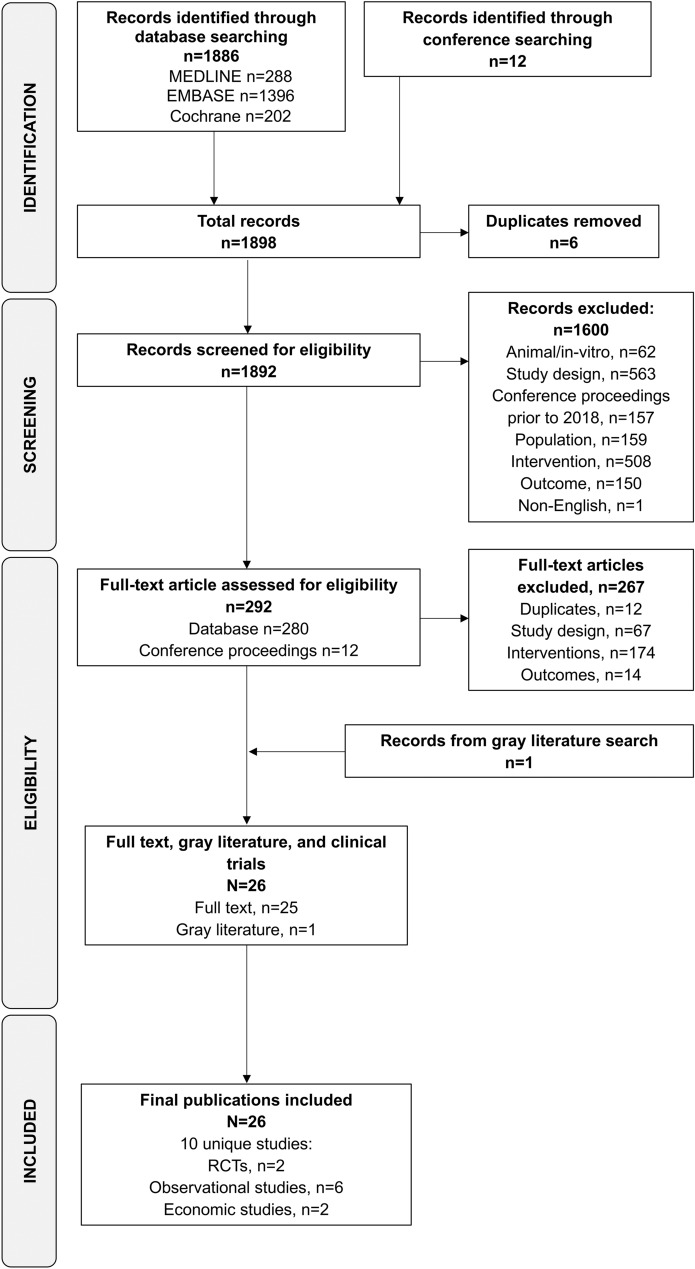
The original and updated database searches identified a total of 1886 publications of connection-enabled insulin platforms and their comparators, while the searches of conference proceedings identified 12 relevant conference abstracts (Fig. 1). Of these, 292 were retained for full-text screening; 267 were excluded, leaving 25 for inclusion. The gray literature search identified one report of interest [27]. Therefore, the final number of publications included was 26, representing 10 unique studies (Fig. 1).
Fig. 1.
PRISMA flow diagram of study selection. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-analyses, RCT randomized controlled trial
A search of the European Union’s Clinical Trials Register did not identify any relevant studies; however, the search of ClinicalTrials.gov identified 24 records of completed (n = 12), ongoing (n = 6), or discontinued/unknown (n = 6) trials of interest, all but one of which were discarded (no results, n = 22; duplicate publications, n = 1). One clinicaltrials.gov entry with a data presented as a conference abstract was used for additional information on one of the included RCTs [28, 29]. One Eli Lilly clinical study report was also included to support one of the included observational studies [30, 31].
Study Characteristics and Interventions/Comparators
Study design details of the ten unique studies are summarized in Supplementary Material Table S3. Two open-label RCTs were identified, both of which assessed a connected insulin cap (Insulclock®); one was fully published [32] and one was a conference abstract [28, 29]. The six observational studies included were prospective cohort studies that assessed connected caps (Insulclock®, GoCap®), connected insulin pens (NovoPen® 6; Bravo pen®, InPen®), or a connected insulin platform (ESYSTA®). Of these, one subgroup analysis (evaluating NovoPen® 6) was fully published [33], four, with an additional subgroup analysis, were conference abstracts [31, 34–37], and the one evaluating a connected insulin platform (ESYSTA®) was a white paper [27]. These core publications were associated with several supporting publications [32, 38–50]. Features of the devices evaluated in these studies are summarized in Table 1.
Table 1.
Features of connection-enabled devices included in this review
| Intervention | Insulclock® | GoCap/Glucose meter | NovoPen 6® | Bravo® pen | InPen® | ESYSTA® |
|---|---|---|---|---|---|---|
| Type of device | Cap | Cap | Reusable pen | Pen | Reusable pen | Platform for reusable pen |
| Mobile app connectivity |
✓ (via Bluetooth) |
✓ (via Bluetooth) |
✓ (via Near Field Communication) |
✓ (via Bluetooth) |
✓ (via Bluetooth) |
|
| Displays last no. of units administered | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Monitors active insulin on-board | ✗ | ✓ | ✓ | ✓ | ✗ | |
| Collects and stores insulin injection dataa | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Insulin injection reminder | ✓ | ✓ | ✗ | ✓ | ✗ | |
| Bolus dose calculator | ✗ | ✗ | ✓ | ✗ | ||
| Integrates with CGM and reports glucose levels | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Mealtime/amount data can be input | ✓ | ✓ | ✗ | ✓ | ✓ | |
| Downloadable reports |
✓ (Insulin units data) |
✓ |
✓ (Insulin dose, time, and date) |
✓ | ✓ |
✓ (Insulin dose, time, and date) |
| Compatible insulins/pens |
KwikPen (insulin lispro), FlexTouch (insulin detemir, aspart [including faster-acting aspart], or degludec) SoloSTAR (insulin glargine) |
Insulin glargine, glulisine | Insulin detemir, aspart (including faster-acting aspart), degludec | Insulin lispro, aspart (including faster-acting aspart) | Any major insulin brand including NPH and premix | |
| Insulin/insulin pen used in identified studies |
KwikPen (insulin lispro) [32] |
SoloSTAR (insulin glargine) | Basal and/or bolus insulinb | Insulin lispro | Not specified | Conventional insulin therapy (ICT or MDI) or insulin pens |
✓ provides data; ✗ does not provide data; CGM continuous glucose monitoring, ICT intensified conventional therapy, MDI multiple daily injection, NPH neutral protamine hagedorn
aIncludes the date, time of day, dose, type of insulin, temperature, and/or duration of insulin injections
bThe majority of participants with a basal insulin pen used insulin degludec (n = 21), with one participant using insulin detemir; of participants with a bolus insulin pen, a total of 79 used insulin aspart, with one participant using human insulin and one participant using faster-acting insulin aspart
Of the two economic studies, one assessed a connected insulin pen (NovoPen® 6) and was fully published [51] with a conference abstract [47] and the other investigated the connected insulin platform (ESYSTA®) and was a conference abstract [52].
The outcomes investigated in all the studies are summarized in Supplementary Material Table S4.
Characteristics of the Participants with Diabetes
Baseline characteristics of the populations of people with diabetes are summarized in Supplementary Material Table S5. Of the studies evaluating Insulclock®, two included people with type 1 diabetes mellitus (T1DM) [32, 36] and one included people with type 2 diabetes mellitus (T2DM) [29]. Single studies evaluated GoCap®, Bravo pen®, InPen®, and ESYSTA® in people with T1DM or T2DM [27, 31, 34, 35], and two parts of one study [40] evaluated NovoPen® 6 (one in adults with T1DM and the other in children and adolescents with T1DM) [33, 37].
Across all studies, people with T2DM were generally older than those with T1DM. Baseline demographics and disease characteristics were infrequently reported in these studies. In studies conducted in adults, 39–56% of people were female and mean age was 39–60 years. Current use of CGM was reported in six studies (Supplementary Material Table S5) [31–35, 37].
Clinical and PROs
Connected Caps
In RCTs, clinical outcomes indicate that the introduction of connected insulin caps resulted in improved glycemic outcomes (Table 2; details are provided in Supplementary Material Table S6) [29, 32]. In 16 people with T1DM, Insulclock® was associated with significant improvement from baseline in CGM metrics in the overall (active use plus masked use) population, including time in range (TIR), time above range (TAR), and glycemic variability (p < 0.05 for all) and mistimed insulin doses (p = 0.032) [32]. There was a numerically greater improvement in HbA1c from baseline to 4 weeks in the overall and active-use populations. In the ten people with full use of the Insulclock®, significant improvements from baseline were observed for TIR and glycemic variability (p < 0.05 for both). Of note, this study showed no difference in clinical outcomes between groups using the Insulclock® device with or without reminders and app alerts (active and masked groups, respectively).
Table 2.
Results of clinical studies evaluating connection-enabled insulin platforms
| Intervention/comparator | Study design/population | Study aim | Method of use | Glycemic/insulin-related outcomes | Patient-reported outcomes |
|---|---|---|---|---|---|
| Connected caps | |||||
| Insulclock® [32] | RCT, open-label, parallel/16 people with T1DM | Clinical impact on glycemic control and treatment compliance and satisfaction with use of device with acoustic and visual plus the Insulclock® app (integrated information on insulin doses, glucose levels, and injection time reminders; active) vs. device with access to dose, time, and duration of injections, without reminders or the Insulclock® app (masked) | Not reported |
Glycemic control improved: TIR increased over time in total study population and active group Glucose SD decreased over time in total study population and masked group Treatment compliance improved: No. of missed and mistimed insulin doses decreased in total study population at end-of-study vs. baseline |
Treatment satisfaction (relating to avoidance hypoglycemia and interference of insulin regimen with work or school) increased in the total study population |
| Insulclock® [28, 29]a | RCT, open-label, crossover/80 people with T2DM | Clinical impact on glycemic control and treatment adherence and satisfaction with use of device with reminders (active) vs. device without feedback (masked) | Not reported |
Glycemic control improved: HbA1c improved vs. baseline in both groups Blood glucose parameters improved vs. baseline (group[s] not specified) Treatment adherence unchanged |
Treatment satisfaction the same with vs. without reminders |
| Insulclock® [36]a | Prospective observational/8 people with T1DM | Impact on duration of insulin injection and treatment satisfaction with use of device with reminder (injection time < 6 s) vs. device without reminder | Not reported | No. of injections < 6 s in duration reduced after vs. before reminder activated | Treatment satisfaction, with self-perceived benefit shown (not specified whether before vs. after reminder or overall) |
| GoCap/Glucose meter [34]a | Prospective, observational/20 people with T1DM or T2DM | Acceptability of device used with connected blood glucose meter to people with diabetes | Insulin dose and SMBG data downloaded for longitudinal tracking | Device easy to use, convenient and useful for diabetes management; allowed access to longitudinal automatically uploaded data by the person with diabetes and HCP | |
| Connected insulin pens | |||||
| NovoPen® 6 [33, 37, 40] | Prospective observational/94 adult people with T1DM | Influence of device on insulin regimen management and glycemic control in people using CGM in a real-world setting; the device was used with injection data overview blinded (could still see CGM data and dose of last injection; baseline) vs. unblinded | Person with diabetes and HCP downloaded, discussed and acted upon pen and CGM findings at each consultation (pen data could not be downloaded outside the HCP’s office) |
Glycemic control improved vs. baseline: TIR increasedb Time in hyperglycemia decreasedb Time in hypoglycemia (glucose < 3 mmol/L) decreasedb Bolus insulin dose increasedb Number of MDB decreasedc |
Not reported |
| Prospective observational/39 children/ adolescents with T1DM | Glycemic parameters following introduction of a connected insulin pen in a pediatric population | Not reported |
Risk of hypoglycemia reduced vs. baseline: Hypoglycemia incidence reduced Time in hypoglycemia reduced TIR unaffected Time in hyperglycemia unaffected Basal insulin dose increased/bolus insulin dose unaffected |
Not reported | |
| InPen® [35]a | Prospective, real-world, observational/482 people with T1DM or T2DM (USA) [conference paper] | Real-world clinical effect of device on key short-term glycemic control in people using CGM | Not reported |
Glycemic control improved vs. before device use: TIR not reduced TBR reduced |
Not reported |
| Bravo® pen [30, 31]a | Prospective, observational/68 people with T1DM or T2DM | Effect on MBD of device with blinded rtCGM vs. unblinded rtCGM | Not reported | Number of MBD reduced with use of rtCGM vs. no use of rtCGM | More likely to have a higher level of confidence in hypoglycemia, less fear of hypoglycemia, increased health problem-solving skills and have a lower illness perception with use of rtCGM vs. baseline |
| Connected insulin platform | |||||
| ESYSTA® [27] | Prospective, observational/215 people with T1DM or T2DM | Impact of device on care of people with poor metabolic control despite specialist support and acceptance of device by people with diabetes and HCPs | Interaction and communication between the people with diabetes and HCP was improved, with simplified documentation; the overview of blood glucose and insulin values was improved for both the person with diabetes and with HCP; self-management was improved |
Glycemic control improved vs. baseline: HbA1c reduced Blood glucose profile improved No increase in hypoglycemic episodes |
Person with diabetes’ and HCP’s views of the device were positive Most people with diabetes and HCPs would recommend the device to others |
CGM continuous glucose monitoring, HbA1c glycated hemoglobin, HCP health care professional, MBD missed bolus dose, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, RCT randomized controlled trial, rtCGM real-time CGM, SD standard deviation, SMBG self-monitoring of blood glucose, TBR time below range, TIR time in range
aConference abstract
bData obtained from 14 people with diabetes
cData obtained from 81 people with diabetes
In the RCT of 80 people with T2DM [28, 29], there was a significant improvement in HbA1c and glycemic variability from baseline to 12 weeks with the use of Insulclock®. People with T2DM reported a slightly higher number of hypoglycemic events during the study period with full use of the Insulclock® device compared to the masked device (29 vs. 25). However, the number of severe hypoglycemic events was equal in both arms (n = 1) [29].
PROs were reported in both RCTs evaluating Insulclock® (Table 2; details are provided in Supplementary Material Table S7). Overall, connected insulin caps were associated with improvement in the satisfaction of people with diabetes. In one RCT, people with T1DM reported improvements from baseline in most items of the Insulin Treatment Satisfaction Questionnaire (ITSQ) after 4 weeks of Insulclock® use, with the largest improvements observed for the items “perception of insulin treatment interference in work/school activities” (p < 0.05 in overall, active, and masked groups) and “potential of current insulin treatment for avoiding severe hypoglycemic episodes” (p < 0.05 in the masked group) [32]. People with T2DM were equally satisfied with Insulclock® with or without feedback, as assessed by the Diabetes Treatment Satisfaction Questionnaire change (DTSQc) survey [29]. Treatment adherence in this study (number of insulin injection irregularities) was reported to be similar for the device with and without feedback (Supplementary Material Table S7).
Similarly, improvements in PROs were observed after introduction of the Insulclock® or the GoCap® in observational studies (Table 2; details are provided in Supplementary Material Table S7). People with T1DM using Insulclock® reported a general benefit at follow-up as assessed by the ITSQ score [36]. Most people with T1DM or T2DM using the Bluetooth-enabled GoCap® found the cap was easy to use and useful in the management of their diabetes [34].
Connected Insulin Pens
Overall, results of observational studies in people with T1DM or T2DM showed improvements in clinical outcomes after introduction of a connected insulin pen (Table 2; details are provided in Supplementary Material Table S6). Use of NovoPen® 6 improved various clinical outcomes, such as HbA1c, missed bolus dose (MBD), TIR, time below range (TBR), and TAR, from baseline and/or blinded use periods (p < 0.05) in adults with T1DM or T2DM [33]. When evaluated in children and adolescents with T1DM, use of the NovoPen® 6 was associated with a significant decrease in incidence of hypoglycemia (p < 0.001) and TBR (p = 0.03) at 12 months [37].
Adults and children with T1DM or T2DM who used the InPen® also reported a significant decrease in TBR 60 days post-InPen® initiation compared with 60 days pre-initiation (p = 0.0002), without a significant decrease in TIR [35].
People with T1DM or T2DM using the Bravo® pen plus blinded or unblinded CGM had fewer MBDs per day, fewer days with MBDs per month, a higher percentage of daily time in TIR, and lower HbA1c levels during the unblinded CGM study period when compared with baseline and to the blinded CGM study period [30, 31]. In this study, people with diabetes were more likely to have a higher confidence in hypoglycemia (measured by the Hypoglycemic Confidence Scale), less fear of hypoglycemia (measured by the Adult Low Blood Sugar Survey), increased health problem-solving skills (measured by the Hypoglycaemia Problem-Solving Scale), and lower illness perception (measured by the Problem Recognition in Illness Self-Management survey) during the unblinded real-time CGM study period than at baseline (Table 2; details are provided in Supplementary Material Table S7) [30, 31]. However, these improvements in PROs, measured using a variety of tools, were not significant.
Connected Insulin Platform
In people with T1DM or T2DM who used the connected insulin system ESYSTA®, there was a significant reduction in HbA1c from baseline to 15 months (p < 0.001), without an increase in the number of hypoglycemic events or insulin dose [27]. These people also had improved QoL as measured by the Short-Form 36, with the greatest improvements observed in “physical pain” and “emotional role function” domains (Table 2; details are provided in Supplementary Material Table S7). The majority of people with diabetes (84%) and health care providers (93%) reported they would recommend the platform to others, and > 75% of people with diabetes continued using the system after the study [27].
Economic Outcomes
The two studies reporting relevant data indicated that use of connected pens and systems is potentially cost saving from an economic perspective (Supplementary Material Table S8) [51, 52].
In people with T1DM, the direct and indirect medical costs per person with diabetes were lower by €11,309 (Swedish Krona [SEK]124,270) and €34,009 (SEK373,725), respectively, for NovoPen 6® versus a nonconnected standard of care over a person with diabetes’ lifetime [51]. Higher treatment costs (due to a higher bolus insulin dose) with the connected insulin pen were offset by a lower cost of complications compared with standard of care. In the cost-effectiveness analysis, NovoPen 6® was a dominant treatment option relative to standard care from a health care payer and a societal perspective. The results of the base-case analysis were supported by the results of the sensitivity analysis, where changes to input parameters and assumptions did not affect the dominance of NovoPen 6® over standard care.
Using Markov model simulations, the use of the connected ESYSTA® platform by people with T1DM or T2DM was associated with €1238.85 per person savings when HbA1c was reduced by 2% (from 8 to 6%) in Germany [52]. The potential savings with the use of ESYSTA® accumulated to up to €31,852.48 for an individual with diabetes over time (time period not specified in the report).
Assessment of RCT and Observational Study Quality
Quality assessments were conducted on fully published studies only, so that enough information was available to complete the assessment.
Using the NICE checklist, the published RCT of Gomez-Peralta and colleagues [32] was ascertained to be of low quality. The study was unclear regarding randomization and similarity of baseline characteristics between groups. There were imbalances between groups in study discontinuations and the authors did not specify how discontinuations were handled [32].
The quality of the two fully published cohort studies, assessed using the Newcastle-Ottawa Scale, was ascertained to be fair [33]. The studies were largely select groups, and none of the studies were truly representative of the general population. However, in these studies, secured records were used for the ascertainment of exposure. The studies were generally small with a limited number of people with diabetes. Follow-up of the cohorts was considered to be adequately long, and all the subjects were accounted for in the assessment of outcomes.
Discussion
This systematic literature review aimed to evaluate clinical, patient-reported, and economic benefits of connected insulin pen devices for T1DM and T2DM. Technologies examined included connected insulin pens/caps/sleeves, connected insulin platforms, and associated mobile applications that can receive insulin dosing data. The review revealed that there were limited primary clinical studies in this field and that although the findings were heterogeneous; generally, the studies showed the potential benefits of connected insulin pens. Given the heterogeneous nature of the findings, we further discuss them in the context of current standard of care and clinical practice.
A total of 10 unique studies were identified: 2 RCTs; 6 observational studies; 2 economic studies in populations of 9 to 482 people with diabetes. The devices identified provide a range of options for data collection and delivery to those with diabetes. A system which facilitates both day-to-day self-care and consultation/discussion with health care professionals to improve the effectiveness of therapy is the “gold standard,” as these two aspects of diabetes care will have varying importance to each individual.
Connected insulin pens and their systems were shown to potentially help reduce suboptimal insulin dosing, and positive results were seen in both children and adults. Studies demonstrate that these systems may help individuals with diabetes to relieve the burden of insulin treatment and to reduce the number of missed bolus injections, which leads to better glycemic control. Satisfaction of people with diabetes with the technologies used was high, suggesting a potential for longer term use of these systems; indeed, one study reported that > 75% of people with diabetes continued to use the ESYSTA® platform after the end of the study [27]. People with diabetes reported increased confidence with and less fear of managing hypoglycemia and, importantly, increased health-related problem-solving skills and understanding of their diabetes goals while using a connected insulin pen [31]. While these differences were not significant, results suggest that further study into PROs associated with connected diabetes systems may provide further positive insights into how these systems ease the emotional burden of diabetes for affected people.
While the current studies show a positive trend toward clinical, economic, and patient-reported benefits, it should be kept in mind when interpreting results that there is a limited number of studies, and most are small in scale. Moreover, the majority of studies were observational and may be subject to bias as a result. However, given that these observational studies reflect real-world experience, they may offer more robust data for evaluation and decision-making regarding digital diabetes tools [53]. This review also highlighted the differences in PROs used across studies, leading to an inconsistency in outcome measures for all aspects of using connected insulin pens. Finally, for each type of technology, only a few studies were available, with inconsistent reporting of outcomes, and not all types of available technology were represented. These deficiencies in the evidence base for connected insulin systems mean there is opportunity for studies in this area to fill those gaps. Of note, the ClinicalTrials.gov search identified several ongoing studies, which suggests that the field is quickly developing in terms of research, and once these studies are complete the evidence base for connected insulin platforms will strengthen, enabling stronger conclusions to be made. The literature search was conducted in May 2021 for studies conducted from 2015, using the common literature databases to ensure full coverage of the available published literature. Since connected pens were not available before 2015, this is the earliest date that relevant publications would be available. This combined with searches of all relevant conferences and the clinical trial databases makes our search comprehensive; however, that the searches were conducted in 2021 is a limitation, because any publications published after this date will not be included. To mitigate the possibility of missing more recent publications, we conducted a non-systematic review of the literature to date, which has shown that no new studies published after May 2021 would meet the criteria for selection. Clearly, there is a need for more primary clinical studies in this field.
When treating diabetes, clinicians must balance the appropriate and timely advancing of insulin treatment with minimizing the risk of overtreatment, often relying on short-term information recalled by the person with diabetes, paper diaries, and incomplete digital information. Devices that can store, transfer, and share data in real time can help overcome these limitations. Features of effective connected insulin pen devices include simplicity of use and data upload/sharing, useful “point-of-care” alerts, and simple and understandable data presentation to facilitate more effective consultations. Of note, current connected insulin pens store only limited information, such as dose and time of dose; dose reminders, bolus calculators, reports, and insulin on board are functions of the associated apps. The expectations of digital health in diabetes are that it will improve outcomes with respect to glucose control (reducing glycemic variability and hypoglycemic events), result in better adherence, provide decision support by allowing people with diabetes and their health care providers to look at trends over time, improve the well-being of the person with diabetes, and reduce disease-related burden on the person with diabetes [18, 54, 55]. The results of the present review support some of these expectations regarding glycemic control and PROs, but few studies elaborated on how the data obtained from the device were used in clinical practice, and as already noted, further investigation is needed. Currently, there are few studies investigating adherence and hypoglycemia rates in people with diabetes using connected diabetes tools, highlighting a need for studies into these outcomes. In addition, some studies included in this review reported unexpected results, such as the studies included herein that did not show substantial differences in outcomes according to the presence or absence of reminders/device feedback [29, 32]. As it would be expected that reminders and/or device feedback would improve outcomes, perhaps further data will provide clarity on this issue.
The participants of the International Panel on Diabetes Digital Technologies highlighted several opportunities for digital health in diabetes, including the potential for a virtual diabetes clinic capable of monitoring health status, addressing concerns, and guiding therapy decisions, where the person with diabetes can collaborate directly with their health care professional [56]. However, obstacles to this kind of approach remain; barriers identified by the panel include lack of interoperability and data compatibility, issues of data ownership and accessibility, deficiencies in health care provider reimbursement and insurance coverage for people with diabetes, and finally a lack of supporting evidence for this kind of approach—something identified by the current review.
Conclusions
Although evidence in this field is still in its early stages, with only two RCTs identified, indications suggest that connected insulin pen systems may reduce suboptimal insulin use and the number of missed insulin doses, potentially leading to better glycemic control in insulin-treated people with diabetes. The available data show that the satisfaction of people with diabetes with connected insulin pen systems is high, and this may lead to improved adherence over time. These connected insulin pen systems could, therefore, be increasingly considered as part of routine clinical care for insulin-treated persons with diabetes who must manage the complexity of their daily insulin routine. Future research focusing on the way data obtained from these devices can be most effectively used alongside other information (e.g., glucose levels, carbohydrate intake, exercise, stress, and illness) is an urgent clinical priority.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study, the journal’s rapid service fee and the open access fee were funded by Eli Lilly and Company.
Medical Writing and Editorial Assistance
The authors acknowledge Rx Communications (Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.
Prior Presentation
This study was presented as a poster at ISPOR-EU 2021: Jamdade V, Liao B, Newson R. Systematic Literature Review of Clinical, Economic, and Patient-Reported Benefits of Connected Insulin Pen Systems. Value Health.2021; 25 (1):S31 (POSB30).
Authorship
All named authors meet the International Committee of Medial Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Birong Liao has made substantial contributions to the study conception, the interpretation of data, and critical revision of the manuscript. Rachel S. Newson has made substantial contributions to the study conception, the design of the work, the interpretation of data, the drafting and critical revision of the manuscript. Vinayak Jamdade has made substantial contributions to the design of the work, the acquisition, analysis, and interpretation of data, the drafting and critical revision of the manuscript. Iain Cranston has made substantial contributions to the study conception, the analysis and interpretation of data, and critical revision of the manuscript.
Disclosures
Birong Liao and Rachel S. Newson are employees of Eli Lilly and Company and are shareholders in the company. Vinayak Jamdade is an employee of Eli Lilly and Company. Iain Cranston: Speaker Panels and/or Clinical Advisory Boards for Novo Nordisk, Eli Lilly and Company, Astra Zeneca, BI, Abbott Diabetes Care, Ascensia, Roche (AccuChek), Insulet (Omnipod) and Dexcom UK. Directorships: Southern Diabetes Medical Services LLP/The AGP Clinical Academy Ltd.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 10th edition. 2021. https://diabetesatlas.org/atlas/tenth-edition/. Accessed Oct 5, 2022.
- 2.Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besancon S, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108072. doi: 10.1016/j.diabres.2020.108072. [DOI] [PubMed] [Google Scholar]
- 3.Ong WM, Chua SS, Ng CJ. Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study. Patient Prefer Adherence. 2014;8:237–246. doi: 10.2147/PPA.S57567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. World J Diabetes. 2017;8(4):120–129. doi: 10.4239/wjd.v8.i4.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61–S70. doi: 10.2337/dc19-S006. [DOI] [PubMed] [Google Scholar]
- 6.Davies M, D’Alessio D, Fradkin J, Kernan W, Mathieu C, Mingrone G. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;2018(61):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 7.Juarez DT, Ma C, Kumasaka A, Shimada R, Davis J. Failure to reach target glycated a1c levels among patients with diabetes who are adherent to their antidiabetic medication. Popul Health Manag. 2014;17(4):218–223. doi: 10.1089/pop.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NgassaPiotie P, Webb EM, Rheeder P. Suboptimal control for patients with type 2 diabetes in the Central Chronic Medicine Dispensing programme in South Africa. Afr J Prim Health Care Fam Med. 2021;13(1):e1–e7. doi: 10.4102/phcfm.v13i1.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes-Walker DJ, Abraham MB, Chee M, Jones TW, group A Glycaemic outcomes in Australasian children and adults with Type 1 Diabetes: failure to meet targets across the age spectrum. Intern Med J. 2021 doi: 10.1111/imj.15426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ. Evaluation of the cascade of diabetes care in the United States, 2005–2016. JAMA Intern Med. 2019;179(10):1376–1385. doi: 10.1001/jamainternmed.2019.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang M, Wang D, Coresh J, Selvin E. Trends in Diabetes Treatment and Control in U.S. Adults, 1999–2018. N Engl J Med. 2021;384(23):2219–2228. doi: 10.1056/NEJMsa2032271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peyrot M, Barnett A, Meneghini L, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29(5):682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peyrot M, Barnett A, Meneghini L, Schumm-Draeger PM. Factors associated with injection omission/non-adherence in the Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabetes Obes Metab. 2012;14(12):1081–1087. doi: 10.1111/j.1463-1326.2012.01636.x. [DOI] [PubMed] [Google Scholar]
- 14.National Institute of Health and Care Excellence (2015) Type 2 diabetes mellitus in adults: management. NICE Guideline 28.
- 15.World Health Organization (2021) Digital health. Geneva, 2021. https://www.who.int/health-topics/digital-health#tab=tab_1. Accessed Aug 4, 2021
- 16.Christian J, Dasgupta N, Jordan M, Juneja M, Nilsen W, Reites J (2018) Digital health and patient registries: today, tomorrow, and the future. In: Gliklich RE DN, Leavy MB, et al. (ed) 21st century patient registries: registries for evaluating patient outcomes: a user’s guide: 3rd edition, Addendum [Internet]. Agency for Healthcare Research and Quality (US), Rockville (MD). [PubMed]
- 17.Drincic A, Prahalad P, Greenwood D, Klonoff DC. Evidence-based mobile medical applications in diabetes. Endocrinol Metab Clin North Am. 2016;45(4):943–965. doi: 10.1016/j.ecl.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyengar V, Wolf A, Brown A, Close K. Challenges in diabetes care: can digital health help address them? Clin Diabetes. 2016;34(3):133–141. doi: 10.2337/diaclin.34.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association Professional Practice Committee 7. Diabetes technology: standards of medical care in diabetes–2022. Diabetes Care. 2022;45(Suppl 1):S97–S112. doi: 10.2337/dc22-S007. [DOI] [PubMed] [Google Scholar]
- 20.Shan R, Sarkar S, Martin SS. Digital health technology and mobile devices for the management of diabetes mellitus: state of the art. Diabetologia. 2019;62(6):877–887. doi: 10.1007/s00125-019-4864-7. [DOI] [PubMed] [Google Scholar]
- 21.Silva BM, Rodrigues JJ, de la Torre DI, López-Coronado M, Saleem K. Mobile-health: a review of current state in 2015. J Biomed Inform. 2015;56:265–272. doi: 10.1016/j.jbi.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2. Chichester: John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence. Single technology appraisal: user guide for company evidence submission template. London; 2017. https://www.nice.org.uk/process/pmg24/chapter/instructions-for-companies. Accessed July 20, 2021.
- 25.National Institute for Health and Care Excellence. Appendix C: Methodology checklist: randomised controlled trials. London; 2012. https://www.nice.org.uk/process/pmg6/resources/the-guidelines-manual-appendices-bi-2549703709/chapter/appendix-c-methodology-checklist-randomised-controlled-trials. Accessed Jul 20, 2021.
- 26.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON; 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Jul 20, 2021.
- 27.Emperra - Digital Diabetes Care. Scientific evaluation of the ESYSTA® S-T-A-R-T project. Potsdam, 2016. https://www.emperra.com/wp-content/uploads/2017/02/esysta_start_whitepaper_en.pdf. Accessed Jul, 2020.
- 28.Clinicaltrials.gov. A randomized study to evaluate the efficacy of Insulclock® in patients with uncontrolled type 2 diabetes (NCT03224234). Bethesda, MD; 2021. https://clinicaltrials.gov/ct2/show/results/NCT03224234?term=insulclock&draw=2&rank=1. Accessed Sep 16, 2021.
- 29.Ramos C, Galindo RJ, Alam MM, Cardona S, Albury BS, Oladejo O, et al. 996-P: A randomized study to evaluate the efficacy of Insulclock pen device in insulin-treated patients with uncontrolled type 2 diabetes. Diabetes. 2020;69(Suppl 1):996-P. doi: 10.2337/db20-996-P. [DOI] [Google Scholar]
- 30.Eli Lilly and Company . Clinical Study Report: OQV_LY8888AT (Bravo Pen) Indianapolis: Eli Lilly and Company; 2018. [Google Scholar]
- 31.Polonsky W, Johnson J, Wolpert H, He X, Kao CY, Meadows E, et al. Missed insulin bolus doses before and after the introduction of rtCGM: the influence of hypoglycaemic fear. Paper presented at the EASD Annual Meeting, 16–20 September. 2019; Barcelona, Spain
- 32.Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, Cruz-Bravo M, Maria-Sanchez C, Poza G, et al. Efficacy of Insulclock in patients with poorly controlled type 1 diabetes mellitus: a pilot, randomized clinical trial. Diabetes Technol Ther. 2020;22(9):686–690. doi: 10.1089/dia.2019.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adolfsson P, Hartvig NV, Kaas A, Moller JB, Hellman J. Increased time in range and improved insulin adherence after introduction of a smart connected insulin pen. Diabetes Technol Ther. 2020;22:709–718. doi: 10.1089/dia.2019.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amante DJ, O'Brien D, Harlan DM, Thompson MJ. Evaluation of patient acceptability of the gocap insulin pen smart cap dose tracking device. J Diabetes Sci Technol. 2018;12(2):426–532. doi: 10.1177/1932296818761742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith M, Gaetano A, Thanasekaran S, Heungyong IG, Lewis J. Smart insulin pens improve time below range in multiple daily insulin therapy. J Manag Care Spec Pharm. 2021;26(10-a):S35. [Google Scholar]
- 36.Gomez-Peralta F, Abreu APC, Gomez-Rodriguez sara S, Cruz-Bravo M, Elvira A. Improving insulin injection technique and patient satisfaction with Insulclock. Diabetes Technol Ther. 2019 doi: 10.1089/dia.2019.2525.abstracts. [DOI] [Google Scholar]
- 37.Adolfsson P, Bjornsson V, Hartvig N, Kaas A, Moller J, Lange E. Reduced number of hypoglycaemic events observed in children after introducing connected insulin pens. Diabetes Technol Ther. 2020;22(Supplement 1):A-1-A-250. doi: 10.1089/dia.2020.2525.abstracts. [DOI] [Google Scholar]
- 38.Adolfsson P, Hartvig NV, Kaas A, Knudsen NN, Mardby AC, Hellman J. 1076-P: Increased time-in-range (TIR) observed after introduction of a connected insulin pen. Diabetes. 2019;68(Suppl 1):1076-P. doi: 10.2337/db19-1076-P. [DOI] [Google Scholar]
- 39.Adolfsson P, Hartvig NV, Kaas A, Knudsen NN, Mardby AC, Moller JB, et al. 126-LB: Improved insulin adherence after introduction of a smart connected insulin pen. Diabetes. 2019;68(Supplement 1):126-LB. doi: 10.2337/db19-126-LB. [DOI] [Google Scholar]
- 40.Catrina S-B, Hartvig NV, Kaas A, Moller J, MÅRdby A-CM, Jendle JH. Type 1 diabetes: Real-world insulin injection patterns [abstract 782]. Paper presented at the Virtual EASD Annual Meeting, 21–25 Sep; 2020.
- 41.Edwards SS, He X, Johnson J, Meadows E, Wang W, Wolpert H, et al. 375-P: Missed bolus doses (MBDs) are associated with reduced time-in-range (TIR): the influence of hypoglycemic fear. Diabetes. 2020;69(Supplement 1):375-P. doi: 10.2337/db20-375-P. [DOI] [Google Scholar]
- 42.Edwards SS, He X, Johnson J, Meadows ES, Wang W, Wolpert H, et al. Key differences with hypoglycaemic fear in people using insulin: the association with missed bolus doses exists for T2D, but not T1D. Diabetologie und Stoffwechsel. 2021;16(Suppl 01):S16–S17. [Google Scholar]
- 43.Edwards SS, Johnson J, Howard W, He X, Kao CY, Meadows E, et al. 83-LB: Missed insulin bolus doses are associated with hypoglycemic fear. Diabetes. 2019;68(Supplement 1):83-LB. doi: 10.2337/db19-83-LB. [DOI] [Google Scholar]
- 44.Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, Cruz-Bravo M, Alcarria E. Insulclock: a novel insulin delivery optimization and tracking system. Diabetes Technol Ther. 2019;21(Supplement 1):A-69. doi: 10.1089/dia.2019.2525.abstracts. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, Ruiz L. Insulclock: a novel insulin delivery optimization and tracking system. Diabetes Technol Ther. 2019;21(4):209–214. doi: 10.1089/dia.2018.0361. [DOI] [PubMed] [Google Scholar]
- 46.Hartvig NV, Hellman J, Kaas A, Knudsen NN, Mardby AC, Moller JB, et al. Improved insulin adherence after introduction of a smart connected insulin pen [abstract 795]. Paper presented at the EASD Annual Meeting, 16–20 September; 2019. Barcelona, Spain.
- 47.Hunt B, Ericsson Å, Gundgaard J, Møller JB, Valentine WJ, Jendle J. Evaluating the long-term cost-effectiveness of introducing a smart insulin pen in standard-of-care treatment of type 1 diabetes in Sweden [abstract 788]. Paper presented at the Virtual EASD Annual Meeting, 21–25 September; 2020.
- 48.Jendle JH, Hartvig NV, Kaas A, Moller J. MÅRdby A-CM, Catrina S-B. 975-P: Effect of late bolus injections on glycemic variability studied by connected pens. Diabetes. 2020;69(Supplement 1):975-P. doi: 10.2337/db20-975-P. [DOI] [Google Scholar]
- 49.Kaas A, Hartvig NV, Hellman J, Knudsen NN, Mardby AC, Adolfsson P. Increased time in range observed after introduction of a connected insulin pen. Paper presented at the EASD Annual Meeting, 16–20 September; 2019. Barcelona, Spain.
- 50.Rodbard D, He X, Wang W, et al. Use of connected insulin pen to evaluate the effects of pre-meal, delayed, missed, and correction boluses on prandial glucose control in T1D and T2D. Diabetes Technol Ther. 2021;22(Supplement):A-44-A-45. [Google Scholar]
- 51.Jendle J, Ericsson A, Gundgaard J, Moller JB, Valentine WJ, Hunt B. Smart insulin pens are associated with improved clinical outcomes at lower cost versus standard-of-care treatment of type 1 diabetes in Sweden: a cost-effectiveness analysis. Diabetes Ther. 2021;12(1):373–388. doi: 10.1007/s13300-020-00980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van de Sand L, Schildt J, Thun S. Health economic analysis of the telemedicine based ESYSTA®-System with a connected smart insulin pen – potential monetary savings from using an integrated diabetes management system. Diabetes Technol Ther. 2019;21(Suppl 1):T1. [Google Scholar]
- 53.Dhruva SS, Ross JS, Desai NR. Real-world evidence: promise and peril for medical product evaluation. P T. 2018;43(8):464–472. [PMC free article] [PubMed] [Google Scholar]
- 54.Kerr D, King F, Klonoff DC. Digital health interventions for diabetes: everything to gain and nothing to lose. Diabetes Spectr. 2019;32(3):226–230. doi: 10.2337/ds18-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sangave NA, Aungst TD, Patel DK. Smart connected insulin pens, caps, and attachments: a review of the future of diabetes technology. Diabetes Spectr. 2019;32(4):378–384. doi: 10.2337/ds18-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phillip M, Bergenstal RM, Close KL, Danne T, Garg SK, Heinemann L, et al. The digital/virtual diabetes clinic: the future is now-recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146–154. doi: 10.1089/dia.2020.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.